Single-Site Catalyst for the Synthesis of Disentangled Ultra-High-Molecular-Weight Polyethylene
Abstract
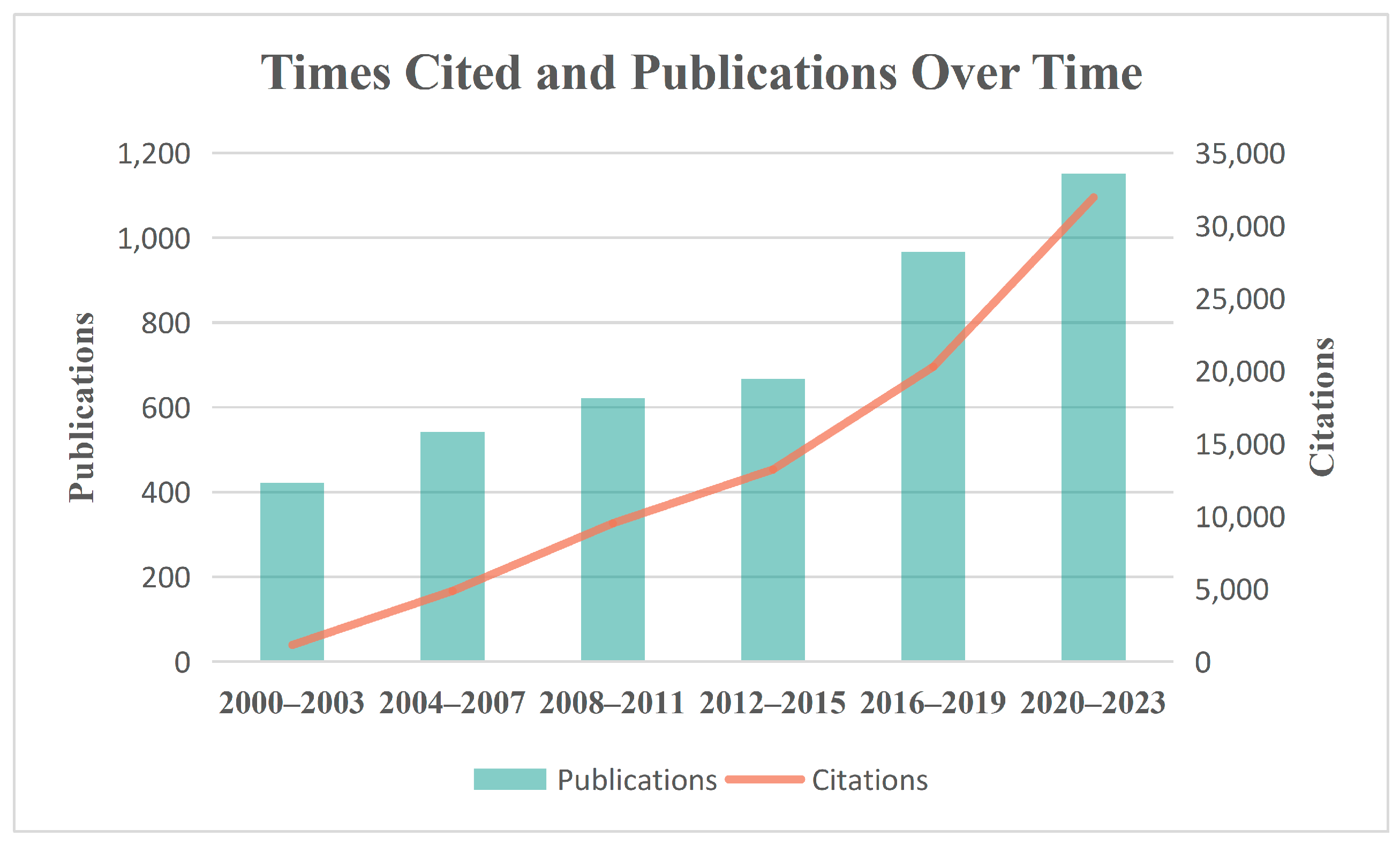
:1. Introduction
2. Homogeneous Single-Site Catalysis for Synthesizing d-UHMWPE
2.1. Homogeneous FI Catalyst for Synthesizing d-UHMWPE
| Entry | Pressure (Bar) | Cocatalyst | Al/Ti | Solvent b | Yield (g) | Activity c | Mw (106 g/mol) | MWD | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1.1 | MAO | 1200 | Toluene | 28.4 | 3.99 | 9.0 | 3.4 | [45] |
| 2 | 2.1 | MAO | 1200 | Toluene | 51.8 | 3.96 | 15.3 | 3.0 | [34] |
| 3 | 4.1 | MAO | 1200 | Toluene | 90.5 | 3.55 | 34.0 | 7.1 | [34] |
| 4 | 1.1 | MAO + 1.0 g BHT | 1200 | Toluene | 35.1 | 4.93 | 7.2 | 2.7 | [45] |
| 5 | 1.1 | PMAO | 2600 | Toluene | 20.0 | 2.9 | 10.7 | 3.6 | [47] |
| 6 | 1.1 | PMAO + 1.2 g BHT | 2600 | Toluene | 26.0 | 3.90 | 10.3 | 3.7 | [47] |
| 7 | 1.1 | MMAO12 | 2600 | Toluene | 23.0 | 3.30 | 7.1 | 6.7 | [47] |
| 8 | 1.1 | MMAO12 + 2.9 g BHT | 2600 | Toluene | 30.0 | 4.30 | 7.1 | 6.7 | [47] |
| 9 | 1.1 | MMAO3A | 2600 | Toluene | 3.0 | 4.00 | n.d. d | [47] | |
| 10 | 1.1 | MMAO3A + 1.6 g BHT | 2600 | Toluene | 30.0 | 4.50 | 8.5 | 3.0 | [47] |
| 11 e | 1.1–1.4 | MAO | 1200 | Toluene | 23.5 | 2.20 | 1.7 | 3.5 | [48] |
| 12 f | 1.1–1.4 | MAO | 1200 | T/H 25/70 | 15.0 | 1.20 | 0.9 | 4.3 | [48] |
| 13 f | 1.1–1.4 | MAO | 1200 | T/H 50/50 | 16.0 | 1.30 | 1.5 | 2 | [48] |
| 14 f | 1.1–1.4 | MAO | 1200 | T/H 75/25 | 30.2 | 2.50 | 2.2 | 3.2 | [48] |
| 15 f | 1.1–1.4 | MAO | 1200 | Heptane | 12.0 | 0.97 | 1.4 | 2.5 | [48] |
2.2. Other Types of Homogeneous Single-Site Catalysts for Synthesizing d-UHMWPE
3. Heterogeneous Single-Site Catalysis for Synthesizing d-UHMWPE
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kurtz, S.M. UHMWPE Biomaterials Handbook, 3rd ed.; Elsevier Academic Press: Amsterdam, The Netherlands, 2009. [Google Scholar] [CrossRef]
- An, L.; Shao, Z.; Armstrong, J.N.; Huang, Y.; Hu, Y.; Li, Z.; Faghihi, D.; Ren, S. Hierarchical structural engineering of ultrahigh-molecular-weight polyethylene. ACS Appl. Mater. Interfaces 2020, 12, 50024–50032. [Google Scholar] [CrossRef] [PubMed]
- Brach del Prever, E.M.; Bistolfi, A.; Bracco, P.; Costa, L. UHMWPE for arthroplasty: Past or future? J. Orthopaed. Traumatol. 2009, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Macuvele, D.L.P.; Nones, J.; Matsinhe, J.V.; Lima, M.M.; Soares, C.; Fiori, M.A.; Riella, H.G. Advances in ultra high molecular weight polyethylene/hydroxyapatite composites for biomedical applications: A brief review. Mater. Sci. Eng. C 2017, 76, 1248–1262. [Google Scholar] [CrossRef] [PubMed]
- Bahçe, E.; Emir, E. Investigation of wear of ultra high molecular weight polyethylene in a soft tissue behaviour knee joint prosthesis wear test simulator. J. Mater. Res. Technol. 2019, 8, 4642–4650. [Google Scholar] [CrossRef]
- Hussain, M.; Naqvi, R.A.; Abbas, N.; Khan, S.M.; Nawaz, S.; Hussain, A.; Zahra, N.; Khalid, M.W. Ultra-high-molecular-weight-polyethylene (UHMWPE) as a promising polymer material for biomedical applications: A concise review. Polymers 2020, 12, 323. [Google Scholar] [CrossRef]
- Stürzel, M.; Mihan, S.; Mülhaupt, R. From multisite polymerization catalysis to sustainable materials and all-polyolefin composites. Chem. Rev. 2016, 116, 1398–1433. [Google Scholar] [CrossRef]
- Kong, D.-C.; Yang, M.-H.; Zhang, X.-S.; Du, Z.-C.; Fu, Q.; Gao, X.-Q.; Gong, J.-W. Control of polymer properties by entanglement: A review. Macromol. Mater. Eng. 2021, 306, 2100536. [Google Scholar] [CrossRef]
- Peng, Q.; Shuai, Y.; Zhou, Q.; Chen, Y.; Dai, J.; Ren, C.; Huang, Z.; Li, W.; Wang, J.; Yang, Y. Reduction of chain entanglement of ethylene polymerization endowed by the dormancy effect of fluorosilicone microdroplets. Ind. Eng. Chem. Res. 2023, 62, 5804–5813. [Google Scholar] [CrossRef]
- Yue, Z.; Wang, N.; Cao, Y.; Li, W.; Dong, C. Reduced entanglement density of ultrahigh-molecular-weight polyethylene favored by the isolated immobilization on the MgCl2 (110) plane. Ind. Eng. Chem. Res. 2020, 59, 3351–3358. [Google Scholar] [CrossRef]
- Huang, Y.-F.; Xu, J.-Z.; Zhang, Z.-C.; Xu, L.; Li, L.-B.; Li, J.-F.; Li, Z.-M. Melt processing and structural manipulation of highly linear disentangled ultrahigh molecular weight polyethylene. Chem. Eng. J. 2017, 315, 132–141. [Google Scholar] [CrossRef]
- Ronca, S.; Forte, G.; Tjaden, H.; Rastogi, S. Solvent-Free Solid-state-processed tapes of ultrahigh-molecular weight polyethylene: Influence of molar mass and molar mass distribution on the tensile properties. Ind. Eng. Chem. Res. 2015, 54, 7373–7381. [Google Scholar] [CrossRef]
- Ronca, S.; Igarashi, T.; Forte, G.; Rastogi, S. Metallic-like thermal conductivity in a lightweight insulator: Solid-state processed ultra high molecular weight polyethylene tapes and films. Polymer 2017, 123, 203–210. [Google Scholar] [CrossRef]
- Kim, T.; Drakopoulos, S.X.; Ronca, S.; Minnich, A.J. Origin of high thermal conductivity in disentangled ultra-high molecular weight polyethylene films: Ballistic phonons within enlarged crystals. Nat. Commun. 2022, 13, 2452. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Di, Y.; Ye, C.; Zhang, L.; Tang, X.; Shu, B.; Yan, X.; Li, W.; Wang, J.; Yang, Y. Morphology evolution and mechanical property enhancement of linear low-density polyethylene by adding disentangled ultrahigh molecular weight polyethylene. Polym. Adv. Technol. 2022, 33, 1047–1056. [Google Scholar] [CrossRef]
- Ferreira, A.E.; Ribeiro, M.R.; Cramail, H.; Lourenço, J.P.; Lorenzo, V.; Pérez, E.; Cerrada, M.L. Extraordinary mechanical performance in disentangled UHMWPE films processed by compression molding. J. Mech. Behav. Biomed. Mater. 2019, 90, 202–207. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, X.; Zhao, S.; Ye, C.; Zhang, Z.; Kuo, S.-W.; Xin, Z. Study on the effects of soluble poss on chain disentanglement in UHMWPE polymerization. Polymer 2022, 244, 124561. [Google Scholar] [CrossRef]
- Bajya, M.; Majumdar, A.; Butola, B.S.; Jasra, R.V. Exploration of disentangled UHMWPE tape as a soft body armour material. Mater. Chem. Phys. 2023, 295, 127162. [Google Scholar] [CrossRef]
- Christakopoulos, F.; Busato, S.P.; Kong, X.; Troisi, E.; Friederichs, N.; Tervoort, T.A. Solid-state extrusion of nascent disentangled ultra-high molecular weight polyethylene. Polym. Eng. Sci. 2024, 64, 3606–3616. [Google Scholar] [CrossRef]
- Fang, X.; Wyatt, T.; Hong, Y.; Yao, D. Gel spinning of UHMWPE fibers with polybutene as a new spin solvent. Polym. Eng. Sci. 2016, 56, 697–706. [Google Scholar] [CrossRef]
- Smith, P.; Lemstra, P.J. Ultra-high-strength polyethylene filaments by solution spinning/drawing. J. Mater. Sci. 1980, 15, 505–514. [Google Scholar] [CrossRef]
- Smith, P.; Chanzy, H.D.; Rotzinger, B.P. Drawing of virgin ultrahigh molecular weight polyethylene: An alternative route to high strength/high modulus materials. J. Mater. Sci. 1987, 22, 523–531. [Google Scholar] [CrossRef]
- Wu, S.-L.; Qiao, J.; Guan, J.; Chen, H.-M.; Wang, T.; Wang, C.; Wang, Y. Nascent disentangled UHMWPE: Origin, synthesis, processing, performances and applications. Eur. Polym. J. 2023, 184, 111799. [Google Scholar] [CrossRef]
- Tan, C.; Chen, C. Nickel catalysts for the synthesis of ultra-high molecular weight polyethylene. Sci. Bull. 2020, 65, 1137–1138. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.-F.; Yan, Y.; Solan, G.A.; Ma, Y.; Sun, W.-H. Recent advancements in N-ligated group 4 molecular catalysts for the (co)polymerization of ethylene. Coord. Chem. Rev. 2020, 411, 213254. [Google Scholar] [CrossRef]
- Antonov, A.A.; Bryliakov, K.P. Post-metallocene catalysts for the synthesis of ultrahigh molecular weight polyethylene: Recent advances. Eur. Polym. J. 2021, 142, 110162. [Google Scholar] [CrossRef]
- Hlatky, G.G. Single-site catalysts for olefin polymerization: Annual review for 1997. Coord. Chem. Rev. 2000, 199, 235–329. [Google Scholar] [CrossRef]
- Heurtefeu, B.; Bouilhac, C.; Cloutet, É.; Taton, D.; Deffieux, A.; Cramail, H. Polymer support of “single-site” catalysts for heterogeneous olefin polymerization. Prog. Polym. Sci. 2011, 36, 89–126. [Google Scholar] [CrossRef]
- Rodrigues, A.-S.; Kirillov, E.; Carpentier, J.-F. Group 3 and 4 single-site catalysts for stereospecific polymerization of styrene. Coord. Chem. Rev. 2008, 252, 2115–2136. [Google Scholar] [CrossRef]
- Kumar, S.; Dholakiya, B.Z.; Jangir, R. Role of organometallic complexes in olefin polymerization: A review report. J. Organomet. Chem. 2021, 953, 122066. [Google Scholar] [CrossRef]
- Alrais, L.; Maksoud, W.A.; Werghi, B.; Bendjeriou-Sedjerari, A.; Abou-Hamad, E.; Hedhili, M.N.; Basset, J.-M. A strategy for high ethylene polymerization performance using titanium single-site catalysts. Chem. Commun. 2023, 59, 12503–12506. [Google Scholar] [CrossRef]
- Stürzel, M.; Kempe, F.; Thomann, Y.; Mark, S.; Enders, M.; Mülhaupt, R. Novel graphene UHMWPE nanocomposites prepared by polymerization filling using single-site catalysts supported on functionalized graphene nanosheet dispersions. Macromolecules 2012, 45, 6878–6887. [Google Scholar] [CrossRef]
- Gote, R.P.; van der Eem, J.; Zhao, J.; Lolage, S.; Traidia, A.; Zhang, Y.; Romano, D.; Rastogi, S. Influence of molecular weight and entanglement density on the creep response of the uniaxially drawn tapes of dis-UHMWPE. Macromolecules 2023, 56, 6903–6919. [Google Scholar] [CrossRef]
- Romano, D.; Tops, N.; Andablo-Reyes, E.; Ronca, S.; Rastogi, S. Influence of polymerization conditions on melting kinetics of low entangled UHMWPE and its implications on mechanical properties. Macromolecules 2014, 47, 4750–4760. [Google Scholar] [CrossRef]
- Pandey, A.; Champouret, Y.; Rastogi, S. Heterogeneity in the distribution of entanglement density during polymerization in disentangled ultrahigh molecular weight polyethylene. Macromolecules 2011, 44, 4952–4960. [Google Scholar] [CrossRef]
- Yao, Y.F.; Rastogi, S.; Xue, H.J.; Chen, Q.; Graf, R.; Verhoef, R. Segmental mobility in the noncrystalline regions of nascent polyethylene synthesized using two different catalytic systems with implications on solid-state deformation. Polymer 2013, 54, 411–422. [Google Scholar] [CrossRef]
- Makio, H.; Kashiwa, N.; Fujita, T. FI catalysts: A new family of high performance catalysts for olefin polymerization. Adv. Synth. Catal. 2002, 344, 477–493. [Google Scholar] [CrossRef]
- Mitani, M.; Saito, J.; Ishii, S.-I.; Nakayama, Y.; Makio, H.; Matsukawa, N.; Matsui, S.; Mohri, J.; Furuyama, R.; Terao, H.; et al. FI catalysts: New olefin polymerization catalysts for the creation of value-added polymers. Chem. Rec. 2004, 4, 137–158. [Google Scholar] [CrossRef]
- Matsugi, T.; Fujita, T. High-performance olefin polymerization catalysts discovered on the basis of a new catalyst design concept. Chem. Soc. Rev. 2008, 37, 1264–1277. [Google Scholar] [CrossRef]
- Mitani, M.; Nakano, T.; Fujita, T. Unprecedented living olefin polymerization derived from an attractive interaction between a ligand and a growing polymer chain. Chem. Eur. J. 2003, 9, 2396–2403. [Google Scholar] [CrossRef]
- Talebi, S.; Duchateau, R.; Rastogi, S.; Kaschta, J.; Peters, G.W.M.; Lemstra, P.J. Molar mass and molecular weight distribution determination of UHMWPE synthesized using a living homogeneous catalyst. Macromolecules 2010, 43, 2780–2788. [Google Scholar] [CrossRef]
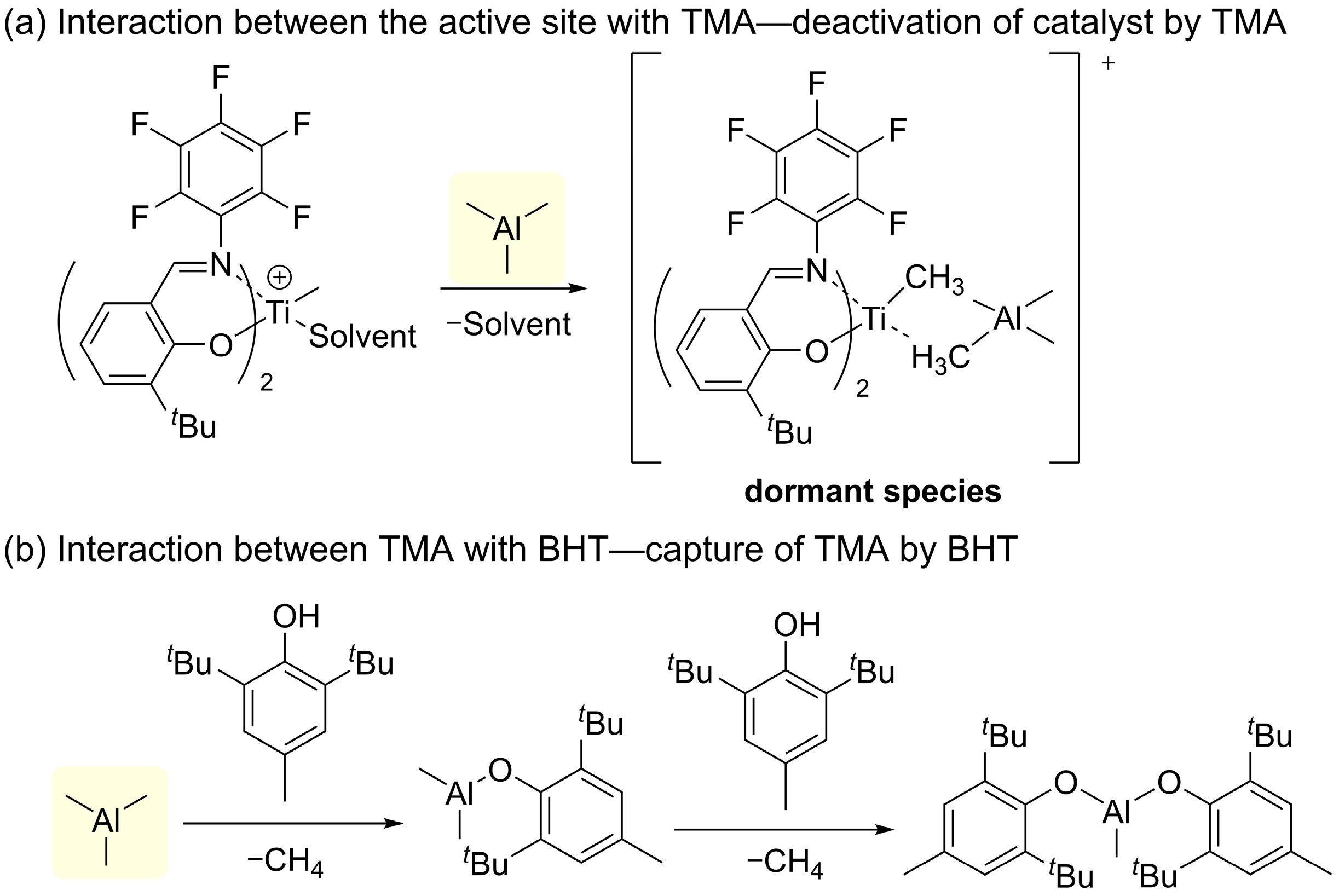
- Busico, V.; Cipullo, R.; Cutillo, F.; Friederichs, N.; Ronca, S.; Wang, B. Improving the performance of methylalumoxane: A facile and efficient method to trap “free” trimethylaluminum. J. Am. Chem. Soc. 2003, 125, 12402–12403. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Zhang, T.; Hou, L.; Qu, S.; Li, X.; Wang, W. The third compound promoted ethylene homopolymerization and copolymerization with 1-octene by using metallocene catalyst. J. Polym. Res. 2024, 31, 158. [Google Scholar] [CrossRef]
- Wang, W.; Guo, T.; Qu, S.; Zhang, T.; Li, X. The third compound promoted copolymerization of ethylene with 4-penten-1-ol by using metallocene catalyst. Macromol. Chem. Phys. 2024, 225, 2400066. [Google Scholar] [CrossRef]
- Romano, D.; Andablo-Reyes, E.A.; Ronca, S.; Rastogi, S. Effect of a cocatalyst modifier in the synthesis of ultrahigh molecular weight polyethylene having reduced number of entanglements. J. Polym. Sci. A Polym. Chem. 2013, 51, 1630–1635. [Google Scholar] [CrossRef]
- Ronca, S.; Romano, D.; Forte, G.; Andablo-Reyes, E. Improving the performance of a catalytic system for the synthesis of ultra high molecular weight polyethylene with a reduced number of entanglements. Adv. Polym. Technol. 2012, 31, 193–204. [Google Scholar] [CrossRef]
- Romano, D.; Andablo-Reyes, E.; Ronca, S.; Rastogi, S. Aluminoxane co-catalysts for the activation of a bis phenoxyimine titanium (IV) catalyst in the synthesis of disentangled ultra-high molecular weight polyethylene. Polymer 2015, 74, 76–85. [Google Scholar] [CrossRef]
- Forte, G.; Ronca, S. Synthesis of disentangled ultra-high molecular weight polyethylene: Influence of reaction medium on material properties. Int. J. Polym. Sci. 2017, 2017, 7431419. [Google Scholar] [CrossRef]
- Romano, D.; Ronca, S.; Rastogi, S. A Hemi-metallocene chromium catalyst with trimethylaluminum-free methylaluminoxane for the synthesis of disentangled ultra-high molecular weight polyethylene. Macromol. Rapid Commun. 2015, 36, 327–331. [Google Scholar] [CrossRef]
- Bodkhe, D.V.; Chikkali, S.H. Ti-iminocarboxylate catalyzed polymerization of ethylene to highly crystalline, disentangled, ultrahigh molecular weight polyethylene. Eur. Polym. J. 2023, 182, 111725. [Google Scholar] [CrossRef]
- Nomura, K.; Sagara, A.; Imanishi, Y. Olefin polymerization and ring-opening metathesis polymerization of norbornene by (arylimido)(aryloxo)vanadium(V) complexes of the type VX2(NAr)(OAr’). Remarkable effect of aluminum cocatalyst for the coordination and insertion and ring-opening metathesis polymerization. Macromolecules 2002, 35, 1583–1590. [Google Scholar] [CrossRef]
- Wang, W.; Nomura, K. Notable effects of aluminum alkyls and solvents for highly efficient ethylene (co)polymerizations catalyzed by (arylimido)-(aryloxo)vanadium complexes. Adv. Synth. Catal. 2006, 348, 743–750. [Google Scholar] [CrossRef]
- Spronck, M.; Klein, A.; Blom, B.; Romano, D. Synthesis of disentangled ultra-high molecular weight polyethylene using vanadium(V)-based catalysts. Z. Anorg. Allg. Chem. 2018, 644, 993–998. [Google Scholar] [CrossRef]
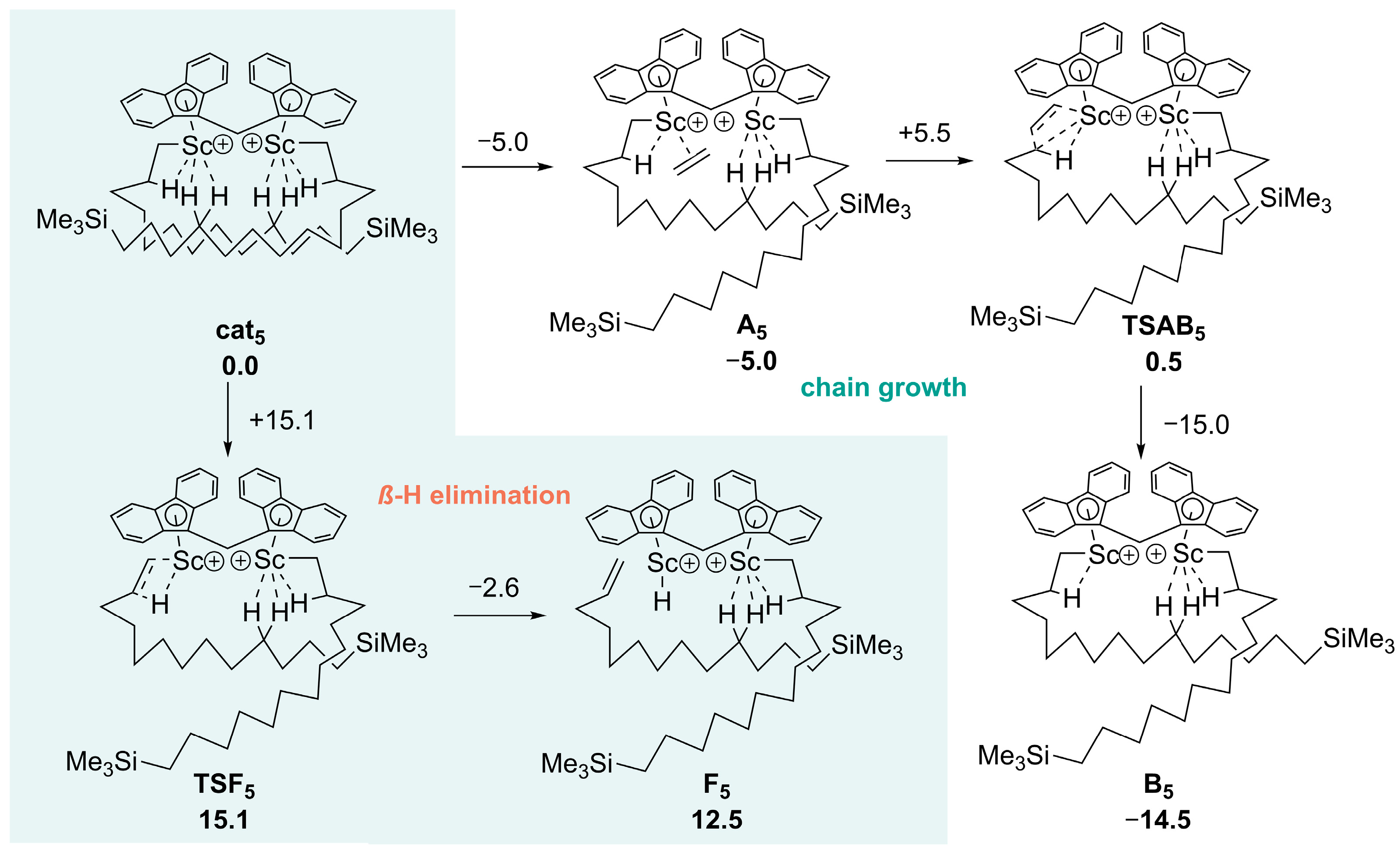
- Zhang, Z.; Kang, X.; Jiang, Y.; Cai, Z.; Li, S.; Cui, D. Access to disentangled ultrahigh molecular weight polyethylene via a binuclear synergic effect. Angew. Chem. Int. Ed. 2023, 62, e202215582. [Google Scholar] [CrossRef] [PubMed]
- Stalzer, M.M.; Delferro, M.; Marks, T.J. Supported single-site organometallic catalysts for the synthesis of high-performance polyolefins. Catal. Lett. 2015, 145, 3–14. [Google Scholar] [CrossRef]
- Ronca, S.; Forte, G.; Tjaden, H.; Yao, Y.; Rastogi, S. Tailoring molecular structure via nanoparticles for solvent-free processing of ultra-high molecular weight polyethylene composites. Polymer 2012, 53, 2897–2907. [Google Scholar] [CrossRef]
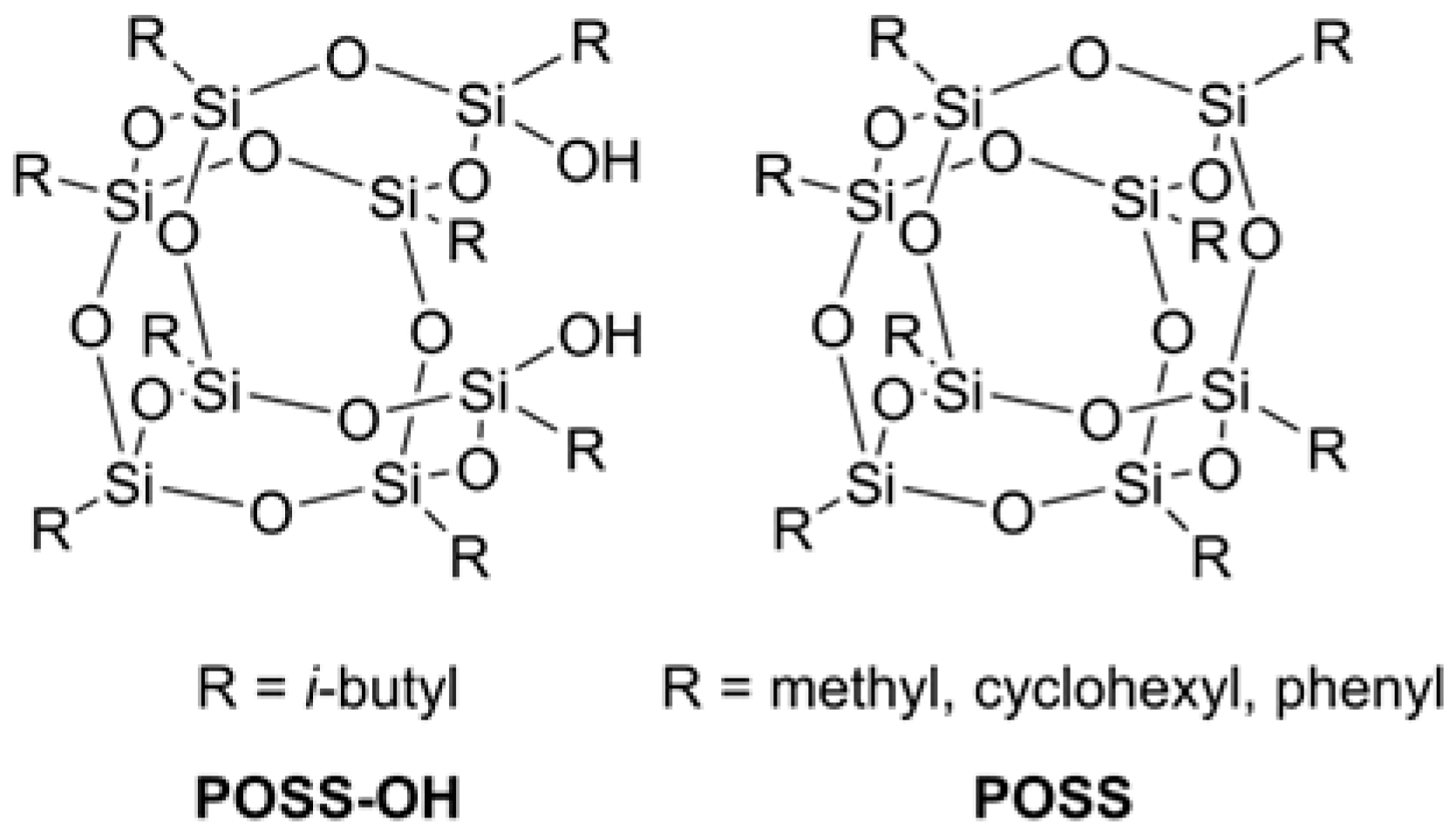
- Li, W.; Guan, C.; Xu, J.; Mu, J.; Gong, D.; Chen, Z.; Zhou, Q. Disentangled UHMWPE/POSS nanocomposites prepared by ethylene in situ polymerization. Polymer 2014, 55, 1792–1798. [Google Scholar] [CrossRef]
- Li, W.; Chen, T.; Guan, C.; Gong, D.; Mu, J.; Chen, Z.; Zhou, Q. Influence of polyhedral oligomeric silsesquioxane structure on the disentangled state of ultrahigh molecular weight polyethylene nanocomposites during ethylene in situ polymerization. Ind. Eng. Chem. Res. 2015, 54, 1478–1486. [Google Scholar] [CrossRef]
- Heidari, A.; Talebi, S.; Rezaei, M.; Keshavarz-Mirzamohamadi, H.; Jafariyeh-Yazdi, E. In situ synthesis of ultrahigh molecular weight polyethylene/graphene oxide nanocomposite using the immobilized single-site catalyst. Polymer Plast. Tech. Eng. 2018, 57, 1313–1324. [Google Scholar] [CrossRef]
- Gote, R.P.; Romano, D.; van der Eem, J.; Zhao, J.; Zhou, F.; Rastogi, S. Unprecedented mechanical properties in linear uhmwpe using a heterogeneous catalytic system. Macromolecules 2023, 56, 361–378. [Google Scholar] [CrossRef]
- Collins Rice, C.G.; Buffet, J.-C.; Turner, Z.R.; O’Hare, D. Supported permethylindenyl titanium catalysts for the synthesis of disentangled ultra-high molecular weight polyethylene (disUHMWPE). Chem. Commun. 2021, 57, 8600–8603. [Google Scholar] [CrossRef]
- Alt, H.G.; Reb, A.; Milius, W.; Weis, A. Amido functionalized ansa half-sandwich dichloride complexes of titanium, zirconium and hafnium with alkyl and ω-alkenyl substituents as homogeneous and self-immobilizing catalyst precursors for ethylene polymerization. J. Organomet. Chem. 2001, 628, 169–182. [Google Scholar] [CrossRef]
- Alt, H.G. The heterogenization of homogeneous metallocene catalysts for olefin polymerization. J. Chem. Soc. Dalton Trans. 1999, 1703–1710. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, S.-L. Self-immobilized polyolefin catalysts: A simple and transitional platform for heterogeneous olefin polymerization. Mater. Today Chem. 2024, 35, 101898. [Google Scholar] [CrossRef]
- Ivancheva, N.I.; Malinskaya, M.Y.; Oleinik, I.I.; Khaikin, S.Y.; Ivanchev, S.S.; Tolstikov, G.A. Features of self-immobilization of titanium phenoxyimine complexes in ethylene polymerization. Dokl. Phys. Chem. 2007, 417, 301–303. [Google Scholar] [CrossRef]
- Ivanchev, S.S.; Vasil’eva, M.Y.; Ivancheva, N.I.; Badaev, V.K.; Oleinik, I.I.; Sviridova, E.V.; Tolstikov, G.A. Polymerization of ethylene with self-immobilizing bis(phenoxyimine) catalytic systems. Polym. Sci. Ser. B 2009, 51, 276–282. [Google Scholar] [CrossRef]
- Ivancheva, N.I.; Badaev, V.K.; Sviridova, E.V.; Nikolaev, D.A.; Oleinik, I.V.; Ivanchev, S.S. Specific features of ethylene polymerization on self-immobilizing catalytic systems based on titanium bis(phenoxy imine) complexes. Russ. J. Appl. Chem. 2011, 84, 118–123. [Google Scholar] [CrossRef]
- Ivancheva, N.I.; Khaikin, S.Y.; Sviridova, E.V.; Fedorov, S.P.; Sanieva, D.V.; Molev, O.V.; Oleinik, I.V.; Ivanchev, S.S. Multicentered self-immobilized ethylene polymerization catalysts based on functionalized titanium halide salicylaldiminate complexes for the synthesis of ultra-high-molecular-weight polyethylene. Russ. J. Appl. Chem. 2012, 85, 1404–1412. [Google Scholar] [CrossRef]
- Oleynik, I.V.; Shundrina, I.K.; Oleyinik, I.I. Highly active titanium(IV) dichloride FI catalysts bearing a diallylamino group for the synthesis of disentangled UHMWPE. Polym. Adv. Technol. 2020, 31, 1921–1934. [Google Scholar] [CrossRef]
- Osichow, A.; Rabe, C.; Vogtt, K.; Narayanan, T.; Harnau, L.; Drechsler, M.; Ballauff, M.; Mecking, S. Ideal polyethylene nanocrystals. J. Am. Chem. Soc. 2013, 135, 11645–11650. [Google Scholar] [CrossRef]
- Mecking, S.; Schnitte, M. Neutral nickel(II) catalysts: From hyperbranched oligomers to nanocrystal-based materials. Acc. Chem. Res. 2020, 53, 2738–2752. [Google Scholar] [CrossRef]
- Kenyon, P.; Wörner, M.; Mecking, S. Controlled polymerization in polar solvents to ultrahigh molecular weight polyethylene. J. Am. Chem. Soc. 2018, 140, 6685–6689. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, P.; Mecking, S. Pentafluorosulfanyl substituents in polymerization catalysis. J. Am. Chem. Soc. 2017, 139, 13786–13790. [Google Scholar] [CrossRef] [PubMed]
- Mecking, S.; Held, A.; Bauers, F.M. Aqueous catalytic polymerization of olefins. Angew. Chem. Int. Ed. 2002, 41, 544–561. [Google Scholar] [CrossRef]
- Wimmer, F.P.; Ebel, V.; Schmidt, F.; Mecking, S. Compartmentalized polymerization in aqueous and organic media to low-entangled ultra high molecular weight polyethylene. Polym. Chem. 2021, 12, 3116–3123. [Google Scholar] [CrossRef]






| Entry | Pre-Catalyst | Temp. (°C) | Pressure (Bar) | Time (Min) | Yield (g) | Activity b | Mw (106 g/mol) | MWD |
|---|---|---|---|---|---|---|---|---|
| 1 | 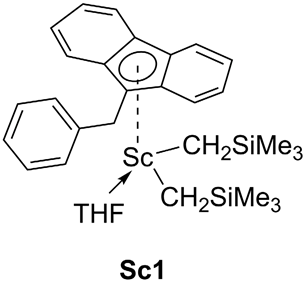 | 80 | 4 | 1 | 28.4 | 0.45 | 0.4 | 2.6 |
| 2 |  | 80 | 4 | 1 | 51.8 | 0.38 | 0.2 | 6.0 |
| 3 |  | 80 | 4 | 1 | 90.5 | 1.42 | 1.2 | 2.0 |
| 4 | 20 | 4 | 1 | 35.1 | 1.80 | 2.8 | 1.9 | |
| 5 | 60 | 4 | 1 | 20.0 | 1.50 | 1.8 | 1.7 | |
| 6 | 120 | 4 | 1 | 26.0 | 5.40 | 1.2 | 1.6 | |
| 7 | 80 | 2 | 1 | 23.0 | 1.80 | 1.3 | 2.9 | |
| 8 | 80 | 6 | 1 | 30.0 | 1.00 | 1.9 | 2.3 | |
| 9 c | 25 | 13 | 10 | 12.0 | 1.82 | 1.5 | 2.6 |
| Entry | Support Type | FI Catalyst (μM) | AlMAO/Ti | AlTIBA (mmol) | Heptane (L) | Yield (g) | Activity b |
|---|---|---|---|---|---|---|---|
| 1 | TiO2 | 11 | 73 | 2 | 0.2 | 7.8 | 6.82 |
| 2 | ZrO2 | 11 | 68 | 2 | 0.2 | 3.0 | 2.45 |
| 3 | Hap | 8.8 | 20 | 2 | 0.2 | 3.0 | 3.03 |
| 4 | CNT | 30 | 133 | 25 | 5.0 | 22.0 | 6.24 |
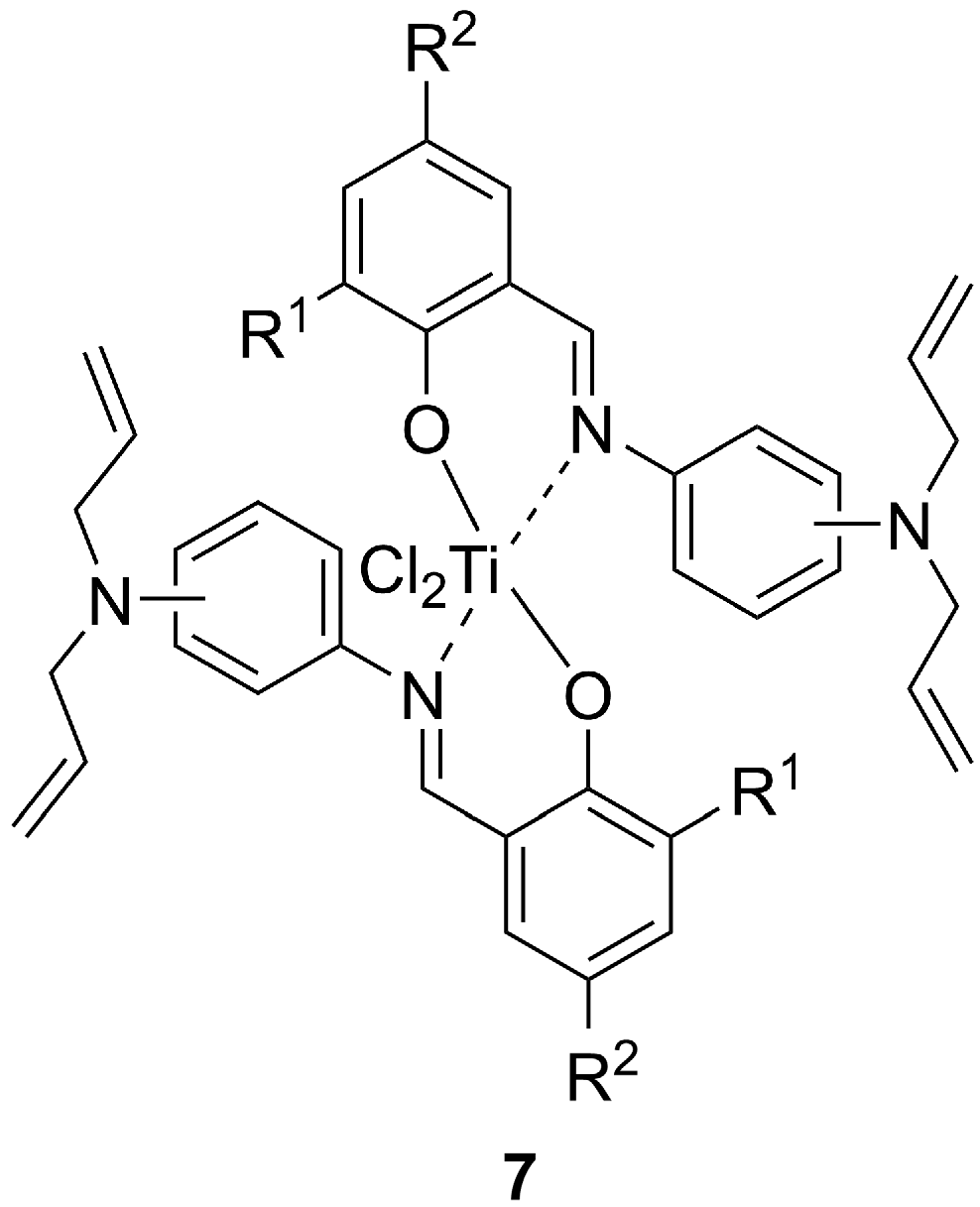
| Entry | Pre-Catalyst | Yield (g) | Activity b | Mw (106 g/mol) | Tm | Χ (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| R1 | R2 | N(Allyl)2 | 1° | 2° | 1° | 2° | ||||
| 1 | t-Bu | H | m | 3.3 | 6.85 | 1.0 | 140.4 | 136.0 | 85.6 | 53.0 |
| 2 | t-Bu | Me | m | 2.7 | 5.65 | 1.3 | 141.0 | 135.4 | 80.8 | 50.0 |
| 3 | t-Bu | OMe | m | 5.2 | 10.80 | 1.4 | 137.5 | 136.0 | 83.3 | 60.6 |
| 4 | t-Bu | t-Bu | m | 2.4 | 5.06 | 2.3 | 143.7 | 136.5 | 83.5 | 52.1 |
| 5 | CMe2(Ph) | H | m | 6.7 | 14.00 | 3.0 | 140.1 | 134.9 | 83.1 | 49.3 |
| 6 | CMe2(Ph) | Me | m | 4.1 | 8.60 | 4.1 | 140.8 | 136.2 | 84.6 | 47.3 |
| 7 | CMe2(Ph) | OMe | m | 2.5 | 5.17 | 2.4 | 139.8 | 135.9 | 85.3 | 53.8 |
| 8 | CMe2(Ph) | t-Bu | m | 5.5 | 11.50 | 2.3 | 140.5 | 136.1 | 84.6 | 48.7 |
| 9 | t-Bu | H | o | 5.2 | 10.90 | 1.1 | 137.7 | 136.2 | 82.8 | 58.1 |
| 10 | t-Bu | Me | o | 3.0 | 6.17 | 1.2 | 137.6, 140.7 | 136.2 | 83.9 | 56.6 |
| 11 | t-Bu | OMe | o | 4.5 | 9.35 | 0.9 | 137.0, 140.4 | 132.8 | 83.1 | 53.5 |
| 12 | t-Bu | t-Bu | o | 4.4 | 9.27 | 1.0 | 139.6, 142.8 | 137.0 | 79.3 | 51.6 |
| 13 | CMe2(Ph) | H | o | 3.4 | 7.04 | 1.5 | 137.5 | 136.6 | 82.9 | 62.6 |
| 14 | CMe2(Ph) | Me | o | 2.1 | 4.29 | 1.0 | 137.6 | 136.9 | 78.7 | 58.2 |
| 15 | CMe2(Ph) | OMe | o | 2.4 | 5.08 | 0.7 | 136.5 | 129.1 | 78.2 | 52.7 |
| 16 | CMe2(Ph) | t-Bu | o | 2.8 | 5.85 | 1.4 | 138.2 | 133.3 | 78.2 | 53.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Qu, S.; Li, X.; Wei, Y.; Li, Q.; Wen, Z.; Guo, Z. Single-Site Catalyst for the Synthesis of Disentangled Ultra-High-Molecular-Weight Polyethylene. Polymers 2025, 17, 95. https://doi.org/10.3390/polym17010095
Chen J, Qu S, Li X, Wei Y, Li Q, Wen Z, Guo Z. Single-Site Catalyst for the Synthesis of Disentangled Ultra-High-Molecular-Weight Polyethylene. Polymers. 2025; 17(1):95. https://doi.org/10.3390/polym17010095
Chicago/Turabian StyleChen, Jian, Shuzhang Qu, Xinwei Li, Yiming Wei, Qian Li, Zhao Wen, and Zifang Guo. 2025. "Single-Site Catalyst for the Synthesis of Disentangled Ultra-High-Molecular-Weight Polyethylene" Polymers 17, no. 1: 95. https://doi.org/10.3390/polym17010095
APA StyleChen, J., Qu, S., Li, X., Wei, Y., Li, Q., Wen, Z., & Guo, Z. (2025). Single-Site Catalyst for the Synthesis of Disentangled Ultra-High-Molecular-Weight Polyethylene. Polymers, 17(1), 95. https://doi.org/10.3390/polym17010095





