Near-Infrared Responsive Composites of Poly-3,4-Ethylenedioxythiophene with Fullerene Derivatives
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. PEDOT Synthesis in the Presence of Fullerenes
2.3. Preparation and Characterization Techniques
3. Results and Discussion
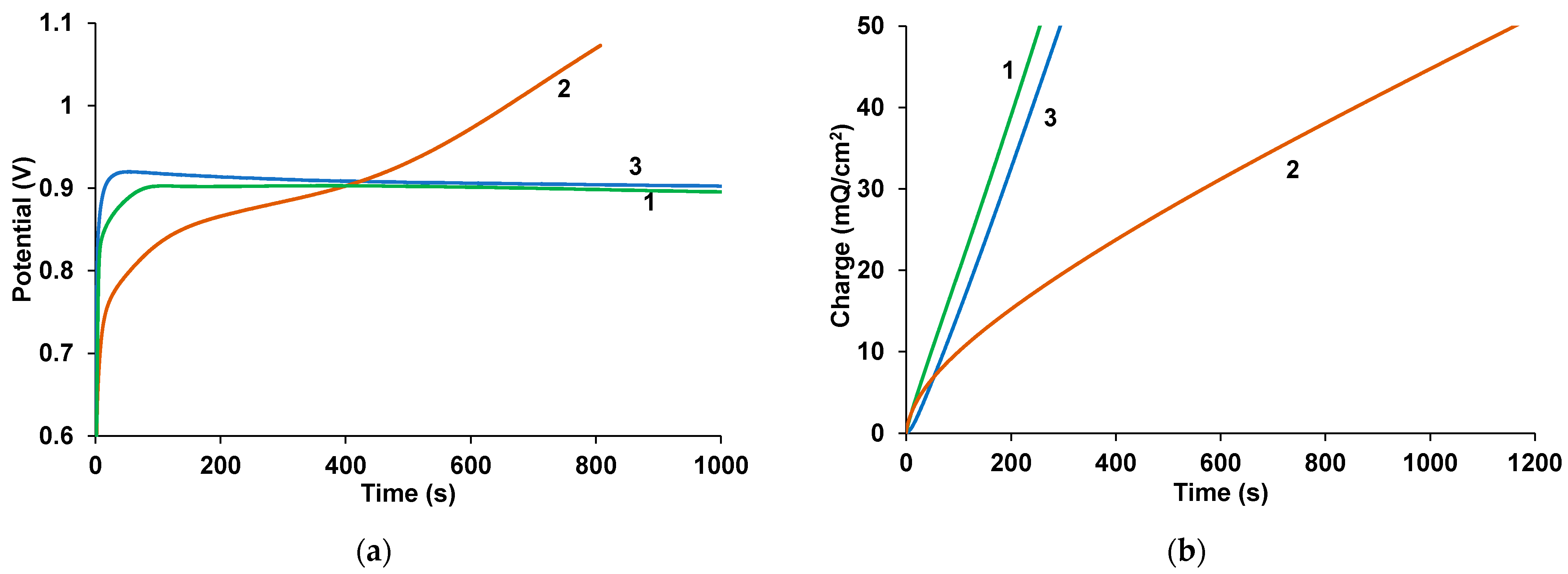
3.1. Electropolymerization of EDOT in the Presence of Fullerenes
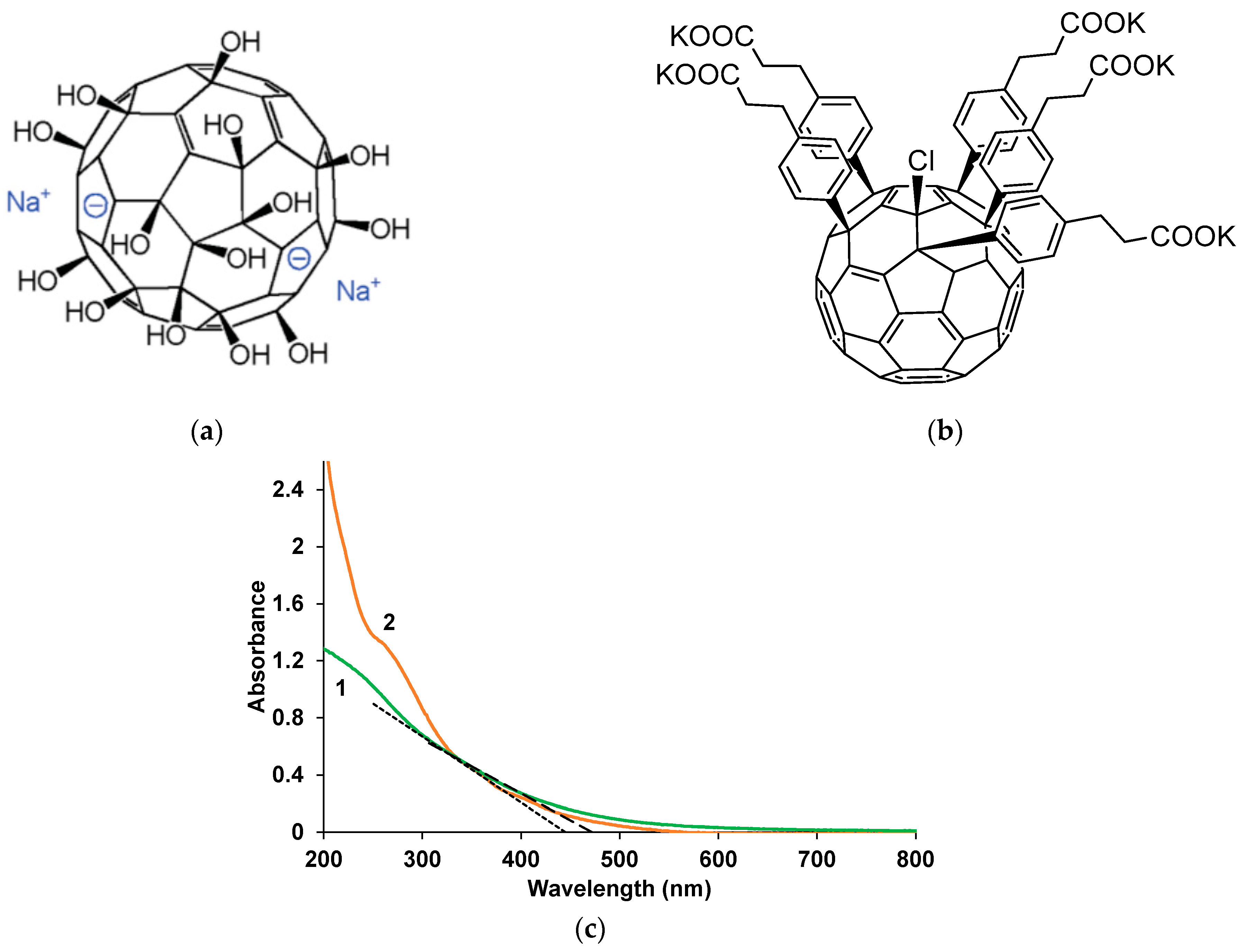
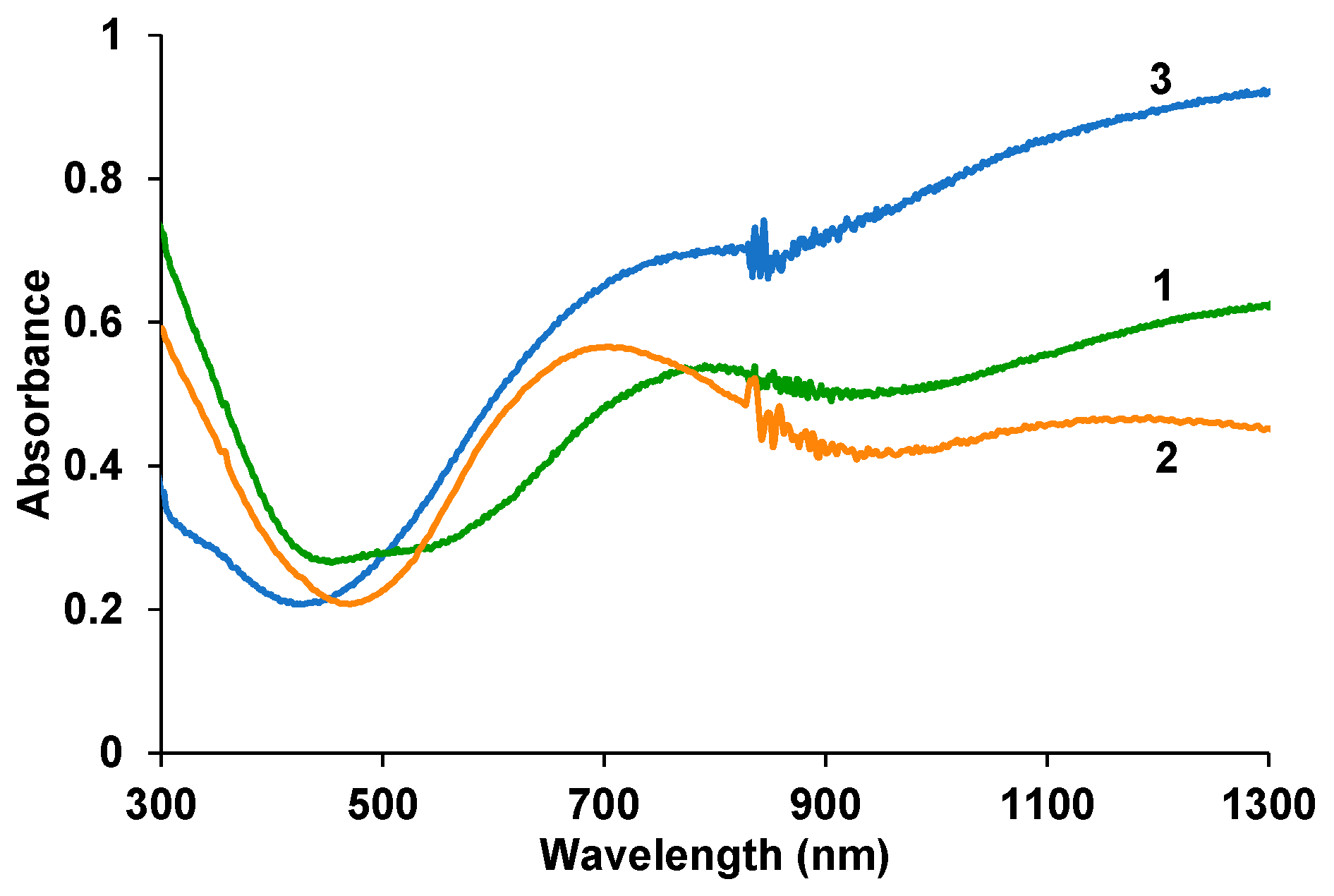
3.2. Electron Absorption Spectroscopy of Composite Films in the UV-Visible and Near-IR Regions
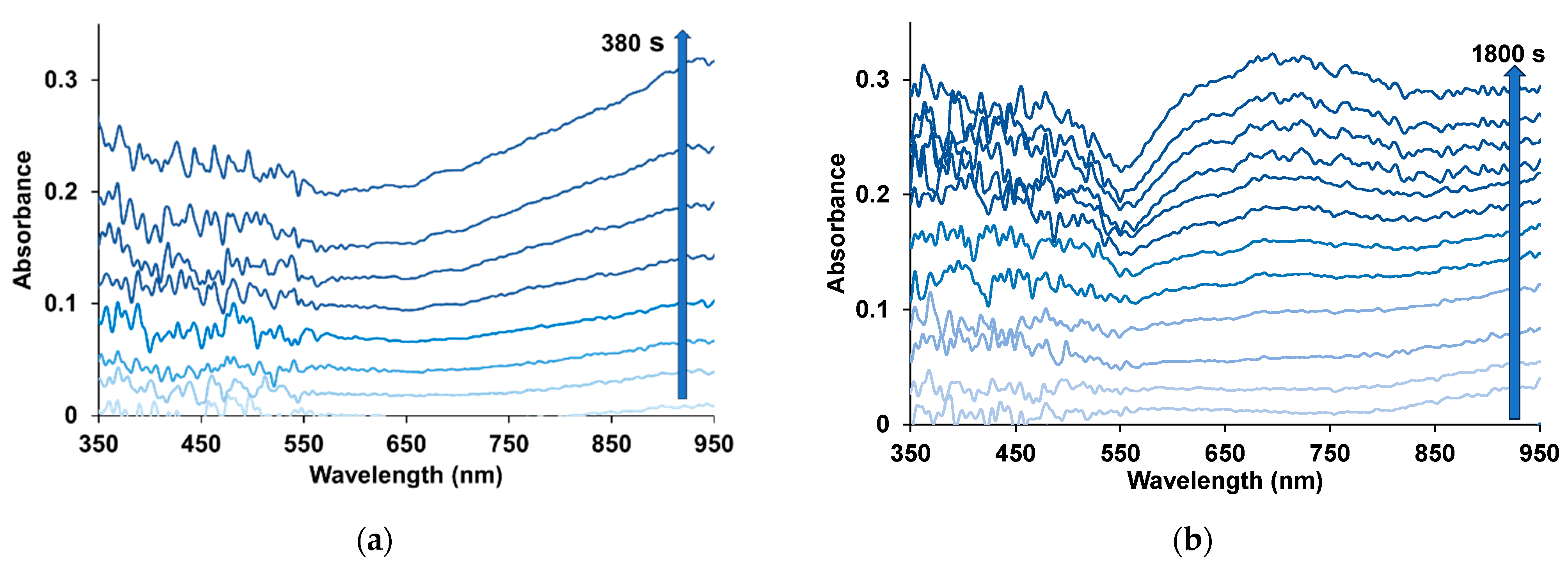
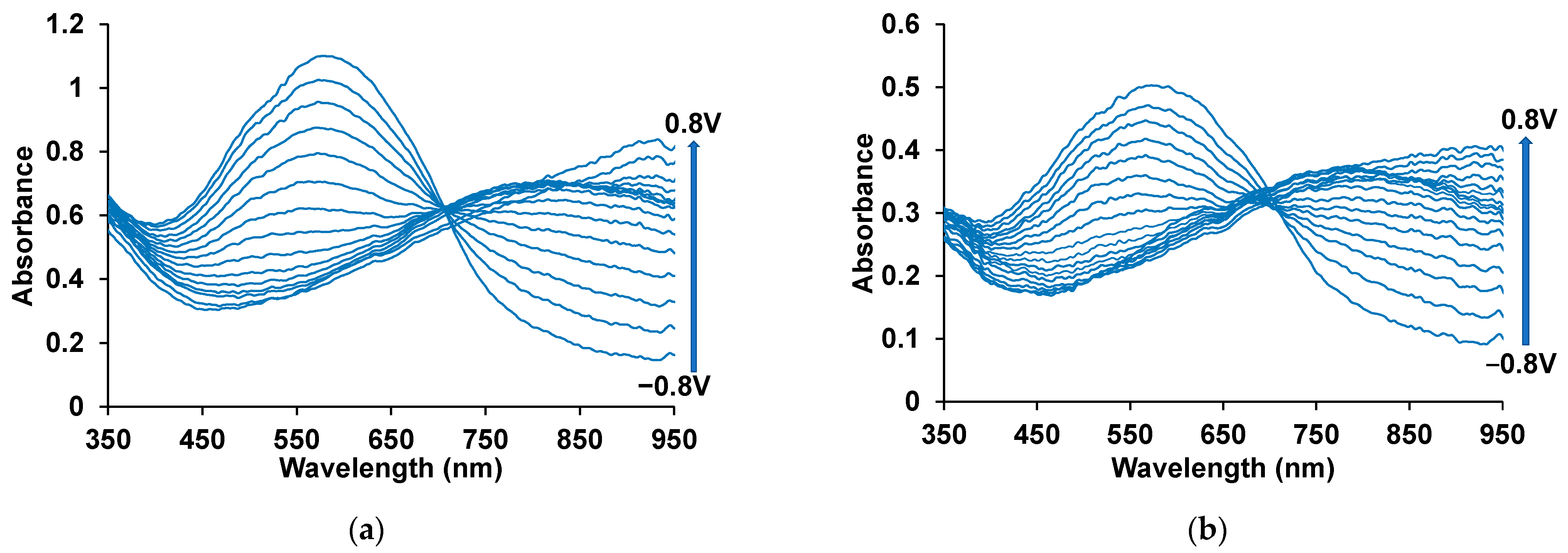
3.3. Spectroelectrochemical Studies
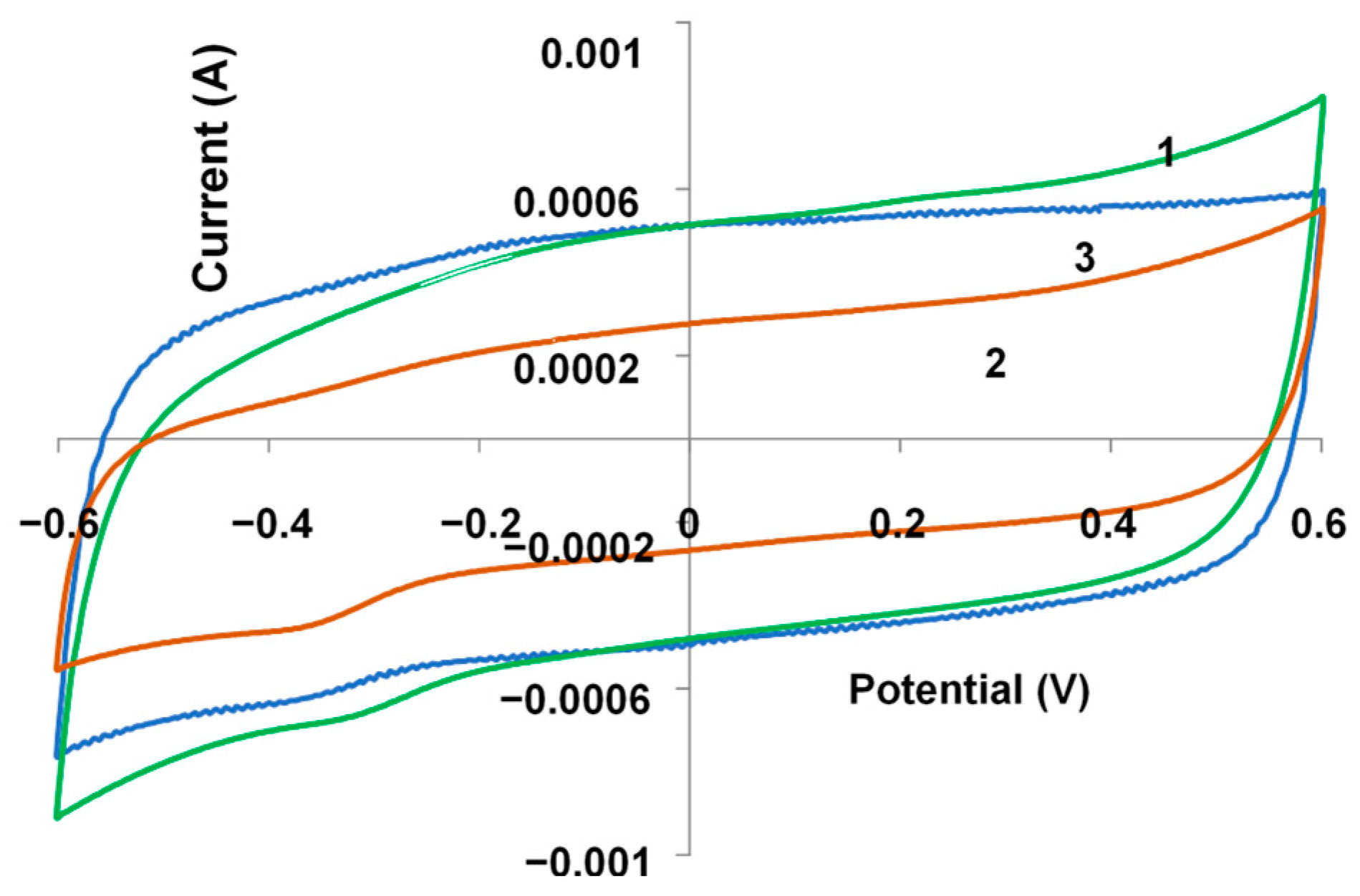
3.4. Electrochemical Studies
3.5. Morphology
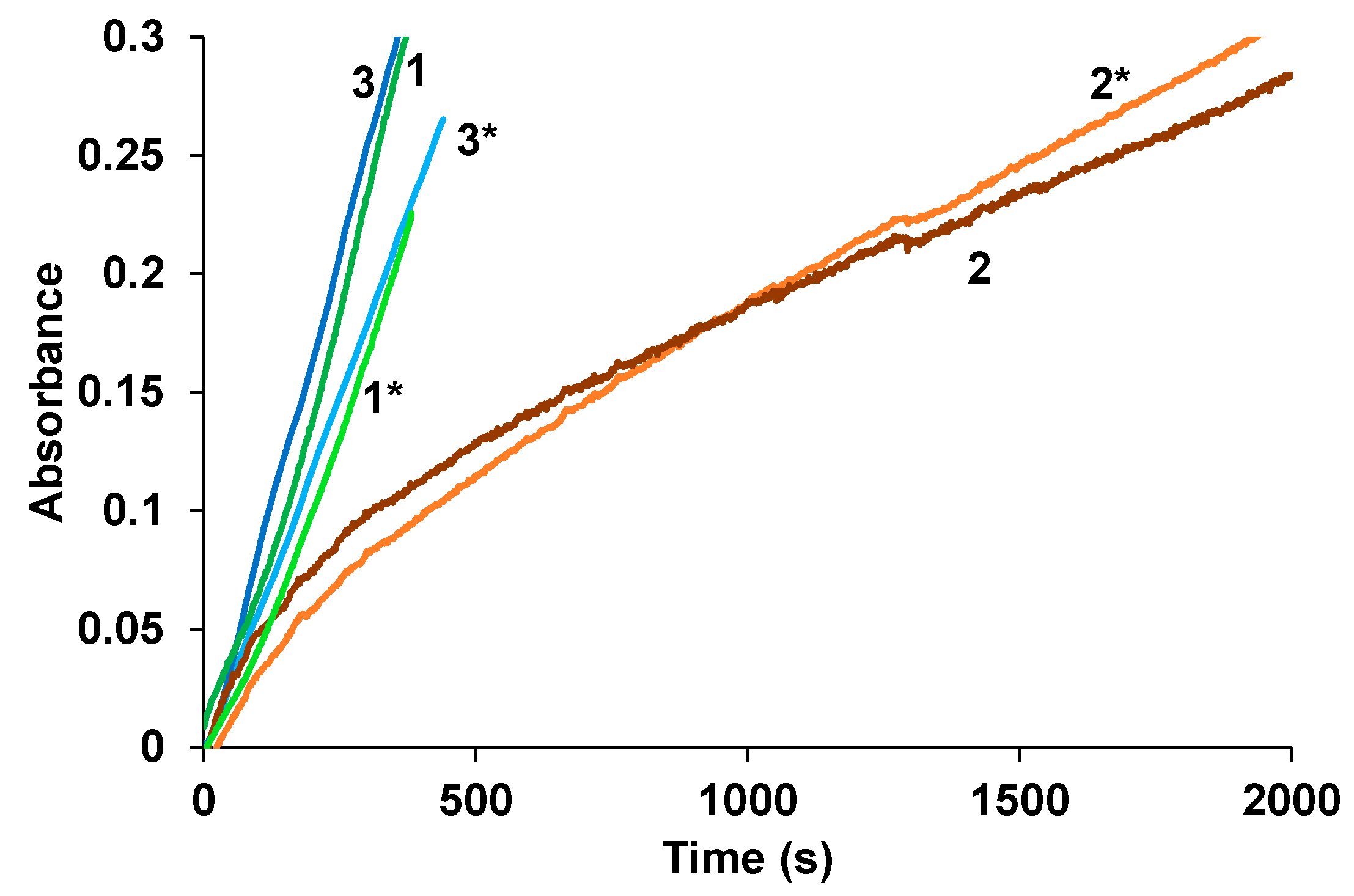
3.6. Near-IR Photoinduced Current
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jolt Oostra, A.; Blom, P.W.M.; Michels, J.J. Prevention of Short Circuits in Solution-Processed OLED Devices. Org. Electron. 2014, 15, 1166–1172. [Google Scholar] [CrossRef]
- Huang, Z.; Zhong, Z.; Peng, F.; Ying, L.; Yu, G.; Huang, F.; Cao, Y. Copper Thiocyanate as an Anode Interfacial Layer for Efficient Near-Infrared Organic Photodetector. ACS Appl. Mater. Interfaces 2021, 13, 1027–1034. [Google Scholar] [CrossRef]
- Fan, B.; Wang, P.; Wang, L.; Shi, G. Polythiophene/Fullerene Bulk Heterojunction Solar Cell Fabricated via Electrochemical Co-Deposition. Sol. Energy Mater. Sol. Cells 2006, 90, 3547–3556. [Google Scholar] [CrossRef]
- Nasybulin, E.; Cox, M.; Kymissis, I.; Levon, K. Electrochemical Codeposition of Poly(Thieno[3,2-b]Thiophene) and Fullerene: An Approach to a Bulk Heterojunction Organic Photovoltaic Device. Synth. Met. 2012, 162, 10–17. [Google Scholar] [CrossRef]
- Reynoso, E.; Durantini, A.M.; Solis, C.A.; Macor, L.P.; Otero, L.A.; Gervaldo, M.A.; Durantini, E.N.; Heredia, D.A. Photoactive Antimicrobial Coating Based on a PEDOT-Fullerene C60polymeric Dyad. RSC Adv. 2021, 11, 23519–23532. [Google Scholar] [CrossRef]
- Suárez, M.B.; Aranda, C.; Macor, L.; Durantini, J.; Heredia, D.A.; Durantini, E.N.; Otero, L.; Guerrero, A.; Gervaldo, M. Perovskite Solar Cells with Versatile Electropolymerized Fullerene as Electron Extraction Layer. Electrochim. Acta 2018, 292, 697–706. [Google Scholar] [CrossRef]
- Dominguez-Alfaro, A.; Jénnifer Gómez, I.; Alegret, N.; Mecerreyes, D.; Prato, M. 2D and 3D Immobilization of Carbon Nanomaterials Into Pedot Via Electropolymerization of a Functional Bis-Edot Monomer. Polymers 2021, 13, 436. [Google Scholar] [CrossRef] [PubMed]
- Alegret, N.; Dominguez-Alfaro, A.; Salsamendi, M.; Gomez, I.J.; Calvo, J.; Mecerreyes, D.; Prato, M. Effect of the Fullerene in the Properties of Thin PEDOT/C60 Films Obtained by Co-Electrodeposition. Inorganica Chim. Acta 2017, 468, 239–244. [Google Scholar] [CrossRef]
- Akiyama, T.; Yoneda, H.; Fukuyama, T.; Sugawa, K.; Yamada, S.; Takechi, K.; Shiga, T.; Motohiro, T.; Nakayama, H.; Kohama, K. Facile Fabrication and Photocurrent Generation Properties of Electrochemically Polymerized Fullerene-Poly(Ethylene Dioxythiophene) Composite Films. Jpn. J. Appl. Phys. 2009, 48, 04C172. [Google Scholar] [CrossRef]
- Dumitriu, C.; Mousavi, Z.; Latonen, R.M.; Bobacka, J.; Demetrescu, I. Electrochemical Synthesis and Characterization of Poly(3,4-Ethylenedioxythiophene) Doped with Sulfonated Calixarenes and Sulfonated Calixarene-Fullerene Complexes. Electrochim. Acta 2013, 107, 178–186. [Google Scholar] [CrossRef]
- Lv, X.; Huang, C.; Tameev, A.; Qian, L.; Zhu, R.; Katin, K.; Maslov, M.; Nekrasov, A.; Zhang, C. Electrochemical Polymerization Process and Excellent Electrochromic Properties of Ferrocene-Functionalized Polytriphenylamine Derivative. Dye. Pigment. 2019, 163, 433–440. [Google Scholar] [CrossRef]
- Gribkova, O.L.L.; Kabanova, V.A.A.; Yagodin, A.V.V.; Averin, A.A.A.; Nekrasov, A.A.A. Water-Soluble Phthalocyanine with Ionogenic Groups as a Molecular Template for Electropolymerization of 3,4-Ethylenedioxythiophene. Russ. J. Electrochem. 2022, 58, 957–967. [Google Scholar] [CrossRef]
- Gribkova, O.L.; Kabanova, V.A.; Kormshchikov, I.D.; Tameev, A.R.; Nekrasov, A.A. Electrodeposition of Photosensitive Layers Based on Conducting Polymers and Zinc Phthalocyaninate, Their Structure and Photoelectrical Properties. Russ. J. Electrochem. 2024, 60, 448–458. [Google Scholar] [CrossRef]
- Kabanova, V.A.; Gribkova, O.L.; Tameev, A.R.; Nekrasov, A.A. Hole Transporting Electrodeposited PEDOT–Polyelectrolyte Layers for Perovskite Solar Cells. Mendeleev Commun. 2021, 31, 454–455. [Google Scholar] [CrossRef]
- Gribkova, O.L.; Sayarov, I.R.; Kabanova, V.A.; Nekrasov, A.A.; Tameev, A.R. Electrodeposited Composite of Poly-3,4-Ethylenedioxythiophene with Fullerenol Photoactive in the Near-IR Range. Russ. J. Electrochem. 2024, 60, 813–822. [Google Scholar] [CrossRef]
- Bobylev, A.G.; Kornev, A.B.; Bobyleva, L.G.; Shpagina, M.D.; Fadeeva, I.S.; Fadeev, R.S.; Deryabin, D.G.; Balzarini, J.; Troshin, P.A.; Podlubnaya, Z.A. Fullerenolates: Metallated Polyhydroxylated Fullerenes with Potent Anti-Amyloid Activity. Org. Biomol. Chem. 2011, 9, 5714–5719. [Google Scholar] [CrossRef]
- Troshin, P.A.; Astakhova, A.S.; Lyubovskaya, R.N. Synthesis of Fullerenols from Halofullerenes. Fuller. Nanotub. Carbon Nanostructures 2005, 13, 331–343. [Google Scholar] [CrossRef]
- Fedorova, N.E.; Klimova, R.R.; Tulenev, Y.A.; Chichev, E.V.; Kornev, A.B.; Troshin, P.A.; Kushch, A.A. Carboxylic Fullerene C60 Derivatives: Efficient Microbicides Against Herpes Simplex Virus And Cytomegalovirus Infections In Vitro. Mendeleev Commun. 2012, 22, 254–256. [Google Scholar] [CrossRef]
- Husebo, L.O.; Sitharaman, B.; Furukawa, K.; Kato, T.; Wilson, L.J. Fullerenols Revisited as Stable Radical Anions. J. Am. Chem. Soc. 2004, 126, 12055–12064. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-J.; Chetyrkina, M.; Wong, C.-W.; Kraevaya, O.A.; Zhilenkov, A.V.; Voronov, I.I.; Wang, P.-H.; Troshin, P.A.; Hsu, S. Identification of Potential Descriptors of Water-Soluble Fullerene Derivatives Responsible for Antitumor Effects on Lung Cancer Cells via QSAR Analysis. Comput. Struct. Biotechnol. J. 2021, 19, 812–825. [Google Scholar] [CrossRef] [PubMed]
- Gribkova, O.L.; Nekrasov, A.A. Spectroelectrochemistry of Electroactive Polymer Composite Materials. Polymers 2022, 14, 3201. [Google Scholar] [CrossRef]
- Garreau, S.; Duvail, J.L.; Louarn, G. Spectroelectrochemical Studies of Poly(3,4-Ethylenedioxythiophene) in Aqueous Medium. Synth. Met. 2001, 125, 325–329. [Google Scholar] [CrossRef]
- Zozoulenko, I.; Singh, A.; Singh, S.K.; Gueskine, V.; Crispin, X.; Berggren, M. Polarons, Bipolarons, And Absorption Spectroscopy of PEDOT. ACS Appl. Polym. Mater. 2019, 1, 83–94. [Google Scholar] [CrossRef]
- Janssen, R.A.J.; Smilowitz, L.; Sariciftci, N.S.; Moses, D. Triplet-State Photoexcitations of Oligothiophene Films and Solutions. J. Chem. Phys. 1994, 101, 1787–1798. [Google Scholar] [CrossRef]
- Peintler-Kriván, E.; Tóth, P.S.; Visy, C. Combination of in Situ UV–Vis-NIR Spectro-Electrochemical and a.c. Impedance Measurements: A New, Effective Technique for Studying the Redox Transformation of Conducting Electroactive Materials. Electrochem. Commun. 2009, 11, 1947–1950. [Google Scholar] [CrossRef]
- Kabanova, V.; Gribkova, O.; Nekrasov, A. Poly(3,4-Ethylenedioxythiophene) Electrosynthesis in the Presence of Mixtures of Flexible-Chain and Rigid-Chain Polyelectrolytes. Polymers 2021, 13, 3866. [Google Scholar] [CrossRef] [PubMed]
- Yamato, H.; Kai, K.I.; Ohwa, M.; Wernet, W.; Matsumura, M. Mechanical, Electrochemical and Optical Properties of Poly(3,4-Ethylenedioxythiophene)/Sulfated Poly(β-Hydroxyethers) Composite Films. Electrochim. Acta 1997, 42, 2517–2523. [Google Scholar] [CrossRef]
- Randriamahazaka, H.; Noël, V.; Chevrot, C. Nucleation and Growth of Poly(3,4-Ethylenedioxythiophene) in Acetonitrile on Platinum under Potentiostatic Conditions. J. Electroanal. Chem. 1999, 472, 103–111. [Google Scholar] [CrossRef]
- Lyutov, V.; Kabanova, V.; Gribkova, O.; Nekrasov, A.; Tsakova, V. Electrochemically Obtained Polysulfonates Doped Poly(3,4-Ethylenedioxythiophene) Films—Effects of the Dopant’s Chain Flexibility and Molecular Weight Studied by Electrochemical, Microgravimetric and XPS Methods. Polymers 2021, 13, 2438. [Google Scholar] [CrossRef]
- Nekrasov, A.A.; Nekrasova, N.V.; Savel’ev, M.A.; Khuzin, A.A.; Barachevsky, V.A.; Tulyabaev, A.R.; Tuktarov, A.R. Electrochemical Investigation of a Photochromic Spiropyran Containing a Pyrrolidinofullerene Moiety. Mendeleev Commun. 2023, 33, 505–508. [Google Scholar] [CrossRef]
- Meskers, S.C.J.; van Duren, J.K.J.; Janssen, R.A.J. Stimulation of Electrical Conductivity in a π-Conjugated Polymeric Conductor with Infrared Light. J. Appl. Phys. 2002, 92, 7041–7050. [Google Scholar] [CrossRef]
- Meskers, S.C.J.; van Duren, J.K.J.; Janssen, R.A.J.; Louwet, F.; Groenendaal, L. Infrared Detectors with Poly(3,4-ethylenedioxy Thiophene)/Poly(Styrene Sulfonic Acid) (PEDOT/PSS) as the Active Material. Adv. Mater. 2003, 15, 613–616. [Google Scholar] [CrossRef]
- Sariciftci, N.S.; Smilowitz, L.; Heeger, A.J.; Wudl, F. Photoinduced Electron Transfer from a Conducting Polymer to Buckminsterfullerene. Science 1992, 258, 1474–1476. [Google Scholar] [CrossRef] [PubMed]




| Counterion | Maximum of the Reduced Form, nm | Maximum of the Polaronic Form, nm | DDCVA | DDEDX |
|---|---|---|---|---|
| NaFl | 575 | 840 | 0.15 | 0.11 |
| KPCF | 573 | 776 | 0.08 | 0.06 |
| PAMPSA | 615 | 878 | 0.25 | 0.84 |
| Eg(opt), eV | From UPS Data | From CV Data | |||||
|---|---|---|---|---|---|---|---|
| EHOMO, eV | Ef, eV | ELUMO PEDOT, eV | ELUMO Fullerene, eV | EHOMO, eV | Eg, eV | ||
| NaFl | 2.78 | −6.6 | −4.8 | ||||
| KPCF | 2.63 | −6.5 | −4.9 | ||||
| PEDOT–PAMPSA | 1.57 | −4.8 | −4.8 | ||||
| PEDOT–NaFl | 1.55 | −2.97 | −4.05 | −4.50 | 1.53 | ||
| PEDOT–KPCF | 1.49 | −2.95 | −4.05 | −4.33 | 1.38 | ||
| PEDOT–NaFl | PEDOT–KPCF | PEDOT–PAMPSA | |
|---|---|---|---|
| Photocurrent current, µA/cm2 | 2.5 | 0.54 | 0.02 |
| Photosensitivity, μA/W | 160 | 36 | 1.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gribkova, O.; Kabanova, V.; Sayarov, I.; Nekrasov, A.; Tameev, A. Near-Infrared Responsive Composites of Poly-3,4-Ethylenedioxythiophene with Fullerene Derivatives. Polymers 2025, 17, 14. https://doi.org/10.3390/polym17010014
Gribkova O, Kabanova V, Sayarov I, Nekrasov A, Tameev A. Near-Infrared Responsive Composites of Poly-3,4-Ethylenedioxythiophene with Fullerene Derivatives. Polymers. 2025; 17(1):14. https://doi.org/10.3390/polym17010014
Chicago/Turabian StyleGribkova, Oxana, Varvara Kabanova, Ildar Sayarov, Alexander Nekrasov, and Alexey Tameev. 2025. "Near-Infrared Responsive Composites of Poly-3,4-Ethylenedioxythiophene with Fullerene Derivatives" Polymers 17, no. 1: 14. https://doi.org/10.3390/polym17010014
APA StyleGribkova, O., Kabanova, V., Sayarov, I., Nekrasov, A., & Tameev, A. (2025). Near-Infrared Responsive Composites of Poly-3,4-Ethylenedioxythiophene with Fullerene Derivatives. Polymers, 17(1), 14. https://doi.org/10.3390/polym17010014








