The Influence of Iron Particles and Polyethylene Glycol on Selected Properties of Polylactide-Based Composites
Abstract
:1. Introduction
2. Materials and Methods
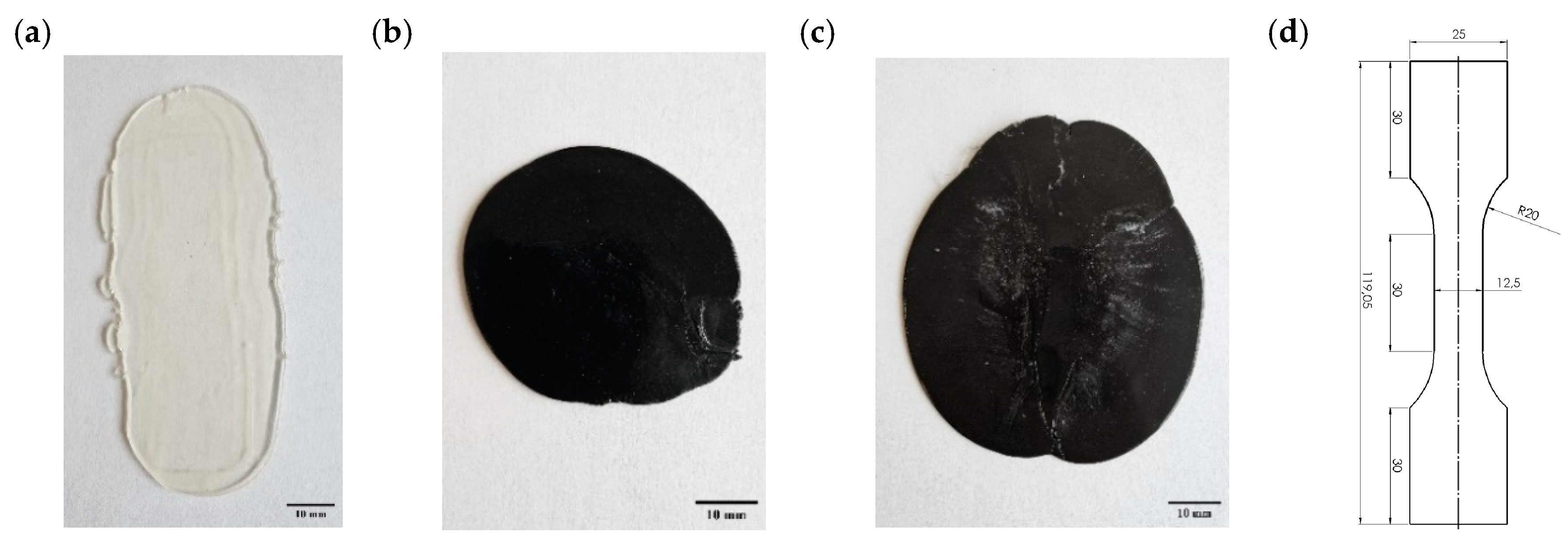
2.1. Samples Preparation
2.2. Hardness, Tensile Tests, and Microstructure Observations
2.3. Density and Weight Loss Tests
2.4. Differential Scanning Calorimetry
2.5. Physicochemical Properties in Saline Solution
3. Results and Discussion
3.1. Tensile and Hardness Properties
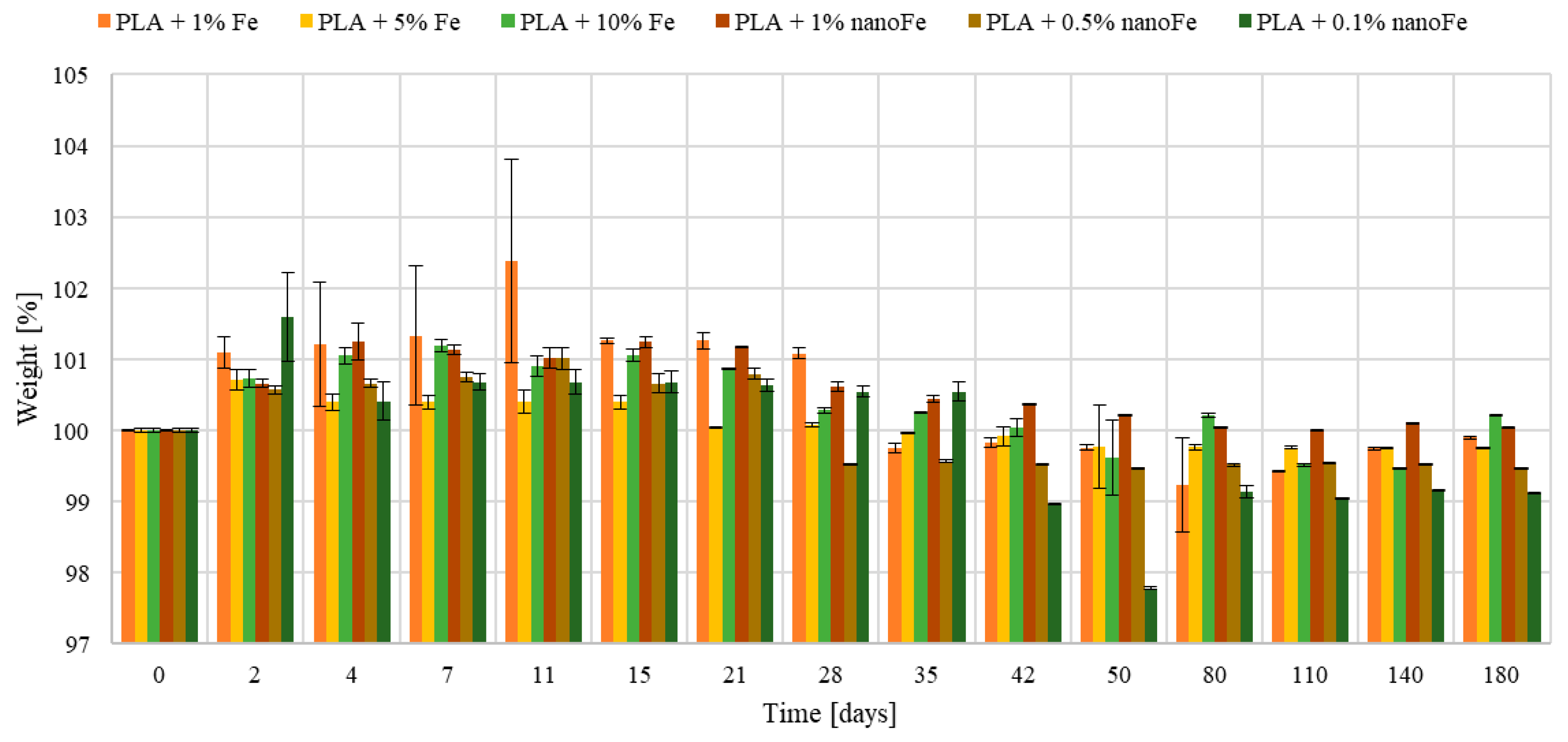
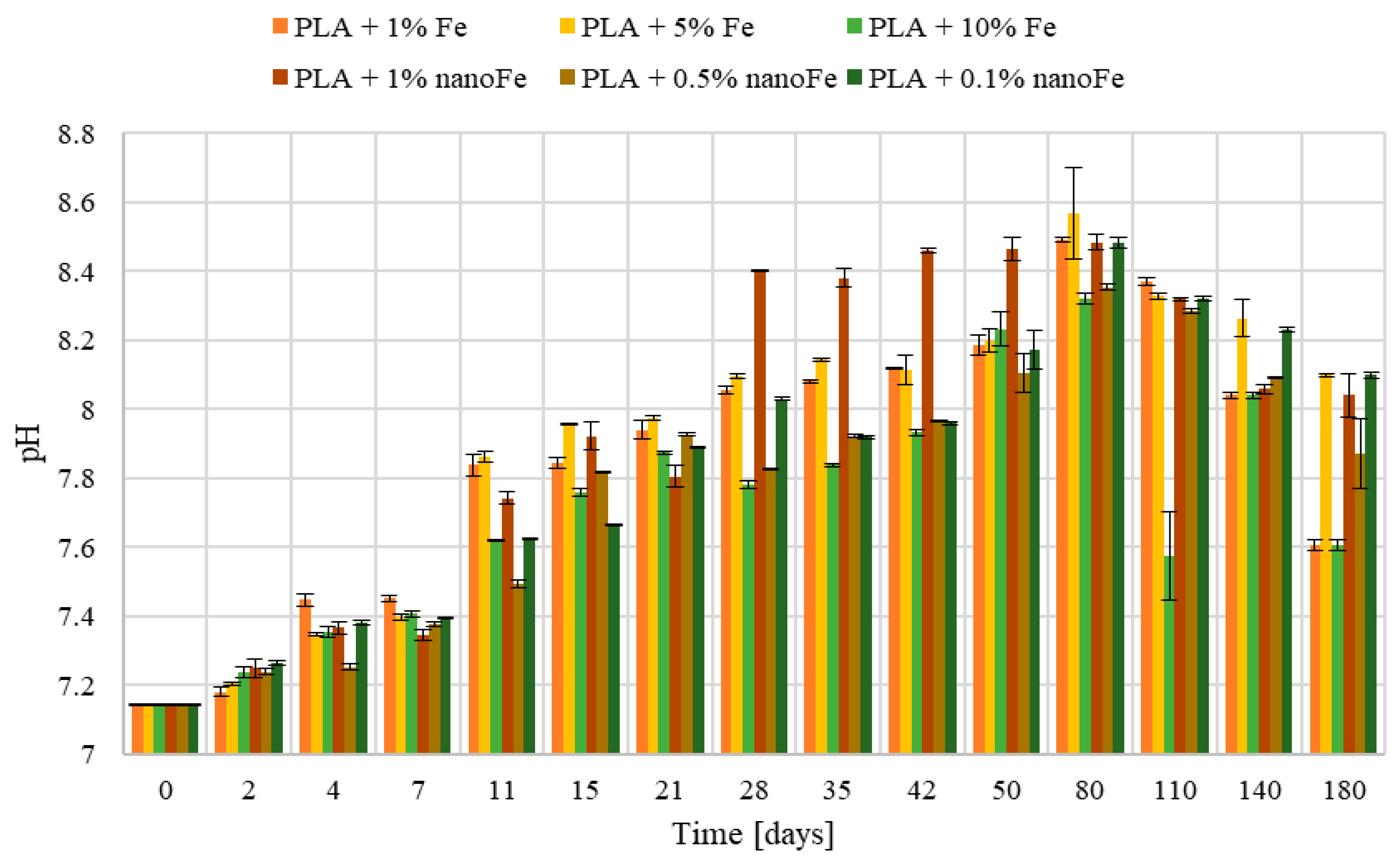
3.2. Mass, pH, and Conductivity Changes
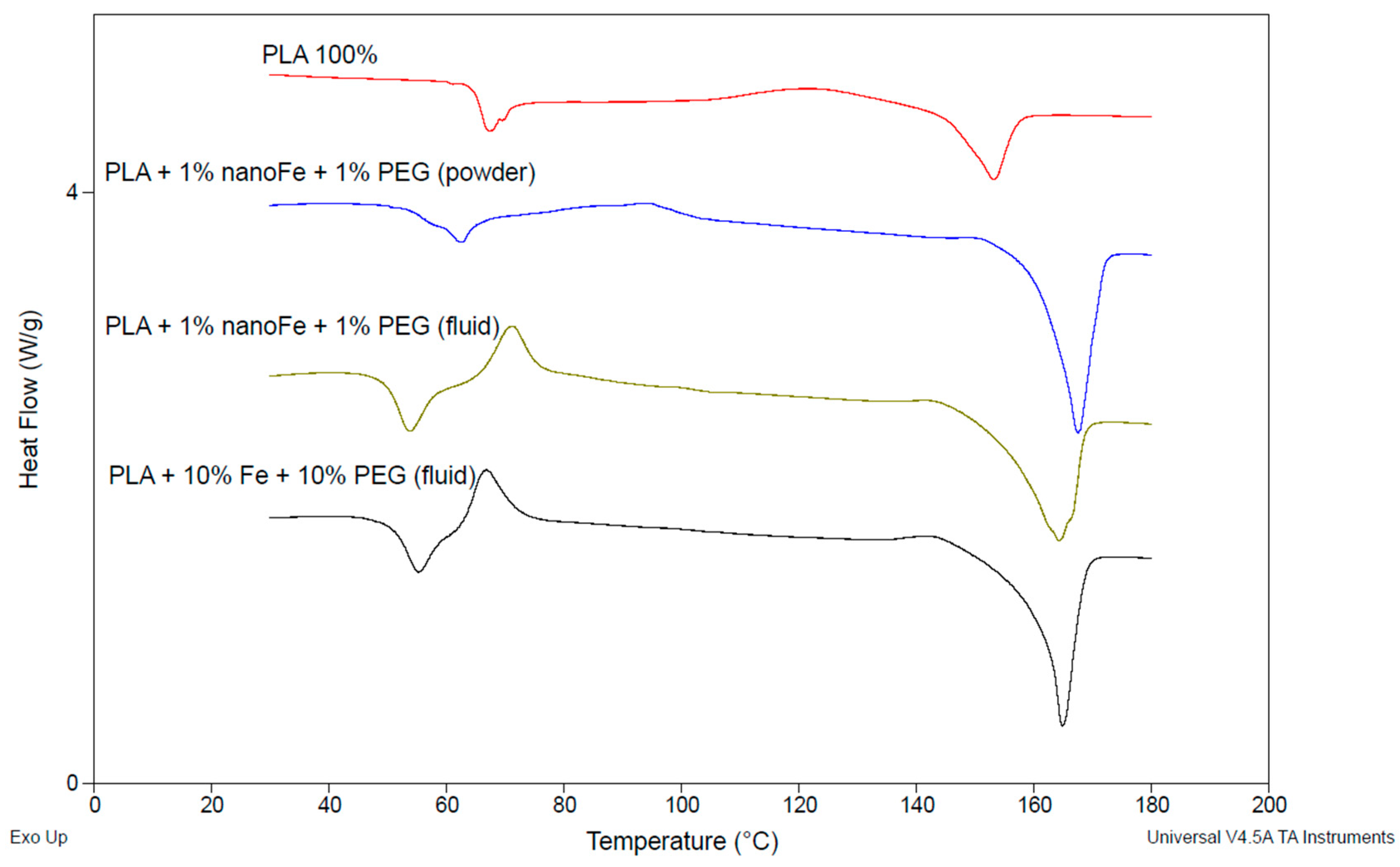
3.3. DSC Test Results
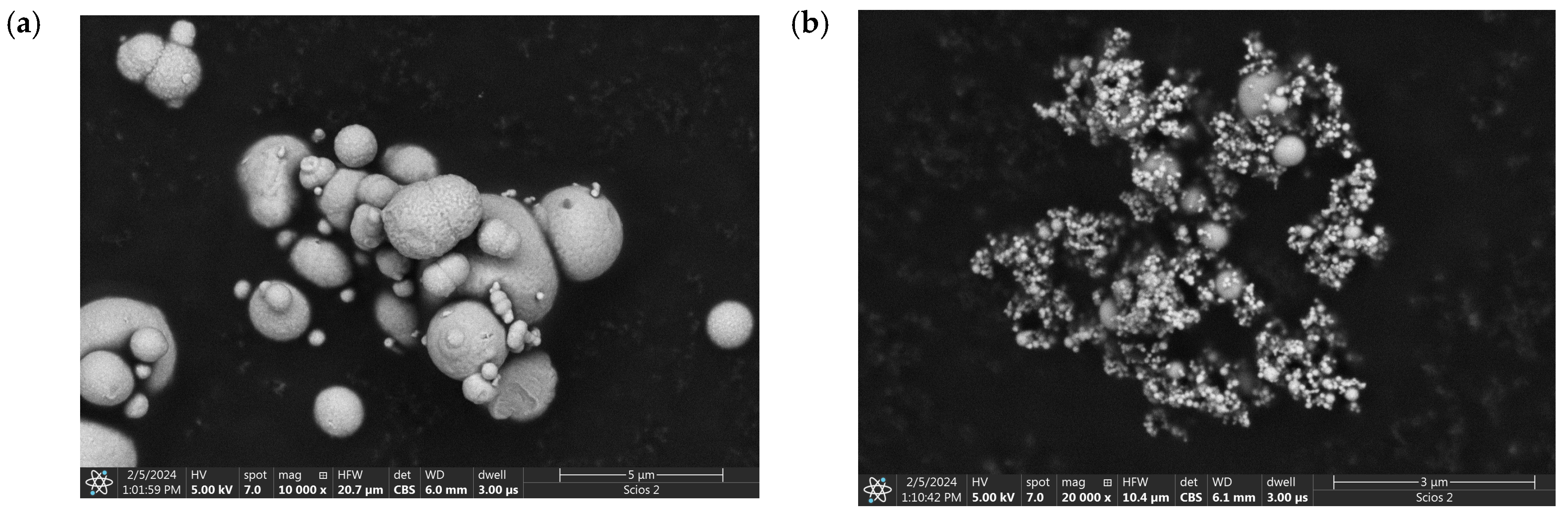
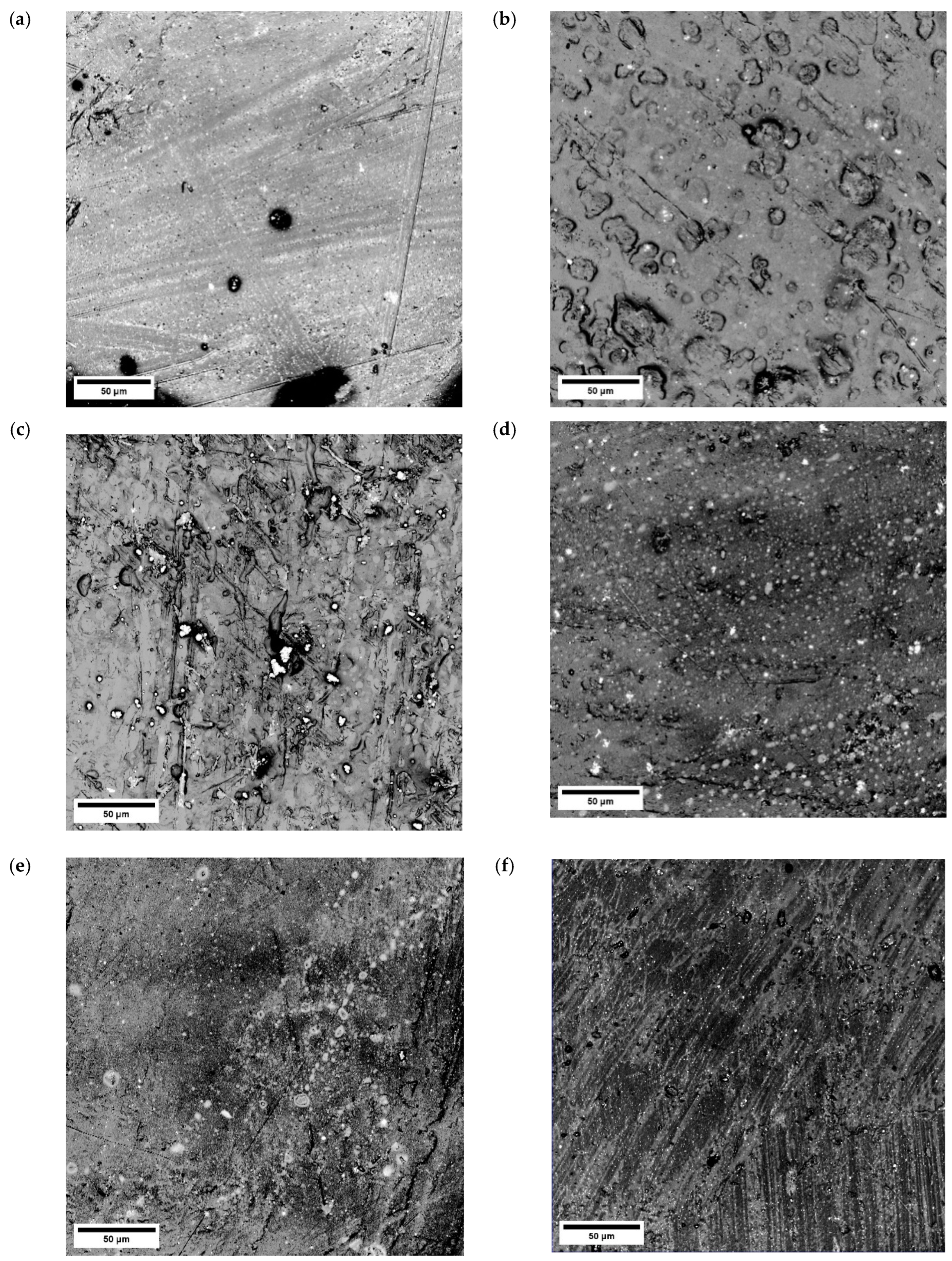
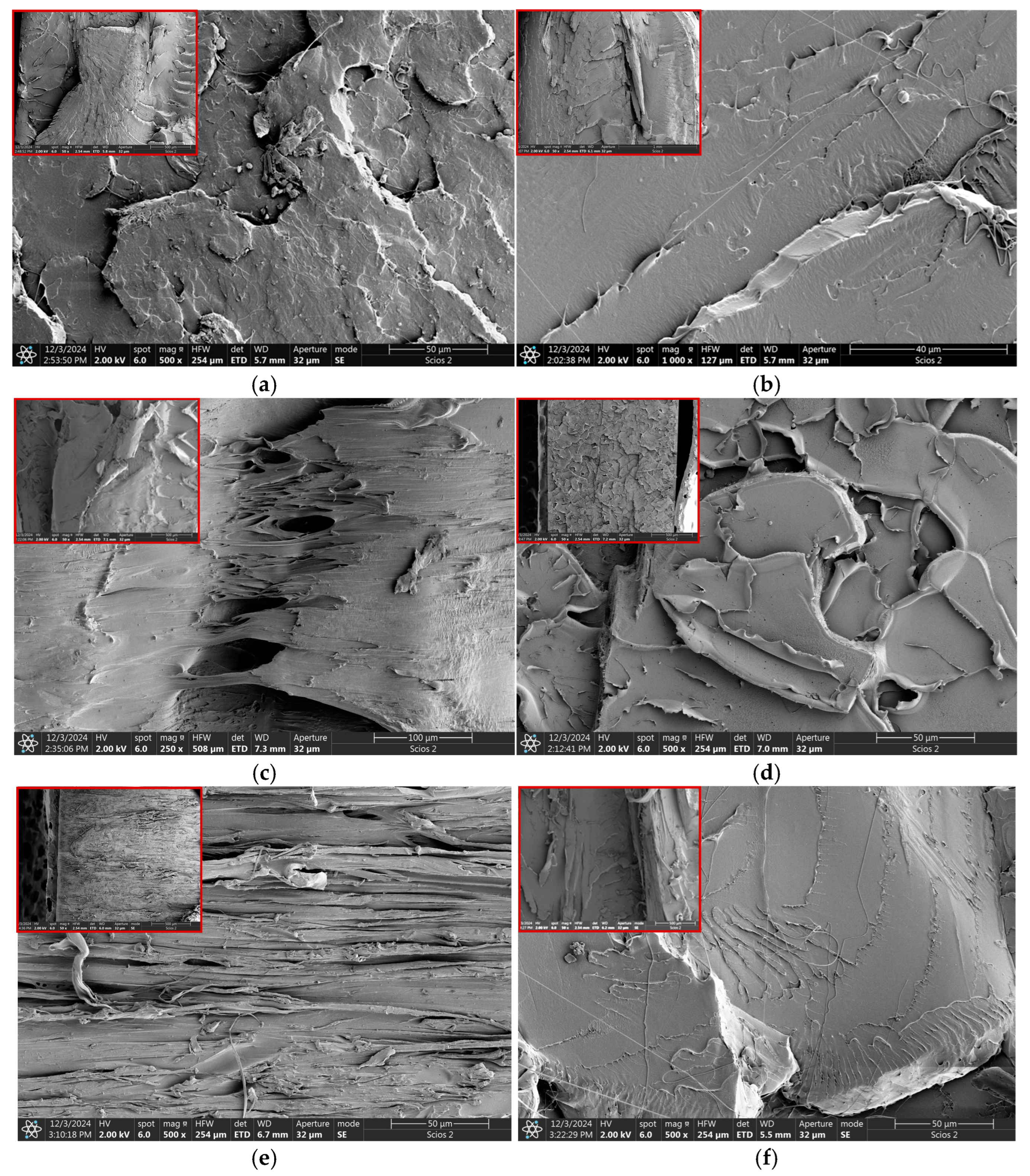
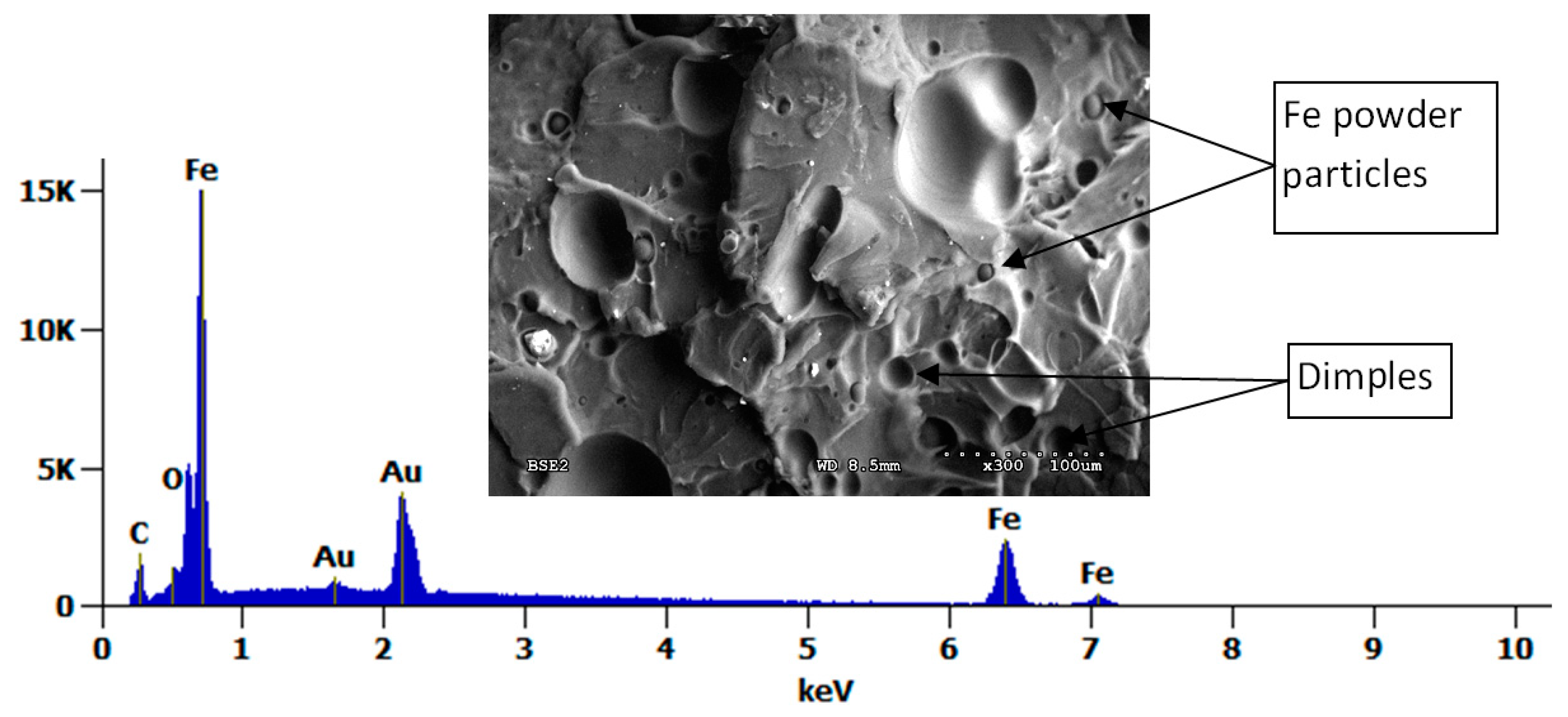
3.4. Microscopic Observations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- DeStefano, V.; Khan, S.; Tabada, A. Applications of PLA in modern medicine. Eng. Regen. 2020, 1, 76–87. [Google Scholar] [CrossRef]
- Perego, G.; Cella, G.D.; Bastioli, C. Effect of molecular weight and crystallinity on poly (lactic acid) mechanical properties. J. Appl. Polym. Sci. 1996, 59, 37–43. [Google Scholar] [CrossRef]
- Hartmann, M.H. High molecular weight polylactic acid polymers. In Biopolymers from Renewable Resources; Springer: Berlin/Heidelberg, Germany, 1998; pp. 367–441. [Google Scholar]
- NatureWorks LLC. Available online: https://www.natureworksllc.com/ (accessed on 27 December 2023).
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef] [PubMed]
- Rasal, R.M.; Janorkar, A.V.; Hirt, D.E. Poly (lactic acid) modifications. Prog. Polym. Sci. 2010, 35, 338–356. [Google Scholar] [CrossRef]
- Gardella, L.; Calabrese, M.; Monticelli, O. PLA maleation: An easy and effective method to modify the properties of PLA/PCL immiscible blends. Colloid Polym. Sci. 2014, 292, 2391–2398. [Google Scholar] [CrossRef]
- López-Camacho, A.; Magaña-García, D.; Grande, M.J.; Carazo-Álvarez, D.; La Rubia, M.D. Preliminary Study of the Bactericide Properties of Biodegradable Polymers (PLA) with Metal Additives for 3D Printing Applications. Bioengineering 2023, 10, 297. [Google Scholar] [CrossRef] [PubMed]
- Bergström, J.S.; Hayman, D. An overview of mechanical properties and material modeling of polylactide (PLA) for medical applications. Ann. Biomed. Eng. 2016, 44, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Zarei, M.; Shabani Dargah, M.; Hasanzadeh Azar, M.; Alizadeh, R.; Mahdavi, F.S.; Sayedain, S.S.; Beheshtizadeh, N. Enhanced bone tissue regeneration using a 3D-printed poly (lactic acid)/Ti6Al4V composite scaffold with plasma treatment modification. Sci. Rep. 2023, 13, 3139. [Google Scholar] [CrossRef]
- Katiyar, P.C.; Singh, B.P.; Chhabra, M.; Parle, D. Innovative Composites for Biomedical Applications: FDM 3D Printing of Finger Splint Using Pla Composite. J. Aeronaut. Mater. 2023, 43, 393–395. [Google Scholar]
- Cordeiro, E.S.; Luna, C.B.B.; da Silva, R.A.; Ramos Wellen, R.M.; de Carvalho, L.H.; de Souza, D.D. On the Production of Poly (Lactic Acid) (PLA) Compounds with Metallic Stearates Based on Zinc, Magnesium and Cobalt. Investigation of Torque Rheometry and Thermal Properties. J. Elastomers Plast. 2023, 55, 805–821. [Google Scholar] [CrossRef]
- Mohammadi-Zerankeshi, M.; Alizadeh, R. 3D-printed PLA-Gr-Mg composite scaffolds for bone tissue engineering applications. J. Mater. Res. Technol. 2023, 22, 2440–2446. [Google Scholar] [CrossRef]
- Shi, D.; Beasock, D.; Fessler, A.; Szebeni, J.; Ljubimova, J.Y.; Afonin, K.A.; Dobrovolskaia, M.A. To PEGylate or not to PEGylate: Immunological properties of nanomedicine’s most popular component, polyethylene glycol and its alternatives. Adv. Drug Deliv. Rev. 2022, 180, 114079. [Google Scholar] [CrossRef]
- de Freitas, C.F.; de Araújo Santos, J.; Pellosi, D.S.; Caetano, W.; Batistela, V.R.; Muniz, E.C. Recent advances of Pluronic-based copolymers functionalization in biomedical applications. Biomater. Adv. 2023, 151, 213484. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, K.; Barone, S.; Soleimani, M. Polyamines and their metabolism: From the maintenance of physiological homeostasis to the mediation of disease. Med. Sci. 2022, 10, 38. [Google Scholar] [CrossRef] [PubMed]
- Langer, R. Biomaterials and biomedical engineering. Chem. Eng. Sci. 1995, 50, 4109–4121. [Google Scholar] [CrossRef]
- Steele, J.A.; Moore, A.C.; St-Pierre, J.P.; McCullen, S.D.; Gormley, A.J.; Horgan, C.C.; Stevens, M.M. In vitro and in vivo investigation of a zonal microstructured scaffold for osteochondral defect repair. Biomaterials 2022, 286, 121548. [Google Scholar] [CrossRef] [PubMed]
- Souto-Lopes, M.; Grenho, L.; Manrique, Y.; Dias, M.M.; Fernandes, M.H.; Monteiro, F.J.; Salgado, C.L. Full physicochemical and biocompatibility characterization of a supercritical CO2 sterilized nano-hydroxyapatite/chitosan biodegradable scaffold for periodontal bone regeneration. Biomater. Adv. 2023, 146, 213280. [Google Scholar] [CrossRef]
- Okolie, O.; Kumar, A.; Edwards, C.; Lawton, L.A.; Oke, A.; McDonald, S.; Njuguna, J. Bio-Based Sustainable Polymers and Materials: From Processing to Biodegradation. J. Compos. Sci. 2023, 7, 213. [Google Scholar] [CrossRef]
- Paul, M.-A.; Delcourt, C.; Alexandre, M.; Degée, P.; Monteverde, F.; Dubois, P. Polylactide/ montmorillonite nanocomposites: Study of the hydrolytic degradation. Polym. Degrad. Stab. 2005, 87, 535–542. [Google Scholar] [CrossRef]
- Oksiuta, Z.; Jalbrzykowski, M.; Mystkowska, J.; Romanczuk, E.; Osiecki, T. Mechanical and thermal properties of polylactide (PLA) composites modified with Mg, Fe, and polyethylene (PE) additives. Polymers 2020, 12, 2939. [Google Scholar] [CrossRef]
- Ray, S.G. Gender Inclusive Game Design: Expanding the Market (Advances in Computer Graphics and Game Development Series); Charles River Media: Hingham, MA, USA, 2003. [Google Scholar]
- Zhou, Q.; Xanthos, M. Nanoclay and crystallinity effects on the hydrolytic degradation of polylactides. Polym. Degrad. Stab. 2008, 93, 1450–1459. [Google Scholar] [CrossRef]
- Tsuji, H.; Ikada, Y. Properties and morphology of poly (L-lactide) 4. Effects of structural parameters on long-term hydrolysis of poly (L-lactide) in phosphatebuffered solution. Polym. Degrad. Stab. 2000, 67, 179–189. [Google Scholar] [CrossRef]
- Schliecker, G.; Schmidt, C.; Fuchs, S.; Kissel, T. Characterization of a homologous series of D, L-lactic acid oligomers; a mechanistic study on the degradation kinetics in vitro. Biomaterials 2003, 24, 3835–3844. [Google Scholar] [CrossRef] [PubMed]
- Bessonova, N.P.; Krasheninnikov, S.V.; Shcherbina, M.A.; Chvalun, S.N. Thermal behavior of crystalline and amorphous HDPE phase in the process of necking at creep deformation. Polym. Test. 2021, 97, 107127. [Google Scholar] [CrossRef]
- Schawe, J.E. Analysis of non-isothermal crystallization during cooling and reorganization during heating of isotactic polypropylene by fast scanning DSC. Thermochim. Acta 2015, 603, 85–93. [Google Scholar] [CrossRef]
- Wunderlich, B. Reversible crystallization and the rigid–amorphous phase in semicrystalline macromolecules. Prog. Polym. Sci. 2003, 28, 383–450. [Google Scholar] [CrossRef]
- Di Lorenzo, M.L.; Righetti, M.C. Crystallization-induced formation of rigid amorphous fraction. Polym. Cryst. 2018, 1, e10023. [Google Scholar] [CrossRef]
- Kurz, R.; Schulz, M.; Scheliga, F.; Men, Y.; Seidlitz, A.; Thurn-Albrecht, T.; Saalwächter, K. Interplay between crystallization and entanglements in the amorphous phase of the crystal-fixed polymer poly (ϵ-caprolactone). Macromolecules 2018, 51, 5831–5841. [Google Scholar] [CrossRef]
- Di Lorenzo, M.L.; Androsch, R. Influence of α′-/α-crystal polymorphism on properties of poly (l-lactic acid). Polym. Int. 2019, 68, 320–334. [Google Scholar] [CrossRef]




| Material | |||
|---|---|---|---|
| PLA 3251D | PLA 3001D | PLA 4043D | |
| Tensile Yield Strength [MPa] | 62 | 48 | 60 |
| Tensile Elongation [%] | 3.5 | 2.5 | 6 |
| Young Modulus [GPa] | 3.5 | 3.6 | 3.6 |
| Heat Distortion Temperature [°C] | 55 | 55 | 55 |
| Melt Flow Rate | 80 | 10–30 | 6 |
| Composite Number | Composition/Indication | Density [g/cm3] |
|---|---|---|
| 1 | Polylactide (99%), iron powder (1% *)/(PLA + 1% Fe) | 1.31 |
| 2 | Polylactide (95%), iron powder (5%)/(PLA + 5% Fe) | 1.62 |
| 3 | Polylactide (90%), iron powder (10%)/(PLA + 10% Fe) | 1.90 |
| 4 | Polylactide (99%), iron nanopowder (1%)/(PLA + 1% nanoFe) | 1.30 |
| 5 | Polylactide (99,5%), iron nanopowder (0.5%)/(PLA + 0.5% nanoFe) | 1.27 |
| 6 | Polylactide (99,9%), iron nanopowder (0.1%)/(PLA + 0.1% nanoFe) | 1.25 |
| 7 | Polylactide, iron nanopowder (1%), polyethylene glycol (1%) (powder)/(PLA + 1% nanoFe + 1% PEG (powder)) | 1.23 |
| 8 | Polylactide, iron nanopowder (1%), polyethylene glycol (1%) (fluid)/(PLA + 1% nanoFe + 1% PEG (fluid)) | 1.23 |
| 9 | Polylactide, iron nanopowder (10%), polyethylene glycol (20%) (fluid)/(PLA + 10% nanoFe + 20% PEG (fluid)) | 1.14 |
| Material | σ [MPa] | Strain ε [%] | Young Modulus E [MPa] | Hardness [°Sh] |
|---|---|---|---|---|
| PLA 100% | 36.0 ± 2.5 | 1.03 ± 0.05 | 3610 ± 76 | 70.1 ± 1.30 |
| PLA + 1% Fe | 24.3 ± 2.1 | 0.63 ± 0.06 | 3585 ± 186 | 70.8 ± 1.61 |
| PLA + 5% Fe | 26.4 ± 2.6 | 0.65 ± 0.08 | 3460 ± 146 | 67.5 ± 0.94 |
| PLA + 10% Fe | 30.0 ± 1.5 | 0.72 ± 0.09 | 3120 ± 169 | 66.8 ± 1.21 |
| PLA + 0.1% nanoFe | 32.6 ± 0.8 | 0.74 ± 0.08 | 3665 ± 170 | 67.2 ± 1.76 |
| PLA + 0.5% nanoFe | 35.5 ± 1.2 | 0.95 ± 0.10 | 3680 ± 125 | 65.1 ± 1.29 |
| PLA + 1.0% nanoFe | 43.6 ± 0.7 | 1.05 ± 0.06 | 3590 ± 210 | 63.8 ± 1.15 |
| PLA + 1% nanoFe + 1% PEG (powder) | 46.0 ± 0.8 | 1.60 ± 0.07 | 3425 ± 130 | 58.7 ± 3.81 |
| PLA + 1% nanoFe + 1% PEG (fluid) | 34.8 ± 1.2 | 1.20 ± 0.13 | 2901 ± 153 | 67.5 ± 2.52 |
| PLA + 10% Fe + 20% PEG (fluid) | 42.1 ± 2.1 | 1.35 ± 0.11 | 3235 ± 171 | 64.1 ± 4.04 |
| Sample | Tg | ΔHc [J/g] | Tm [°C] | ΔHm [J/g] | Xc [%] | ||
|---|---|---|---|---|---|---|---|
| Onset [°C] | Midpoint [°C] | ΔCp [J/(°C·g)] | |||||
| Pure PLA | 45.24 | 50.21 | 0.7318 | 3.42 | 153.25 | 19.91 | 17.60 |
| PLA + 10% Fe | 66.17 | 67.34 | 0.9784 | 29.52 | 170.11 | 59.37 | 31.85 |
| PLA + 5% Fe | 67.08 | 68.30 | 1.1420 | 30.39 | 171.95 | 54.20 | 25.41 |
| PLA + 1% Fe | 65.70 | 66.32 | 0.7750 | 32.42 | 169.69 | 51.32 | 20.71 |
| PLA + 1.0% nanoFe | 60.98 | 63.18 | 0.5109 | 14.16 | 169.53 | 56.23 | 44.89 |
| PLA + 0.5% nanoFe | 62.16 | 63.05 | 0.4432 | 12.49 | 169.68 | 53.58 | 43.85 |
| PLA + 0.1% nanoFe | 60.71 | 61.97 | 0.5382 | 26.99 | 169.96 | 50.44 | 25.02 |
| Sample | Tg | ΔHc [J·g−1] | Tm [°C] | ΔHm [J·g−1] | Xc [%] | ||
|---|---|---|---|---|---|---|---|
| Onset [°C] | Midpoint [°C] | ΔCp [J·(°C·g)−1] | |||||
| PLA | 45.24 | 50.21 | 0.7318 | 3.42 | 153.25 | 19.91 | 17.60 |
| PLA + 1% nanoFe + 1% PEG (powder) | 57.64 | 59.45 | 0.4681 | 11.17 | 167.52 | 56.12 | 47.97 |
| PLA + 1% nanoFe + 1% PEG (fluid) | 49.70 | 51.65 | 1.3000 | 19.08 | 164.22 | 55.36 | 38.72 |
| PLA + 10% Fe + 20% PEG (fluid) | 50.86 | 54.35 | 1.9710 | 17.70 | 164.89 | 57.29 | 42.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oksiuta, Z.; Nowińska, D.; Joka Yildiz, M.; Jałbrzykowski, M. The Influence of Iron Particles and Polyethylene Glycol on Selected Properties of Polylactide-Based Composites. Polymers 2025, 17, 146. https://doi.org/10.3390/polym17020146
Oksiuta Z, Nowińska D, Joka Yildiz M, Jałbrzykowski M. The Influence of Iron Particles and Polyethylene Glycol on Selected Properties of Polylactide-Based Composites. Polymers. 2025; 17(2):146. https://doi.org/10.3390/polym17020146
Chicago/Turabian StyleOksiuta, Zbigniew, Dominika Nowińska, Magdalena Joka Yildiz, and Marek Jałbrzykowski. 2025. "The Influence of Iron Particles and Polyethylene Glycol on Selected Properties of Polylactide-Based Composites" Polymers 17, no. 2: 146. https://doi.org/10.3390/polym17020146
APA StyleOksiuta, Z., Nowińska, D., Joka Yildiz, M., & Jałbrzykowski, M. (2025). The Influence of Iron Particles and Polyethylene Glycol on Selected Properties of Polylactide-Based Composites. Polymers, 17(2), 146. https://doi.org/10.3390/polym17020146







