Hydrogels from Renewable Resources: Advances in 3D Networks Based on Cellulose and Hemicellulose
Abstract
1. Introduction
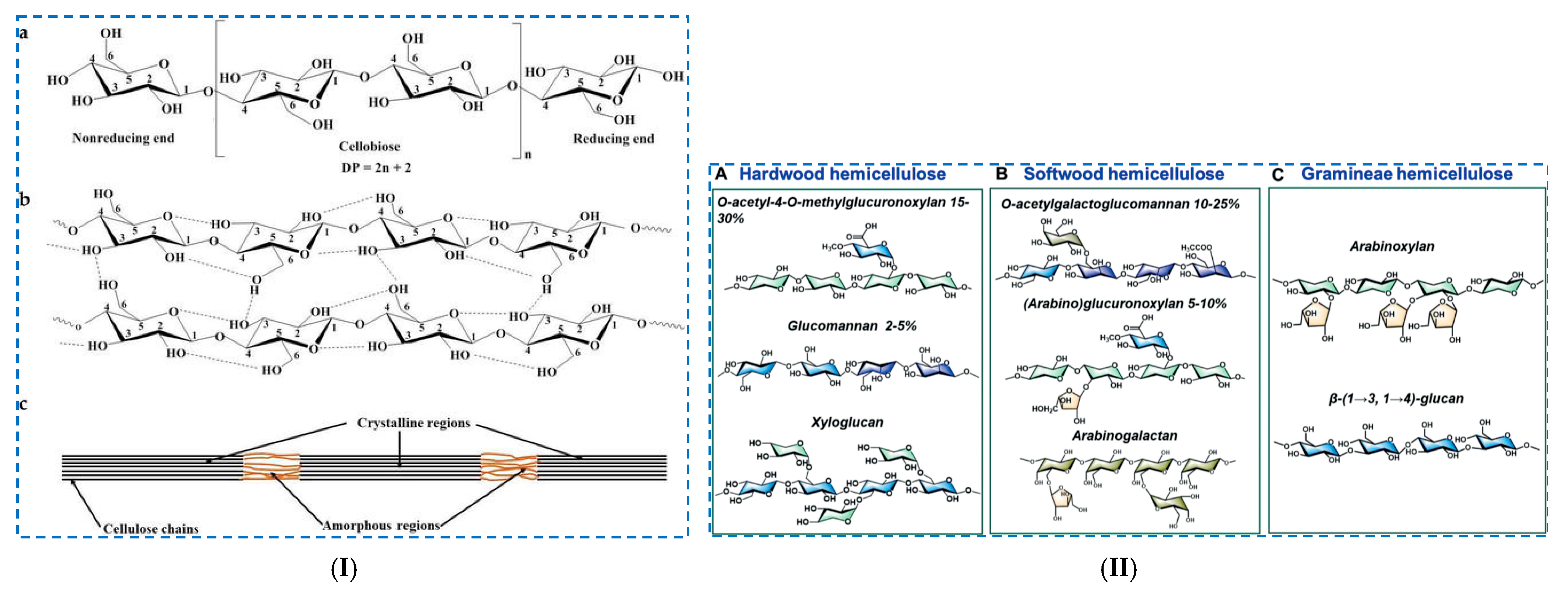
2. Renewable Biopolymers: Structure and Properties
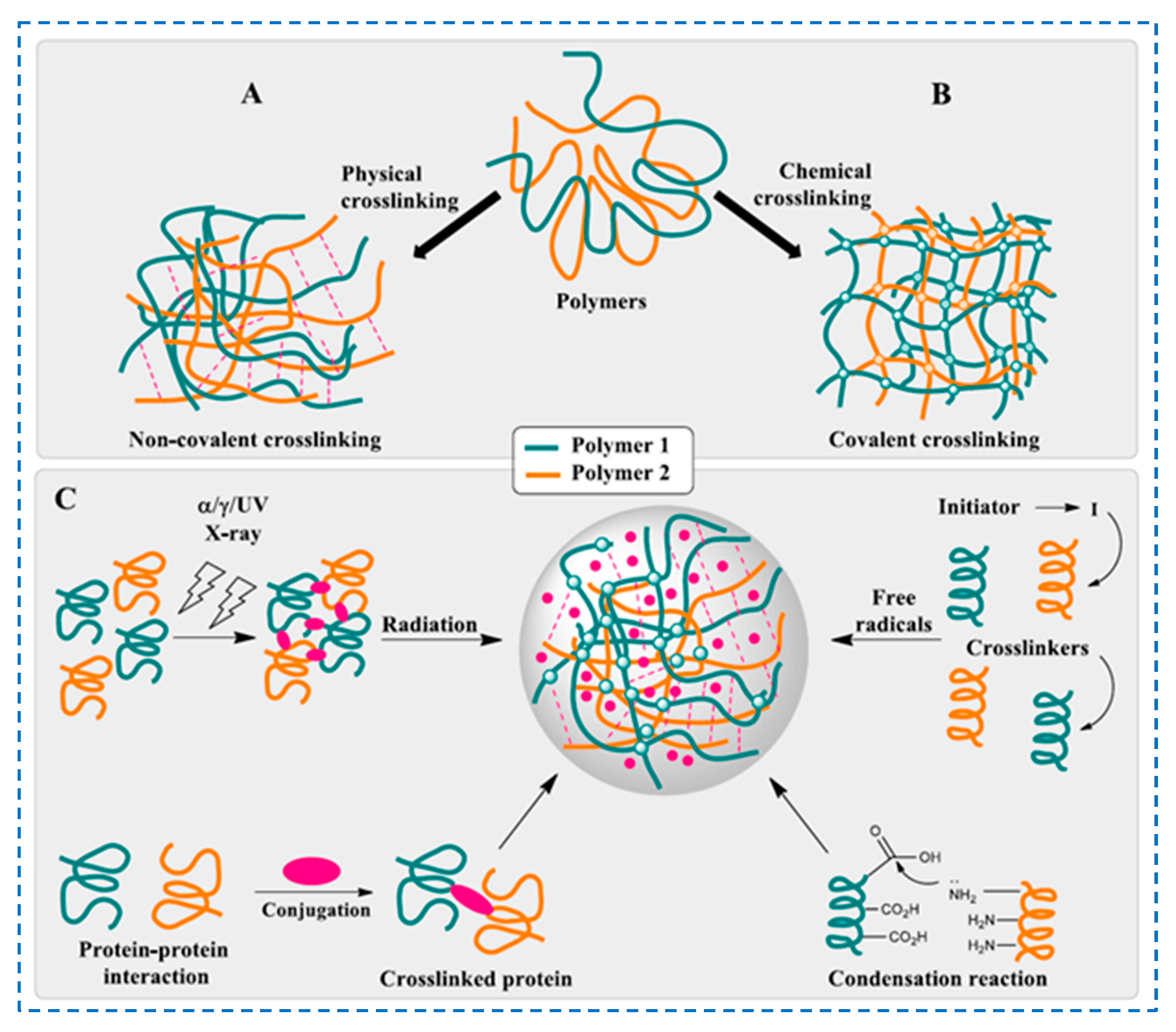
3. Hydrogel Design and Synthesis Methods
- Physical crosslinking—reversible hydrogel—represents a hydrophilic polymer network made either by physical entanglement of polymer chains or by non-covalent interactions.
- Chemical crosslinking—irreversible hydrogel—represents the polymer network made by covalent crosslinking of polymer chains by means of appropriate chemical crosslinking agents.
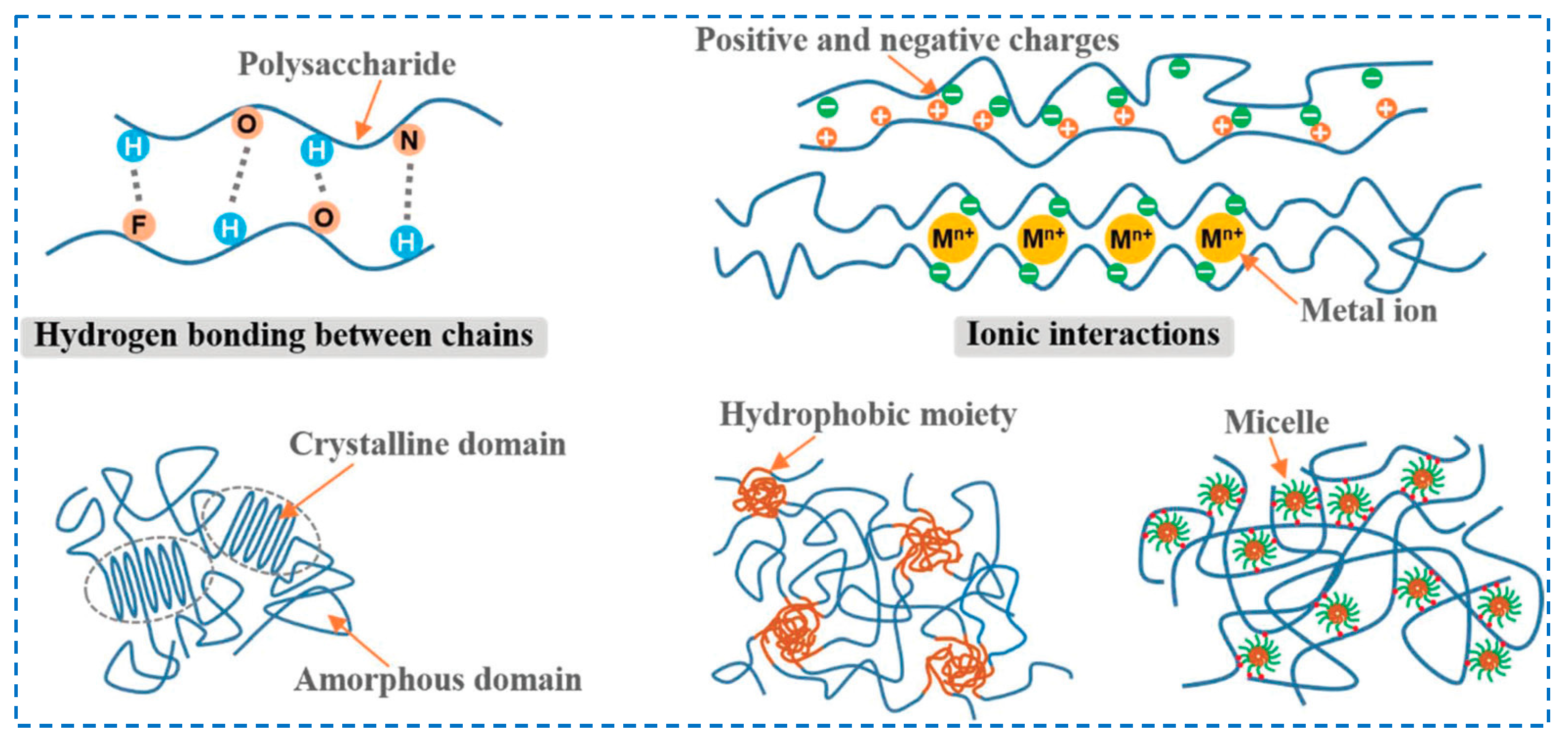
3.1. Physical Crosslinking
3.1.1. Cellulose-Based Hydrogels
Hydrogen Bonding
Freeze–Thaw Method
Ionic Interactions
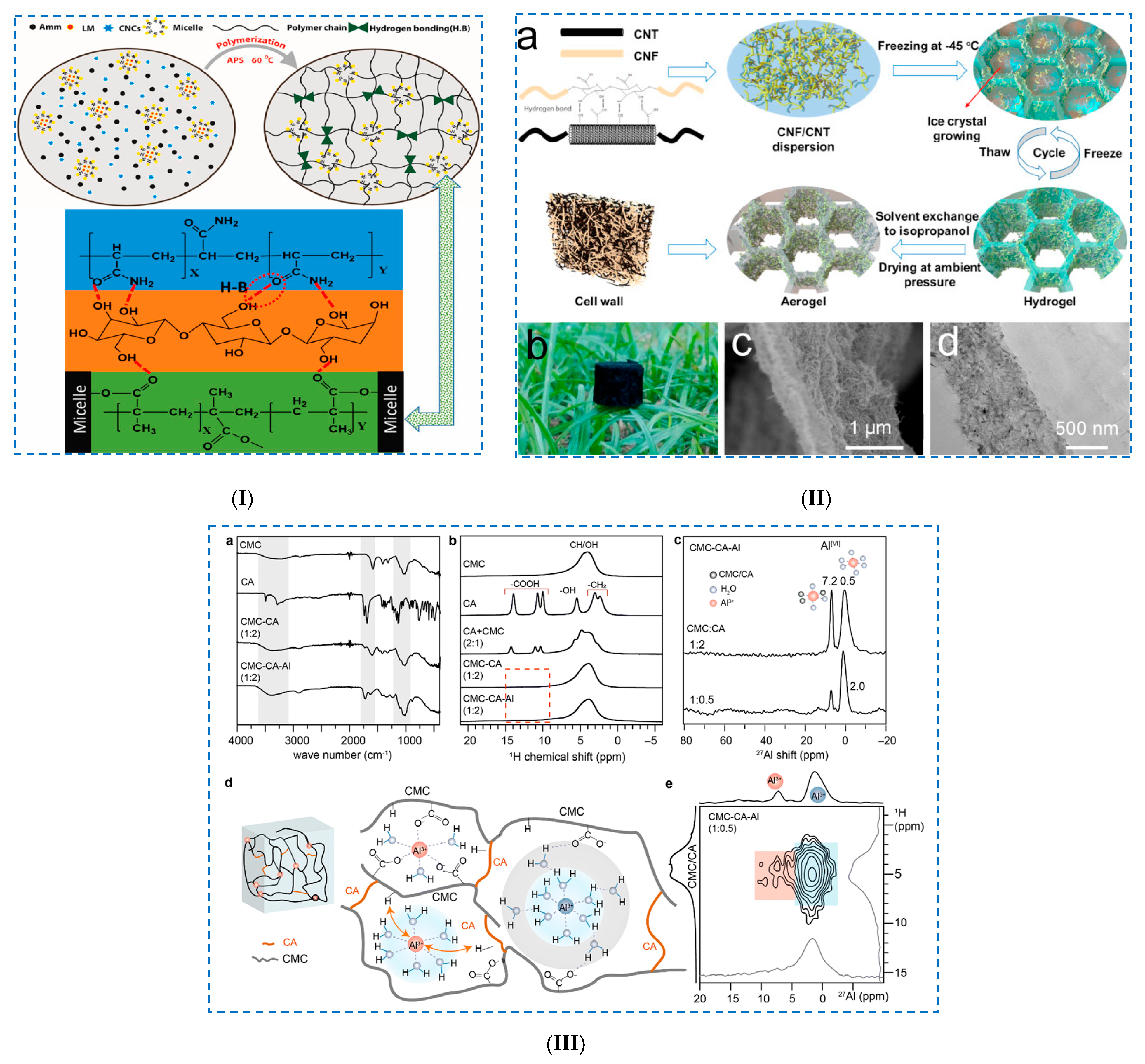
3.1.2. Hemicellulose-Based Hydrogels
Hydrogen Bonding
Freeze–Thaw Method
Ionic Interactions
3.2. Chemical Crosslinking
3.2.1. Cellulose-Based Hydrogels
Ring-Opening Reactions
Polymerization Reactions
Schiff Base Reaction
3.2.2. Hemicellulose-Based Hydrogels
Ring-Opening Reactions
Small Molecule Chemical Crosslinking
Polymerization Reactions
Schiff Base Reaction
Thiol-Ene Reaction
4. Hydrogels’ Performances
4.1. Swelling Behavior
4.2. Mechanical Properties
4.3. Biocompatibility and Biodegradability
4.4. Property Comparative Analysis and Challenges
5. Biomedical Applications of Hydrogels Based on Renewable Biopolymers
5.1. Drug Delivery
5.1.1. Cellulose-Based Hydrogels
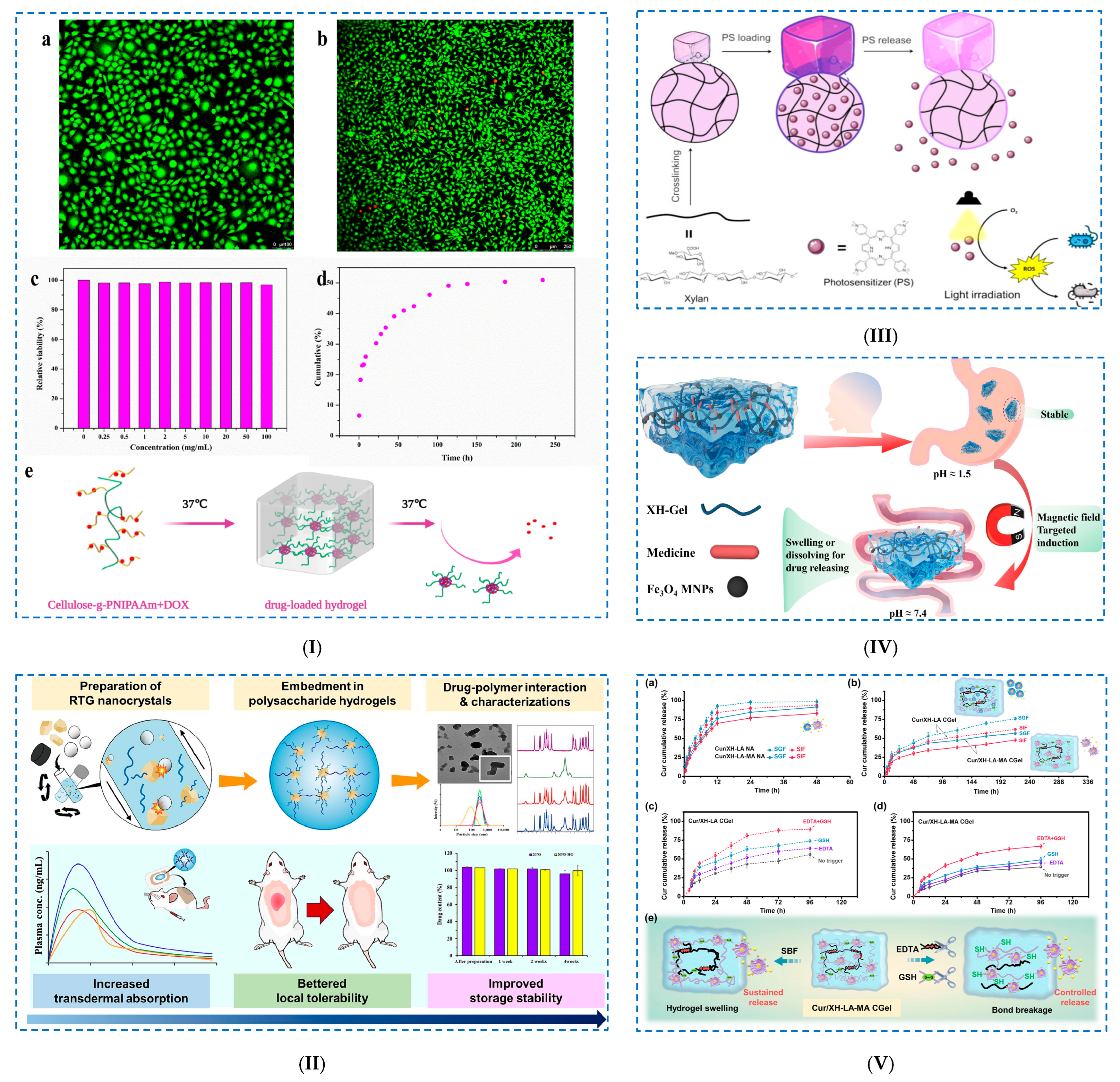
5.1.2. Hemicellulose-Based Hydrogels
5.2. Wound Healing
5.2.1. Cellulose-Based Hydrogels
5.2.2. Hemicellulose-Based Hydrogels

5.3. Tissue Engineering
5.3.1. Cellulose-Based Hydrogels
5.3.2. Hemicellulose-Based Hydrogels

6. Conclusions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Mujtaba, M.; Fraceto, L.F.; Fazeli, M.; Mukherjee, S.; Savassa, S.M.; de Medeiros, G.A.; do Espírito Santo Pereira, A.; Mancini, S.D.; Lipponen, J.; Vilaplana, F. Lignocellulosic Biomass from Agricultural Waste to the Circular Economy: A Review with Focus on Biofuels, Biocomposites and Bioplastics. J. Clean. Prod. 2023, 402, 136815. [Google Scholar] [CrossRef]
- Mignogna, D.; Szabó, M.; Ceci, P.; Avino, P. Biomass Energy and Biofuels: Perspective, Potentials, and Challenges in the Energy Transition. Sustainability 2024, 16, 7036. [Google Scholar] [CrossRef]
- Osman, A.I.; Farghali, M.; Ihara, I.; Elgarahy, A.M.; Ayyad, A.; Mehta, N.; Hoong Ng, K.; Abd El-Monaem, E.M.; Eltaweil, A.S.; Hosny, M.; et al. Materials, Fuels, Upgrading, Economy, and Life Cycle Assessment of the Pyrolysis of Algal and Lignocellulosic Biomass: A Review. Environ. Chem. Lett. 2023, 21, 1419–1476. [Google Scholar] [CrossRef]
- Dutta, D.; Sit, N. A Comprehensive Review on Types and Properties of Biopolymers as Sustainable Bio-Based Alternatives for Packaging. Food Biomacromol. 2024, 1, 58–87. [Google Scholar] [CrossRef]
- Chen, C.; Xi, Y.; Weng, Y. Recent Advances in Cellulose-Based Hydrogels for Tissue Engineering Applications. Polymers 2022, 14, 3335. [Google Scholar] [CrossRef]
- Kaur, R.; Pathak, L.; Vyas, P. Biobased Polymers of Plant and Microbial Origin and Their Applications-A Review. Biotechnol. Sustain. Mater. 2024, 1, 13. [Google Scholar] [CrossRef]
- Sultana, S.; Sonia, Z.A.; Mahmud, M.; Mottakin, M.; Haider, J.B.; Ahmed, S.; Hossen, M.M. An Investigation of Cellulose, Hemicellulose and Lignin Co-Extraction from Water Hyacinth. Adv. J. Chem. A 2024, 7, 75–88. [Google Scholar] [CrossRef]
- Eleutério, T.; Trota, M.J.; Meirelles, M.G.; Vasconcelos, H.C. A Review of Natural Fibers: Classification, Composition, Extraction, Treatments, and Applications. Fibers 2025, 13, 119. [Google Scholar] [CrossRef]
- Cheng, H.; Fang, Y.; Chen, B.; Gong, J.; Wang, P. Hemicellulose Extraction and Applications as Hydrogels: A Review. J. Appl. Polym. Sci. 2025, 149, e57190. [Google Scholar] [CrossRef]
- Nanda, D.; Behera, D.; Pattnaik, S.S.; Behera, A.K. Advances in Natural Polymer-Based Hydrogels: Synthesis, Applications, and Future Directions in Biomedical and Environmental Fields. Discov. Polym. 2025, 2, 6. [Google Scholar] [CrossRef]
- Vázquez-Rivas, E.; Desales-Guzmán, L.A.; Pacheco-Sánchez, J.H.; Burillo-Amezcua, S.G. Cellulose-Based Hybrid Hydrogels for Tissue Engineering Applications: A Sustainable Approach. Gels 2025, 11, 438. [Google Scholar] [CrossRef]
- Zhao, B.; Li, H.; Tian, K.; Su, Y.; Zou, Z. Synthesis and Antitumor Activity of Bagasse Xylan Derivatives Modified by Graft-Esterification and Cross-Linking. Int. J. Biol. Macromol. 2023, 253, 126867. [Google Scholar] [CrossRef]
- Melo-Silveira, R.F.; Viana, R.L.S.; Sabry, D.A.; da Silva, R.A.; Machado, D.; Nascimento, A.K.L.; Scortecci, K.C.; Ferreira-Halder, C.V.; Sassaki, G.L.; Rocha, H.A.O. Antiproliferative Xylan from Corn Cobs Induces Apoptosis in Tumor Cells. Carbohydr. Polym. 2019, 210, 245–253. [Google Scholar] [CrossRef]
- Li, K.; Li, S.; Wang, D.; Li, X.; Wu, X.; Liu, X.; Du, G.; Li, X.; Qin, X.; Du, Y. Extraction, Characterization, Antitumor and Immunological Activities of Hemicellulose Polysaccharide from Astragalus radix Herb Residue. Molecules 2019, 24, 3644. [Google Scholar] [CrossRef] [PubMed]
- Borovkova, V.S.; Malyar, Y.N.; Sudakova, I.G.; Chudina, A.I.; Skripnikov, A.M.; Fetisova, O.Y.; Kazachenko, A.S.; Miroshnikova, A.V.; Zimonin, D.V.; Ionin, V.A.; et al. Molecular Characteristics and Antioxidant Activity of Spruce (Picea abies) Hemicelluloses Isolated by Catalytic Oxidative Delignification. Molecules 2022, 27, 266. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Cui, J.; Liu, F.; Liang, C.; Feng, S.; Sun, Y.; Gao, W.; Guo, Y.; Zhang, B.; Huang, W. A 4D Printed Adhesive, Thermo-Contractile, and Degradable Hydrogel for Diabetic Wound Healing. Adv. Healthc. Mater. 2024, 13, e2303499. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yao, S.; Mao, X.; Fang, Z.; Yang, C.; Zhang, Y. Thermosensitive Hydrogel Coupled with Sodium Ascorbyl Phosphate Promotes Human Umbilical Cord-Derived Mesenchymal Stem Cell-Mediated Skin Wound Healing in Mice. Sci. Rep. 2023, 13, 11909. [Google Scholar] [CrossRef]
- Liu, D.; Li, L.; Shi, B.L.; Shi, B.; Li, M.D.; Qiu, Y.; Zhao, D.; Shen, Q.D.; Zhu, Z.Z. Ultrasound-Triggered Piezocatalytic Composite Hydrogels for Promoting Bacterial-Infected Wound Healing. Bioact. Mater. 2023, 24, 96–111. [Google Scholar] [CrossRef]
- Butenko, S.; Nagalla, R.R.; Guerrero-Juarez, C.F.; Palomba, F.; David, L.M.; Nguyen, R.Q.; Gay, D.; Almet, A.A.; Digman, M.A.; Nie, Q.; et al. Hydrogel Crosslinking Modulates Macrophages, Fibroblasts, and Their Communication, During Wound Healing. Nat. Commun. 2024, 15, 6820. [Google Scholar] [CrossRef]
- Hu, J.; Li, L.; Li, Z.; Yang, L.; Ren, X.; Cheng, Y.; Li, Y.; Huang, Q. Fabricating Water-Resistant and Stimuli Responsive Smart Hydrogels via Iminoboronate Chemistry. Adv. Funct. Mater. 2024, 34, 2411234. [Google Scholar] [CrossRef]
- Yang, X.; Ci, Y.; Zhu, P.; Chen, T.; Li, F.; Tang, Y. Preparation and Characterization of Cellulose-Chitosan/β-FeOOH Composite Hydrogels for Adsorption and Photocatalytic Degradation of Methyl Orange. Int. J. Biol. Macromol. 2024, 274, 133201. [Google Scholar] [CrossRef]
- Liu, C.; Lei, F.; Li, P.; Jiang, J.; Wang, K. Borax Crosslinked Fenugreek Galactomannan Hydrogel as Potential Water-Retaining Agent in Agriculture. Carbohydr. Polym. 2020, 236, 116100. [Google Scholar] [CrossRef]
- Yu, J.; Yun, J.; Zang, S.; Han, M.; Sun, X.; Wang, Z.; Zhou, Y.; Khan, A.; An, M.; Li, J.; et al. Hydrogel Fiber Fabric Combining Rapid Water Transport, Thermal Localization, and Large-Scale Production for Ultra-High Salt-Resistant Solar Desalination. Nano Energy 2023, 117, 108847. [Google Scholar] [CrossRef]
- Khaksar-Baghan, N.; Koochakzaei, A. An Overview of Gel-Based Cleaning Approaches for Art Conservation. Herit. Sci. 2024, 12, 248. [Google Scholar] [CrossRef]
- Liao, G.; Sun, E.; Kana, E.B.G.; Huang, H.; Sanusi, I.A.; Qu, P.; Jin, H.; Liu, J.; Shuai, L. Renewable Hemicellulose-Based Materials for Value-Added Applications. Carbohydr. Polym. 2024, 341, 122351. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.K.; Yang, Y.; Koirala, G.R.; Oh, S.; Kim, T. From Lab to Wearables: Innovations in Multifunctional Hydrogel Chemistry for Next-Generation Bioelectronic Devices. Biomaterials 2024, 310, 122632. [Google Scholar] [CrossRef]
- Dassanayake, R.S.; Acharya, S.; Abidi, N. Biopolymer-Based Materials from Polysaccharides: Properties, Processing, Characterization and Sorption Applications. In Advanced Sorption Process Applications; Edebali, S., Ed.; IntechOpen: London, UK, 2018; Chapter 1; pp. 1–24. [Google Scholar] [CrossRef]
- Rao, J.; Lv, Z.; Chen, G.; Peng, F. Hemicellulose: Structure, Chemical Modification, and Application. Prog. Polym. Sci. 2023, 140, 101675. [Google Scholar] [CrossRef]
- Nawaz, H.; He, A.; Wu, Z.; Wang, X.; Jiang, Y.; Ullah, A.; Xu, F.; Xie, F. Revisiting Various Mechanistic Approaches for Cellulose Dissolution in Different Solvent Systems: A Comprehensive Review. Int. J. Biol. Macromol. 2024, 273, 133012. [Google Scholar] [CrossRef]
- Khomutinnikov, N.V.; Govyazin, I.O.; Ivanov, G.E.; Fedorova, E.M.; Makarov, I.S.; Vinogradov, M.I.; Kulichikhin, V.G. Experimental Study on the Manufacturing of Functional Paper with Modified by N-Methylmorpholine-N-oxide Surfaces. Polymers 2023, 15, 692. [Google Scholar] [CrossRef]
- Makarov, I.S.; Budaeva, V.V.; Gismatulina, Y.A.; Kashcheyeva, E.I.; Zolotukhin, V.N.; Gorbatova, P.A.; Sakovich, G.V.; Vinogradov, M.I.; Palchikova, E.E.; Levin, I.S.; et al. Preparation of Lyocell Fibers from Solutions of Miscanthus Cellulose. Polymers 2024, 16, 2915. [Google Scholar] [CrossRef]
- Przypis, M.; Wawoczny, A.; Gillner, D. Biomass and Cellulose Dissolution—The Important Issue in Renewable Materials Treatment. Appl. Sci. 2023, 13, 1055. [Google Scholar] [CrossRef]
- Shu, L.; Zhang, X.-F.; Wang, Z.; Yao, J. Structure Reorganization of Cellulose Hydrogel by Green Solvent Exchange for Potential Plastic Replacement. Carbohydr. Polym. 2022, 275, 118695. [Google Scholar] [CrossRef] [PubMed]
- Parajó, J.J.; Santiago-Alonso, A.; Vallet, P.; Teijeira, T.; Emeterio, R.S.; Villanueva, M.; Salgado, J. Comprehensive Analysis of the Acute Toxicity of Ionic Liquids Using Microtox® Bioassays. Appl. Sci. 2024, 14, 2480. [Google Scholar] [CrossRef]
- Nasution, H.; Harahap, H.; Dalimunthe, N.F.; Ginting, M.H.S.; Jaafar, M.; Tan, O.O.H.; Aruan, H.K.; Herfananda, A.L. Hydrogel and Effects of Crosslinking Agent on Cellulose-Based Hydrogels: A Review. Gels 2022, 8, 568. [Google Scholar] [CrossRef]
- Tudoroiu, E.E.; Dinu-Pîrvu, C.E.; Albu Kaya, M.G.; Popa, L.; Anuța, V.; Prisada, R.M.; Ghica, M.V. An Overview of Cellulose Derivatives-Based Dressings for Wound-Healing Management. Pharmaceuticals 2021, 14, 1215. [Google Scholar] [CrossRef]
- Ghilan, A.; Nicu, R.; Ciolacu, D.E.; Ciolacu, F. Insight into the Latest Medical Applications of Nanocellulose. Materials 2023, 16, 4447. [Google Scholar] [CrossRef]
- Nicu, R.; Ciolacu, F.; Ciolacu, D.E. Advanced Functional Materials Based on Nanocellulose for Pharmaceutical/Medical Applications. Pharmaceutics 2021, 13, 1125. [Google Scholar] [CrossRef]
- Sofiah, A.G.N.; Pasupuleti, J.; Samykano, M.; Kadirgama, K.; Koh, S.P.; Tiong, S.K.; Pandey, A.K.; Yaw, C.T.; Natarajan, S.K. Harnessing Nature’s Ingenuity: A Comprehensive Exploration of Nanocellulose from Production to Cutting-Edge Applications in Engineering and Sciences. Polymers 2023, 15, 3044. [Google Scholar] [CrossRef]
- Hemraz, U.D.; Lam, E.; Sunasee, R. Recent Advances in Cellulose Nanocrystals-Based Antimicrobial Agents. Carbohydr. Polym. 2023, 315, 120987. [Google Scholar] [CrossRef]
- Ciolacu, D.E.; Nicu, R.; Ciolacu, F. Natural Polymers in Heart Valve Tissue Engineering: Strategies, Advances and Challenges. Biomedicines 2022, 10, 1095. [Google Scholar] [CrossRef]
- Yoshimi, Y.; Yu, L.; Cresswell, R.; Guo, X.; Echevarría-Poza, A.; Lyczakowski, J.J.; Dupree, R.; Kotake, T.; Dupree, P. Glucomannan Engineering Highlights Roles of Galactosyl Modification in Fine-Tuning Cellulose-Glucomannan Interaction in Arabidopsis Cell Walls. Nat. Commun. 2025, 16, 1235. [Google Scholar] [CrossRef]
- Ainani, A.F.; Darmawan; Rubiyanto, J.T.; Ardian, M.N.; Habiba, W.N.; Syarifuddin, A.; Dirpan, A. Hemicellulose-Based Hydrogel Composite: Enhanced Properties and Diverse Applications. Carbohydr. Polym. Technol. Appl. 2024, 8, 100558. [Google Scholar] [CrossRef]
- Condò, I.; Giannitelli, S.M.; Lo Presti, D.; Cortese, B.; Ursini, O. Overview of Dynamic Bond Based Hydrogels for Reversible Adhesion Processes. Gels 2024, 10, 442. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Yang, X.; Song, W. Bioinspired Supramolecular Hydrogel from Design to Applications. Small Methods 2024, 8, 2300753. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Ma, C.; Xu, Y.; Du, L.; Yang, X. Food Gels Based on Polysaccharide and Protein: Preparation, Formation Mechanisms, and Delivery of Bioactive Substances. Gels 2024, 10, 735. [Google Scholar] [CrossRef]
- Hu, H.; Xu, F.J. Rational Design and Latest Advances of Polysaccharide-Based Hydrogels for Wound Healing. Biomater. Sci. 2020, 8, 2084. [Google Scholar] [CrossRef]
- Rumon, M.M.H.; Rahman, M.S.; Akib, A.A.; Sohag, M.S.; Rakib, M.R.A.; Khan, M.A.R.; Yesmin, F.; Shakil, M.S.; Khan, M.M.R. Progress in Hydrogel Toughening: Addressing Structural and Crosslinking Challenges for Biomedical Applications. Discov. Mater. 2025, 5, 5. [Google Scholar] [CrossRef]
- Hu, W.; Wang, Z.; Xiao, Y.; Zhag, S.; Wang, J. Advances in Crosslinking Strategies of Biomedical Hydrogels. Biomater. Sci. 2019, 7, 843. [Google Scholar] [CrossRef]
- Chen, L.; Xu, J.; Zhu, M.; Zeng, Z.; Song, Y.; Zhang, Y.; Zhang, X.; Deng, Y.; Xiong, R.; Huang, C. Self-Healing Polymers Through Hydrogen-Bond Cross-Linking: Synthesis and Electronic Applications. Mater. Horiz. 2023, 10, 4000–4032. [Google Scholar] [CrossRef]
- Ren, Y.; Dong, X. Dynamic Polymeric Materials via Hydrogen-Bond Cross-Linking: Effect of Multiple Network Topologies. Prog. Polym. Sci. 2024, 158, 101890. [Google Scholar] [CrossRef]
- Yu, H.; Xiao, Q.; Qi, G.; Chen, F.; Tu, B.; Zhang, S.; Li, Y.; Chen, Y.; Yu, H.; Duan, P. A Hydrogen Bonds-Crosslinked Hydrogels with Self-Healing and Adhesive Properties for Hemostatic. Front. Bioeng. Biotechnol. 2022, 10, 855013. [Google Scholar] [CrossRef]
- Xie, Z.; Hu, B.L.; Li, R.W.; Zhang, Q. Hydrogen Bonding in Self-Healing Elastomers. ACS Omega 2021, 6, 9319–9333. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, S.J.K.; Huang, Z.; Chen, X.; Sala, R.L.; McCune, J.A.; Malliaras, G.G.; Scherman, O.A. Highly Stretchable Dynamic Hydrogels for Soft Multilayer Electronics. Sci. Adv. 2024, 10, eadn5142. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Chen, A. Application of Supramolecular Hydrogel in Supercapacitors: Opportunities and Challenges. Aggregate 2024, 5, e581. [Google Scholar] [CrossRef]
- Fuchs, S.; Shariati, K.; Ma, M. Specialty Tough Hydrogels and Their Biomedical Applications. Adv. Healthc. Mater. 2020, 9, 1901396. [Google Scholar] [CrossRef]
- Kundu, R.; Mahada, P.; Chhirang, B.; Das, B. Cellulose Hydrogels: Green and Sustainable Soft Biomaterials. Curr. Res. Green Sustain. Chem. 2022, 5, 100252. [Google Scholar] [CrossRef]
- Bernal-Chávez, S.A.; Romero-Montero, A.; Hernández-Parra, H.; Peña-Corona, S.I.; Del Prado-Audelo, M.L.; Alcalá-Alcalá, S.; Cortés, H.; Kiyekbayeva, L.; Sharifi-Rad, J.; Leyva-Gómez, G. Enhancing Chemical and Physical Stability of Pharmaceuticals Using Freeze-Thaw Method: Challenges and Opportunities for Process Optimization Through Quality by Design Approach. J. Biol. Eng. 2023, 17, 35. [Google Scholar] [CrossRef]
- Waresindo, W.X.; Luthfianti, H.R.; Priyanto, A.; Hapidin, D.A.; Edikresnha, D.; Aimon, A.H.; Suciati, T.; Khairurrijal, K. Freeze–Thaw Hydrogel Fabrication Method: Basic Principles, Synthesis Parameters, Properties, and Biomedical Applications. Mater. Res. Express 2023, 10, 024003. [Google Scholar] [CrossRef]
- Yang, L.; Wang, H.; Yang, Y.; Li, Y. Self-Healing Cellulose-Based Hydrogels: From Molecular Design to Multifarious Applications. Carbohydr. Polym. 2025, 347, 122738. [Google Scholar] [CrossRef]
- dos Santos Carvalho, J.D.; Rabelo, R.S.; Hubinger, M.D. Thermo-Rheological Properties of Chitosan Hydrogels with Hydroxypropyl Methylcellulose and Methylcellulose. Int. J. Biol. Macromol. 2022, 209, 367–375. [Google Scholar] [CrossRef]
- Xue, L.; An, R.; Zhao, J.; Qiu, M.; Wang, Z.; Ren, H.; Yu, D.; Zhu, X. Self-Healing Hydrogels: Mechanisms and Biomedical Applications. MedComm 2025, 6, e70181. [Google Scholar] [CrossRef]
- Hao, Y.; Ji, H.; Gao, L.; Qu, Z.; Zhao, Y.; Chen, J.; Wang, X.; Ma, X.; Zhang, G.; Zhang, T. Self-Assembled Carrier-Free Formulations Based on Medicinal and Food Active Ingredients. Biomater. Sci. 2024, 12, 6253–6273. [Google Scholar] [CrossRef] [PubMed]
- Gabrielli, V.; Baretta, R.; Pilot, R.; Ferrarini, A.; Frasconi, M. Insights into the Gelation Mechanism of Metal-Coordinated Hydrogels by Paramagnetic NMR Spectroscopy and Molecular Dynamics. Macromolecules 2022, 55, 450–461. [Google Scholar] [CrossRef]
- Khare, E.; Holten-Andersen, N.; Buehler, M.J. Transition-Metal Coordinate Bonds for Bioinspired Macromolecules with Tunable Mechanical Properties. Nat. Rev. Mater. 2021, 6, 421–436. [Google Scholar] [CrossRef]
- Ghilan, A.; Nita, L.E.; Pamfil, D.; Simionescu, N.; Tudorachi, N.; Rusu, D.; Rusu, A.G.; Bercea, M.; Rosca, I.; Ciolacu, D.E.; et al. One-Step Preparation of Carboxymethyl Cellulose—Phytic Acid Hydrogels with Potential for Biomedical Applications. Gels 2022, 8, 647. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhai, Z.; Yao, Y.; Stant, J.C.; Landrum, S.L.; Bortner, M.J.; Frazier, C.E.; Edgar, K.J. Oxidized Hydroxypropyl Cellulose/Carboxymethyl Chitosan Hydrogels Permit pH-Responsive, Targeted Drug Release. Carbohydr. Polym. 2023, 300, 120213. [Google Scholar] [CrossRef]
- Khan, M.; Shah, L.A.; Rahman, T.U.; Yoo, H.M.; Ye, D.; Vacharasin, J. Cellulose Nanocrystals Boosted Hydrophobic Association in Dual Network Polymer Hydrogels as Advanced Flexible Strain Sensor for Human Motion Detection. J. Mech. Behav. Biomed. Mater. 2023, 138, 105610. [Google Scholar] [CrossRef]
- Bishnoi, P.; Siwal, S.S.; Kumar, V.; Thakur, V.K. Cellulose-Based Smart Materials: Novel Synthesis Techniques, Properties and Applications in Energy Storage and Conversion Devices. Electron 2024, 2, e42. [Google Scholar] [CrossRef]
- Zainal, S.H.; Mohd, N.H.; Suhaili, N.; Anuar, F.H.; Lazim, A.M.; Othaman, R. Preparation of Cellulose-Based Hydrogel: A Review. J. Mater. Res. Technol. 2021, 10, 935–952. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, H.; Guo, M.; Zhao, M.; Liu, Y.; Zhang, D.; Terrones, M.; Wang, Y. Large-Scale Preparation of Electrically Conducting Cellulose Nanofiber/Carbon Nanotube Aerogels: Ambient-Dried, Recyclable, and 3D-Printable. Carbon 2022, 194, 23–33. [Google Scholar] [CrossRef]
- Huang, Z.; Qin, R.; Zhang, H.; Guo, M.; Zhang, D.; Gao, C.; Gao, F.; Chen, X.; Terrones, M.; Wang, Y. Ambient-Drying to Construct Unidirectional Cellulose Nanofibers/ Carbon Nanotubes Aerogel with Ultra-Lightweight, Robust, and Superior Microwave Absorption Performance. Carbon 2023, 212, 118150. [Google Scholar] [CrossRef]
- Shu, L.; Zhang, X.F.; Wu, Y.; Wang, Z.; Yao, J. Facile Fabrication of Strong and Conductive Cellulose Hydrogels with Wide Temperature Tolerance for Flexible Sensors. Int. J. Biol. Macromol. 2023, 240, 124438. [Google Scholar] [CrossRef]
- Thomas, N.; Moussaoui, S.; Reyes-Suárez, B.; Lafon, O.; Manjunatha Reddy, G.N. Dual Cross-Linked Cellulose Based Hydrogel Films. Mater. Adv. 2024, 5, 9210–9219. [Google Scholar] [CrossRef]
- Liu, C.; Wang, Y.; Shi, S.; Zheng, Y.; Ye, Z.; Liao, J.; Sun, Q.; Dang, B.; Shen, X. Myelin Sheath-Inspired Hydrogel Electrode for Artificial Skin and Physiological Monitoring. ACS Nano 2024, 18, 27420–27432. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Fu, C.; Alam, N.; Ni, Y.; Chen, L.; Huang, L.; Hu, H. Preparation of Hemicellulose Nanoparticle-Containing Ionic Hydrogels with High Strength, Self-Healing, and UV Resistance and Their Applications as Strain Sensors and Asymmetric Pressure Sensors. Biomacromolecules 2022, 23, 2272–2279. [Google Scholar] [CrossRef]
- Li, F.; Zhang, Z.; Wang, X.; Yin, X.; Fu, M.; Qin, T.; Ji, X.; Yang, G.; Sun, S. A Physical Crosslinked Ph-Sensitive Hydrogel Based on Hemicellulose/Graphene Oxide for Controlled Oral Drug Delivery. Int. J. Biol. Macromol. 2025, 289, 138875. [Google Scholar] [CrossRef]
- Zhang, W.; Wen, J.Y.; Ma, M.G.; Li, M.F.; Peng, F.; Bian, J. Anti-Freezing, Water-Retaining, Conductive, And Strain-Sensitive Hemicellulose/Polypyrrole Composite Hydrogels for Flexible Sensors. J. Mater. Res. Technol. 2021, 14, 555–566. [Google Scholar] [CrossRef]
- Tohamy, H.A.S. Carboxymethyl Hemicellulose Hydrogel as a Fluorescent Biosensor for Bacterial and Fungal Detection with DFT and Molecular Docking Studies. Sci. Rep. 2025, 15, 741. [Google Scholar] [CrossRef]
- Bustamante-Torres, M.; Romero-Fierro, D.; Arcentales-Vera, B.; Palomino, K.; Magaña, H.; Bucio, E. Hydrogels Classification According to the Physical or Chemical Interactions and as Stimuli-Sensitive Materials. Gels 2021, 7, 182. [Google Scholar] [CrossRef]
- Xue, X.; Hu, Y.; Wang, S.; Chen, X.; Jiang, Y.; Su, J. Fabrication of Physical and Chemical Crosslinked Hydrogels for Bone Tissue Engineering. Bioact. Mater. 2022, 12, 327–339. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, K.; Yang, Y.; Kim, M.S.; Lee, C.H.; Zhang, R.; Xu, T.; Choi, S.E.; Si, C. Hemicellulose-Based Hydrogels for Advanced Applications. Front. Bioeng. Biotechnol. 2023, 10, 1110004. [Google Scholar] [CrossRef] [PubMed]
- de Siqueira, E.C.; de França, J.A.A.; de Souza, R.F.M.; da Silva Leoterio, D.M.; Cordeiro, J.N.; Doboszewski, B. Mechanisms of the Chemical Crosslinking to Obtain the Hydrogels: Synthesis, Conditions of Crosslinking and Biopharmaceutical Applications. Res. Soc. Dev. 2023, 12, e18312943072. [Google Scholar] [CrossRef]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental Concepts of Hydrogels: Synthesis, Properties, and Their Applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef] [PubMed]
- Alavarse, E.A.C.; Frachini, E.C.G.; da Silva, R.L.C.G.; Lima, V.H.; Shavandi, A.; Petri, D.F.S. Crosslinkers for Polysaccharides and Proteins: Synthesis Conditions, Mechanisms, and Crosslinking Efficiency, A Review. Int. J. Biol. Macromol. 2022, 202, 558–596. [Google Scholar] [CrossRef]
- Thivya, P.; Akalya, S.; Sinija, V.R. A Comprehensive Review on Cellulose-Based Hydrogel and its Potential Application in the Food Industry. Appl. Food Res. 2022, 2, 100161. [Google Scholar] [CrossRef]
- Zou, P.; Yao, J.; Cui, Y.N.; Zhao, T.; Che, J.; Yang, M.; Li, Z.; Gao, C. Advances in Cellulose-Based Hydrogels for Biomedical Engineering: A Review Summary. Gels 2022, 8, 364. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Islam, M.; Hasan, M.K.; Nam, K.W. A Comprehensive Review of Radiation-Induced Hydrogels: Synthesis, Properties, and Multidimensional Applications. Gels 2024, 10, 381. [Google Scholar] [CrossRef]
- Hiroki, A.; Taguchi, M. Development of Environmentally Friendly Cellulose Derivative-Based Hydrogels for Contact Lenses Using a Radiation Crosslinking Technique. Appl. Sci. 2021, 11, 9168. [Google Scholar] [CrossRef]
- Bonetti, L.; De Nardo, L.; Fare, S. Crosslinking Strategies in Modulating Methylcellulose Hydrogel Properties. Soft Matter 2023, 19, 7869. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, T.; Liu, K.; Zhu, L.; Miao, C.; Chen, T.; Gao, M.; Wang, J.; Si, C. Modulation and Mechanisms of Cellulose-Based Hydrogels for Flexible Sensors. SusMat 2024, 5, e255. [Google Scholar] [CrossRef]
- Rizwan, M.; Gilani, S.R.; Durani, A.I.; Naseem, S. Materials Diversity of Hydrogel: Synthesis, Polymerization Process and Soil Conditioning Properties in Agricultural Field. J. Adv. Res. 2021, 17, 15–40. [Google Scholar] [CrossRef]
- Thang, N.H.; Chien, T.B.; Cuong, D.X. Polymer-Based Hydrogels Applied in Drug Delivery: An Overview. Gels 2023, 9, 523. [Google Scholar] [CrossRef] [PubMed]
- Luu, T.; Gristwood, K.; Knight, J.C.; Jörg, M. Click Chemistry: Reaction Rates and Their Suitability for Biomedical Applications. Bioconjug. Chem. 2024, 35, 715–731. [Google Scholar] [CrossRef]
- Cabrera-Quiñones, N.C.; López-Méndez, L.J.; Cruz-Hernández, C.; Guadarrama, P. Click Chemistry as an Efficient Toolbox for Coupling Sterically Hindered Molecular Systems to Obtain Advanced Materials for Nanomedicine. Int. J. Mol. Sci. 2025, 26, 36. [Google Scholar] [CrossRef] [PubMed]
- Ghosal, K.; Bhattacharyya, S.K.; Mishra, V.; Zuilhof, H. Click Chemistry for Biofunctional Polymers: From Observing to Steering Cell Behavior. Chem. Rev. 2024, 124, 13216–13300. [Google Scholar] [CrossRef] [PubMed]
- Roca-Arroyo, A.F.; Gutierrez-Rivera, J.A.; Morton, L.D.; Castilla-Casadiego, D.A. Hydrogel Network Architecture Design Space: Impact on Mechanical and Viscoelastic Properties. Gels 2025, 11, 588. [Google Scholar] [CrossRef]
- Zhang, Z.; Song, Q.; Jin, Y.; Feng, Y.; Li, J.; Zhang, K. Advances in Schiff Base and Its Coating on Metal Biomaterials—A Review. Metals 2023, 13, 386. [Google Scholar] [CrossRef]
- Raju, S.K.; Settu, A.; Thiyagarajan, A.; Rama, D.; Sekar, P.; Kumar, S. Biological Applications of Schiff Bases: An Overview. GSC Biol. Pharm. Sci. 2022, 21, 203–215. [Google Scholar] [CrossRef]
- Quan, L.; Xin, Y.; Wu, X.; Ao, Q. Mechanism of Self-Healing Hydrogels and Application in Tissue Engineering. Polymers 2022, 14, 2184. [Google Scholar] [CrossRef]
- Naranjo-Alcazar, R.; Bendix, S.; Groth, T.; Ferrer, G.G. Research Progress in Enzymatically Cross-Linked Hydrogels as Injectable Systems for Bioprinting and Tissue Engineering. Gels 2023, 9, 230. [Google Scholar] [CrossRef]
- Qi, Y.; Wang, F.; Liu, J.; Wang, C.; Liu, Y. Enzyme-Mediated Hydrogelation for Biomedical Applications: A Review. Int. J. Biol. Macromol. 2025, 311, 143379. [Google Scholar] [CrossRef]
- Nicu, R.; Lisa, G.; Darie-Nita, R.N.; Avadanei, M.I.; Bargan, A.; Rusu, D.; Ciolacu, D.E. Tailoring the Structure and Physico-Chemical Features of Cellulose-Based Hydrogels Using Multi-Epoxy Crosslinking Agents. Gels 2024, 10, 523. [Google Scholar] [CrossRef]
- Yan, W.; Zhou, T.; Han, L.; Zhu, M.; Cheng, Z.; Li, D.; Ren, F.; Wang, K.; Lu, X. Conductive Cellulose Bio-Nanosheets Assembled Biostable Hydrogel for Reliable Bioelectronics. Adv. Funct. Mater. 2021, 31, 201046. [Google Scholar] [CrossRef]
- Ciolacu, D.E.; Rusu, D.; Darie-Nita, R.N.; Tîmpu, D.; Ciolacu, F. Influence of Gel Stage from Cellulose Dissolution in NaOH-Water System on the Performances of Cellulose Allomorphs-Based Hydrogels. Gels 2022, 8, 410. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, D.F.B.A.; Wasli, M.E.; Tan, C.S.Y.; Musa, Z.; Chin, S.F. Eco-Friendly Cellulose-Based Hydrogels Derived from Wastepapers as a Controlled-Release Fertilizer. Chem. Biol. Technol. Agric. 2023, 10, 36. [Google Scholar] [CrossRef]
- Wei, Y.; Xiang, L.J.; Zhu, P.H.; Qian, Y.; Zhao, B.; Chen, G. Multifunctional Organohydrogel-Based Ionic Skin for Capacitance and Temperature Sensing toward Intelligent Skin-Like Devices. Chem. Mater. 2021, 33, 8623–8634. [Google Scholar] [CrossRef]
- Blažic, R.; Marušić, K.; Vidović, E. Swelling and Viscoelastic Properties of Cellulose-Based Hydrogels Prepared by Free Radical Polymerization of Dimethylaminoethyl Methacrylate in Cellulose Solution. Gels 2023, 9, 94. [Google Scholar] [CrossRef]
- Liu, X.; Shen, J.; Wang, Y.; Li, M.; Fu, S. Photoinduced Metal-Free Atom Transfer Radical Polymerization for the Modification of Cellulose with Poly(N-isopropylacrylamide) to Create Thermo-Responsive Injectable Hydrogels. Int. J. Mol. Sci. 2024, 25, 2867. [Google Scholar] [CrossRef]
- Kang, M.; Oderinde, O.; Han, X.; Fu, G.; Zhang, Z. Development of Oxidized Hydroxyethyl Cellulose-Based Hydrogel Enabling Unique Mechanical, Transparent and Photochromic Properties for Contact Lenses. Int. J. Biol. Macromol. 2021, 183, 1162–1173. [Google Scholar] [CrossRef]
- Ling, Q.; Ke, T.; Liu, W.; Ren, Z.; Zhao, L.; Gu, H. Tough, Repeatedly Adhesive, Cyclic Compression-Stable, and Conductive Dual-Network Hydrogel Sensors for human Health Monitoring. Ind. Eng. Chem. Res. 2021, 60, 18373–18383. [Google Scholar] [CrossRef]
- Yin, H.; Song, P.; Chen, X.; Huang, Q.; Huang, H. A Self-Healing Hydrogel Based on Oxidized Microcrystalline Cellulose and Carboxymethyl Chitosan as Wound Dressing Material. Int. J. Mol. Sci. 2022, 221, 1606–1617. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Guo, Z.; Wang, Z.; Fan, Z.; Cheng, H.; Xie, Y. Study on Preparation and Properties of Hemicellulose/Chitosan Composite Hydrogels. China Pulp Pap. 2022, 41, 34–40. [Google Scholar] [CrossRef]
- Elkihel, A.; Christie, C.; Vernisse, C.; Ouk, T.S.; Lucas, R.; Chaleix, V.; Sol, V. Xylan-Based Cross-Linked Hydrogel for Photodynamic Antimicrobial Chemotherapy. ACS Appl. Bio Mater. 2021, 4, 7204–7212. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Xu, H.; Hu, Q.; Yang, Y.; Ni, S.; Peng, F.; Jin, X. High Stretchable and Tough Xylan-G-Gelatin Hydrogel via the Synergy of Chemical Cross-Linking and Salting Out for Strain Sensors. Int. J. Biol. Macromol. 2024, 261, 129759. [Google Scholar] [CrossRef]
- Liu, X.; Chang, M.; Zhang, H.; Ren, J. Fabrication of Bentonite Reinforced Dopamine Grafted Carboxymethyl Xylan Cross-Linked with Polyacrylamide Hydrogels with Adhesion Properties. Colloids Surf. A Physicochem. Eng. Asp. 2022, 647, 129024. [Google Scholar] [CrossRef]
- Sun, X.F.; Xie, Y.; Shan, S.; Li, W.; Sun, L. Chemically-Crosslinked Xylan/Graphene Oxide Composite Hydrogel for Copper Ions Removal. J. Polym. Environ. 2022, 30, 3999–4013. [Google Scholar] [CrossRef]
- Hou, Y.; Deng, B.; Wang, S.; Ma, Y.; Long, X.; Wang, F.; Qin, C.; Liang, C.; Yao, S. High-Strength, High-Water-Retention Hemicellulose-Based Hydrogel and Its Application in Urea Slow Release. Int. J. Mol. Sci. 2023, 24, 9208. [Google Scholar] [CrossRef]
- Rodríguez-Ramírez, C.A.; Tasqu, J.E.; Garcia, N.L.; D’Accorso, N.B. Hemicelluloses Hydrogel: Synthesis, Characterization, and Application in Dye Removal. Int. J. Biol. Macromol. 2023, 253, 127010. [Google Scholar] [CrossRef]
- Hu, L.; Xie, Y.; Gao, S.; Shi, X.; Lai, C.; Zhang, D.; Lu, C.; Liu, Y.; Du, L.; Fang, X.; et al. Strain-Induced Orientation Facilitates the Fabrication of Highly Stretchable and Tough Xylan-Based Hydrogel for Strain Sensors. Carbohydr. Polym. 2023, 312, 120827. [Google Scholar] [CrossRef]
- Li, Y.; Yao, M.; Luo, Y.; Li, J.; Wang, Z.; Liang, C.; Qin, C.; Huang, C.; Yao, S. Polydopamine-Reinforced Hemicellulose-Based Multifunctional Flexible Hydrogels for Human Movement Sensing and Self-Powered Transdermal Drug Delivery. ACS Appl. Mater. Interfaces 2023, 15, 5883–5896. [Google Scholar] [CrossRef]
- Shen, F.; Ge, W.; Ling, H.; Yang, Y.; Chen, R.; Wang, X. Hemicellulose-Based Nanoaggregate-Incorporated Biocompatible Hydrogels with Enhanced Mechanical Properties and Sustained Controlled Curcumin Release Behaviors. Int. J. Biol. Macromol. 2024, 259, 129445. [Google Scholar] [CrossRef]
- Long, J.; Zhou, G.; Yu, X.; Xu, J.; Hu, L.; Pranovich, A.; Yong, Q.; Xie, Z.H.; Xu, C. Harnessing Chemical Functionality of Xylan Hemicellulose Towards Carbohydrate Polymer-Based pH/Magnetic Dual-Responsive Nanocomposite Hydrogel for Drug Delivery. Carbohydr. Polym. 2024, 343, 122461. [Google Scholar] [CrossRef]
- Guo, Z.; Fang, Y.; Wang, Z. High-Strength Hemicellulose-Based Conductive Composite Hydrogels Reinforced by Hofmeister Effect. BioResources 2024, 19, 7708–7722. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, W.; Koppolu, R.; van Bochove, B.; Seppälä, J.; Hupa, L.; Willför, S.; Xu, C.; Wang, X. Injectable Thiol-Ene Hydrogel of Galactoglucomannan and Cellulose Nanocrystals in Delivery of Therapeutic Inorganic Ions with Embedded Bioactive Glass Nanoparticles. Carbohydr. Polym. 2022, 276, 118780. [Google Scholar] [CrossRef] [PubMed]
- Aswathy, S.H.; NarendraKumar, U.; Manjubala, I. Physicochemical Properties of Cellulose-Based Hydrogel for Biomedical Applications. Polymers 2022, 14, 4669. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Choudhary, P.; Majeed, A.; Guleria, S.; Kumar, M.; Rana, A.K.; Rajauria, G. Cellulose Based Membranes, Hydrogels and Aerogels for Water Treatment Application. Ind. Crops Prod. 2025, 225, 120474. [Google Scholar] [CrossRef]
- Zhang, Y.; Kobayashi, K.; Wada, M. Comparative Analysis of the Structures and Properties of Cellulose Hydrogels Prepared Using Different Solvent Systems. Cellulose 2025, 32, 2337–2351. [Google Scholar] [CrossRef]
- Martin, S.S.; Gan, L.; Zhang, L.; Yang, X.; Tan, Z.; Shi, H.; Long, L.; Li, H. Cellulose Nanocrystal-Based Intelligent Hydrogels: Innovations, Challenges, and Prospective Application in Advanced Wound Healing. Int. J. Biol. Macromol. 2025, 316, 144752. [Google Scholar] [CrossRef]
- Li, X.; Wan, C.; Tao, T.; Chai, H.; Huang, Q.; Chai, Y.; Wu, Y. An Overview of the Development Status and Applications of Cellulose-Based Functional Materials. Cellulose 2024, 31, 61–99. [Google Scholar] [CrossRef]
- Rumon, M.M.H. Advances in Cellulose-Based Hydrogels: Tunable Swelling Dynamics and Their Versatile Real-Time Applications. RSC Adv. 2025, 15, 11688–11729. [Google Scholar] [CrossRef]
- Zainuddin, N.A.; Pandey, M.; Rahman, S.A.; Bahrin, R.; Mohamad, N. A Systematic Literature Review of Hemicellulose-Based Hydrogels for Drug Delivery System-A Review. Curr. Trends Biotechnol. Pharm. 2024, 18, 11–24. [Google Scholar] [CrossRef]
- Rumon, M.M.H.; Akib, A.A.; Sarkar, S.D.; Khan, M.A.R.; Uddin, M.M.; Nasrin, D.; Roy, C.K. Polysaccharide-Based Hydrogels for Advanced Biomedical Engineering Applications. ACS Polym. Au 2024, 4, 463–486. [Google Scholar] [CrossRef]
- Das, A.; Ringu, T.; Ghosh, S.; Pramanik, N. A Comprehensive Review on Recent Advances in Preparation, Physicochemical Characterization, and Bioengineering Applications of Biopolymers. Polym. Bull. 2023, 80, 7247–7312. [Google Scholar] [CrossRef]
- Nyamayaro, K.; Hatzikiriakos, S.G.; Mehrkhodavandi, P. Utilizing Cellulose-Based Conducting Hydrogels in Iontronics. RSC Sustain. 2023, 1, 1369–1385. [Google Scholar] [CrossRef]
- Zhang, Z.; Lu, Y.; Gao, S.; Wu, S. Sustainable and Efficient Wastewater Treatment Using Cellulose-Based Hydrogels: A Review of Heavy Metal, Dye, and Micropollutant Removal Applications. Separations 2025, 12, 72. [Google Scholar] [CrossRef]
- Yadav, R.; Kumar, R.; Kathpalia, M.; Ahmed, B.; Dua, K.; Gulati, M.; Singh, S.; Singh, P.J.; Kumar, S.; Shah, R.M.; et al. Innovative Approaches to Wound Healing: Insights into Interactive Dressings and Future Directions. J. Mater. Chem. B. 2024, 12, 7977–8006. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Aljohani, K.; Aljohani, B.S.; Lal, B.; Jadeja, Y.; Ballal, S.; Chahar, M.; Suman, R. Innovations in Cellulose-Based Hydrogels for Enhanced Wastewater Treatment Through Adsorption. Int. J. Biol. Macromol. 2025, 303, 140660. [Google Scholar] [CrossRef]
- Le, V.T.; Joo, S.W.; Berkani, M.; Mashifana, T.; Kamyab, H.; Wang, C.; Vasseghian, Y. Sustainable Cellulose-Based Hydrogels for Water Treatment and Purification. Ind. Crops Prod. 2023, 205, 117525. [Google Scholar] [CrossRef]
- Lu, P.; Ruan, D.; Huang, M.; Tian, M.; Zhu, K.; Gan, Z.; Xiao, Z. Harnessing the Potential of Hydrogels for Advanced Therapeutic Applications: Current Achievements and Future Directions. Signal Transduct. Target. Ther. 2024, 9, 166. [Google Scholar] [CrossRef]
- Yin, B.; Gosecka, M.; Bodaghi, M.; Crespy, D.; Youssef, G.; Dodda, J.M.; Wong, S.H.D.; Imran, A.B.; Gosecki, M.; Jobdeedamrong, A.; et al. Engineering Multifunctional Dynamic Hydrogel for Biomedical and Tissue Regenerative Applications. Chem. Eng. J. 2024, 487, 150403. [Google Scholar] [CrossRef]
- Romano, S.; Yazdanpanah, S.; Petillo, O.; Conte, R.; Sepe, F.; Peluso, G.; Calarco, A. Sustainable Hydrogels for Medical Applications: Biotechnological Innovations Supporting One Health. Gels 2025, 11, 559. [Google Scholar] [CrossRef] [PubMed]
- Ciolacu, D.E.; Nicu, R.; Suflet, D.M.; Rusu, D.; Darie-Nita, R.N.; Simionescu, N.; Cazacu, G.; Ciolacu, F. Multifunctional Hydrogels Based on Cellulose and Modified Lignin for Advanced Wounds Management. Pharmaceutics 2023, 15, 2588. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, M.; Geng, Z.; Liu, Y. Functional Hydrogels for the Repair and Regeneration of Tissue Defects. Front. Bioeng. Biotechnol. 2023, 16, 1190171. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yun, Z.; Song, W.; Yu, T.; Xue, W.; Liu, Q.; Sun, X. Highly Oriented Hydrogels for Tissue Regeneration: Design Strategies, Cellular Mechanisms, and Biomedical Applications. Theranostics 2024, 14, 1982–2035. [Google Scholar] [CrossRef]
- Liu, X.; Hu, Y.; Ju, Y.; Yang, P.; Shen, N.; Yang, A.; Wu, R.; Fang, B.; Liu, L. Immunomodulatory Hydrogels for Tissue Repair and Regeneration. APL Mater. 2024, 12, 080603. [Google Scholar] [CrossRef]
- Zhao, L.; Luo, B.; Gao, S.; Liu, Y.; Lai, C.; Zhang, D.; Guan, W.; Wang, C.; Chu, F. Stretchable, Self-Adhesive, and Conductive Hemicellulose-Based Hydrogels as Wearable Strain Sensors. Int. J. Biol. Macromol. 2024, 282, 137313. [Google Scholar] [CrossRef]
- Chibrikov, V.; Pieczywek, P.M.; Cybulska, J.; Zdunek, A. The Effect of Hemicelluloses on Biosynthesis, Structure and Mechanical Performance of Bacterial Cellulose-Hemicellulose Hydrogels. Sci. Rep. 2024, 14, 21671. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, Y.; Wu, K.; Liu, Y.; Lu, H.; Liang, B. Preparation of Hydrogels with Self-Reinforced Mechanical Properties Using Ball-Milled Microcrystalline Cellulose and Regenerated Cellulose from Deep Eutectic Solvent. New J. Chem. 2024, 48, 7405–7412. [Google Scholar] [CrossRef]
- Dou, Y.; Wang, X.; Liu, Z.; Kong, F.; Wang, S. Effect of Different Modified Lignins on the Properties of Xylan Composite Hydrogels. J. Appl. Polym. Sci. 2024, 141, e54910. [Google Scholar] [CrossRef]
- Lyu, Z.; Rao, J.; Qi, X.; Bai, Z.; Jia, S.; Su, Z.; Peng, F. Facile Approach for Preparation of Xylan-based Double-network Hydrogels. Pap. Biomater. 2022, 7, 19–27. [Google Scholar] [CrossRef]
- Wang, C.; Bai, J.; Tian, P.; Xie, R.; Duan, Z.; Lv, Q.; Tao, Y. The Application Status of Nanoscale Cellulose-Based Hydrogels in Tissue Engineering and Regenerative Biomedicine. Front. Bioeng. Biotechnol. 2021, 9, 732513. [Google Scholar] [CrossRef]
- Ciolacu, D.E. Biochemical Modification of Lignocellulosic Biomass. In Biomass as Renewable Raw Material to Obtain Bioproducts of High-Tech Value; Popa, V., Volf, I., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; Chapter 9; pp. 315–350. [Google Scholar] [CrossRef]
- Ramírez Brenes, R.G.; Chaves, L.d.S.; Bojorge, N.; Pereira, N., Jr. Endo-Exoglucanase Synergism for Cellulose Nanofibril Production Assessment and Characterization. Molecules 2023, 28, 948. [Google Scholar] [CrossRef]
- Garcia-Garcia, A.; Muñana-González, S.; Lanceros-Mendez, S.; Ruiz-Rubio, L.; Alvarez, L.P.; Vilas-Vilela, J.L. Biodegradable Natural Hydrogels for Tissue Engineering, Controlled Release, and Soil Remediation. Polymers 2024, 16, 2599. [Google Scholar] [CrossRef]
- Zhang, P.; Chen, Z.; Zhou, L.; Gao, J.; Zheng, H.; Lin, H.; Zhu, G.; Qin, X.; Cao, W. Carboxymethyl Cellulose and Carboxymethyl Chitosan-Based Composite Nanogel as a Stable Delivery Vehicle for Oyster Peptides: Characterization, Absorption and Transport Mechanism. Food Chem. 2024, 442, 138464. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, A.; Mishra, D.K. Xylan-Based Hydrogels: A Polymeric Carrier for Sustained and Targeted Delivery of Drugs. In Drug Formulation Design; Shukla, R., Kuznetsov, A., Ali, A., Eds.; IntechOpen: London, UK, 2022; Chapter 6; pp. 1–203. [Google Scholar] [CrossRef]
- Li, T.; Sun, W.; Qian, D.; Wang, P.; Liu, X.; He, C.; Chang, T.; Liao, G.; Zhang, J. Plant-Derived Biomass-Based Hydrogels for Biomedical Applications. Trends Biotechnol. 2025, 43, 4. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.M.; Branciforti, M.C.; Brienzo, M. Biodegradation of Hemicellulose-Cellulose-Starch-Based Bioplastics and Microbial Polyesters. Recycling 2021, 6, 22. [Google Scholar] [CrossRef]
- Pei, W.; Yu, Y.; Wang, P.; Zheng, L.; Lan, K.; Jin, Y.; Yong, Q.; Huang, C. Research Trends of Bio-Application of Major Components in Lignocellulosic Biomass (Cellulose, Hemicellulose and Lignin) in Orthopedics Fields Based on the Bibliometric Analysis: A Review. Int. J. Biol. Macromol. 2024, 267, 131505. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, T.; Dong, C.; Pan, X. Biomedical Applications of Hemicellulose-Based Hydrogels. Curr. Med. Chem. 2020, 27, 4647–4659. [Google Scholar] [CrossRef]
- Gong, J.; Hou, L.; Ching, Y.C.; Ching, K.Y.; Hai, N.G.; Chuah, C.H. A Review of Recent Advances of Cellulose-Based Intelli-gent-Responsive Hydrogels as Vehicles for Controllable Drug Delivery System. Int. J. Biol. Macromol. 2024, 264, 130525. [Google Scholar] [CrossRef]
- Ding, H.; Tan, P.; Fu, S.; Tian, X.; Zhang, H.; Ma, X.; Gu, Z.; Luo, K. Preparation and Application of pH-Responsive Drug Delivery Systems. J. Contr. Release. 2022, 348, 206–238. [Google Scholar] [CrossRef]
- Ahmadpour, F.; Ganjali, F.; Radinekiyan, F.; Eivazzadeh-Keihan, R.; Salimibani, M.; Bahreinizad, H.; Mahdavi, M.; Maleki, A. Fabrication and Characterization of a Novel Magnetic Nanostructure Based on Pectin–Cellulose Hydrogel for in Vitro Hyperthermia During Cancer Therapy. RSC Adv. 2024, 14, 13676–13684. [Google Scholar] [CrossRef]
- Nicu, R.; Ciolacu, D.E.; Petrovici, A.-R.; Rusu, D.; Avadanei, M.; Mihaila, A.C.; Butoi, E.; Ciolacu, F. 3D Matrices for Enhanced Encapsulation and Controlled Release of Anti-Inflammatory Bioactive Compounds in Wound Healing. Int. J. Mol. Sci. 2023, 24, 4213. [Google Scholar] [CrossRef]
- Tang, Z.; Yang, Y.; Pan, Y.; Yu, M.; Lin, X.; Mondal, A.K. Biocompatible, Injectable, and Self-Healing Poly(Nvinylpyrrolidone)/Carboxymethyl Cellulose Hydrogel for Drug Release. ACS Omega 2024, 9, 5854–5861. [Google Scholar] [CrossRef]
- Asadi, M.; Salehi, Z.; Akrami, M.; Hosseinpour, M.; Jockenhövel, S.; Ghazanfari, S. 3D Printed pH-Responsive Tablets Containing N-Acetylglucosamine-Loaded Methylcellulose Hydrogel for Colon Drug Delivery Applications. Int. J. Pharm. 2023, 645, 123366. [Google Scholar] [CrossRef]
- Xue, H.; Zhu, C.; Wang, Y.; Gu, Q.; Shao, Y.; Jin, A.; Zhang, X.; Lei, L.; Li, Y. Stimulus-Responsive Cellulose Hydrogels in Biomedical Applications and Challenges. Mater. Today Bio 2025, 32, 101814. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Seo, J.H.; Jeong, M.Y.; Yang, I.G.; Kim, J.S.; Kim, J.H.; Ho, M.J.; Jin, S.G.; Choi, M.K.; Choi, Y.S.; et al. Carboxymethyl Cellulose-Based Rotigotine Nanocrystals-Loaded Hydrogel for Increased Transdermal Delivery with Alleviated Skin Irritation. Carbohydr. Polym. 2024, 338, 122197. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Gu, K.; Sun, K.; Wang, X.; Yao, S.; Mo, X.; Long, S.; Lan, T.; Qin, C. Preparation and Swelling Behaviors of High-Strength Hemicellulose-g-Polydopamine Composite Hydrogels. Materials 2021, 14, 186. [Google Scholar] [CrossRef]
- Davodabadi, F.; Sargazi, S.; Baino, F. Recent Advances in Hydrogel-Based Drug Delivery Systems for Enhanced Cancer Therapy: A Review. Mater. Today Commun. 2025, 48, 113615. [Google Scholar] [CrossRef]
- Bi, H.; Zhang, X.; Wang, Q.; Yong, Q.; Xu, W.; Xu, M.; Xu, C.; Wang, X. Dynamic reversible disulfide bonds hydrogel of thiolated galactoglucomannan/cellulose nanofibril with self-healing property for protein release. Ind. Crops Prod. 2023, 206, 117615. [Google Scholar] [CrossRef]
- Suhail, M.; Alamgir; Wahab, A.; Eggers, T.; Ahmad, Z.; Shehzad, K.; Iqbal, M.Z. Magnetically Responsive Hydrogel Systems: Fundamental Features, Emerging Applications, and Future Horizons. Coord. Chem. Rev. 2025, 543, 216916. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, X.; Zhang, Q.; Zhang, D.; Xie, X.; Zhou, H.; Wu, Z.; Liu, R.; Pang, J. Review of Konjac Glucomannan Structure, Properties, Gelation Mechanism, and Application in Medical Biology. Polymers 2023, 15, 1852. [Google Scholar] [CrossRef]
- Wu, H.; Bu, N.; Chen, J.; Chen, Y.; Sun, R.; Wu, C.; Pang, J. Construction of Konjac Glucomannan/Oxidized Hyaluronic Acid Hydrogels for Controlled Drug Release. Polymers 2022, 14, 927. [Google Scholar] [CrossRef] [PubMed]
- Bukatuka, C.F.; Mbituyimana, B.; Xiao, L.; Qaed Ahmed, A.A.; Qi, F.; Adhikari, M.; Shi, Z.; Yang, G. Recent Trends in the Application of Cellulose-Based Hemostatic and Wound Healing Dressings. J. Funct. Biomater. 2025, 16, 151. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.M.; Simões, M.; Vitorino, C.; Mascarenhas-Melo, F. Physical Crosslinking of Hydrogels: The Potential of Dynamic and Reversible Bonds in Burn Care. Coord. Chem. Rev. 2025, 542, 216868. [Google Scholar] [CrossRef]
- Gürsoy, E.N.; Sener, K.; Külahci, M.B.; Balabanli, K.B.; Cevher, Ş.C. Modern Strategies in Wound Healing: The Rise of Bacterial Cellulose Dressings. Adv. Therap. 2025, 8, e00072. [Google Scholar] [CrossRef]
- Li, B.; Cao, P.F.; Saito, T.; Sokolov, A.P. Intrinsically Self-Healing Polymers: From Mechanistic Insight to Current Challenges. Chem. Rev. 2022, 123, 701–735. [Google Scholar] [CrossRef]
- Sarkhel, S.; Jaiswal, A. Emerging Frontiers in In Situ Forming Hydrogels for Enhanced Hemostasis and Accelerated Wound Healing. ACS Appl. Mater. Interfaces 2024, 16, 61503–61529. [Google Scholar] [CrossRef]
- Gounden, V.; Singh, M. Hydrogels and Wound Healing: Current and Future Prospects. Gels 2024, 10, 43. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, J.; Li, D.; Cheng, F. The Recent Progress of the Cellulose-Based Antibacterial Hydrogel. Gels 2024, 10, 109. [Google Scholar] [CrossRef]
- Mo, M.; Wu, C.; Chen, Y. Bacterial Cellulose-Based Superabsorbent Hydrogel for Wet Wound Dressing. Molecules 2025, 30, 737. [Google Scholar] [CrossRef]
- Premarathna, A.D.; Ahmed, T.A.E.; Rjabovs, V.; Hammami, R.; Critchley, A.T.; Tuvikene, R.; Hincke, M.T. Immunomodulation By Xylan and Carrageenan-Type Polysaccharides from Red Seaweeds: Anti-Inflammatory, Wound Healing, Cytoprotective, and Anticoagulant Activities. Int. J. Biol. Macromol. 2024, 260, 129433. [Google Scholar] [CrossRef]
- Murphy, E.J.; Fehrenbach, G.W.; Abidin, I.Z.; Buckley, C.; Montgomery, T.; Pogue, R.; Murray, P.; Major, I.; Rezoagli, E. Polysaccharides—Naturally Occurring Immune Modulators. Polymers 2023, 15, 2373. [Google Scholar] [CrossRef]
- Guamba, E.; Vispo, N.S.; Whitehead, D.C.; Singh, A.K.; Santos-Oliveira, R.; Niebieskikwiat, D.; Zamora-Ledezma, C.; Alexis, F. Cellulose-Based Hydrogels Towards an Antibacterial Wound Dressing. Biomater. Sci. 2023, 11, 3461–3468. [Google Scholar] [CrossRef]
- Yi, X.; He, J.; Wei, X.; Li, H.; Liu, X.; Cheng, F. A Mussel Inspired Multifunctional Hydrogel Reinforced by Bacterial Cellulose for Wound Healing: Sustained Drug Release, Enhanced Adhesion and Self-Healing Property. Cellulose 2023, 30, 6523–6538. [Google Scholar] [CrossRef]
- Deng, L.; Li, F.; Han, Z.; Qu, X.; Li, J.; Zhou, Z.; Chen, S.; Wang, H.; Lv, X. Bacterial Cellulose-Based Hydrogel with Regulated Rehydration and Enhanced Antibacterial Activity for Wound Healing. Int. J. Biol. Macromol. 2024, 267, 131291. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.; Liu, X.; Wang, X.; Peng, F.; Ren, J. Mussel-Inspired Adhesive Hydrogels Based on Biomass-Derived Xylan and Tannic Acid Cross-Linked with Acrylic Acid with Antioxidant and Antibacterial Properties. J. Mater. Sci. 2021, 56, 14729–14740. [Google Scholar] [CrossRef]
- Khan, M.U.A.; Haider, S.; Raza, M.A.; Shah, S.A.; Razak, S.I.A.; Kadir, M.R.A.; Subhan, F.; Haider, A. Smart and Ph-Sensitive rGO/Arabinoxylan/Chitosan Composite for Wound Dressing: In-Vitro Drug Delivery, Antibacterial Activity, and Biological Activities. Int. J. Biol. Macromol. 2021, 192, 820–831. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Bano, S.; Poojary, S.S.; Priyadarshi, R.; Choudhary, A.; Kumar, D.; Negi, Y.S. Comparative Analysis of TiO2 and Ag Nanoparticles on Xylan/Chitosan Conjugate Matrix for Wound Healing Application. Int. J. Polym. Mater. Biomater. 2022, 71, 376–385. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Wang, C.; Yuan, X.Q.; Yan, H.H.; Li, C.B.; Wang, C.H.; Xie, X.R.; Hou, G.G. Multifunctional Xyloglucan-Containing Electrospun Nanofibrous Dressings for Accelerating Infected Wound Healing. Int. J. Biol. Macromol. 2023, 247, 125504. [Google Scholar] [CrossRef]
- Xu, Y.; Hu, J.; Bi, D.; Su, W.; Hu, L.; Ma, Y.; Zhu, M.; Wu, M.; Huang, Y.; Yu, E.; et al. A Bioactive Xyloglucan Polysaccharide Hydrogel Mechanically Enhanced by Pluronic F127 Micelles for Promoting Chronic Wound Healing. Int. J. Biol. Macromol. 2024, 277, 134102. [Google Scholar] [CrossRef]
- Liu, Y.; Teng, J.; Huang, R.; Zhao, W.; Yang, D.; Ma, Y.; Wei, H.; Chen, H.; Zhang, J.; Chen, J. Injectable Plant-Derived Polysaccharide Hydrogels with Intrinsic Antioxidant Bioactivity Accelerate Wound Healing by Promoting Epithelialization and Angiogenesis. Int. J. Biol. Macromol. 2024, 266, 131170. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Yu, S.; Zhang, Y.; Zhang, H.; Ma, Y.; Xu, M.; An, P.; Zhou, Y.; Halila, S.; Wei, Y.; et al. Injectable Chitosan/Xyloglucan Composite Hydrogel with Mechanical Adaptivity and Endogenous Bioactivity for Skin Repair. Carbohydr. Polym. 2023, 313, 120904. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Q.; Liao, Q.; Meldrum, O.W.; Guo, L.; Wang, K.; Zhang, S.; Liu, Y.; Chen, X.; Zhu, J.; Li, L. Mechanical Properties and Wound Healing Potential of Bacterial Cellulose-Xyloglucan-Dextran Hydrogels. Carbohydr. Polym. 2023, 321, 121268. [Google Scholar] [CrossRef] [PubMed]
- Falsafi, S.R.; Topuz, F.; Rostamabadi, H. Dialdehyde Carbohydrates-Advanced Functional Materials for Biomedical Applications. Carbohydr. Polym. 2023, 321, 121276. [Google Scholar] [CrossRef]
- Chen, G.; Han, T.; Xiang, Z.; Song, T. Controlling Size and Stabilization of Silver Nanoparticles for Use in Optimized Chitosan-Dialdehyde Xylan Wound Dressings. Cellulose 2022, 29, 5833–5851. [Google Scholar] [CrossRef]
- Pan, X.; Zong, Q.; Liu, C.; Wu, H.; Fu, B.; Wang, Y.; Sun, W.; Zhai, Y. Konjac Glucomannan Exerts Regulatory Effects on Macrophages and its Applications in Biomedical Engineering. Carbohydr. Polym. 2024, 345, 122571. [Google Scholar] [CrossRef]
- Zhuang, K.; Shu, X.; Xie, W. Konjac Glucomannan-Based Composite Materials: Construction, Biomedical Applications, and Prospects. Carbohydr. Polym. 2024, 344, 122503. [Google Scholar] [CrossRef]
- Ren, M.; Wang, X.; Ouyang, X.; Ling, J.; Wang, N. Protocatechuic Acid Grafted Chitosan/Oxidized Glucomannan Hydrogel with Antimicrobial and Anti-Inflammatory Effects for Enhancing Wound Repair. Int. J. Biol. Macromol. 2024, 281, 136514. [Google Scholar] [CrossRef]
- Xu, S.; Yan, S.; You, J.; Wu, X. Antibacterial Micelles-Loaded Carboxymethyl Chitosan/Oxidized Konjac Glucomannan Composite Hydrogels for Enhanced Wound Repairing. ACS Appl. Mater. Interfaces 2024, 16, 13563–13572. [Google Scholar] [CrossRef]
- Mashudi, S.; Saifullah, T.; Handoko, L.; Purnomo, R.A. A Novel Dressing for Wound Care Based on Konjac Glucomannan. Biomed. J. Sci. Tech. Res. 2022, 47, 2. [Google Scholar] [CrossRef]
- Hao, H.; Li, D. Facile Fabrication of Functional Hyaluronic Acid-/Konjac Glucomannan-Based Injectable Hydrogel as Wound Closure and Anti-Microbial Material for the Treatment of Burn Wound Healing. J. Mater. Res. 2024, 39, 2258–2271. [Google Scholar] [CrossRef]
- Li, H.; Liang, X.; Chen, Y.; Liu, K.; Fu, X.; Zhang, C.; Wang, X.; Yang, J. Synergy of Antioxidant and M-2 polarization in Polyphenol-Modified Konjac Glucomannan Dressing for Remodeling Wound Healing Microenvironment. Bioeng. Transl. Med. 2023, 8, e10398. [Google Scholar] [CrossRef]
- Varguez-Catzim, P.; Hernández-Aburto, M.; Rodriguez-Canto, W.; Hunh-Ibarra, M.; Aguilar-Vega, M.; Claudio-Rizo, J.A.; González-Díaz, M.O. Tailoring Membrane Technology with Galactomannan for Enhanced Biocompatibility and Antibacterial Action. Int. J. Biol. Macromol. 2025, 286, 138320. [Google Scholar] [CrossRef]
- Lima, I.C.; Castro, R.R.; Adjafre, B.L.; Sousa, S.H.A.F.; de Paula, D.G.; Alves, A.P.N.N.; Silva, P.G.B.; Assreuy, A.M.S.; Mota, M.R.L. Galactomannan of Delonix Regia Seeds Modulates Cytokine Expression and Oxidative Stress Eliciting Anti-Inflammatory and Healing Effects in Mice Cutaneous Wound. Int. J. Biol. Macromol. 2022, 203, 342–349. [Google Scholar] [CrossRef]
- Souza, A.A.; Ribeiro, K.A.; Seixas, J.R.P.C.; Neto, J.C.S.; Santiago, M.G.P.F.; Aragão-Neto, A.C.; Lima-Ribeiro, M.H.M.; Borba, E.F.O.; Silva, T.G.; Kennedy, J.F.; et al. Effects Including Photobiomodulation of Galactomannan Gel From Cassia Grandis Seeds in the Healing Process of Second-Degree Burns. Int. J. Biol. Macromol. 2023, 251, 126213. [Google Scholar] [CrossRef] [PubMed]
- de Seixas, J.R.; Ribeiro, K.A.; de Souza, A.A.; de Seixas, J.R.P.C.; Ribeiro, K.A.; de Souza, A.A.; da Silva, C.E.; Pedra-Fixe, M.G.; Lima-Ribeiro, M.H.M.; da Neto, J.C.S.; et al. Hydrogels Based on Galactomannan and κ-Carrageenan Containing Immobilized Biomolecules for In Vivo Thermal-Burn Wound Treatment. Int. J. Biol. Macromol. 2024, 270, 132379. [Google Scholar] [CrossRef]
- Yuyu, E.; Chang, Z.; Su, W.; Li, W.; Li, P.; Lei, F.; Yao, X.; Yuan, S.; Li, J.; Zhang, F.; et al. Multi-Functional Gleditsia Sinensis Galactomannan-Based Hydrogel With Highly Stretchable, Adhesive, and Antibacterial Properties as Wound Dressing for Accelerating Wound Healing. Int. J. Biol. Macromol. 2024, 283, 137279. [Google Scholar] [CrossRef]
- Meretsky, C.R.; Polychronis, A.; Liovas, D.; Schiuma, A.T. Advances in Tissue Engineering and Its Future in Regenerative Medicine Compared to Traditional Reconstructive Techniques: A Comparative Analysis. Cureus 2024, 16, e68872. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Song, S.; Ren, X.; Zhang, J.; Lin, Q.; Zhao, Y. Supramolecular Adhesive Hydrogels for Tissue Engineering Applications. Chem. Rev. 2022, 122, 5604–5640. [Google Scholar] [CrossRef]
- Liu, H.; Hu, Y.; Liu, Y.; Hu, R.; Wu, X.; Li, B. A Review of Recent Advances in Biomedical Applications of Smart Cellulose-Based Hydrogels. Int. J. Biol. Macromol. 2023, 253, 127149. [Google Scholar] [CrossRef]
- Naranđa, J.; Bračič, M.; Maver, U.; Trojner, T. Recent Advancements in Smart Hydrogel-Based Materials in Cartilage Tissue Engineering. Materials 2025, 18, 2576. [Google Scholar] [CrossRef]
- Malekpour, K.; Hazrati, A.; Khosrojerdi, A.; Roshangar, L.; Ahmadi, M. An Overview to Nanocellulose Clinical Application: Biocompatibility and Opportunities in Disease Treatment. Regen. Ther. 2023, 24, 630–641. [Google Scholar] [CrossRef]
- Sreedharan, M.; Vijayamma, R.; Liyaskina, E.; Revin, V.V.; Ullah, M.W.; Shi, Z.; Yang, G.; Grohens, Y.; Kalarikkal, N.; Khan, A.K.; et al. Nanocellulose-Based Hybrid Scaffolds for Skin and Bone Tissue Engineering: A 10-Year Overview. Biomacromolecules 2024, 25, 2136–2155. [Google Scholar] [CrossRef]
- Tamo, A.K. Nanocellulose-Based Hydrogels as Versatile Materials with Interesting Functional Properties for Tissue Engineering Applications. J. Mater. Chem. B 2024, 12, 7692–7759. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Dai, L.; Wei, J.; Zhu, X.; Li, J.; Chen, Z.; Ni, Y. Nanocellulose-Based Hydrogels as Versatile Drug Delivery Vehicles: A Review. Int. J. Biol. Macromol. 2022, 222, 830–843. [Google Scholar] [CrossRef] [PubMed]
- Lan, X.; Ma, Z.; Szojka, A.R.A.; Kunze, M.; Mulet-Sierra, A.; Vyhlidal, M.J.; Boluk, Y.; Adesida, A.B. TEMPO-Oxidized Cellulose Nanofiber-Alginate Hydrogel as a Bioink for Human Meniscus Tissue Engineering. Front. Bioeng. Biotechnol. 2021, 9, 766399. [Google Scholar] [CrossRef] [PubMed]
- Shrivastav, P.; Pramanik, S.; Vaidya, G.; Abdelgawad, M.A.; Ghoneim, M.M.; Singh, A.; Abualsoud, B.M.; Amaral, L.S.; Abourehab, M.A.S. Bacterial Cellulose as a Potential Biopolymer in Biomedical Applications: A State-Of-The-Art Review. J. Mater. Chem. B 2022, 10, 3199–3241. [Google Scholar] [CrossRef]
- Li, B.; Zhang, L.; Wang, D.; Peng, F.; Zhao, X.; Liang, C.; Li, H.; Wang, H. Thermosensitive -Hydrogel-Coated Titania Nanotubes with Controlled Drug Release and Immunoregulatory Characteristics for Orthopedic Applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 122, 111878. [Google Scholar] [CrossRef]
- Tohidi, H.; Maleki, N.; Simchi, A. Conductive, Injectable, and Self-Healing Collagen-Hyaluronic Acid Hydrogels Loaded with Bacterial Cellulose and Gold Nanoparticles for Heart Tissue Engineering. Int. J. Biol. Macromol. 2024, 280, 135749. [Google Scholar] [CrossRef]
- Ai, J.; Li, K.; Li, J.; Yu, F.; Ma, J. Super Flexible, Fatigue Resistant, Self-Healing PVA/Xylan/Borax Hydrogel with Dual-Crosslinked Network. Int. J. Biol. Macromol. 2021, 172, 66–73. [Google Scholar] [CrossRef]
- Ali, A.; Hasan, A.; Negi, Y.S. Effect of Carbon Based Fillers on Xylan/Chitosan/Nano-Hap Composite Matrix for Bone Tissue Engineering Application. Int. J. Biol. Macromol. 2022, 197, 1–11. [Google Scholar] [CrossRef]
- Jiang, L.; Yao, F.; Zhang, E.; Yu, Q.; Yu, C.; Chen, Z.; Chen, J.; Yue, Z.; Che, P.; Li, J.; et al. Combined Treatment of Xyloglucan Derivative Hydrogel and Anti-C5a Receptor Antibody in Preventing Peritoneal Adhesion. Acta Biomater. 2022, 151, 163–173. [Google Scholar] [CrossRef]
- Ma, N.; Cheung, D.Y.; Butcher, J.T. Incorporating Nanocrystalline Cellulose into a Multifunctional Hydrogel for Heart Valve Tissue Engineering Applications. J. Biomed. Mater. Res. 2022, 110, 76–91. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Xie, Y.; Zhu, H.; Zheng, X.; Hou, R.; Shi, Z.; Jing Li, J.; Yang, Q. Highly Electroactive Tissue Engineering Scaffolds Based on Nanocellulose/Sulfonated Carbon Nanotube Composite Hydrogels for Myocardial Tissue Repair. Biomacromolecules 2023, 24, 5989–5997. [Google Scholar] [CrossRef] [PubMed]
- Hou, R.; Xie, Y.; Song, R.; Bao, J.; Shi, Z.; Xiong, C.; Yang, Q. Nanocellulose/Polypyrrole Hydrogel Scaffolds with Mechanical Strength and Electrical Activity Matching Native Cardiac Tissue for Myocardial Tissue Engineering. Cellulose 2024, 31, 4247–4262. [Google Scholar] [CrossRef]
- Liu, X.; Hu, H.; Ma, J.; Wang, B. Mineralized Cellulose Nanofibers Reinforced Bioactive Hydrogel Remodels the Osteogenic and Angiogenic Microenvironment for Enhancing Bone Regeneration. Carbohydr. Polym. 2025, 357, 123480. [Google Scholar] [CrossRef]
- Maturavongsadit, P.; Narayanan, L.K.; Chansoria, P.; Shirwaiker, R.; Benhabbour, S.R. Cell-Laden Nanocellulose/Chitosan-Based Bioinksfor 3D Bioprinting and Enhanced Osteogenic Cell Differentiation. ACS Appl. Biol. Mater. 2021, 4, 2342–2353. [Google Scholar] [CrossRef]
- Sedighim, S.; Chen, Y.; Xu, C.; Mohindra, R.; Liu, H.; Agrawal, D.K.; Thankam, F.G. Carboxymethyl Cellulose–Alginate Interpenetrating Hydroxy Ethyl Methacrylate Crosslinked Polyvinyl Alcohol Reinforced Hybrid Hydrogel Templates with Improved Biological Performance for Cardiac Tissue Engineering. Biotechnol. Bioeng. 2023, 120, 819–835. [Google Scholar] [CrossRef]
- Maharjana, B.; Parka, J.; Kaliannagoundera, V.K.; Awasthia, G.P.; Joshia, M.K.; Parka, C.H.; Kim, C.S. Regenerated Cellulose Nanofiber Reinforced Chitosan Hydrogel Scaffolds for Bone Tissue Engineering. Carbohydr. Polym. 2021, 251, 117023. [Google Scholar] [CrossRef]
- Catori, D.M.; Fragal, E.H.; Messias, I.; Garcia, F.P.; Nakamura, C.V.; Rubira, A.F. Development of Composite Hydrogel Based on Hydroxyapatite Mineralization over Pectin Reinforced with Cellulose Nanocrystal. Int. J. Biol. Macromol. 2021, 167, 726–735. [Google Scholar] [CrossRef]
- Li, X.; Yang, B.; Pang, Y.; Zhu, S.; Guan, L.; Lin, Q.; Li, L.; Zhu, Y.; Whittaker, A.K.; Yang, B.; et al. Mussel-Inspired Antimicrobial Hydrogel with Cellulose Nanocrystals/Tannic Acid Modified Silver Nanoparticles for Enhanced Calvarial Bone Regeneration. Int. J. Biol. Macromol. 2024, 270, 132419. [Google Scholar] [CrossRef]
- Sinna, J.; Jeencham, R.; Mueangkhot, P.; Sophon, S.; Noralak, P.; Raksapakdee, R.; Numpaisal, P.-o.; Ruksakulpiwat, Y. Development of Poly(vinyl alcohol) Grafted Glycidyl Methacrylate/Cellulose Nanofiber Injectable Hydrogels for Meniscus Tissue Engineering. Polymers 2023, 15, 4230. [Google Scholar] [CrossRef] [PubMed]

| Structure/Properties | Cellulose | Hemicellulose |
|---|---|---|
| Source Abundance | Highly available | Highly available |
| Processing can be expensive | Less used | |
| Structure | Linear | Branched |
| β-(1→4)-linked glucose chains | Heterogeneous | |
| Functional Groups | Hydroxyl | Hydroxyl |
| – | Acetyl | |
| Crystalline Structure | Crystalline | Amorphous |
| Chemical Reactivity | Low | Higher |
| Stable polymer backbone | Contains various functional groups | |
| Solubility | Insoluble in water (native) | Soluble in water/alkaline |
| Its derivatives are more soluble | – | |
| Swelling Capacity | High | Moderate to high |
| Abundant hydroxyl groups | Varies with composition | |
| Mechanical Strength | Strong | Moderate |
| Biodegradability | High | High |
| Type of Hydrogel | Preparation Method | Hydrogel Composition and Structure | Hydrogel Characteristics | Ref. | |
|---|---|---|---|---|---|
| Composition | Network | ||||
| Cellulose hydrogels | Hydrogen bonding | - CMC/PA | - hydrogen bonds between CMC and PA. | - highly porous network; - fine and interconnected pores; - SDE = 374%. | [66] |
| - Ox-HPC/CMCS | - H-bonds and Schiff base reaction. | - smaller and uniform pore sizes; - gelation time: 30 min (24% Ox-HPC/6% CMCS). | [67] | ||
| - CNC/PAM/LMC | - dually crosslinked hydrophobic-associated hydrogels: - (i) hydrophobic-associated micelles based on LMC; - (ii) radical polymerization of Amm and LMC; - (iii) H-bonds: CNCs and hydrophobically associated hydrogel. | - fracture strain (1.5% CNCs): 34%; - fracture stress (1.5% CNCs): 342%; - sensitivity: GF = 19.25 at 700% strain; - dissipated energy: 10.9 kJ/m3; - conductivity: 22.97 mS/cm. | [68] | ||
| Freeze–thaw method | - CNF/CNT | - composite aerogel; - freeze–thaw cycles: entanglement of CNTs and CNFs. | - ⍴ = 0.0519 g·cm−3; - average pore sizes: 19.32 nm; - surface area: 157.24 m2/g; - conductivity: 30.95 S/cm. | [71] | |
| - CNF/CNT | - anisotropic aerogel; - multiple unidirectional freeze–thaw cycles. | - ⍴ = 0.0296 g·cm−3; - porosity: 98.1–98.6%; - shrinkages: 11.03%; - stress: 329.8 kPa at 75% strain. | [72] | ||
| Ionic interactions | - cotton cellulose/PVA | - ionic conductive hydrogels: - (i) hydrogen bonds: cellulose and PVA; - (ii) ionic interactions: Zn2+/Ca2+ and OH groups of cellulose. | - tensile strength: 0.30 MPa, - compressive strength: 2.05 MPa at 70% strain; - elongation at break: 130%; - conductivity of 8.16 S m−1. | [73] | |
| - CMC | - dual-crosslinked network: - (i) coordinated aqua-complexes by Al3+; - (ii) ionic crosslinking: aqua–complexes with the –COO– groups of CMC and CA; - (iii) H-bonds: aqua–complexes with -COOH groups of CMC and CA. | - high thermal stability; - CMC–CA–Al hydrogels: absorption/exchange properties and pH-responsive behavior;- adsorption capacity Li+ ions = 50 mg/g sorbent; - adsorption capacity Cs+ ions = 3–12.2 mg/g sorbent. | [74] | ||
| - PEDOT/SCNF/Al(TFSI)3/PAA/TOCNFs/Al3+ ions | - interpenetrating core−sheath-structured conductive nanofibers incorporated in a physically crosslinked polyelectrolyte; - hydrogel network: electrostatic interactions, H-bonds, hydrophobic interaction and static π-π stacking. | - conductivity: 7.1 S/m; - stretchability: 770%; - self-healing efficiency: 80% after 2 h at room temperature; - sensitivity: 0.14 kPa−1; - self-adhesion: 28 kPa (pigskin). | [75] | ||
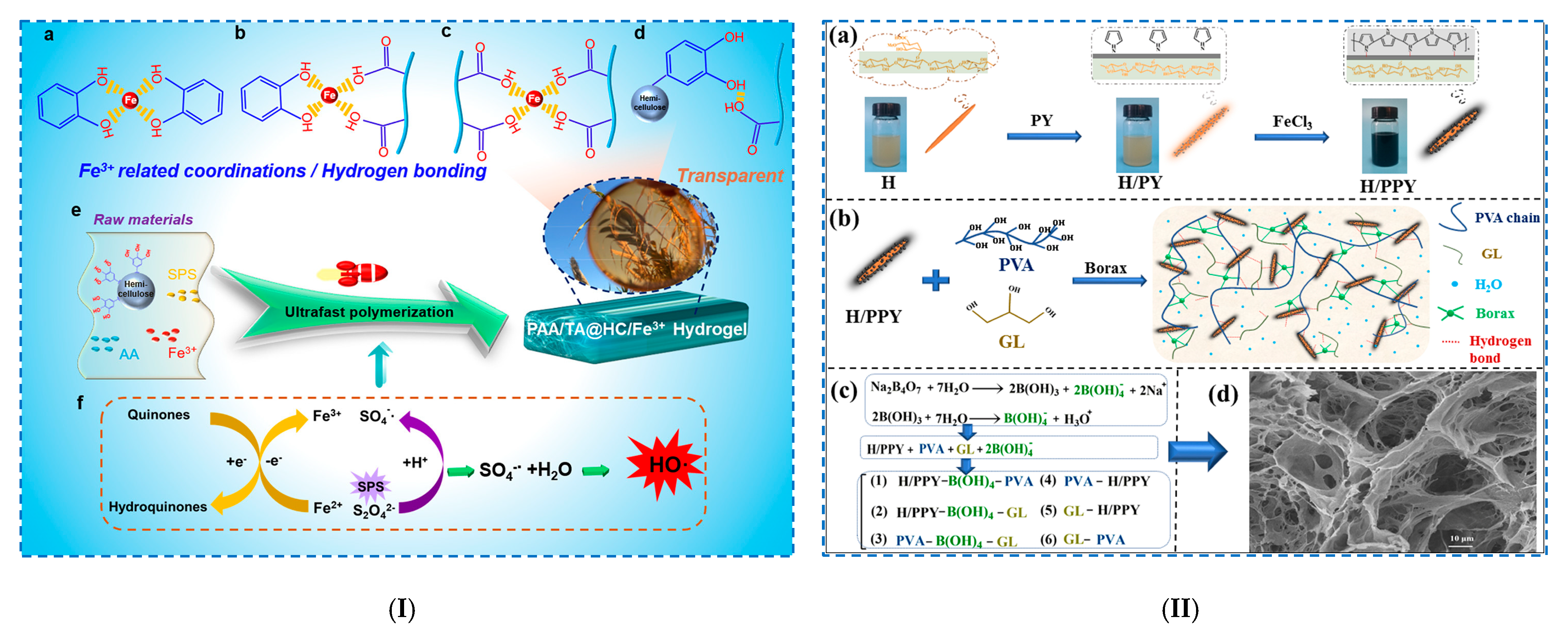
| Hemicellulose hydrogels | Hydrogen bonding | - TA@HC/PAA | - PAA/TA@HC/Fe3+ hydrogel: - (i) H-bonds between hydrophilic groups of components; - (ii) reversible interfacial interactions with Fe3+ (metal complexes); - (iii) free radical polymerization of AA. | - tensile stress: 115 kPa; - deformation: 5600%; - toughness: 4400 kJ/m3; - adhesion strength: 8.5 kPa (pigskin), 12.6 kPa (plastic) and 17.8 kPa (paper). | [76] |
| - HC/GO | - HC-based hydrogels: - (i) H-bonds: between the OH groups (HC) and carboxyl groups (GO); - (ii) hydrogen bonds: between the OH groups (HC) and OH groups (GO). | - interconnected 3D porous network; - excellent mechanical properties; - non-toxic and biocompatible for cells. | [77] | ||
| Freeze–thaw method | - H/PPY/B/GL/PVA | - PVA/B–GL–H/PPY hydrogel: strong H-bonds between PVA, H/PPY and GL; - freeze–thaw method (2 cycles): a multifunctional conductive composite hydrogel. | - tensile strain: 1094.9%; - stress: 480.6 kPa; - compressive stress: 1635.4 kPa; - toughness: 2.82 MJ/m3. | [78] | |
| Ionic interactions | - CM–Hemi/N–CDs | - CM–Hemi@Ca hydrogel: ionic bonds between Ca2+ and the COO– groups in CM-Hemi; - network of CM–Hemi@Ca–N–CD hydrogel: amide bonds between the COOH groups of CM–Hemi and the NH2 groups on the N–CD surface. | - pore size: 1.63–2.46 µm; - good binding interactions with proteins; - bond length: 1.92 A° (Staphylococcus aureus and Candida albicans); - bond length: 2.01 A° (Escherichia coli). | [79] | |
| Type of Hydrogel | Preparation Method | Hydrogel Composition and Structure | Hydrogel Characteristics | Ref. | |
|---|---|---|---|---|---|
| Composition | Network | ||||
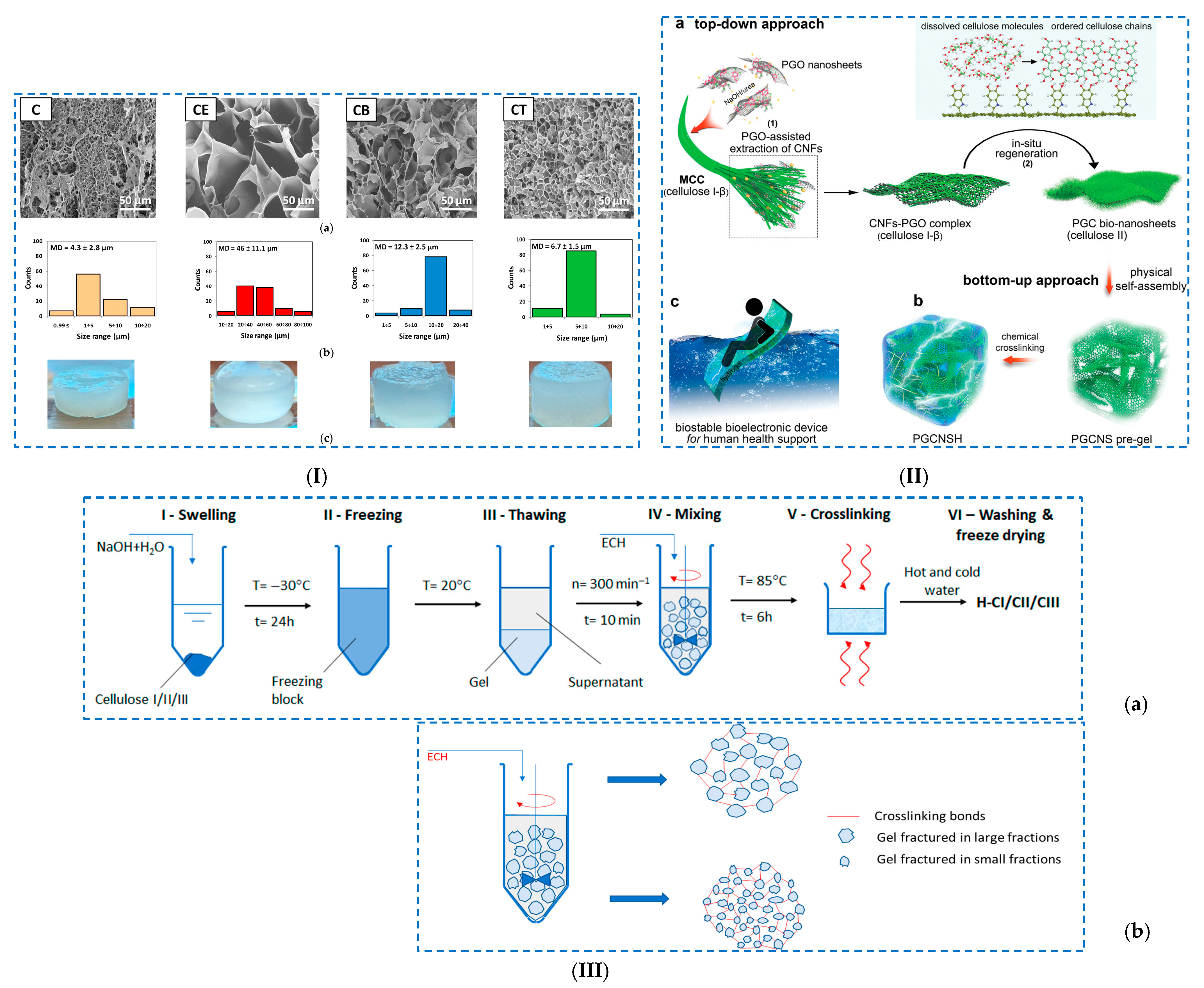
| Cellulose hydrogels | Ring-opening reactions | - cellulose | - cellulose-based hydrogels chemically crosslinking with different epoxy crosslinkers: (i) ECH, (ii) BDDE and (iii) TMPTGE. | - Q = 1030% (CE); 710%; 580% (CB); 470% (CT); - thermal stability: 372 °C (CE); 403 °C (CB); 388 °C (CT). | [103] |
| - CNFs/PGO | - CNF-PGO complex: H-bonds; - PGC bio-nanosheets: physical self-assembly (freeze–thaw treatment of CNF-PGO); - PGCNSH hydrogel: chemical crosslinking of PGC bio-nanosheets with ECH. | - excellent mechanical performances; - long-term underwater mechanical stability; - electrical conductivity (30 days): >6 S/m; - sensitivity (GF): 1.76 in the strain range of 15–20%. | [104] | ||
| - CI, CII or CIII | - cellulose-based hydrogels chemically crosslinked with ECH. | - Q = 2128% (H-CI); 2440% (H-CII); 1926% (H-CIII); - H-CIII: stronger and stiffer structure; - H-CII: softer, stable structure. | [105] | ||
| - cellulose/CMC | - cellulose-based hydrogel chemically crosslinked with ECH. | - Q = 2055%; - maximum soil moisture: 36.5% (topsoil); 30.1% (wet clayey soil); 23.4% (sandy soil). | [106] | ||
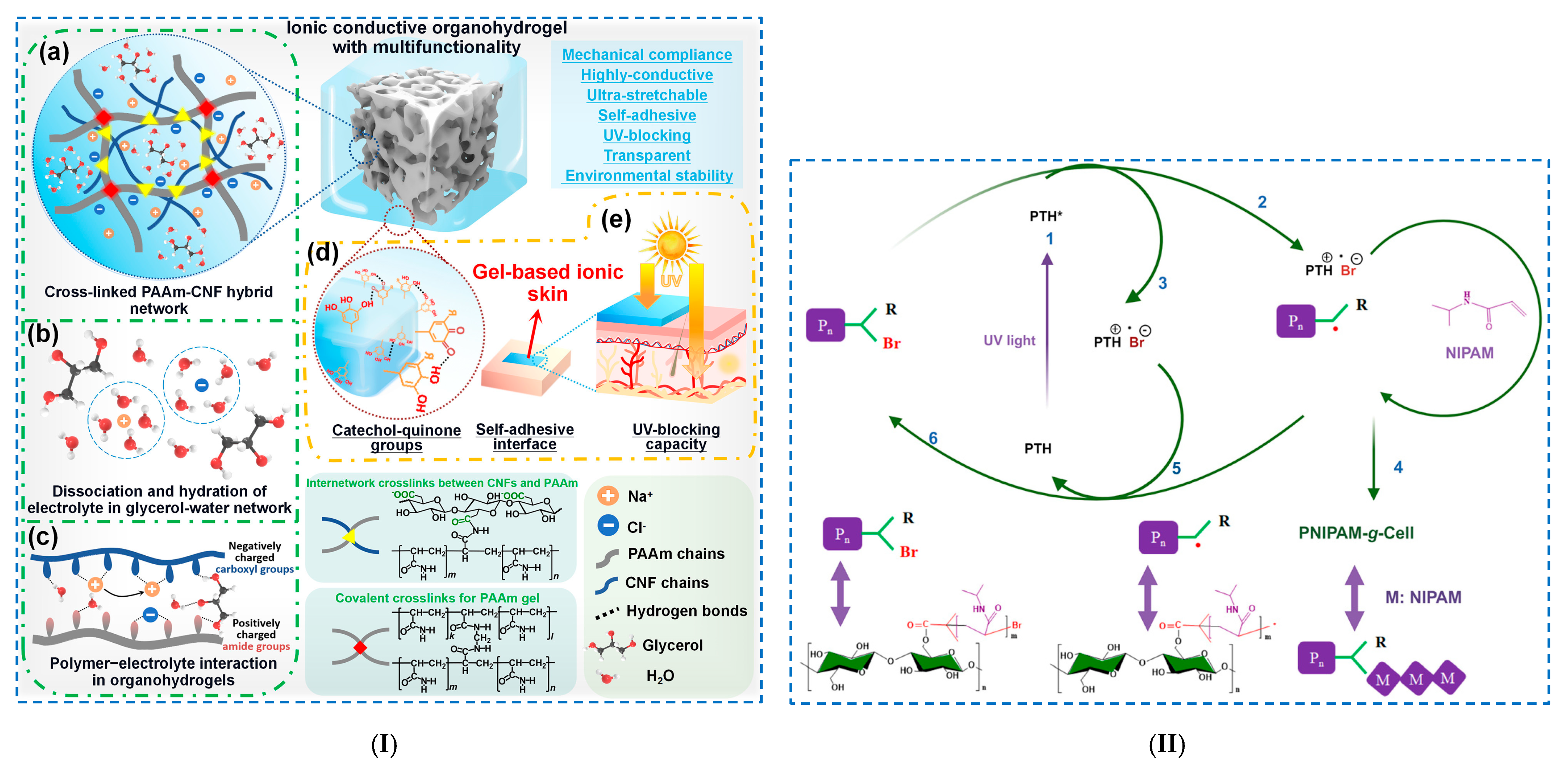
| Polymerization reactions | - CNF/PAAm/TA | - ionic organohydrogel PAAm-CNF-TA-NaCl: - (i) PAAm/CNF as hybrid skeleton; - (ii) TA-functionalized interface; - (iii) electrolytes (NaCl) dissolved in a binary solvent glycerol–water system. | - Young’s modulus: 23 kPa; - mechanical strength: 86 kPa; - ionic conductivity: 2.7 S/m; - self-adhesiveness: 103 N/m; - UV-blocking efficiency: 99.9%. | [107] | |
| - cellulose/PDMAEMA | - hydrogel: free radical polymerization of DMAEMA and MBA in cellulose solution. | - ERD (% of the initial Q): 8–10% (1-1); 24.5–32.5% (1-3); 40.0–44.8% (1-5). | [108] | ||
| - cellulose–IBBr/NIPAM | - PNIPAM-g-Cell copolymer: photoinduced metal-free atom transfer radical polymerization; - cellulose–IBBr as macroinitiator and PTH as catalyst. | - high microporosity and well-defined structure; - pore size: 100 μm. | [109] | ||
| Schiff base reaction | - OHEC/HEA/DSPO | - P(HEA-co-AAm): free radical polymerization of OHEC, AAM, HEA and DSPO; - P(HEA-co-AAm)-OHEC-DSPO composite hydrogel: - (i) Schiff base bonds: OHEC and P(HEA-co-AAm); - (ii) H-bonds: P(HEA-co-AAm) and OHEC; - (iii) boron–oxygen bridges: borax and HEA. | - dual stimuli-response hydrogels; - T: >93% (DSPO < 2 × 10−4 mM); - compressive strength: 8.5 MPa; - Q: increase with increasing pH values; - non-toxicity (L929 cells); - intelligent contact lenses. | [110] | |
| - DCMC/AG/PAA | - dual network hydrogels: - (i) first layer: Schiff reaction between DCMC and AG; - (ii) second layer: free radical polymerization of acrylic acid (AA). | - compressive strain: 50%; - tensile strain: 550%; - cyclic compression: ≥10,000 times; - GF = 8.1; - adhesion: ≥10 times. | [111] | ||
| - OMCC/CMCS | - hydrogel synthesis based on Schiff base reaction. | - SR = 31.18 g/g (water, OMCM-22); - SR = 40 g/g (pH 9.0, OMCM-22); - stress: 25 kPa at 68% strain (OMCM-79). | [112] | ||
| Hemicellulose hydrogels | Ring-opening reactions | - CHC/CS | - hydrogel’s network: - (i) dissolving CHC and CS in an alkali/urea/water system; - (ii) chemical crosslinking of CHC and CS with ECH. | - pore size: 200 μm; - compressive strength: 325 kPa. | [113] |
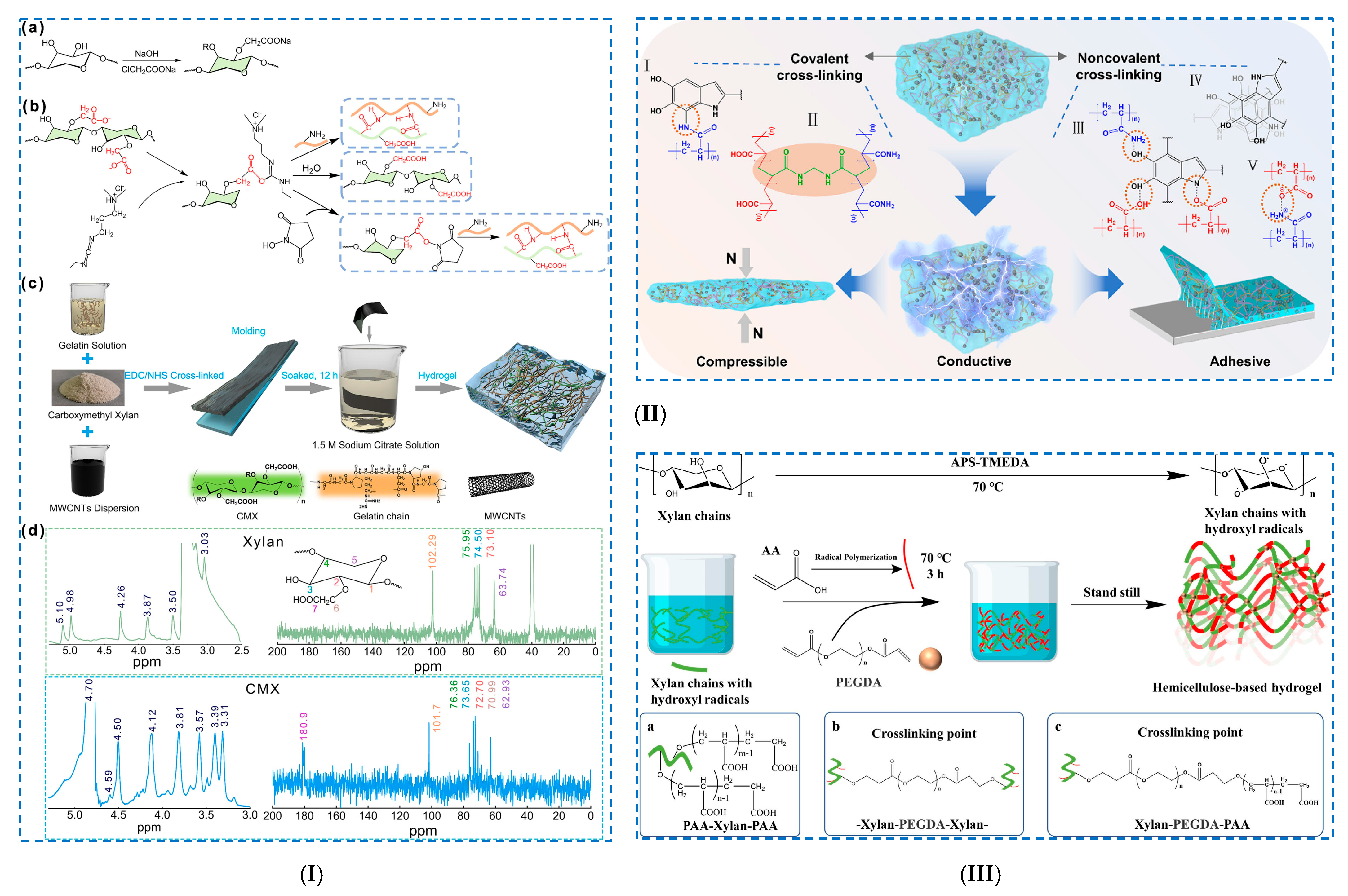
| Small molecule chemical crosslinking | - beech xylan/DTPA | - hydrogel: one-step reaction by covalent crosslinking of xylan. | - SR: >62; - SR depending on pH; - photocytotoxicity against S. aureus, P. aeruginosa, E. coli and B. cereus. | [114] | |
| - CMX/G/OCNT | - CMX-g-G/OCNT hydrogel: - (i) covalent amide bonds: CMX (–COOH groups) and G (–NH2 groups); intermolecular H-bonding; - (ii) salting-out treatment Na3cit; - (iii) doping with OCNT. | CMX-g-G hydrogel: - tensile strain: 1.54 MPa; - tensile stress, 324%; - toughness: 2.31 MJ m−3; CMX-g-G/OCNT hydrogel: - tensile strength: 1.63 MPa. | [115] | ||
| Polymerization reactions | - CMX–DA/PAM/bentonite | - hydrogel’s network: - (i) free radical polymerization (PAM); - (ii) grafting of PAM onto the CMX-DA; - (ii) physical interactions: bentonite with polymer chains. | - SR: 14.39 g/g; - compressive strength: 218.29 kPa; - tensile strength: 42.17 kPa; - elongation at break: 436%; - adhesion properties (SFE, mJ/m2): 62.78 (glass), 64.87 (plastic) and 67.39 (metal). | [116] | |
| - xylan-MAH/GO-VTEO | - GO–VTEO–xylan–MAH hydrogel: free radical polymerization. | - SR depending on pH; - pH = 5 (the optimum pH for the adsorption of Cu2+ ions) - maximum adsorption of Cu2+: 228 mg/g. | [117] | ||
| - HC/CSN/PAA | - HC-CSN-Fe3+ hydrogel: - (i) free radical copolymerization (PAA); - (ii) grafting of HC onto PAA; - (iii) complexing Fe3+ with the amino groups of CSN. | - water retention in the soil (48 h): 97.54%; - sustained urea release; - antioxidant performance: 76.75%; - UV blocking efficiency: 92.2%. | [118] | ||
| - HC/Pam | - HC–g–Am–BIS–BT hydrogels prepared by free radical graft copolymerization (HC acts as a crosslinking agent): - (i) covalent bonds between HC and Am; - (ii) H-bonds between HC and Am–BIS–BT. | - uniform and regular network structure; - maximum MB adsorption: 140.66 mg/g; | [119] | ||
| - DAGMA/PAM/HC | - PAM-DM-HC hydrogels: - (i) synthesis of DAGMA: from DA and GMA; - (ii) synthesis of the PAM: polymerization process of AM; - (iii) hydrogel: copolymerization process between PAM and HC. | - tensile strength: 0.34 MPa; - tensile strain: 2098.4%; - toughness: 3.79 ± 0.95 MJ/m3; Hydrogels with MXene: - strength: 0.51 MPa; - toughness: 5.95 ± 1.19 MJ/m3. | [120] | ||
| - HC/NP/PAM/PAC | - P(AM-AC)-HC-NP hydrogel: - (i) non-covalent bonds and electrostatic interactions: between the amino groups (PAM), carboxyl groups (PAC) and catechol group (NP); - (ii) grafting copolymerization of HC with acrylamide (AM) and acrylic acid (AC). | - compressive strain: 88%; - compression stress: 650 kPa; - stable mechanical properties: after 1000 cycles of cyclic compression; - good adhesion on different surfaces; - stable electrochemical properties. | [121] | ||
| - XH–LA/[P(AA-co-DMAEMA)]/sodium alginate | - composite hydrogel: a double network formed by alginate/Ca2+ and P(AA-co-DMAEMA) with hemicellulose-based nanoaggregates: - (i) vinyl-functionalized amphiphilic hemicellulose derivative (XH-LA-MA); - (ii) ionically crosslinked sodium alginate/Ca2+ network; - (iii) covalently crosslinked P(AA-co-DMEMA) network; - (iv) XH-LA-MA participates in the hydrogel through covalent bonds. | - denser pore structure; - robust mechanical stability; - sustained drug delivery in load-bearing environments. | [122] | ||
| - XH/PAA/Fe3O4 MNPs | - Fe3O4@XH-Gel nanocomposite hydrogel: - (i) free radical polymerization of PAA; - (ii) grafting AA onto XH chains (PAA–Xylan–PAA); - (iii) crosslinking reaction between the polymers (Xylan–PEGDA–Xylan); - (iv) crosslinking reactions between PEGDA and PAA (Xylan–PEGDA–PAA). | - pH-responsive characteristics; - SR = 5.0 (pH = 2); - SR = 48.9 (pH = 8); - magnetic responsiveness characteristics. | [123] | ||
| Schiff base reaction | - DAX/gelatin/PVOH | - PVOH/gelatin-DAX hydrogel (I–PGD): - (i) chemically crosslinked network: Schiff base reaction (DAX and gelatin); - (ii) physically crosslinked network: PVOH; - synthesis of the Hofmeister-enhanced conductive composite hydrogel (H–PGD): the induction of polymer chain aggregation by ions during salinization. | - tensile strength: 3.02 MPa; - elongation: 330.95%; - factor of roughly (60 times): 1.79 MPa; - ionic conductivity: 5.4 × 10−3 S/m; - electrode specific capacitance: 186 F/g. | [124] | |
| Thiol-ene reaction | - GGMMA/CNCs–SH | - GGMMA/CNCs-SH hydrogel: thiol-ene crosslinking reaction between GGMMA and CNCs–SH. | - mechanical stiffness: 12.35 kPa; - sustained release of Si and Ca ions/species. | [125] | |
| Cellulose Hydrogels | Hemicellulose Hydrogels | |
|---|---|---|
| Advantages | Most abundant natural polymer | Wide abundance in nature |
| Biodegradability | Biodegradability | |
| Biocompatibility | Biocompatibility | |
| Non-toxicity | Non-toxicity | |
| Non-immunogenic | Non-immunogenic | |
| – | Anti-inflammatory properties | |
| – | Antioxidant properties | |
| – | Protective barrier against bacterial infection | |
| High swelling ability | Good swelling capacity | |
| Excellent mechanical properties | Low-to-moderate mechanical strength | |
| Cost-effectiveness | Cost-effectiveness | |
| Disadvantages | Not biodegradable in human body (lack of specific enzyme) | Not biodegradable in human body (lack of specific enzyme) |
| – | Lack of reproducibility (batch variability) | |
| – | Poor stability over time | |
| Low cell adhesion | Low cell adhesion | |
| Absence of antimicrobial properties | – |
| Hydrogel Type | Hydrogel Network | Characteristics | Biological Properties | Ref. | |
|---|---|---|---|---|---|
| Drug Release | Biocompatibility/ Antibacterial/ Anti-Inflammatory | ||||
| Cellulose hydrogels | - Ox-HPC/CMCS | - physically crosslinked hydrogels; - injectable; - pH-responsive; - self-healing properties. | - drug: phenylalanine; - drug release: 60% (12.2 h, pH 7.4); - drug release: 60% (8.3 h, pH 6.8). | - | [67] |
| - MC/PLA/EURFS100 | - physically crosslinked hydrogels; - pH-responsive; - colon-targeting oral drug delivery. | - drug: GlcNAc; EURFS100/PLA (70/30 w/w)-MC (3% w/v); - drug release: 90% (29 h); - drug release: <20% (stomach and small intestine). | - L929 cells; - no cytotoxicity (hydrogels with or without drug, 1 day). | [167] | |
| - C/D | - chemically crosslinked hydrogels; - anti-inflammatory properties. | - BAC: PF; - BAC release: 45.01% (14 days, D hydrogel); - BAC release: 20.9% (14 days, C hydrogel). | - hVIC and hVEC; - cell viability: >80%; - anti-inflammatory effects: reduced interleukin IL-6 and chemokine MCP-1. | [165] | |
| - cellulose/PDMAEMA | - chemically crosslinked hydrogels. | - antibacterial properties: due to PDMAEMA and Ag+ ions; - Ag content: 4 wt% (1-3 CP); - Ag content: 3.1 wt% (1-5 CP). | - | [108] | |
| - Na.CMC | - physically crosslinked hydrogels; - transdermal drug delivery. | - drug: RTG; - drug release: 95% (120 min, pH 7.4, NS-HG). - drug release: >90% (15 min, pH 7.4, ME-HG). | - SD rats (male, 220 ± 20 g, 6-week-old); - ME-HG: induced skin erythema (2 days); - NS-HG: non-toxic, good biocompatibility. | [169] | |
| - cellulose–IBBr/NIPAM | - chemically crosslinked hydrogels; - injectable; - thermo-responsive. | - drug: DOX; - drug release (PNIPAM-g-Cell): stable for 240 h. | - L929 cells; - cell viability: >96.9% (100 mg/mL). | [109] | |
| - CMC/PVP | - chemically crosslinked hydrogels; - injectable; - self-healing properties. | - drug: 4-ASA; - drug release: 50% (pH 2); - drug release: 70% (pH 7.4). | - NIH-3T3 cells; - cell viability: 95% (5 days). | [166] | |
| Hemicellulose hydrogels | - HC/GO | - physically crosslinked hydrogels; - pH-sensitive; - oral drug delivery. | - drug: VB12; - drug release: 30% (12 h, pH 1.7); - drug release: 85% (12 h, pH 7.4). | - HEK 293 T cells; - cell viability: >90% (0.05 mg/mL); - cell viability: 80% (0.1 mg/mL). | [77] |
| - KGM/OHA | - physically crosslinked hydrogels. | - drug: EGCG; - drug release: 62.44 ± 1.97% (10 h, KO-3). | - | [175] | |
| - bagasse HC/PDA | - chemically crosslinked hydrogels; - pH-sensitive hydrogels. | - drug: methylene blue; - drug release: 62.82% (composite hydrogel); - drug release: 47.77% (hemicellulose hydrogel). | - | [170] | |
| - beech XH | - chemically crosslinked hydrogels; - good thermal stability (xyl-2). | - drug: TMPyP; - drug release: 88% (10 h, pH 7.4). | - photocytotoxicity against S. aureus, P. aeruginosa, E. coli and B. cereus; - dark conditions: no cytotoxicity; - light irradiation: inhibition S. aureus (20.7 ± 1.5 mm). | [114] | |
| - XH/PAA/Fe3O4 MNPs | - chemically crosslinked hydrogels; - pH/magnetic dual-responsive. | - drugs: acetylsalicylic acid and theophylline; - acetylsalicylic acid release: 90% (6 h, pH 7.4); - theophylline release: 80% (6 h, pH 7.4). | - L929 cells; - cell viability: 82.50% (5 wt% Fe3O4 MNPs); - cell viability: 111.73% (15 wt% Fe3O4 MNPs). | [123] | |
| - XH–LA/[P(AA-co-DMAEMA)]/sodium alginate | - chemically crosslinked hydrogels; - stimulus responsiveness. | - drug: Cur; - drug release: 93% (22 h, Cur/XH-LA); - drug release: 69–77% (48 h, Cur/XH-LA-MA). | - NIN/3T3 cells; - cell viability: 88% (CGel); - cell viability: 85% (XH-LA CGel, XH-LA-MA CGel). | [122] | |
| - CNCs–SH/GGMMA | - chemically crosslinked hydrogels; - injectable; - in situ sustained release of therapeutic ions. | - therapeutic agent: BaGNP and Cu-BaGNP; - therapeutic ions: Si, Ca, Cu; - Si ion release: rapid (3 days) and slow (the next 11 days); - Ca ion release: 14 days; - Cu ion release: no detectable. | - | [125] | |
| Hydrogel Type | Hydrogel Network | Characteristics | Biological Properties | Ref. | |
|---|---|---|---|---|---|
| Biocompatibility/ Wound Healing | Drug Release/Antimicrobial/ Anti-Inflammatory | ||||
| Cellulose hydrogels | - cellulose | - physically crosslinked hydrogels; - antimicrobial activity. | - ex vivo model: pig skin; - inhibit bacteria growth: pigskin covered with F53 (72 h). | - antibacterial activities: against E. coli and S. aureus; - inhibit bacteria growth: F53 (pitahaya) and F12 (tomato). | [186] |
| - C/LE | - chemically crosslinked hydrogels; - antimicrobial activity; - adhesion properties; - oral dressings. | - hGF cells; - cell viability: >100% (CLE samples); - cell viability: >99% (P-CLE samples). | - drug: PrHy; - drug release: 68% PrHy (1440 min, P-CLE 7); - drug release: 34% PrHy (1440 min, P-CLE); - antimicrobial activity: against E. coli and S. aureus. | [143] | |
| - OMCC/CMCS | - chemically crosslinked hydrogels; - pH sensitivity; - self-healing properties; - hemostatic effects. | - GES-1 cell; - cell viability: >85% (non-toxicity); - in vitro blood compatibility: human blood; - BCI: 0.037 (30 min). | - BAC: rutin; - the entrapment efficiency: 45.76% (OMCM-54); - BAC release: 47.77% (1 h, pH 7.0, OMCM-54); - BAC release: 90.22% (12 h). | [112] | |
| - BC/ PVA | - chemically crosslinked hydrogels; - antimicrobial activity. | - L929 cells; - cell viability: 96.9% (PBC4 and PBC5); - cell viability: 86% (PBC3). | - antibacterial activities: against E. coli and S. aureus; - inhibition zone diam.: 3 cm (S. aureus) and 2.6 cm (E. coli). | [183] | |
| - PDA@BC/ PVA via PB. | - chemically crosslinked hydrogels; - antibacterial activity; - tissue adhesiveness; - self-healing property. | - L929 cells; - cell viability: >85% (24 h); - in vivo model: Kunming mice (female, 5–6 weeks); - wound closure: 97.1% (PB-PDA@BC/Doxy, 14 days). | - drug: Doxy; - drug release: 88.3% (42 h, PB-PDA@BC1.5); - adhesion strength to skins: 4.7 ± 0.3 to 5.4 ± 0.4 kPa; - antimicrobial activity: 94% (against E. coli and S. aureus). | [187] | |
| - DBC/ QCS | - chemically crosslinked hydrogels; - antibacterial activity. | - L929 cells; - cell viability: >90%; - in vivo model: SD rats (female, 6–8 weeks); - wound healing: 14 days (thicker granulation). | - drug: PFD; - drug release: slowly within 14 days (DBC/QCS/PFD); - antibacterial activity: against E. coli (80.8%) and S. aureus (81.3%). | [188] | |
| Hemicellulose hydrogels | - xylan-PAA-TA | - chemically crosslinked hydrogels; - adhesion property; - antioxidant activity; - antibacterial ability. | - NIH-3T3 cells; - xylan-PAA-TA hydrogels showed the best cell affinity. | - antibacterial activity: against S. aureus; - antioxidant activity: 94.12% (xylan-PAA-TA3). | [189] |
| - ARX/CS/rGO | - chemically crosslinked hydrogels; - pH-responsive; - antibacterial activity. | - MC3T3-E1 cells; - cell viability: 91% (ATC-4); - increased cell adhesion and proliferation. | - drug: silver-sulphadiazine; - drug release: 93.1% (pH 7.4) and 58.3% (pH 6.4); - antimicrobial activity: against P. aeruginosa, E. faecalis and E. coli. | [190] | |
| - XG/ BC/ D | - physically crosslinked hydrogels. | - male Sprague Dawley mice (7–8 weeks old); - wound healing area: 98.3% (14 days, BC-D); - wound healing area: 96.98% (14 days, BC-XG-D); - tissue thickness: 15.4 mm (BC-D) and 15.2 mm (BC-XG-D). | - | [191] | |
| - XG/QCS/PVA/COL | - chemically crosslinked hydrogels; - antibacterial activity; - promote angiogenesis. | - L929 and HaCaT cells; - cell viability: >100% (PCQX-M); - average proliferation rates: 192.99% (HaCaT) and 133.83% (L929); - in vivo model: BALB/c mice (male, 8 weeks old); - formation of granulation tissue deposition of collagen. | - antibacterial activity: against E. coli (96.74%, PCQX-M) and S. aureus (99.49%, PCQX-M). | [192] | |
| - XG/F127DA | - chemically crosslinked hydrogels; - antibacterial activity; -anti-inflammatory properties; - promote angiogenesis. | - L929 cells; - cell viability: 95%; - in vivo model: 45 male C57BL/6 mice; - reduce the production of pro-inflammatory cytokines (IFN-γ, TNF-α, IL-1β, IL-6 and IL-12); - wound closure rate: 98.30 ± 1.83% (3 days). | - antimicrobial activity: against S. aureus and P. aeruginosa. | [193] | |
| - XG/OP | - chemically crosslinked hydrogels; - injectability; - self-healing properties; - angiogenic activity. | - L929 and HUVECs cells; - cell viability: 110.6% (L929 cell) and >80% (HUVECs); - in vivo model: male SD rats (8–10 weeks age); - average healing rate: 94.5% (14 days). | - in vitro antioxidant capacity (the scavenging rate of DPPH): 73.9%; - anti-inflammatory properties; - hemolysis rate: 0.7% (hemostatic properties). | [194] | |
| - OXG/CSMA | - chemically crosslinked hydrogels; - injectability; - self-healing capability. | - NIH/3T3; - cell viability: 214.78 ± 6.49% (XCUV); - ICR mice (male, 8-week-old); - wound closure rate: 73.71% ± 3.54 (XCUV, 5 days); - epidermis thicknesses: 49.76 ± 6.71 μm (XCUV, 21 days); - granulation tissue: 247.84 ± 7.83 μm (XCUV, 5 days). | - | [195] | |
| - KGM/HA | - chemically crosslinked hydrogels; -antibacterial properties; - anti-biofilm efficacy. | - NIH/3T3 cells; - cell viability: 91.6 ± 4.55% (HAKGM, 72 h); - burn wound healing ratio: 98 ± 4.9% (HAKGM, 12 days); - burn wound shrinking rate: 90% (12 days). | - antimicrobial activity: against E. coli (75%) and S. aureus (98%); - inhibition of biofilm formation: 92.4 ± 4.3% (HAKGM). | [204] | |
| - KGM/GA | - chemically crosslinked hydrogels; - antioxidant properties. | - L929 cells, Raw264.7 cells; - cell viability: 95% (L929); - cell viability: 80% (Raw 264.7); - wound healing area: 31.1 ± 0.85% (KGM-GA, 3 days); - KGM-GA has the ability to regulate M2 macrophages. | - H2O2 scavenging activity: KGM-GA showed excellent ROS scavenging activities; - intracellular ROS level: after treatment with KGM-GA, the ROS in L929 cells was reduced from 35% to less than 20%. | [205] | |
| - GA/PVA | - chemically crosslinked hydrogels; - anti-inflammatory effects. | - fibroblasts, monocytes and osteoblasts; - cell viability: 185% (fibroblasts, 48 h, PVA/GA = 80:20); - cell viability: 133% (osteoblasts, 48 h, PVA/GA = 70:30); - PVA/GA = 80:20: decreasing MCP-1 cytokine secretion. | - antibacterial activity: against E. coli (52 to 72%). | [206] | |
| - GM/ κC; - lactoferrin or Cramoll. | - physically crosslinked hydrogels; - topical sustained release system. | - Wistar albino rats (Male, 8–10 weeks); - reduction in lesion area (14 days): 36.05% (HC), 45.49% (HL) and 66.10% (HLC). - HLC: a better level of epithelial recovery with typical collagen distribution, stratified re-epithelialization and thicker keratinization layer formation. | - biomolecule (Cramoll and lactoferrin) release: optimized lesion contraction. | [209] | |
| - OGM/HA/AM/MEDA | - chemically crosslinked hydrogels; - self-healing properties; - antibacterial performance. | - GES-1 cells; - cell viability: 100% (72 h); - SD rats (male, 6–8 weeks); - reduction in wound area: 90% (O4H4M4A, 7 days); - skin damage area: 0.14% (14 days). | - antibacterial activity (O4H4M4A): against E. coli and S. aureus; - OD600: 0.04 (S. aureus) and 0.05 (E. coli). | [210] | |
| Hydrogel Type | Hydrogel Network | Characteristics | Biological Properties | Ref. |
|---|---|---|---|---|
| Biocompatibility/Anti-Inflammatory/ Drug Release/Antibacterial | ||||
| Cellulose hydrogels | - oxidized BC (OBCS)/AuNPs | - thermosensitive hydrogel; - injectable; - self-healing capacity; - heart TE applications. | - H9C2 cells; - cell viability: >92% (14 days); - self-healing efficiency: 80%; - electrical conductivity: 0.03 to 0.6 S/m; - BPM = 47; - FPA = 5.47 μv. | [222] |
| - TEMPO—oxidized cellulose (TCNF)/ALG | - potential bioinks; - 3D-bioprinted meniscus-like tissue constructs; - fibrocartilage-like tissue. | - hMFC cells; - hMFC-deposited ECM was observed in bioinks (6 weeks); - TCNF/ALG bioinks (7030, 8020): support the development of a more inner meniscus fibrocartilage-like phenotype (presence of sGAG, collagen I and II); - COL (bovine) bioink: a more outer meniscus phenotype. | [219] | |
| - TEMPO-modified NCC (mNCC)/MeGel | - 3D-bioprinted self-standing tubular structure; - TE heart valves. | - HADMSC cells; - cell viability: >7 days; - the most cell spreading: 2.0 mNG (mNCC-MeGel); - HADMSC encapsulated within mNG: decreased αSMA expression and increased vimentin and aggrecan expression. | [226] | |
| - TEMPO- oxidized CNF (TOCN)/SCNT | - myocardial TE scaffold. | - L929 cells and H9C2 cells; - RGR of L929: 95% (>5 days)—good biocompatibility and non-cytotoxicity to myocardial cells; - growth of H9C2 cardiomyocyte (7 days): significantly increasing numbers of cells; - conductivity: 5.2 × 10−6 to 6.2 × 10−2 S/cm. | [227] | |
| - TEMPO—oxidized CNF (TOCN)/PPy | - myocardial TE scaffold. | - H9C2 cells and L929 fibroblasts; - RGR of L929: >90% (3 days)—composite conductive hydrogel has no cytotoxicity; - growth of H9C2 cardiomyocyte (7 days): the number of cells increased and interconnected to form a network; - hydrogel significantly increased the expression levels of cardiac-specific proteins cTnT and cx-43. | [228] | |
| - TEMPO-oxidized BC (m-TOBC)/MSN | - bioactive hydrogel scaffolds for bone defect repair; - bone tissue reconstruction and repair. | - HUVEC cells; - cell proliferation rate: the highest for GelMA/m-TOBC/DMSN; - wound closure rate: 65.5% (GelMA/m-TOBC/DMSN, 24 h); - enhanced in vitro mineralized matrix deposition and osteoblastic alkaline phosphatase expression; - promote angiogenesis (enhanced cell migration, tube formation and upregulated angiogenic gene expression levels). | [229] | |
| - HPMC/CS/Gyl | - osteogenesis capacity; - thermosensitive hydrogel; - antibacterial characteristics; - orthopedic implants. | - MC3T3-E1 osteoblasts and RAW264.7 cells; - released Gly: stimulated macrophage polarization to the pro-inflammatory M1 phenotype; - enhanced osteogenesis and immunoregulatory antibacterial activities; - drug: Sim; - Sim amount release (10 h): Sim@CGHH(sol) much higher than Sim@CGHH (gel); - antimicrobial activity: against E. coli (75%) and S. aureus (98%); - in vivo antibacterial activities: Sim@CGHH has significantly higher antibacterial activity. | [221] | |
| - HEC/CS/BGP/CNCs | - 3D complex bone tissue-engineered constructs; - bone TE applications. | - MC3T3-E1 cells; - good cell viability and proliferation in the bioprinted scaffolds (7 days); - CS + 1.5% CNC scaffolds exhibited the fastest osteogenesis; - CS−CNC scaffold: accelerated activity of alkaline phosphatase, calcium mineralization and collagen formation in ECM. | [230] | |
| - CMC/ALG/HEMA/AA/(PVA) | - cardiac TE applications. | - H9c2 cells; - cell viability: 101.48 ± 1.89% (CAHA2A) and 89.61 ± 3.22% (CAHA2AP); - ECM content (15 days): 1.84± 0.23% (CAHA2A) and 1.88 ± 0.08% (CAHA2AP); - both hydrogel scaffolds: deliver cells to the local environment and promote anterograde and retrograde movement. | [231] | |
| - regenerated cellulose (rCL)/CS | - electrospun regenerated nanofibers; - bone TE applications. | - MC3T3-E1 cells; - rCL/CS enhanced MC3T3-E1 cell attachment and proliferation; - rCL/CS improved bioactivity (Ca/P ratio = 1.83); - rCL/CS increased ALP and ARS activity. | [232] | |
| - CNCs/Hap/pectin | - bone regeneration applications. | - L929 cells; - all hydrogels are non-toxic and biocompatible; - biomimetic method: HAp formation similar to biological hydroxyapatite. | [233] | |
| - CNCs/TA/AgNPs (CNCs/TA@AgNPs)/PVA/PDA@HAP | - bioactive hydrogel; - antibacterial properties; - osteogenic capabilities; - bone TE applications. | - BMSC cells; - cell viability: 95%; - PVA-Ag-PHA: supports BMSC adhesion and proliferation, and enhances the osteogenic differentiation of cells; - Sprague Dawley rats (3 weeks old); - PVA-Ag-PHA: exhibited a large amount of newly forming bone in the center of the bone defects (after implanting for 4 weeks); - PVA-Ag-PHA: promotes bone tissue healing over 8 weeks; - antimicrobial activity: against S. aureus and E. coli. | [234] | |
| - CNF/PVA/GMA | - soft and flexible hydrogel; - injectable; - meniscus TE applications. | - CSPCs; - cell viability (24 h): >95% (PVA-g-GMA and PVA-g-MA/CNF injectable hydrogels) is noncytotoxic; - cell viability (14 days): 81% (10%PVA-g-GMA/0.5%CNF) and 78% (10%PVA-g-GMA/0.7%CNF)—good cell adhesion and viability in the long-term culture. | [235] | |
| Hemicellulose hydrogels | - Xylan/CS/Hap/(GO/RGO) | - bone TE applications. | - MG-63 cells; - X/C/HAp/0.5%RGO: the highest cell viability; - samples with GO or RGO content: capable of osteointegration; - samples with RGO content: larger apatite morphology. | [224] |
| - mXG/anti-C5aRab | - prevent postoperative peritoneal adhesions. | - HPMC cells; - cell viability: 100% (48 h); - KM mice (6 weeks old); - mXG/anti-C5aRab hydrogel: provided an adhesion barrier while preventing an excessive inflammatory response. | [225] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciolacu, D.E. Hydrogels from Renewable Resources: Advances in 3D Networks Based on Cellulose and Hemicellulose. Polymers 2025, 17, 2760. https://doi.org/10.3390/polym17202760
Ciolacu DE. Hydrogels from Renewable Resources: Advances in 3D Networks Based on Cellulose and Hemicellulose. Polymers. 2025; 17(20):2760. https://doi.org/10.3390/polym17202760
Chicago/Turabian StyleCiolacu, Diana Elena. 2025. "Hydrogels from Renewable Resources: Advances in 3D Networks Based on Cellulose and Hemicellulose" Polymers 17, no. 20: 2760. https://doi.org/10.3390/polym17202760
APA StyleCiolacu, D. E. (2025). Hydrogels from Renewable Resources: Advances in 3D Networks Based on Cellulose and Hemicellulose. Polymers, 17(20), 2760. https://doi.org/10.3390/polym17202760






