Constructing a Broad-Pore-Domain Structure of Adsorbents for Acteoside Adsorption
Abstract
:1. Introduction
2. Experimental Section
3. Results and Discussion
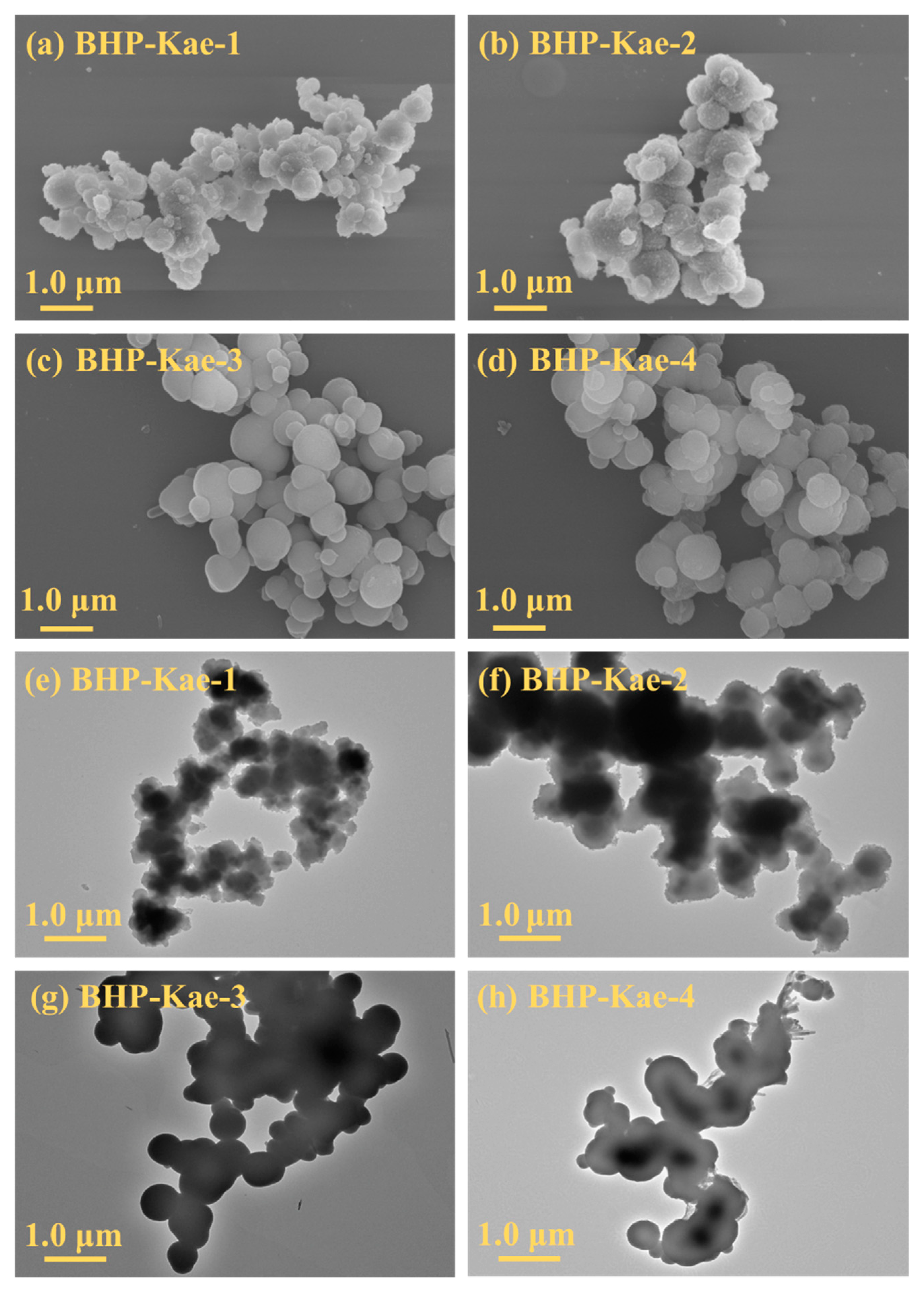
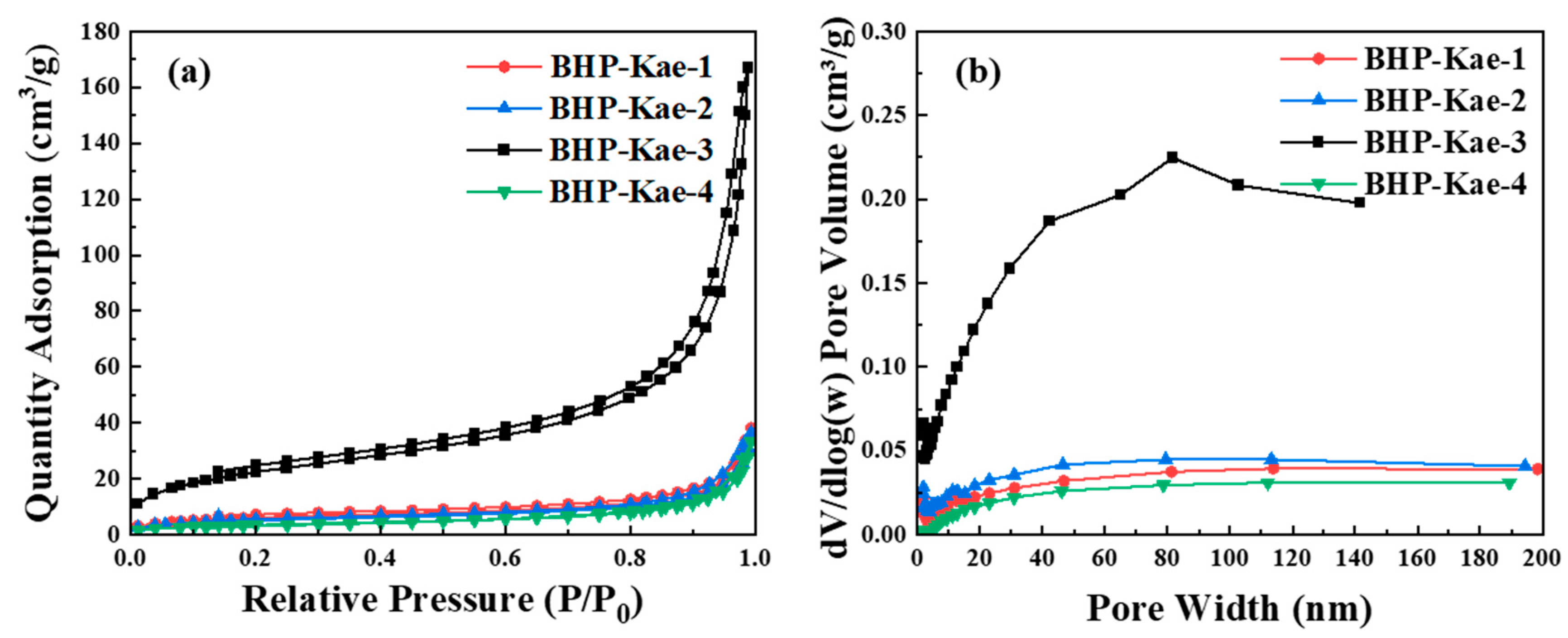
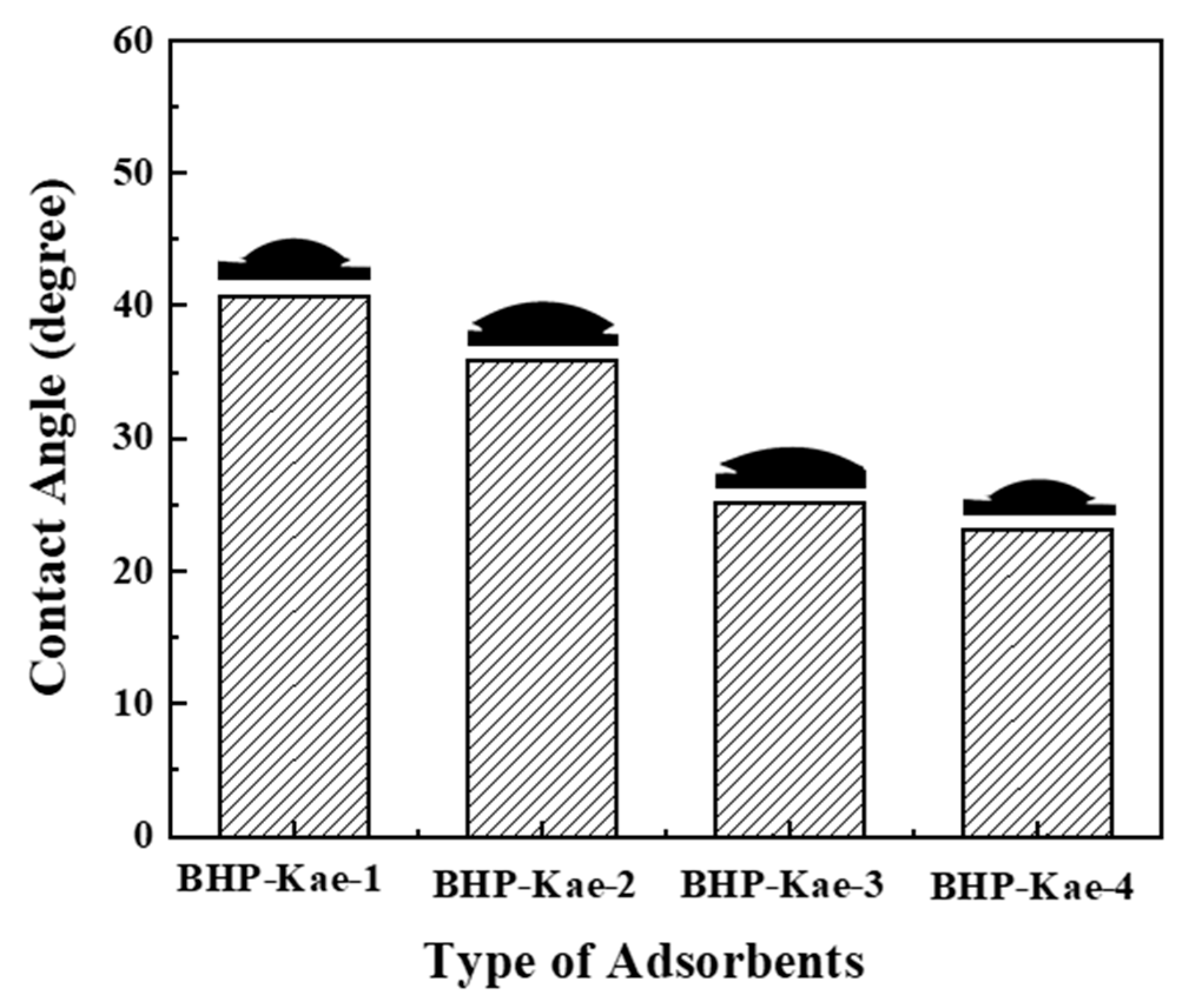
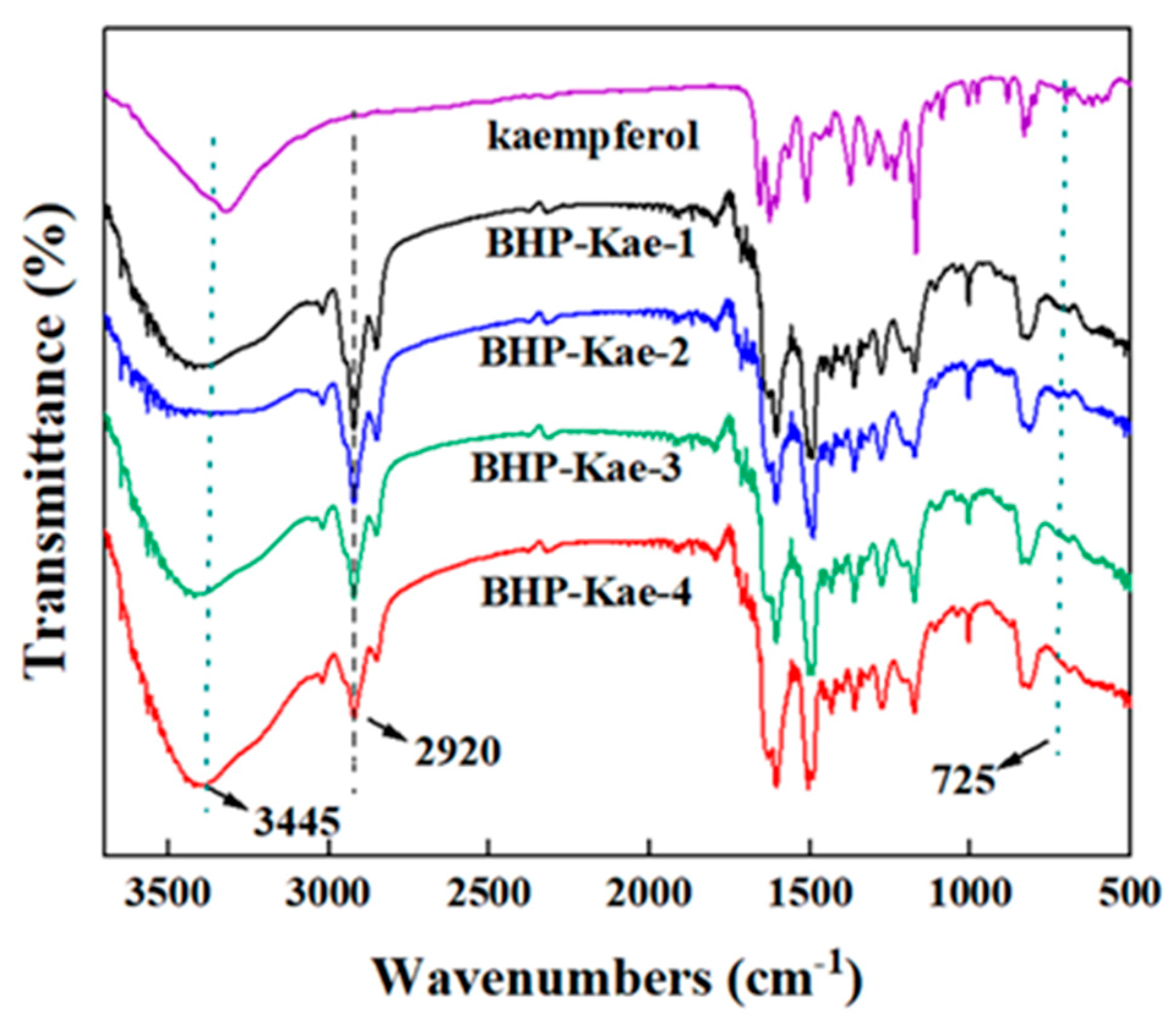
3.1. Characterization of BHP-Kae
3.2. Adsorption Evaluation
3.2.1. Effect of Adsorbent Reaction Time
3.2.2. Effect of ACT Concentration
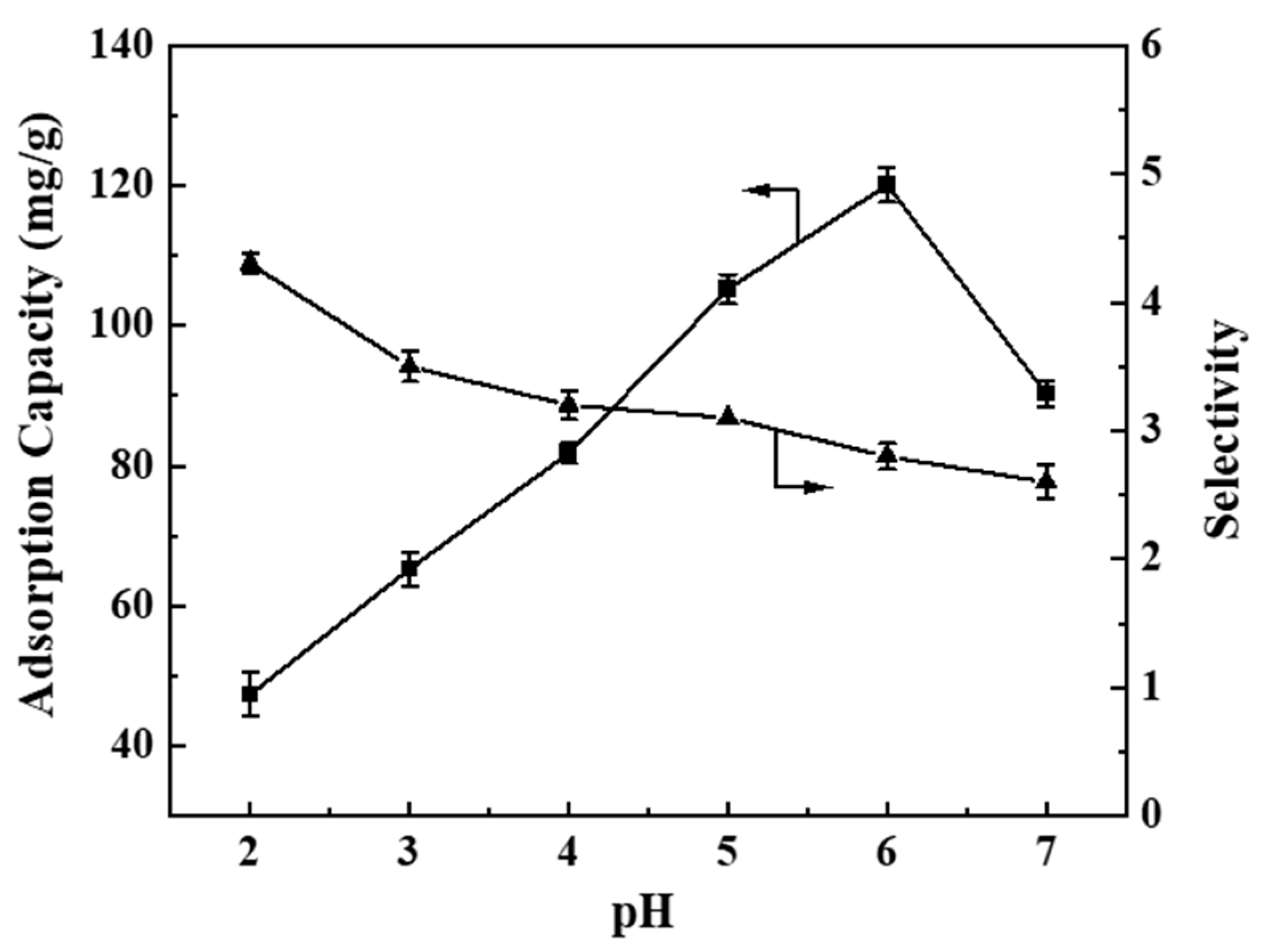
3.2.3. Effect of pH
3.2.4. Influence of Adsorption Temperature
3.3. Study on Adsorption Kinetics of BHP-Kae-3
3.4. Thermodynamic Study on Adsorption of BHP-Kae-3
3.5. Adsorption Mechanism
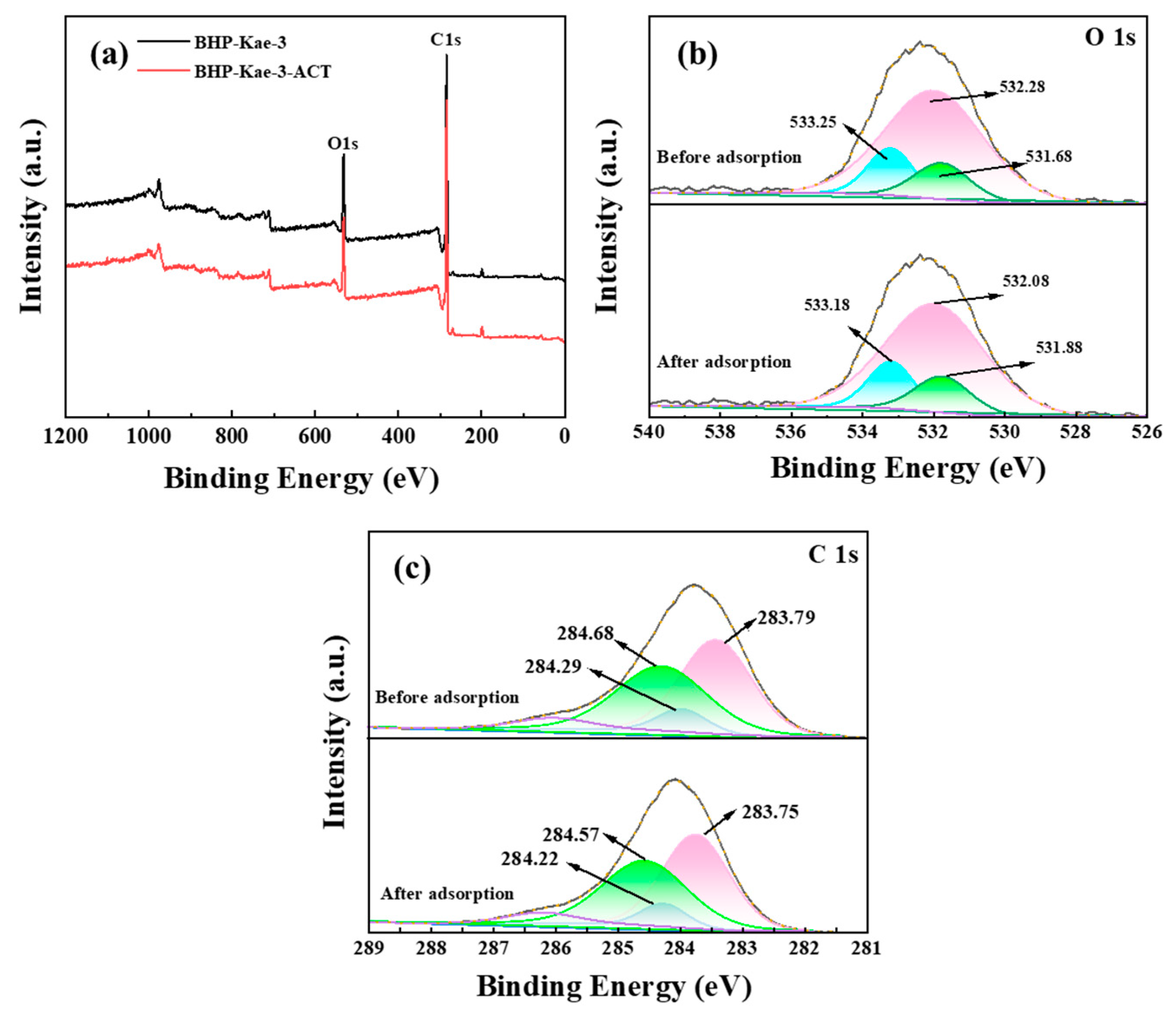
3.5.1. XPS Analysis
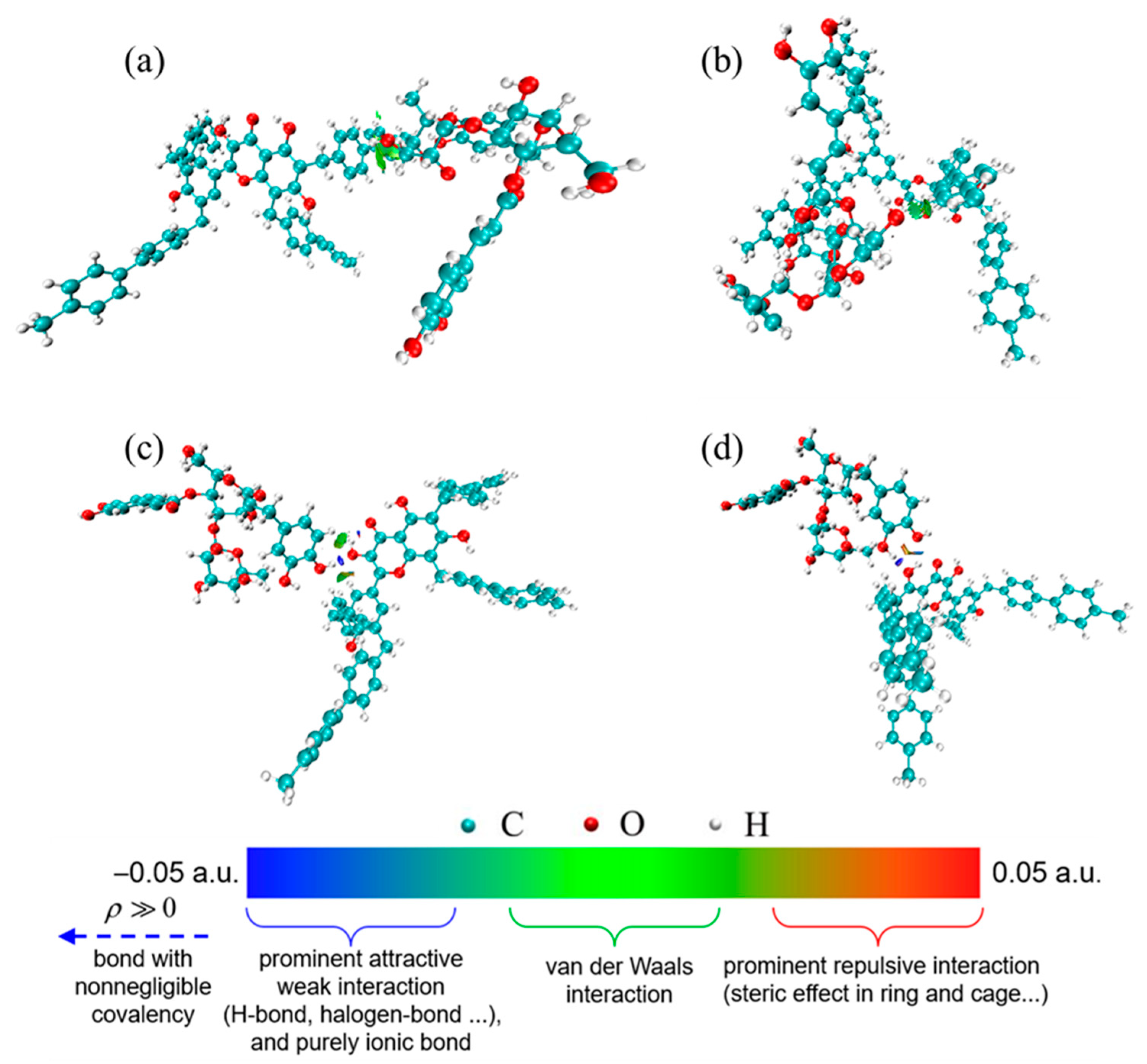
3.5.2. Molecular Simulation
3.6. Study on Regeneration Ability of BHP-Kae-3
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, F.; Zhuo, B.; Wang, S.; Lou, J.; Zhang, Y.; Chen, Q.; Shi, Z.; Song, Y.; Tu, P. Atriplex canescens: A new host for Cistanche deserticola. Heliyon 2021, 7, e07368. [Google Scholar] [CrossRef]
- Piwowarczyk, R.; Ochmian, I.; Lachowicz, S.; Kapusta, I.; Sotek, Z.; Błaszak, M. Phytochemical parasite-host relations and interactions: A Cistanche armena case study. Sci. Total Environ. 2020, 716, 137071. [Google Scholar] [CrossRef]
- Miao, Y.; Zhang, X.; Pei, J.; Liu, C.; Huang, L. Adaptive bacterial and fungal matching between a parasitic plant and its host: A case of Cistanche deserticola and Haloxylon ammodendron. Ind. Crops Prod. 2023, 191, 115932. [Google Scholar] [CrossRef]
- Jiang, Y.; Tu, P.-F. Analysis of chemical constituents in Cistanche species. J. Chromatogr. A 2009, 1216, 1970–1979. [Google Scholar] [CrossRef]
- Dong, J.; Li, J.; Liu, Y.; Cui, L.; Liu, X.; Wang, G.; Wang, Q.; Criddle, D.N.; Tu, P.; Li, C. A comparative study of the anti-fatigue activity of extracts from different parts of Cistanche tubulosa (Schenk) Wight. J. Tradit. Chin. Med. Sci. 2024, 11, 222–231. [Google Scholar] [CrossRef]
- Li, Z.; Lin, H.; Gu, L.; Gao, J.; Tzeng, C.-M. Herba Cistanche (Rou Cong-Rong): One of the Best Pharmaceutical Gifts of Traditional Chinese Medicine. Front. Pharmacol. 2016, 7, 41. [Google Scholar] [CrossRef]
- Fu, Z.; Fan, X.; Wang, X.; Gao, X. Cistanches Herba: An overview of its chemistry, pharmacology, and pharmacokinetics property. J. Ethnopharmacol. 2018, 219, 233–247. [Google Scholar] [CrossRef]
- Zhou, S.; Feng, D.; Zhou, Y.; Duan, H.; Jiang, Y.; Yan, W. Analysis of the active ingredients and health applications of Cistanche. Front. Nutr. 2023, 10, 1101182. [Google Scholar] [CrossRef]
- Gu, C.; Yang, X.; Huang, L. Cistanches Herba: A Neuropharmacology Review. Front. Pharmacol. 2016, 7, 289. [Google Scholar] [CrossRef]
- Jiang, H.; Ma, R.; Ji, F.; Liu, Y.; Wang, B.; Fu, S.; Ma, L.; Wang, S.; Liu, C.; Guo, Z.; et al. Structure characterization of polysaccharides from Cistanche deserticola and their neuroprotective effects against oxidative stress in slow transit constipation mice. Int. J. Biol. Macromol. 2024, 260, 129527. [Google Scholar] [CrossRef]
- Cheng, N.; Wang, H.; Hao, H.; Rahman, F.-U.; Zhang, Y. Research progress on polysaccharide components of Cistanche deserticola as potential pharmaceutical agents. Eur. J. Med. Chem. 2023, 245, 114892. [Google Scholar] [CrossRef]
- Li, M.; Tian, X.; Li, X.; Mao, T.; Liu, T. Anti-fatigue activity of gardenia yellow pigment and Cistanche phenylethanol glycosides mixture in hypoxia. Food Biosci. 2021, 40, 100902. [Google Scholar] [CrossRef]
- Guo, Y.; Cao, L.; Zhao, Q.; Zhang, L.; Chen, J.; Liu, B.; Zhao, B. Preliminary characterizations, antioxidant and hepatoprotective activity of polysaccharide from Cistanche deserticola. Int. J. Biol. Macromol. 2016, 93, 678–685. [Google Scholar] [CrossRef]
- Yan, J.; Wang, H.; Wang, H.; Bian, Y.; Wang, K.; Zhai, X.; Li, Y.; Wu, K.; Wang, W.; Li, J.; et al. Quantitative analysis and hepatoprotective mechanism of Cistanche deserticola Y. C. Ma against alcohol-induced liver injury in mice. Biomed. Pharmacother. 2023, 162, 114719. [Google Scholar] [CrossRef]
- Ran, Z.; Ju, B.; Cao, L.; Hou, Q.; Wen, L.; Geng, R.; Liao, Y.; Hu, J.; Yang, J. Microbiome—Metabolomics analysis reveals the potential effect of verbascoside in alleviating cognitive impairment in db/db mice. Food Funct. 2023, 14, 3488–3508. [Google Scholar] [CrossRef]
- Zhang, S.; Gong, F.; Liu, J.; Liu, T.; Yang, J.; Hu, J. A novel PHD2 inhibitor acteoside from Cistanche tubulosa induces skeletal muscle mitophagy to improve cancer-related fatigue. Biomed. Pharmacother. 2022, 150, 113004. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, N.; Luo, K.; Liu, Y.; Bai, Z.; Tang, S. Recent advances of innovative and high-efficiency stationary phases for chromatographic separations. TrAC Trends Anal. Chem. 2022, 153, 116647. [Google Scholar] [CrossRef]
- Sajid, M.; Płotka-Wasylka, J. Combined extraction and microextraction techniques: Recent trends and future perspectives. TrAC Trends Anal. Chem. 2018, 103, 74–86. [Google Scholar] [CrossRef]
- Li, L.; Tsao, R.; Yang, R.; Liu, C.; Young, J.C.; Zhu, H. Isolation and purification of phenylethanoid glycosides from Cistanche deserticola by high-speed counter-current chromatography. Food Chem. 2008, 108, 702–710. [Google Scholar] [CrossRef]
- Nie, F.; Feng, C.; Ahmad, N.; Tian, M.; Liu, Q.; Wang, W.; Lin, Z.; Li, C.; Zhao, C. A new green alternative solvent for extracting echinacoside and acteoside from Cistanche deserticola based on ternary natural deep eutectic solvent. J. Ind. Eng. Chem. 2023, 118, 499–510. [Google Scholar] [CrossRef]
- Park, H.I.; Kang, J.; Park, J.-H.; Park, J.C.; Park, J.; Lee, K.B.; Lee, C.H. One-pot synthesis of novel porous carbon adsorbents derived from poly vinyl chloride for high methane adsorption uptake. Chem. Eng. J. 2022, 440, 135867. [Google Scholar] [CrossRef]
- Ioni, Y.; Sapkov, I.; Kirsanova, M.; Dimiev, A.M. Flame modified graphene oxide: Structure and sorption properties. Carbon 2023, 212, 118122. [Google Scholar] [CrossRef]
- Kayan, A. Inorganic-organic hybrid materials and their adsorbent properties. Adv. Compos. Hybrid Mater. 2019, 2, 34–45. [Google Scholar] [CrossRef]
- Abdul, G.; Zhu, X.; Chen, B. Structural characteristics of biochar-graphene nanosheet composites and their adsorption performance for phthalic acid esters. Chem. Eng. J. 2017, 319, 9–20. [Google Scholar] [CrossRef]
- Yang, D.-H.; Tao, Y.; Ding, X.; Han, B.-H. Porous organic polymers for electrocatalysis. Chem. Soc. Rev. 2022, 51, 761–791. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, Y.; Meng, N.; Liao, Y. Photothermal Conversion Porous Organic Polymers: Design, Synthesis, and Applications. Small Methods 2024, 8, 2301554. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, Y.-W. Creation and bioapplications of porous organic polymer materials. J. Mater. Chem. B 2017, 5, 9278–9290. [Google Scholar] [CrossRef]
- Huang, J.; Turner, S.R. Hypercrosslinked Polymers: A Review. Polym. Rev. 2018, 58, 1–41. [Google Scholar] [CrossRef]
- Yang, Y.; Tan, B.; Wood, C.D. Solution-processable hypercrosslinked polymers by low cost strategies: A promising platform for gas storage and separation. J. Mater. Chem. A 2016, 4, 15072–15080. [Google Scholar] [CrossRef]
- Wang, X.; Mu, P.; Zhang, C.; Chen, Y.; Zeng, J.; Wang, F.; Jiang, J.-X. Control Synthesis of Tubular Hyper-Cross-Linked Polymers for Highly Porous Carbon Nanotubes. ACS Appl. Mater. Interfaces 2017, 9, 20779–20786. [Google Scholar] [CrossRef]
- Long, C.; Li, Y.; Yu, W.; Li, A. Adsorption characteristics of water vapor on the hypercrosslinked polymeric adsorbent. Chem. Eng. J. 2012, 180, 106–112. [Google Scholar] [CrossRef]
- Wang, R.; Lei, Y.; Ke, L.; Bai, L.; Zheng, Y.; Xu, Z.; Li, T.; Chen, S.; Zhang, D. Construction of self-healing multifunctional hyper-crosslinked hyperbranched polymer@metal-organic polyhedra (HHMOP) membranes. Eur. Polym. J. 2024, 211, 112966. [Google Scholar] [CrossRef]
- Lavine, M.S. Processable cross-linked polymers. Science 2017, 356, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Leveson-Gower, R.B.; Roelfes, G. Biocatalytic Friedel-Crafts Reactions. ChemCatChem 2022, 14, e202200636. [Google Scholar] [CrossRef]
- Gu, Y.; Son, S.U.; Li, T.; Tan, B. Hypercrosslinked Polymers: Low-Cost Hypercrosslinked Polymers by Direct Knitting Strategy for Catalytic Applications. Adv. Funct. Mater. 2021, 31, 2170082. [Google Scholar] [CrossRef]
- Li, M.; Shi, L.; Liu, Y.; Li, S.; Cui, W.; Li, W.; Zhi, Y.; Shan, S.; Miao, Y. A dual-ionic hyper-crosslinked polymer for efficient CO2 fixation and conversion. Chem. Eng. J. 2024, 481, 148550. [Google Scholar] [CrossRef]
- Sun, L.; Xu, G.; Tu, Y.; Zhang, W.; Hu, X.; Yang, P.; Wu, D.; Liang, Y.; Wei, D.; Li, A.; et al. Multifunctional porous β-cyclodextrin polymer for water purification. Water Res. 2022, 222, 118917. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, T.; Wang, X.; Chen, J.; Fan, M.; Shi, Z.; Liu, J.; Xu, L.; Zang, Y. Construction of hydroxyl-functionalized hyper-crosslinked networks from polyimide for highly efficient iodine adsorption. iScience 2024, 27, 108993. [Google Scholar] [CrossRef]
- James, A.M.; Harding, S.; Robshaw, T.; Bramall, N.; Ogden, M.D.; Dawson, R. Selective Environmental Remediation of Strontium and Cesium Using Sulfonated Hyper-Cross-Linked Polymers (SHCPs). ACS Appl. Mater. Interfaces 2019, 11, 22464–22473. [Google Scholar] [CrossRef]
- Yuan, D.; Zhang, S.; Tan, J.; Dai, Y.; Wang, Y.; He, Y.; Liu, Y.; Zhao, X.; Zhang, M.; Zhang, Q. Highly efficacious entrapment of Th (IV) and U (VI) from rare earth elements in concentrated nitric acid solution using a phosphonic acid functionalized porous organic polymer adsorbent. Sep. Purif. Technol. 2020, 237, 116379. [Google Scholar] [CrossRef]
- Yuan, D.; Zhang, S.; Xiang, Z.; He, Y.; Wang, Y.; Liu, Y.; Zhao, X.; Zhou, X.; Zhang, Q. Highly Efficient Removal of Thorium in Strong HNO3 Media Using a Novel Polymer Adsorbent Bearing a Phosphonic Acid Ligand: A Combined Experimental and Density Functional Theory Study. ACS Appl. Mater. Interfaces 2019, 11, 24512–24522. [Google Scholar] [CrossRef]
- Chen, M.; Xiao, J.; El-Seedi, H.R.; Woźniak, K.S.; Daglia, M.; Little, P.J.; Weng, J.; Xu, S. Kaempferol and atherosclerosis: From mechanism to medicine. Crit. Rev. Food Sci. Nutr. 2022, 64, 2157–2175. [Google Scholar] [CrossRef] [PubMed]
- Barreca, D.; Trombetta, D.; Smeriglio, A.; Mandalari, G.; Romeo, O.; Felice, M.R.; Gattuso, G.; Nabavi, S.M. Food flavonols: Nutraceuticals with complex health benefits and functionalities. Trends Food Sci. Technol. 2021, 117, 194–204. [Google Scholar] [CrossRef]
- Yang, L.; Gao, Y.; Bajpai, V.K.; El-Kammar, H.A.; Simal-Gandara, J.; Cao, H.; Cheng, K.-W.; Wang, M.; Arroo, R.R.J.; Zou, L.; et al. Advance toward isolation, extraction, metabolism and health benefits of kaempferol, a major dietary flavonoid with future perspectives. Crit. Rev. Food Sci. Nutr. 2023, 63, 2773–2789. [Google Scholar] [CrossRef]
- Xu, M.; Wang, J.; Zhang, L.; Wang, Q.; Liu, W.; An, Y.; Hao, L.; Wang, C.; Wang, Z.; Wu, Q. Construction of hydrophilic hypercrosslinked polymer based on natural kaempferol for highly effective extraction of 5-nitroimidazoles in environmental water, honey and fish samples. J. Hazard. Mater. 2022, 429, 128288. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Casareto, M.; Mowbray, M.; Henry, R.; Hayden, J.; Rubloff, G.; Lee, S.B.; Gregorczyk, K.E. On-Wafer Wide-Pore Anodic Aluminum Oxide. J. Electrochem. Soc. 2023, 170, 063507. [Google Scholar] [CrossRef]
- Ai, Y.; Liu, Y.; Lan, W.; Jin, J.; Xing, J.; Zou, Y.; Zhao, C.; Wang, X. The effect of pH on the U (VI) sorption on graphene oxide (GO): A theoretical study. Chem. Eng. J. 2018, 343, 460–466. [Google Scholar] [CrossRef]
- Cao, S.; Yin, W.; Yang, B.; Zhu, Z.; Sun, H.; Sheng, Q.; Chen, K. Insights into the influence of temperature on the adsorption behavior of sodium oleate and its response to flotation of quartz. Int. J. Min. Sci. Technol. 2022, 32, 399–409. [Google Scholar] [CrossRef]
- Yi, M.; Cheng, Y.; Wang, Z.; Wang, C.; Hu, B.; He, X. Effect of particle size and adsorption equilibrium time on pore structure characterization in low pressure N2 adsorption of coal: An experimental study. Adv. Powder Technol. 2020, 31, 4275–4281. [Google Scholar] [CrossRef]
- Jang, J.Y.; Kim, Y.K.; Ko, Y.-J.; Son, S.U. Hollow Microporous Organic Polymer@Hypercrosslinked Polymer Catalysts Bearing N-Heterocyclic Carbene-Iron Species for the Synthesis of Biomass-Derived Polymer Platforms. ACS Sustain. Chem. Eng. 2024, 12, 8880–8889. [Google Scholar] [CrossRef]
- Li, Q.; Li, S.; Yu, C.; Zhan, Z.; Cheng, G.; Tan, B.; Ren, S. Hypercrosslinked polymer membranes via interfacial polymerization for organic dye separations. Commun. Mater. 2024, 5, 255. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Wang, S.; Feng, H.; Pang, F.; Li, H.; Liu, B.; Zang, X.; Zhang, S.; Wang, Z. Construction of a sulfonic acid functionalized hypercrosslinked polymer for the adsorption of rubidium and cesium. J. Water Process Eng. 2024, 68, 106321. [Google Scholar] [CrossRef]
- Alahabadi, A.; Hosseini-Bandegharaei, A.; Moussavi, G.; Amin, B.; Rastegar, A.; Karimi-Sani, H.; Fattahi, M.; Miri, M. Comparing adsorption properties of NH4Cl-modified activated carbon towards chlortetracycline antibiotic with those of commercial activated carbon. J. Mol. Liq. 2017, 232, 367–381. [Google Scholar] [CrossRef]
- Yousatit, S.; Rungruangwattanachot, W.; Yuwawanitchakorn, N.; Nuntang, S.; Punyapalakul, P.; Ngamcharussrivichai, C. Amine-Functionalized Natural Rubber/Mesostructured Silica Nanocomposites for Adsorptive Removal of Clofibric Acid in Aqueous Phase. Molecules 2023, 28, 2330. [Google Scholar] [CrossRef]
- Jabar, J.M.; Adebayo, M.A.; Taleat, T.A.A.; Yılmaz, M.; Rangabhashiyam, S. Ipoma batatas (sweet potato) leaf and leaf-based biochar as potential adsorbents for procion orange MX-2R removal from aqueous solution. J. Anal. Appl. Pyrolysis 2025, 185, 106876. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Chen, Q. Independent gradient model based on Hirshfeld partition: A new method for visual study of interactions in chemical systems. J. Comput. Chem. 2022, 43, 539–555. [Google Scholar] [CrossRef]
- Lefebvre, C.; Rubez, G.; Khartabil, H.; Boisson, J.-C.; Contreras-García, J.; Hénon, E. Accurately extracting the signature of intermolecular interactions present in the NCI plot of the reduced density gradient versus electron density. Phys. Chem. Chem. Phys. 2017, 19, 17928–17936. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Nie, F.; Feng, C.; Tian, M.; Yu, M.; Zhao, C.; Fu, Y. Magnetic dual-template molecularly imprinted polymers for separation and enrichment of echinacoside and acteoside from Cistanche deserticola Y. C. Ma. Chem. Eng. Res. Des. 2022, 182, 719–732. [Google Scholar] [CrossRef]
- Liu, B.; Ouyang, J.; Yuan, X.; Wang, L.; Zhao, B. Adsorption properties and preparative separation of phenylethanoid glycosides from Cistanche deserticola by use of macroporous resins. J. Chromatogr. B 2013, 937, 84–90. [Google Scholar] [CrossRef]
- Fan, Y.; Xu, C.; Li, J.; Zhang, L.; Yang, L.; Zhou, Z.; Zhu, Y.; Zhao, D. Ionic liquid-based microwave-assisted extraction of verbascoside from Rehmannia root. Ind. Crop Prod. 2018, 124, 59–65. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ru, W.; Liu, J.; Xiong, F.; Sun, Y.; Zhang, Y.; Li, Y.; Lv, Y.; Li, X. Constructing a Broad-Pore-Domain Structure of Adsorbents for Acteoside Adsorption. Polymers 2025, 17, 79. https://doi.org/10.3390/polym17010079
Ru W, Liu J, Xiong F, Sun Y, Zhang Y, Li Y, Lv Y, Li X. Constructing a Broad-Pore-Domain Structure of Adsorbents for Acteoside Adsorption. Polymers. 2025; 17(1):79. https://doi.org/10.3390/polym17010079
Chicago/Turabian StyleRu, Weibo, Jiaxing Liu, Feng Xiong, Yu Sun, Yong Zhang, Yipei Li, Yin Lv, and Xueqin Li. 2025. "Constructing a Broad-Pore-Domain Structure of Adsorbents for Acteoside Adsorption" Polymers 17, no. 1: 79. https://doi.org/10.3390/polym17010079
APA StyleRu, W., Liu, J., Xiong, F., Sun, Y., Zhang, Y., Li, Y., Lv, Y., & Li, X. (2025). Constructing a Broad-Pore-Domain Structure of Adsorbents for Acteoside Adsorption. Polymers, 17(1), 79. https://doi.org/10.3390/polym17010079





