Calcium Phosphate (CaP) Composite Nanostructures on Polycaprolactone (PCL): Synergistic Effects on Antibacterial Activity and Osteoblast Behavior
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Prepartion of SBF Solution
2.3. Hydrothermal Synthesis of CaP Nanostructures
2.4. Comprehensive Characterization of the “PCL_CaP” Nanostructured Surface: Structure, Chemical Composition, and Wettability
2.4.1. Morphological Analysis
2.4.2. Chemical Bonding Analysis
2.4.3. Surface Wettability Analysis
2.5. Antibacterial Test of “PCL_CaP” Nanostructured Surfaces
2.5.1. Live/Dead Staining for Bacterial Viability
2.5.2. Colony-Forming Unit (CFU) Counting
2.5.3. SEM Imaging for Bacterial Morphology
2.6. Cell Viability Test
2.6.1. Pre-Osteoblast Culture and Differentiation
2.6.2. Pre-Osteoblast Proliferation
2.6.3. Alkaline Phosphatase (ALP) Activity
2.7. Statistical Analysis
3. Results and Discussion
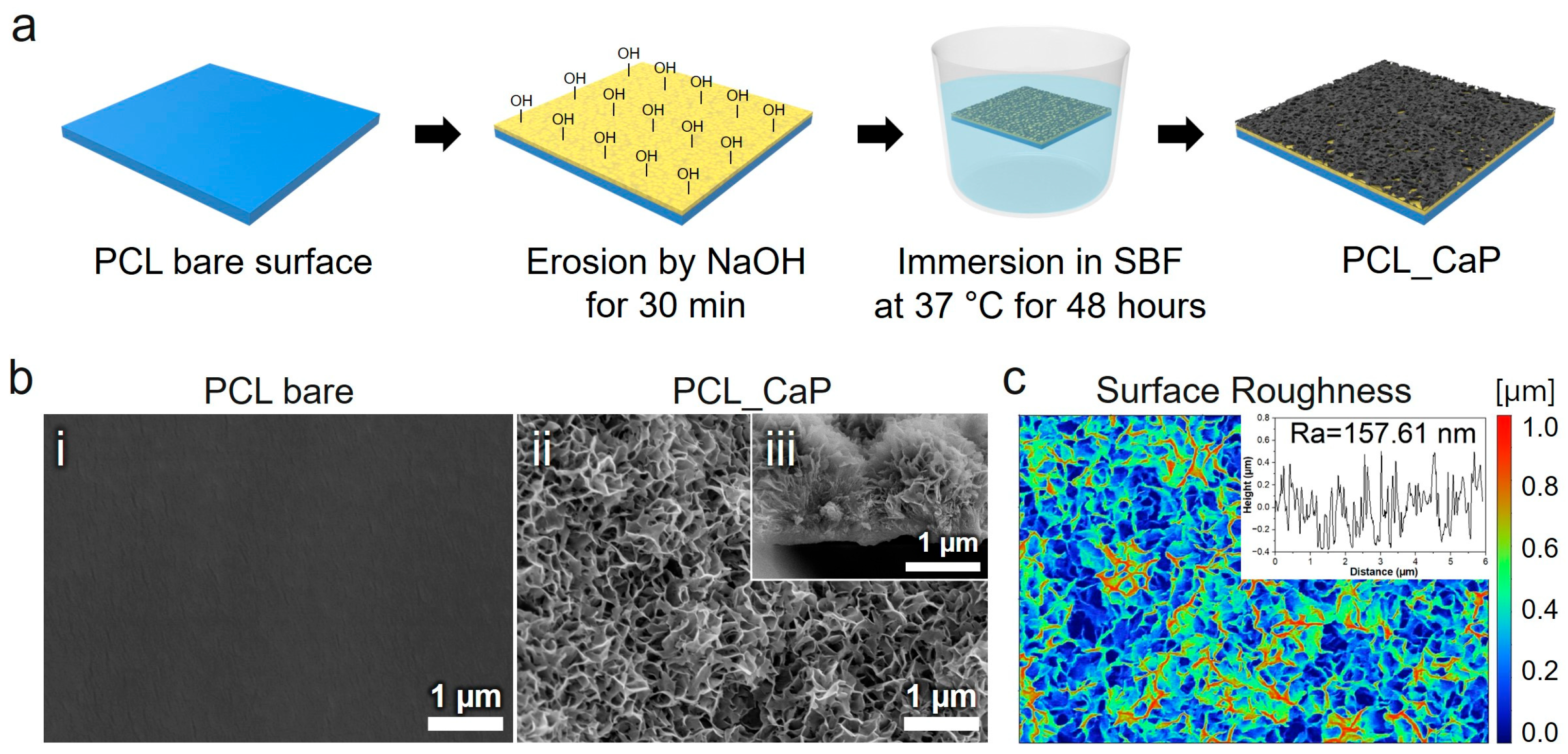
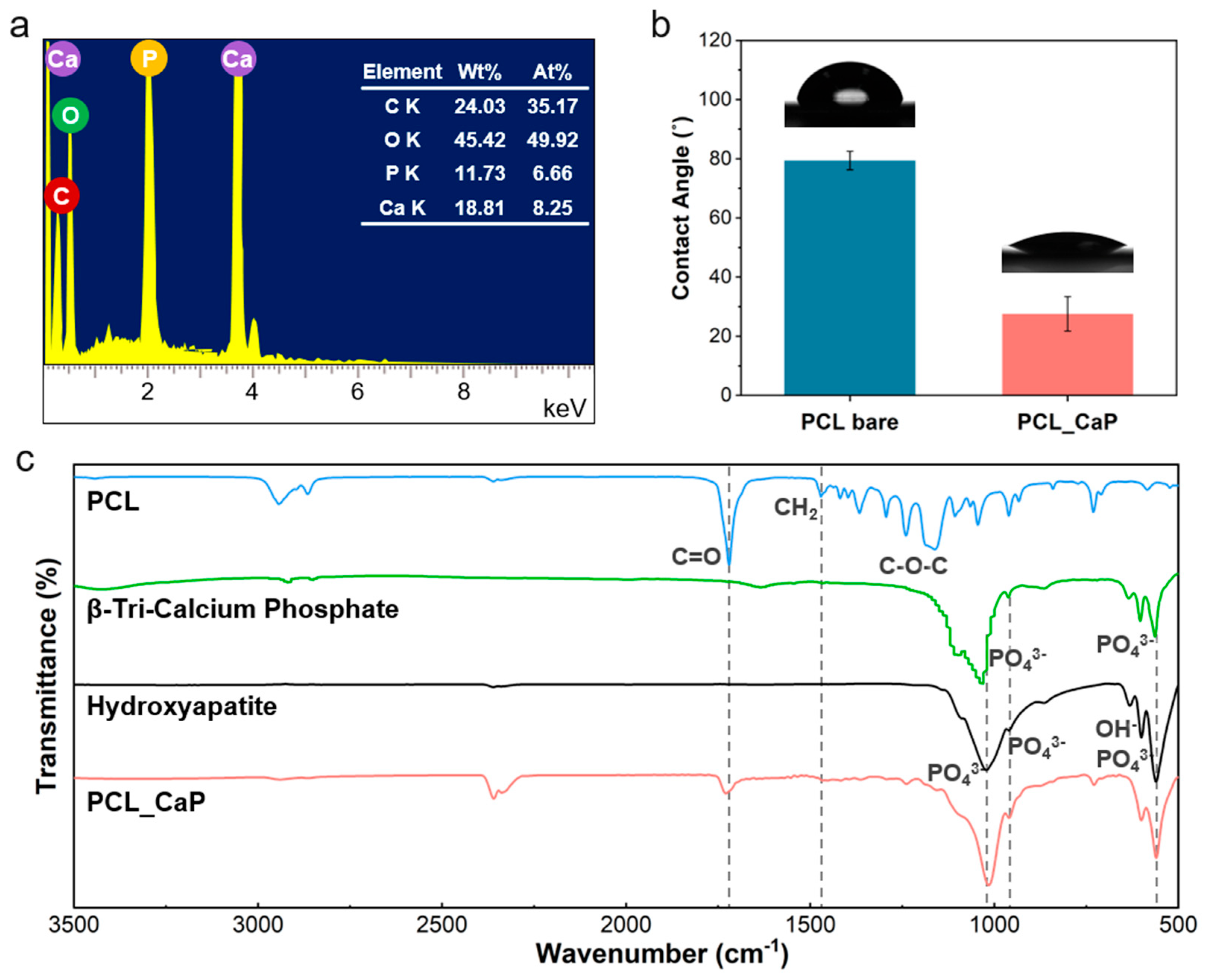
3.1. Characterization of “PCL_CaP” Nanostructured Surfaces
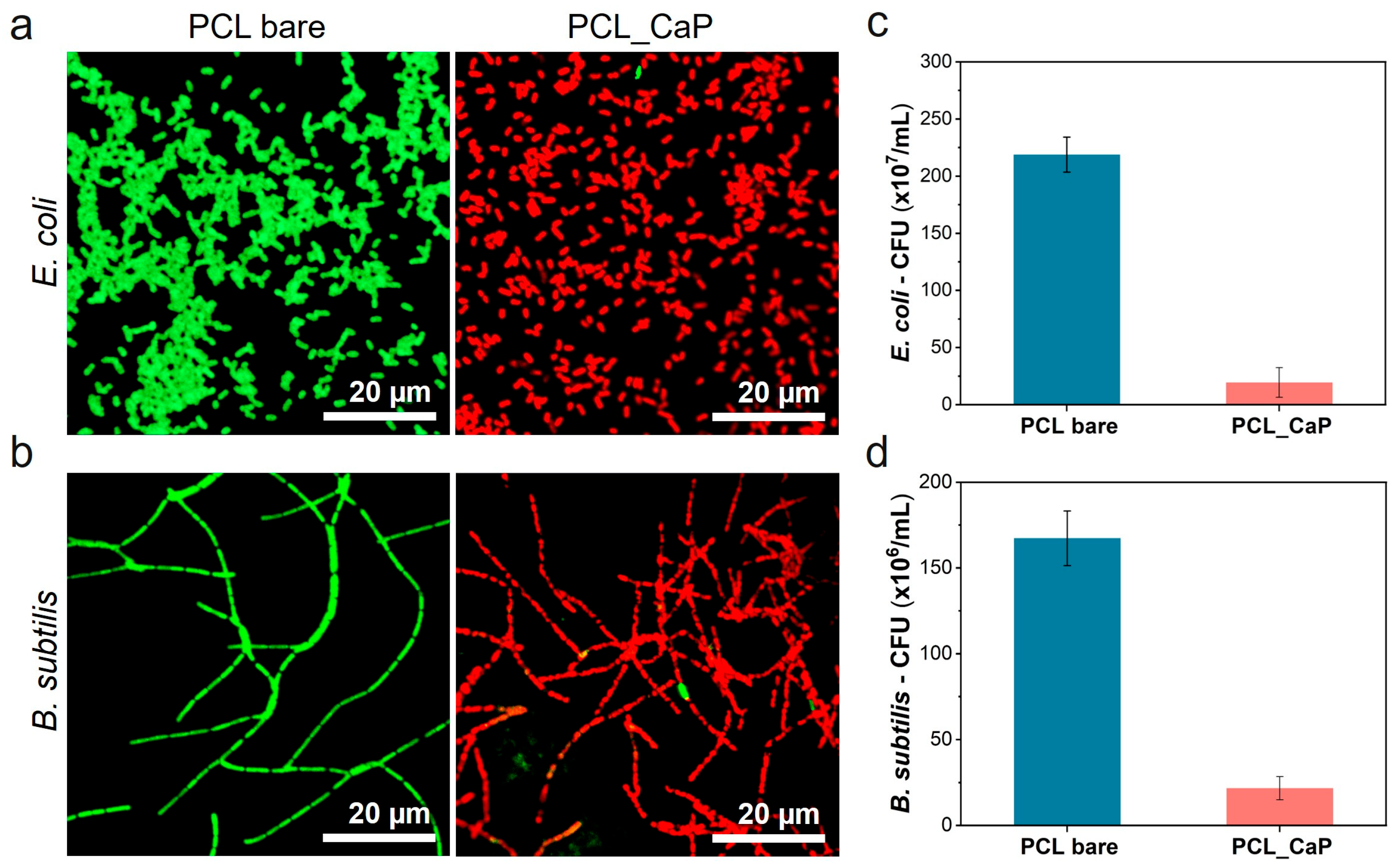
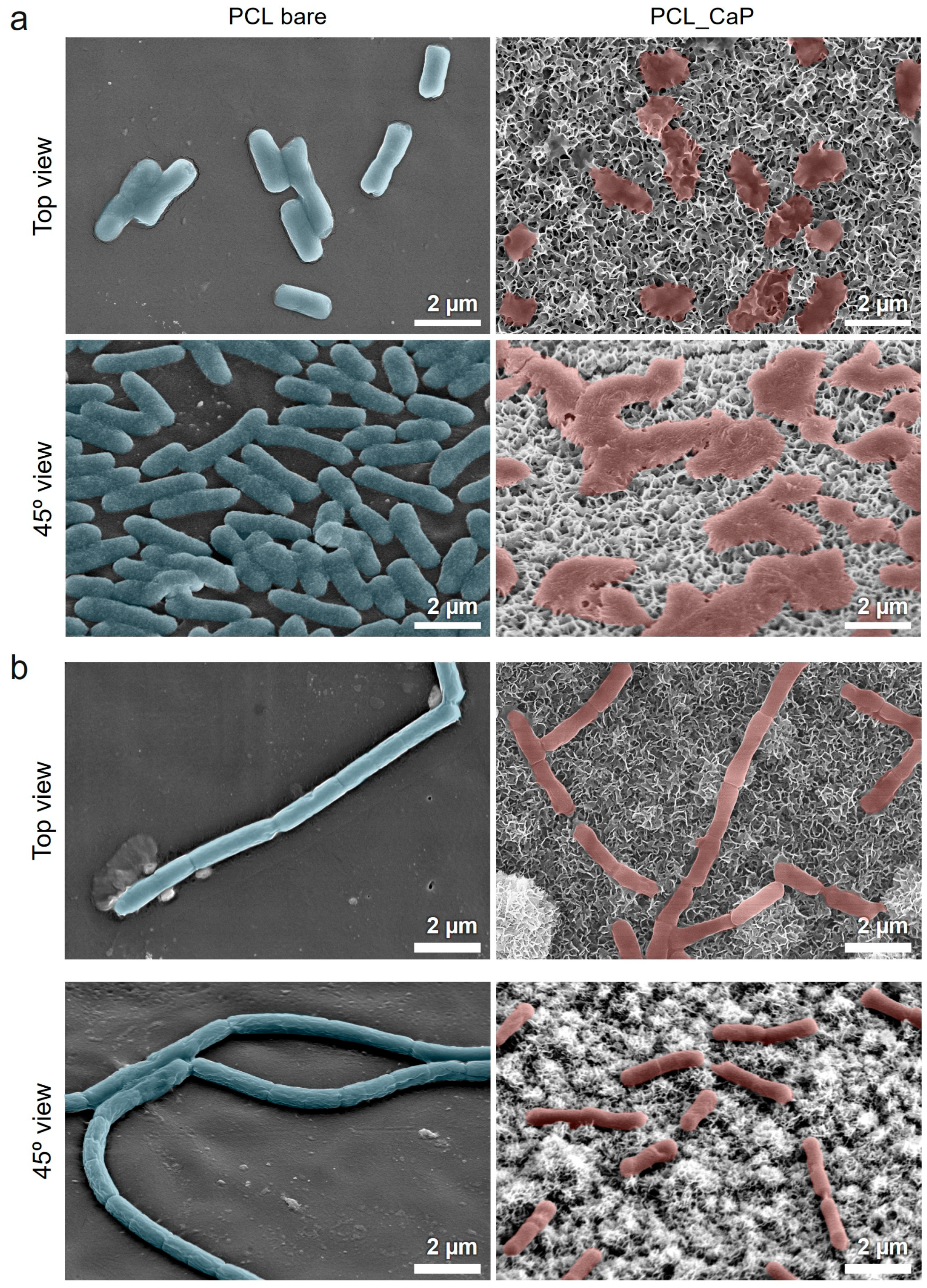
3.2. Evaluation of the Antibacterial Properties of “PCL_CaP” Nanostructured Surfaces
3.3. Pre-Osteoblast Behavior of “PCL_CaP” Nanostructured Surfaces
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Pina, S.; Oliveira, J.M.; Reis, R.L. Natural-Based Nanocomposites for Bone Tissue Engineering and Regenerative Medicine: A Review. Adv. Mater. 2015, 27, 1143–1169. [Google Scholar] [CrossRef] [PubMed]
- Roohani-Esfahani, S.I.; Nouri-Khorasani, S.; Lu, Z.; Appleyard, R.; Zreiqat, H. The Influence Hydroxyapatite Nanoparticle Shape and Size on the Properties of Biphasic Calcium Phosphate Scaffolds Coated with Hydroxyapatite-PCL Composites. Biomaterials 2010, 31, 5498–5509. [Google Scholar] [CrossRef] [PubMed]
- Park, S.A.; Lee, S.H.; Kim, W.D. Fabrication of Porous Polycaprolactone/Hydroxyapatite (PCL/HA) Blend Scaffolds Using a 3D Plotting System for Bone Tissue Engineering. Bioprocess Biosyst. Eng. 2011, 34, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Milovac, D.; Gallego Ferrer, G.; Ivankovic, M.; Ivankovic, H. PCL-Coated Hydroxyapatite Scaffold Derived from Cuttlefish Bone: Morphology, Mechanical Properties and Bioactivity. Mater. Sci. Eng. C 2014, 34, 437–445. [Google Scholar] [CrossRef]
- Venturini Helaehil, J.; Lourenço, C.B.; Huang, B.; Venturini Helaehil, L.; Xavier De Camargo, I.; Chiarotto, G.B.; Santamaria, M., Jr.; Bártolo, P.; Ferreira Caetano, G. In Vivo Investigation of Polymer-Ceramic PCL/HA and PCL/β-TCP 3D Composite Scaffolds and Electrical Stimulation for Bone Regeneration. Polymers 2022, 14, 65. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, J.H.; Shim, J.H.; Hwang, N.S.; Heo, C.Y. Bioactive Calcium Phosphate Materials and Applications in Bone Regeneration. Biomater. Res. 2019, 23, 4. [Google Scholar] [CrossRef]
- Tolmacheva, N.; Bhattacharyya, A.; Noh, I. Calcium Phosphate Biomaterials for 3D Bioprinting in Bone Tissue Engineering. Biomimetics 2024, 9, 2. [Google Scholar] [CrossRef]
- Furko, M.; Balázsi, K.; Balázsi, C. Calcium Phosphate Loaded Biopolymer Composites—A Comprehensive Review on the Most Recent Progress and Promising Trends. Coatings 2023, 13, 360. [Google Scholar] [CrossRef]
- Ciapetti, G.; Ambrosio, L.; Savarino, L.; Granchi, D.; Cenni, E.; Baldini, N.; Pagani, S.; Guizzardi, S.; Causa, F.; Giunti, A. Osteoblast Growth and Function in Porous Poly ε-Caprolactone Matrices for Bone Repair: A Preliminary Study. Biomaterials 2003, 24, 3815–3824. [Google Scholar] [CrossRef]
- Guarino, V.; Causa, F.; Netti, P.A.; Ciapetti, G.; Pagani, S.; Martini, D.; Baldini, N.; Ambrosio, L. The Role of Hydroxyapatite as Solid Signal on Performance of PCL Porous Scaffolds for Bone Tissue Regeneration. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 86, 548–557. [Google Scholar] [CrossRef]
- Cipitria, A.; Skelton, A.; Dargaville, T.R.; Dalton, P.D.; Hutmacher, D.W. Design, Fabrication and Characterization of PCL Electrospun Scaffolds—A Review. J. Mater. Chem. 2011, 21, 9419–9453. [Google Scholar] [CrossRef]
- Stafin, K.; Śliwa, P.; Piątkowski, M. Towards Polycaprolactone-Based Scaffolds for Alveolar Bone Tissue Engineering: A Biomimetic Approach in a 3D Printing Technique. Int. J. Mol. Sci. 2023, 24, 16180. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, Y.; Zhou, Y.; Chen, J.; Wan, Q. The Application of Polycaprolactone in Three-Dimensional Printing Scaffolds for Bone Tissue Engineering. Polymers 2021, 13, 2754. [Google Scholar] [CrossRef] [PubMed]
- Chastain, S.R.; Kundu, A.K.; Dhar, S.; Calvert, J.W.; Putnam, A.J. Adhesion of Mesenchymal Stem Cells to Polymer Scaffolds Occurs via Distinct ECM Ligands and Controls Their Osteogenic Differentiation. J. Biomed. Mater. Res.—A 2006, 78, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.S.; Gwak, S.J.; Cho, Y.S. Fabrication of Polycaprolactone/Nano Hydroxyapatite (Pcl/Nha) 3d Scaffold with Enhanced in Vitro Cell Response via Design for Additive Manufacturing (Dfam). Polymers 2021, 13, 1394. [Google Scholar] [CrossRef]
- Valerini, D.; Tammaro, L.; Vitali, R.; Guillot, G.; Rinaldi, A. Sputter-Deposited Ag Nanoparticles on Electrospun Pcl Scaffolds: Morphology, Wettability and Antibacterial Activity. Coatings 2021, 11, 345. [Google Scholar] [CrossRef]
- Veerachamy, S.; Yarlagadda, T.; Manivasagam, G.; Yarlagadda, P.K. Bacterial Adherence and Biofilm Formation on Medical Implants: A Review. Proc. Inst. Mech. Eng. H 2014, 228, 1083–1099. [Google Scholar] [CrossRef]
- Gallo, J.; Holinka, M.; Moucha, C.S. Antibacterial Surface Treatment for Orthopaedic Implants. Int. J. Mol. Sci. 2014, 15, 13849–13880. [Google Scholar] [CrossRef]
- Xu, Q.; Xu, Z.; Jiang, X.; Yarmolenko, M.A.; Rogachev, A.A.; Rogachev, A.V. Antibacterial Coatings Based on Polycaprolactone and Polyurethane with Prolonged Release of Ciprofloxacin. Surf. Coat. Technol. 2021, 405, 126584. [Google Scholar] [CrossRef]
- Yu, J.; Zhou, M.; Zhang, L.; Wei, H. Antibacterial Adhesion Strategy for Dental Titanium Implant Surfaces: From Mechanisms to Application. J. Funct. Biomater. 2022, 13, 169. [Google Scholar] [CrossRef]
- Soares, Í.; Faria, J.; Marques, A.; Ribeiro, I.A.C.; Baleizão, C.; Bettencourt, A.; Ferreira, I.M.M.; Baptista, A.C. Drug Delivery from PCL/Chitosan Multilayer Coatings for Metallic Implants. ACS Omega 2022, 7, 23096–23106. [Google Scholar] [CrossRef] [PubMed]
- Al-Dulaijan, Y.A.; Cheng, L.; Weir, M.D.; Melo, M.A.S.; Liu, H.; Oates, T.W.; Wang, L.; Xu, H.H.K. Novel Rechargeable Calcium Phosphate Nanocomposite with Antibacterial Activity to Suppress Biofilm Acids and Dental Caries. J. Dent. 2018, 72, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Menotti, F.; Scutera, S.; Coppola, B.; Longo, F.; Mandras, N.; Cavallo, L.; Comini, S.; Sparti, R.; Fiume, E.; Cuffini, A.M.; et al. Tuning of Silver Content on the Antibacterial and Biological Properties of Poly(ɛ-Caprolactone)/Biphasic Calcium Phosphate 3D-Scaffolds for Bone Tissue Engineering. Polymers 2023, 15, 3618. [Google Scholar] [CrossRef] [PubMed]
- Fosca, M.; Streza, A.; Antoniac, I.V.; Vadalà, G.; Rau, J.V. Ion-Doped Calcium Phosphate-Based Coatings with Antibacterial Properties. J. Funct. Biomater. 2023, 14, 250. [Google Scholar] [CrossRef]
- Rau, J.; Fosca, M.; Graziani, V.; Egorov, A.; Zobkov, Y.; Fedotov, A.; Ortenzi, M.; Caminiti, R.; Baranchikov, A.; Komlev, V. Silver-Doped Calcium Phosphate Bone Cements with Antibacterial Properties. J. Funct. Biomater. 2016, 7, 10. [Google Scholar] [CrossRef]
- Odonkor, S.T.; Addo, K.K. Bacteria Resistance to Antibiotics: Recent Trends and Challenges. Int. J. Biol. Med. Res. 2011, 2, 1204–1210. [Google Scholar]
- Ivanova, E.P.; Hasan, J.; Webb, H.K.; Truong, V.K.; Watson, G.S.; Watson, J.A.; Baulin, V.A.; Pogodin, S.; Wang, J.Y.; Tobin, M.J.; et al. Natural Bactericidal Surfaces: Mechanical Rupture of Pseudomonas Aeruginosa Cells by Cicada Wings. Small 2012, 8, 2489–2494. [Google Scholar] [CrossRef]
- Iglesias-Fernandez, M.; Buxadera-Palomero, J.; Sadowska, J.M.; Espanol, M.; Ginebra, M.P. Implementation of Bactericidal Topographies on Biomimetic Calcium Phosphates and the Potential Effect of Its Reactivity. Biomater. Adv. 2022, 136, 212797. [Google Scholar] [CrossRef]
- Li, K.; Chen, J.; Xue, Y.; Ding, T.; Zhu, S.; Mao, M.; Zhang, L.; Han, Y. Polymer Brush Grafted Antimicrobial Peptide on Hydroxyapatite Nanorods for Highly Effective Antibacterial Performance. Chem. Eng. J. 2021, 423, 130133. [Google Scholar] [CrossRef]
- Hollister, S.J. Porous Scaffold Design for Tissue Engineering. Nat. Mater. 2005, 4, 518–524. [Google Scholar] [CrossRef]
- Rezaei, A.; Mohammadi, M.R. In Vitro Study of Hydroxyapatite/Polycaprolactone (HA/PCL) Nanocomposite Synthesized by an in Situ Sol-Gel Process. Mater. Sci. Eng. C 2013, 33, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T.; Takadama, H. How Useful Is SBF in Predicting in Vivo Bone Bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Janmohammadi, M.; Nourbakhsh, M.S.; Bahraminasab, M.; Tayebi, L. Effect of Pore Characteristics and Alkali Treatment on the Physicochemical and Biological Properties of a 3D-Printed Polycaprolactone Bone Scaffold. ACS Omega 2023, 8, 7378–7394. [Google Scholar] [CrossRef] [PubMed]
- Sinkhonde, D. Quantitative Study on Surface Porosity and Roughness Parameters of Mineral and Organic Admixtures Based on Multi-Scale Characterisation Techniques. Clean. Mater. 2023, 7, 100166. [Google Scholar] [CrossRef]
- Ganbaatar, S.E.; Kim, Y.M.; Kim, H.K.; Cho, Y.S.; Park, H.H. Evaluation of Antibacterial Activity on Nanoline-Array Surfaces with Different Spacing. Colloids Surf. B Biointerfaces 2025, 245, 114242. [Google Scholar] [CrossRef]
- Chauhan, N.; Gupta, P.; Arora, L.; Pal, D.; Singh, Y. Dexamethasone-Loaded, Injectable Pullulan-Poly(Ethylene Glycol) Hydrogels for Bone Tissue Regeneration in Chronic Inflammatory Conditions. Mater. Sci. Eng. C 2021, 130, 112463. [Google Scholar] [CrossRef]
- Guo, Z.; Jiang, N.; Chen, C.; Zhu, S.; Zhang, L.; Li, Y. Surface Bioactivation through the Nanostructured Layer on Titanium Modified by Facile HPT Treatment. Sci. Rep. 2017, 7, 4155. [Google Scholar] [CrossRef]
- Li, Y.; Yu, Z.; Ai, F.; Wu, C.; Zhou, K.; Cao, C.; Li, W. Characterization and Evaluation of Polycaprolactone/Hydroxyapatite Composite Scaffolds with Extra Surface Morphology by Cryogenic Printing for Bone Tissue Engineering. Mater. Des. 2021, 205, 109712. [Google Scholar] [CrossRef]
- Falde, E.J.; Yohe, S.T.; Colson, Y.L.; Grinstaff, M.W. Superhydrophobic Materials for Biomedical Applications. Biomaterials 2016, 104, 87–103. [Google Scholar] [CrossRef]
- Bouiahya, K.; Oulguidoum, A.; Laghzizil, A.; Shalabi, M.; Nunzi, J.M.M.; Masse, S.; Nunzi, J.M. Hydrophobic Chemical Surface Functionalization of Hydroxyapatite Nanoparticles for Naphthalene Removal. Colloids Surf. A Physicochem. Eng. Asp. 2020, 595, 124706. [Google Scholar] [CrossRef]
- Kim, H.K.; Cho, Y.S. Fabrication of a Superhydrophobic Surface via Spraying with Polystyrene and Multi-Walled Carbon Nanotubes. Colloids Surf. A Physicochem. Eng. Asp. 2015, 465, 77–86. [Google Scholar] [CrossRef]
- Lyu, J.S.; Lee, J.S.; Han, J. Development of a Biodegradable Polycaprolactone Film Incorporated with an Antimicrobial Agent via an Extrusion Process. Sci. Rep. 2019, 9, 20236. [Google Scholar] [CrossRef] [PubMed]
- Saleh, A.T.; Ling, L.S.; Hussain, R. Injectable Magnesium-Doped Brushite Cement for Controlled Drug Release Application. J. Mater. Sci. 2016, 51, 7427–7439. [Google Scholar] [CrossRef]
- Hayhurst, E.J.; Kailas, L.; Hobbs, J.K.; Foster, S.J. Cell Wall Peptidoglycan Architecture in Bacillus subtilis. Biol. Sci. 2008, 105, 14603–14608. [Google Scholar] [CrossRef]
- Linklater, D.P.; Baulin, V.A.; Juodkazis, S.; Crawford, R.J.; Stoodley, P.; Ivanova, E.P. Mechano-Bactericidal Actions of Nanostructured Surfaces. Nat. Rev. Microbiol. 2021, 19, 8–22. [Google Scholar] [CrossRef]
- Lee, M.S.; Hussein, H.R.; Chang, S.W.; Chang, C.Y.; Lin, Y.Y.; Chien, Y.; Yang, Y.P.; Kiew, L.V.; Chen, C.Y.; Chiou, S.H.; et al. Nature-Inspired Surface Structures Design for Antimicrobial Applications. Int. J. Mol. Sci. 2023, 24, 1348. [Google Scholar] [CrossRef]
- Zhang, G.; Meredith, T.C.; Kahne, D. On the Essentiality of Lipopolysaccharide to Gram-Negative Bacteria. Curr. Opin. Microbiol. 2013, 16, 779–785. [Google Scholar] [CrossRef]
- Cho, Y.S.; Hong, M.W.; Jeong, H.J.; Lee, S.J.; Kim, Y.Y.; Cho, Y.S. The Fabrication of Well-Interconnected Polycaprolactone/Hydroxyapatite Composite Scaffolds, Enhancing the Exposure of Hydroxyapatite Using the Wire-Network Molding Technique. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 2315–2325. [Google Scholar] [CrossRef]
- Wong, J.Y.; Leach, J.B.; Brown, X.Q. Balance of Chemistry, Topography, and Mechanics at the Cell-Biomaterial Interface: Issues and Challenges for Assessing the Role of Substrate Mechanics on Cell Response. Surf. Sci. 2004, 570, 119–133. [Google Scholar] [CrossRef]
- Wang, K.; Cai, L.; Jesse, S.; Wang, S. Poly(ε-Caprolactone)-Banded Spherulites and Interaction with MC3T3-E1 Cells. Langmuir 2012, 28, 4382–4395. [Google Scholar] [CrossRef]
- Golub, E.E.; Boesze-Battaglia, K. The Role of Alkaline Phosphatase in Mineralization. Curr. Orthop. 2007, 18, 444–448. [Google Scholar] [CrossRef]
- Ribeiro, N.; Sousa, S.R.; Monteiro, F.J. Influence of Crystallite Size of Nanophased Hydroxyapatite on Fibronectin and Osteonectin Adsorption and on MC3T3-E1 Osteoblast Adhesion and Morphology. J. Colloid Interface Sci. 2010, 351, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.S.; Choi, S.; Lee, S.H.; Kim, K.K.; Cho, Y.S. Assessments of Polycaprolactone/Hydroxyapatite Composite Scaffold with Enhanced Biomimetic Mineralization by Exposure to Hydroxyapatite via a 3D-Printing System and Alkaline Erosion. Eur. Polym. J. 2019, 113, 340–348. [Google Scholar] [CrossRef]
- Griffith, L.G.; Naughton, G. Tissue Engineering—Current Challenges and Expanding Opportunities. Science 2002, 295, 1009–1014. [Google Scholar] [CrossRef]

| Order | Reagent | Amount |
|---|---|---|
| 1 | NaCl | 40.175 g |
| 2 | NaHCO3 | 1.775 g |
| 3 | KCl | 1.125 g |
| 4 | K2HPO4·3H2O | 1.155 g |
| 5 | MgCl2·6H2O | 1.555 g |
| 6 | 1M HCl | 195 mL |
| 7 | CaCl2 | 1.46 g |
| 8 | Na2SO4 | 0.36 g |
| 9 | Tris | 30.59 g |
| 10 | 1M HCl | 0~25 mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ganbaatar, S.E.; Kim, H.-K.; Kang, N.-U.; Kim, E.C.; U, H.J.; Cho, Y.-S.; Park, H.-H. Calcium Phosphate (CaP) Composite Nanostructures on Polycaprolactone (PCL): Synergistic Effects on Antibacterial Activity and Osteoblast Behavior. Polymers 2025, 17, 200. https://doi.org/10.3390/polym17020200
Ganbaatar SE, Kim H-K, Kang N-U, Kim EC, U HJ, Cho Y-S, Park H-H. Calcium Phosphate (CaP) Composite Nanostructures on Polycaprolactone (PCL): Synergistic Effects on Antibacterial Activity and Osteoblast Behavior. Polymers. 2025; 17(2):200. https://doi.org/10.3390/polym17020200
Chicago/Turabian StyleGanbaatar, Suvd Erdene, Hee-Kyeong Kim, Nae-Un Kang, Eun Chae Kim, Hye Jin U, Young-Sam Cho, and Hyun-Ha Park. 2025. "Calcium Phosphate (CaP) Composite Nanostructures on Polycaprolactone (PCL): Synergistic Effects on Antibacterial Activity and Osteoblast Behavior" Polymers 17, no. 2: 200. https://doi.org/10.3390/polym17020200
APA StyleGanbaatar, S. E., Kim, H.-K., Kang, N.-U., Kim, E. C., U, H. J., Cho, Y.-S., & Park, H.-H. (2025). Calcium Phosphate (CaP) Composite Nanostructures on Polycaprolactone (PCL): Synergistic Effects on Antibacterial Activity and Osteoblast Behavior. Polymers, 17(2), 200. https://doi.org/10.3390/polym17020200







