Synthesis and Characterization of PLA/Biochar Bio-Composites Containing Different Biochar Types and Content
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of PLA and Its Bio-Composites
2.3. Intrinsic Viscosity
2.4. Gel Permeation Chromatography-Size Exclusion Chromatography (GPC/SEC)
2.5. Nuclear Magnetic Resonance (NMR)
2.6. Fourier-Transformed Infra-Red Spectroscopy (FTIR)
2.7. Transmission Electron Microscopy (TEM)
2.8. X-Ray Diffraction (XRD)
2.9. Differential Scanning Calorimetry (DSC)
2.10. Dielectric Relaxation Spectroscopy (DRS)
2.11. Mechanical Properties
2.12. Color Measurement
2.13. Enzymatic Hydrolysis
2.14. Water Contact Angle
2.15. Scanning Electron Microscopy (SEM)
2.16. Thermogravimetric Analysis (TGA)
3. Results
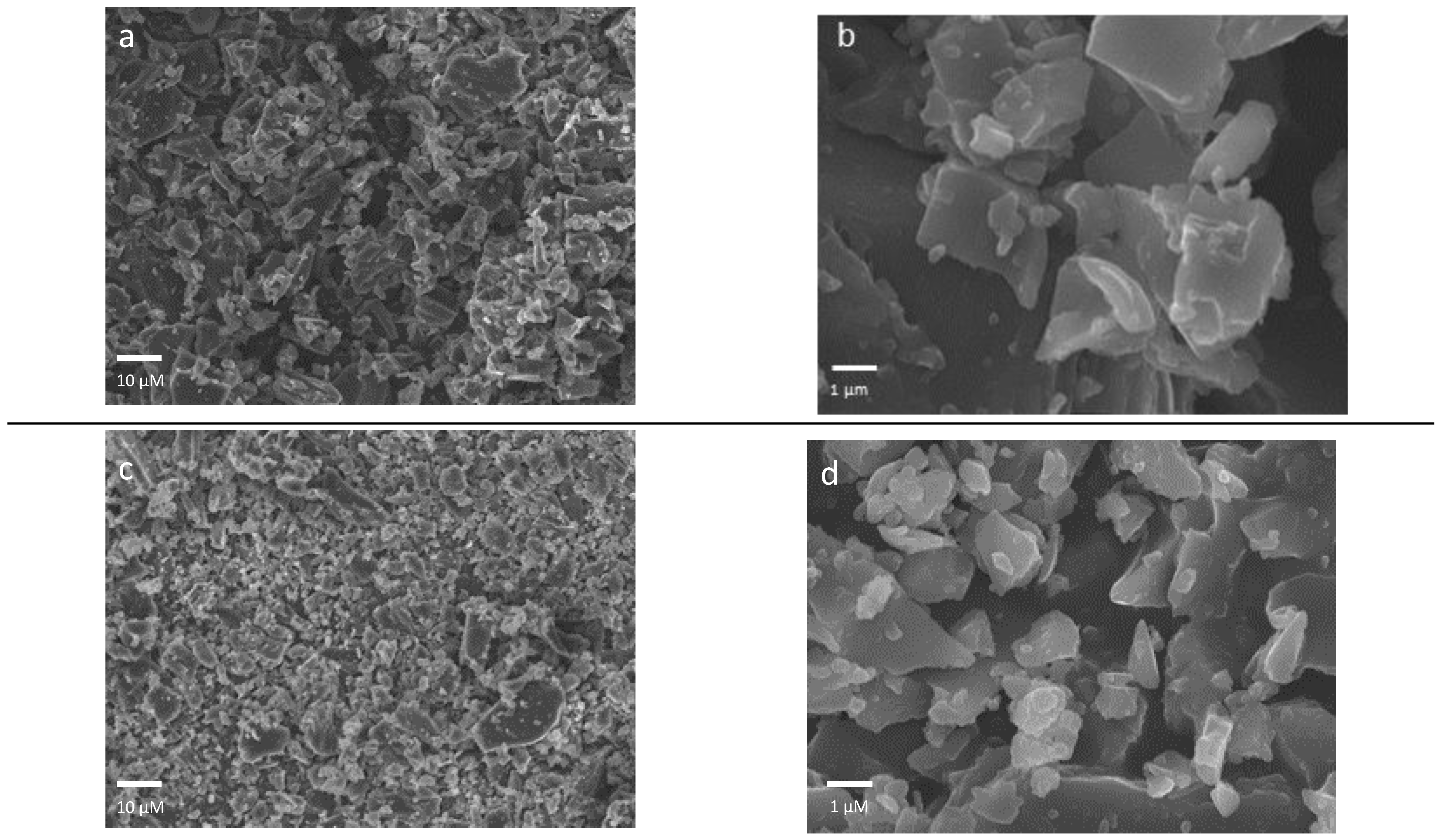
3.1. Synthesis and Morphological Characterization of Biochar
3.2. Synthesis and Characterization of In Situ Prepared PLA/BC Bio-Composites
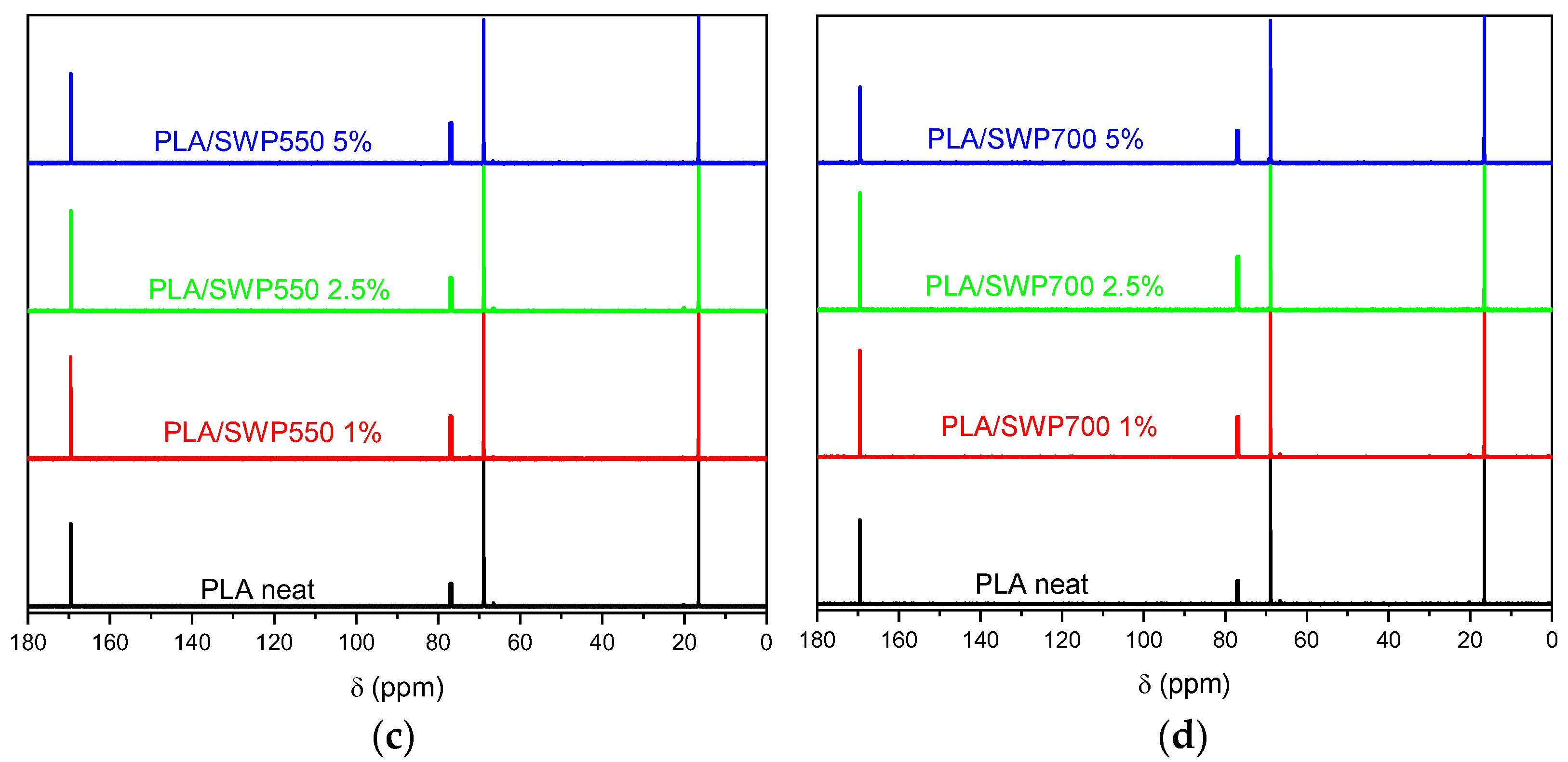
3.3. Structural Characterization of Biochar and PLA/BC Bio-Composites
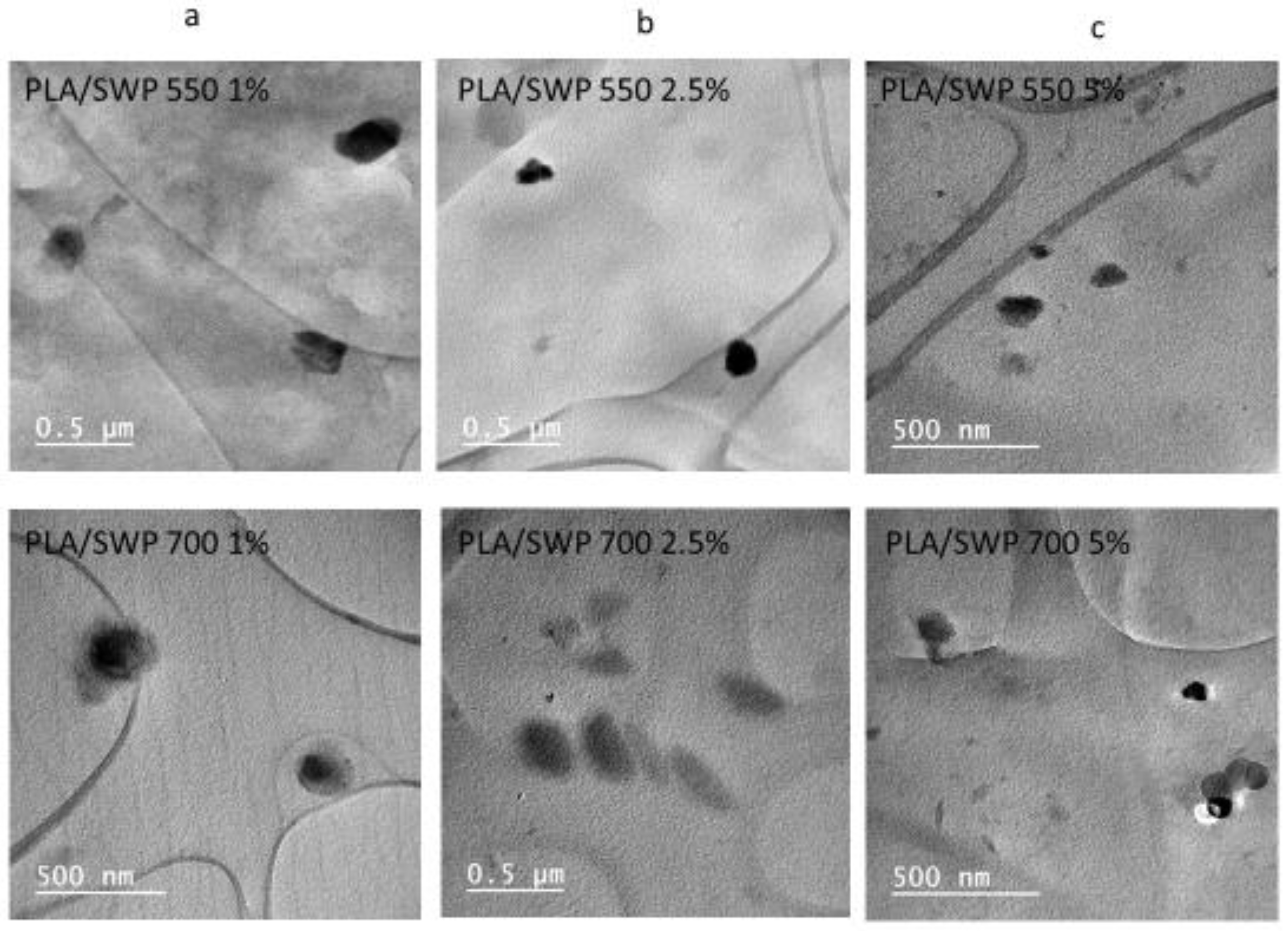
3.4. Biochar Dispersion Studied by TEM
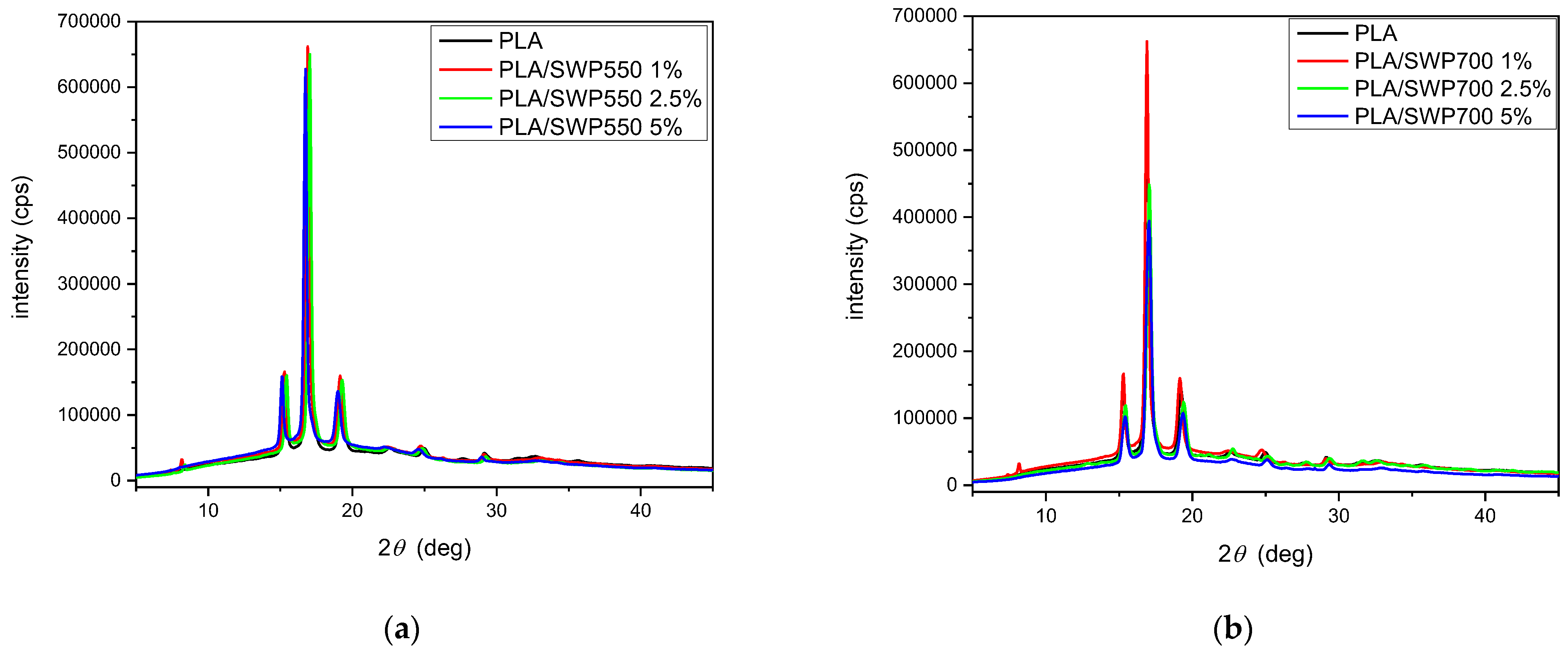
3.5. XRD
3.6. Thermal Transitions (DSC)
3.7. Molecular Mobility (DRS)
3.8. Mechanical Properties of PLA/BC Bio-Composites
3.9. Effect of Biochar Content on PLA Coloration
3.10. Enzymatic Hydrolysis
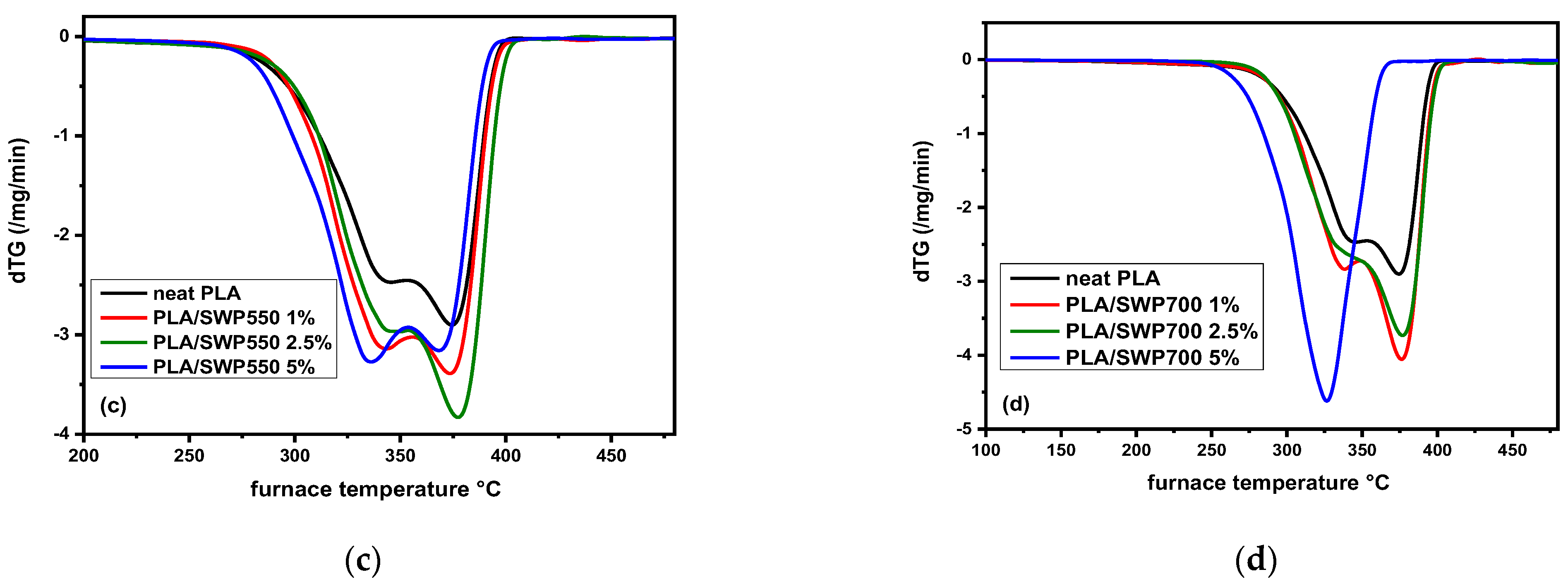
3.11. Thermal Degradation Studies of PLA/BC Bio-Composites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bachmann, M.; Zibunas, C.; Hartmann, J.; Tulus, V.; Suh, S.; Guillén-gosálbez, G.; Bardow, A. Towards Circular Plastics within Planetary Boundaries. Nat. Sustain. 2023, 6, 599–610. [Google Scholar] [CrossRef]
- Balla, E.; Daniilidis, V.; Karlioti, G.; Kalamas, T.; Stefanidou, M.; Bikiaris, N.D.; Vlachopoulos, A.; Koumentakou, I.; Bikiaris, D.N. Poly(Lactic Acid): A Versatile Biobased Polymer for the Future with Multifunctional Properties-from Monomer Synthesis, Polymerization Techniques and Molecular Weight Increase to PLA Applications. Polymers 2021, 13, 1822. [Google Scholar] [CrossRef] [PubMed]
- Bikiaris, N.D.; Koumentakou, I.; Samiotaki, C.; Meimaroglou, D.; Varytimidou, D.; Karatza, A.; Kalantzis, Z.; Roussou, M.; Bikiaris, R.D.; Papageorgiou, G.Z. Recent Advances in the Investigation of Poly(Lactic Acid) (PLA) Nanocomposites: Incorporation of Various Nanofillers and Their Properties and Applications. Polymers 2023, 15, 1196. [Google Scholar] [CrossRef]
- Vlachopoulos, A.; Karlioti, G.; Balla, E.; Daniilidis, V.; Kalamas, T.; Stefanidou, M.; Bikiaris, N.D.; Christodoulou, E.; Koumentakou, I.; Karavas, E.; et al. Poly(Lactic Acid)-Based Microparticles for Drug Delivery Applications: An Overview of Recent Advances. Pharmaceutics 2022, 14, 359. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Deng, S.; Chen, P.; Ruan, R. Polylactic Acid (PLA) Synthesis and Modifications: A Review. Front. Chem. China 2009, 4, 259–264. [Google Scholar] [CrossRef]
- Bioplastics Market Data. Available online: https://www.european-bioplastics.org/market/ (accessed on 3 September 2021).
- Androsch, R.; Iqbal, H.M.N.; Schick, C. Non-Isothermal Crystal Nucleation of Poly (l-Lactic Acid). Polymer 2015, 81, 151–158. [Google Scholar] [CrossRef]
- Armentano, I.; Bitinis, N.; Fortunati, E.; Mattioli, S.; Rescignano, N.; Verdejo, R.; Lopez-Manchado, M.A.; Kenny, J.M. Multifunctional Nanostructured PLA Materials for Packaging and Tissue Engineering. Prog. Polym. Sci. 2013, 38, 1720–1747. [Google Scholar] [CrossRef]
- Mortazavi, B.; Hassouna, F.; Laachachi, A.; Rajabpour, A.; Ahzi, S.; Chapron, D.; Toniazzo, V.; Ruch, D. Experimental and Multiscale Modeling of Thermal Conductivity and Elastic Properties of PLA/Expanded Graphite Polymer Nanocomposites. Thermochim. Acta 2013, 552, 106–113. [Google Scholar] [CrossRef]
- Saeidlou, S.; Huneault, M.A.; Li, H.; Park, C.B. Poly(Lactic Acid) Crystallization. Prog. Polym. Sci. 2012, 37, 1657–1677. [Google Scholar] [CrossRef]
- Pradhan, R.; Misra, M.; Erickson, L.; Mohanty, A. Compostability and Biodegradation Study of PLA-Wheat Straw and PLA-Soy Straw Based Green Composites in Simulated Composting Bioreactor. Bioresour. Technol. 2010, 101, 8489–8491. [Google Scholar] [CrossRef]
- Trivedi, A.K.; Gupta, M.K.; Singh, H. PLA Based Biocomposites for Sustainable Products: A Review. Adv. Ind. Eng. Polym. Res. 2023, 6, 382–395. [Google Scholar] [CrossRef]
- Sha, L.; Chen, Z.; Chen, Z.; Zhang, A.; Yang, Z. Polylactic Acid Based Nanocomposites: Promising Safe and Biodegradable Materials in Biomedical Field. Int. J. Polym. Sci. 2016, 2016, 686915411. [Google Scholar] [CrossRef]
- Bartoli, M.; Arrigo, R.; Malucelli, G.; Tagliaferro, A.; Duraccio, D. Recent Advances in Biochar Polymer Composites. Polymers 2022, 14, 2506. [Google Scholar] [CrossRef] [PubMed]
- Arrigo, R.; Bartoli, M.; Malucelli, G. Poly(Lactic Acid)–Biochar Biocomposites: Effect of Processing and Filler Content on Rheological. Polymers 2020, 12, 892. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Charpak, Y.D.; Trabold, T.A.; Lewis, C.L.; Diaz, C.A. Biochar-Filled Plastics: Effect of Feedstock on Thermal and Mechanical Properties. Biomass Convers. Biorefinery 2022, 12, 4349–4360. [Google Scholar] [CrossRef]
- Kane, S.; Van Roijen, E.; Ryan, C.; Miller, S. Reducing the Environmental Impacts of Plastics While Increasing Strength: Biochar Fillers in Biodegradable, Recycled, and Fossil-Fuel Derived Plastics. Compos. Part C Open Access 2022, 8, 100253. [Google Scholar] [CrossRef]
- Zouari, M.; Devallance, D.B.; Marrot, L. Effect of Biochar Addition on Mechanical Properties, Thermal Stability, and Water Resistance of Hemp-Polylactic Acid (PLA) Composites. Materials 2022, 15, 2271. [Google Scholar] [CrossRef] [PubMed]
- Vidal, J.L.; Yavitt, B.M.; Wheeler, M.D.; Kolwich, J.L.; Donovan, L.N.; Sit, C.S.; Hatzikiriakos, S.G.; Jalsa, N.K.; MacQuarrie, S.L.; Kerton, F.M. Biochar as a Sustainable and Renewable Additive for the Production of Poly(ε-Caprolactone) Composites. Sustain. Chem. Pharm. 2022, 25, 100586. [Google Scholar] [CrossRef]
- Aboughaly, M.; Babaei-Ghazvini, A.; Dhar, P.; Patel, R.; Acharya, B. Enhancing the Potential of Polymer Composites Using Biochar as a Filler: A Review. Polymers 2023, 15, 3981. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Preparation, Modification and Environmental Application of Biochar: A Review. J. Clean. Prod. 2019, 227, 1002–1022. [Google Scholar] [CrossRef]
- Qambrani, N.A.; Rahman, M.M.; Won, S.; Shim, S.; Ra, C. Biochar Properties and Eco-Friendly Applications for Climate Change Mitigation, Waste Management, and Wastewater Treatment: A Review. Renew. Sustain. Energy Rev. 2017, 79, 255–273. [Google Scholar] [CrossRef]
- Zhang, Q.; Khan, M.U.; Lin, X.; Cai, H.; Lei, H. Temperature Varied Biochar as a Reinforcing Filler for High-Density Polyethylene Composites. Compos. Part B Eng. 2019, 175, 107151. [Google Scholar] [CrossRef]
- Das, C.; Tamrakar, S.; Kiziltas, A.; Xie, X. Incorporation of Biochar to Improve Mechanical, Thermal and Electrical Properties of Polymer Composites. Polymers 2021, 13, 2663. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Lei, H.; Cai, H.; Han, X.; Lin, X.; Qian, M.; Zhao, Y.; Huo, E.; Villota, E.M.; Mateo, W. Improvement on the Properties of Microcrystalline Cellulose/Polylactic Acid Composites by Using Activated Biochar. J. Clean. Prod. 2020, 252, 119898. [Google Scholar] [CrossRef]
- She, D.; Dong, J.; Zhang, J.; Liu, L.; Sun, Q.; Geng, Z.; Peng, P. Development of Black and Biodegradable Biochar/Gutta Percha Composite Films with High Stretchability and Barrier Properties. Compos. Sci. Technol. 2019, 175, 1–5. [Google Scholar] [CrossRef]
- Giorcelli, M.; Khan, A.; Pugno, N.M.; Rosso, C.; Tagliaferro, A. Biochar as a Cheap and Environmental Friendly Filler Able to Improve Polymer Mechanical Properties. Biomass Bioenergy 2019, 120, 219–223. [Google Scholar] [CrossRef]
- Tengku Yasim-Anuar, T.A.; Yee-Foong, L.N.; Lawal, A.A.; Ahmad Farid, M.A.; Mohd Yusuf, M.Z.; Hassan, M.A.; Ariffin, H. Emerging Application of Biochar as a Renewable and Superior Filler in Polymer Composites. RSC Adv. 2022, 12, 13938–13949. [Google Scholar] [CrossRef] [PubMed]
- Infurna, G.; Botta, L.; Ingargiola, I.; Maniscalco, M.; Caputo, G.; Dintcheva, N.T. Biochar from Digestate Pyrolysis as a Filler for Biopolymer Blends: Effect of Blend Composition. J. Polym. Environ. 2023, 32, 1921–1963. [Google Scholar] [CrossRef]
- Papadopoulou, K.; Klonos, P.A.; Kyritsis, A.; Mašek, O.; Wurzer, C.; Tsachouridis, K.; Anastasiou, A.D.; Bikiaris, D.N. Synthesis and Study of Fully Biodegradable Composites Based on Poly(Butylene Succinate) and Biochar. Polymers 2023, 15, 1049. [Google Scholar] [CrossRef]
- Papadopoulou, K.; Ainali, N.M.; Mašek, O.; Bikiaris, D.N. Biochar as a UV Stabilizer: Its Impact on the Photostability of Poly(Butylene Succinate) Biocomposites. Polymers 2024, 16, 3080. [Google Scholar] [CrossRef] [PubMed]
- Mašek, O.; Wolfram, B.; Roy-Poirier, A.; Lowe, W.; Peters, C.; Brownsort, P.; Mignard, D.; Pritchard, C.; Masek, O.; Buss, W.; et al. Consistency of Biochar Properties over Time and Production Scales: A Characterisation of Standard Materials. J. Anal. Appl. Pyrolysis 2018, 132, 200–210. [Google Scholar] [CrossRef]
- Herrmann, G. Dynamics of Composite Materials, 1st ed.; Schönhals, A., Szymoniak, P., Eds.; Springer: Berlin, Germany, 2022; Volume 5, ISBN 9783030897222. [Google Scholar]
- Savvidis, G.; Karanikas, V.; Zarkogianni, M.; Eleftheriadis, I.; Nikolaidis, N.; Tsatsaroni, E. Screen-Printing of Cotton with Natural Pigments: Evaluation of Color and Fastness Properties of the Prints. J. Nat. Fibers 2017, 14, 326–334. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee Recommendations for Performing Kinetic Computations on Thermal Analysis Data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Available online: https://kinetics.netzsch.com/en/ (accessed on 6 September 2018).
- Papadopoulou, K.; Tarani, E.; Ainali, N.M.; Chrissafis, K.; Wurzer, C.; Mašek, O.; Bikiaris, D.N. The Effect of Biochar Addition on Thermal Stability and Decomposition Mechanism of Poly(Butylene Succinate) Bionanocomposites. Molecules 2023, 28, 5330. [Google Scholar] [CrossRef]
- Jurczyk, S.; Andrzejewski, J.; Piasecki, A.; Musioł, M.; Rydz, J.; Kowalczuk, M. Mechanical and Rheological Evaluation of Polyester-Based Composites Containing Biochar. Polymers 2024, 16, 1231. [Google Scholar] [CrossRef]
- Makri, S.P.; Xanthopoulou, E.; Valera, M.A.; Mangas, A.; Marra, G.; Ruiz, V.; Koltsakidis, S.; Tzetzis, D.; Zoikis Karathanasis, A.; Deligkiozi, I.; et al. Poly(Lactic Acid) Composites with Lignin and Nanolignin Synthesized by In Situ Reactive Processing. Polymers 2023, 15, 2386. [Google Scholar] [CrossRef] [PubMed]
- Pérez, J.M.; Ruiz, C.; Fernández, I. Synthesis of a Biodegradable PLA: NMR Signal Deconvolution and End-Group Analysis. J. Chem. Educ. 2022, 99, 1000–1007. [Google Scholar] [CrossRef]
- Inkinen, S.; Hakkarainen, M.; Albertsson, A.C.; Södergård, A. From Lactic Acid to Poly(Lactic Acid) (PLA): Characterization and Analysis of PLA and Its Precursors. Biomacromolecules 2011, 12, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Infurna, G.; Botta, L.; Maniscalco, M.; Morici, E.; Caputo, G.; Marullo, S.; D’Anna, F.; Dintcheva, N.T. Biochar Particles Obtained from Agricultural Carob Waste as a Suitable Filler for Sustainable Biocomposite Formulations. Polymers 2022, 14, 3075. [Google Scholar] [CrossRef] [PubMed]
- de Jesus Duarte, S.; Glaser, B.; Paiva de Lima, R.; Pelegrino Cerri, C.E. Chemical, Physical, and Hydraulic Properties as Affected by One Year of Miscanthus Biochar Interaction with Sandy and Loamy Tropical Soils. Soil Syst. 2019, 3, 24. [Google Scholar] [CrossRef]
- Füllbrandt, M.; Purohit, P.J.; Schönhals, A. Combined FTIR and Dielectric Investigation of Poly(Vinyl Acetate) Adsorbed on Silica Particles. Macromolecules 2013, 46, 4626–4632. [Google Scholar] [CrossRef]
- Terzopoulou, Z.; Klonos, P.A.; Kyritsis, A.; Tziolas, A.; Avgeropoulos, A.; Papageorgiou, G.Z.; Bikiaris, D.N. Interfacial Interactions, Crystallization and Molecular Mobility in Nanocomposites of Poly(Lactic Acid) Filled with New Hybrid Inclusions Based on Graphene Oxide and Silica Nanoparticles. Polymer 2019, 166, 1–12. [Google Scholar] [CrossRef]
- Wurm, A.; Ismail, M.; Kretzschmar, B.; Pospiech, D.; Schick, C. Retarded Crystallization in Polyamide/Layered Silicates Nanocomposites Caused by an Immobilized Interphase. Macromolecules 2010, 43, 1480–1487. [Google Scholar] [CrossRef]
- Klonos, P.A.; Papadopoulos, L.; Papageorgiou, G.Z.; Kyritsis, A.; Pissis, P.; Bikiaris, D.N. Interfacial Interactions, Crystallization, and Molecular Dynamics of Renewable Poly(Propylene Furanoate) in Situ Filled with Initial and Surface Functionalized Carbon Nanotubes and Graphene Oxide. J. Phys. Chem. C 2020, 124, 10220–10234. [Google Scholar] [CrossRef]
- Lin, Y.; Liu, L.; Xu, G.; Zhang, D.; Guan, A.; Wu, G. Interfacial Interactions and Segmental Dynamics of Poly(Vinyl Acetate)/Silica Nanocomposites. J. Phys. Chem. C 2015, 119, 12956–12966. [Google Scholar] [CrossRef]
- Klonos, P.A.; Bikiaris, R.D.; Terzopoulou, Z.; Mouchlianiti, K.; Tsachouridis, K.; Anastasiou, A.D.; Kyritsis, A.; Kyzas, G.Z. Structure-Properties Relationships in New Polymer Nanocomposites Based on the Renewable Poly(Butylene Succinate) Filled with Low Amounts of Nanoparticles of 1-3D Geometries. Polymer 2024, 296, 126841. [Google Scholar] [CrossRef]
- Madkour, S.; Szymoniak, P.; Heidari, M.; Von Klitzing, R.; Schönhals, A. Unveiling the Dynamics of Self-Assembled Layers of Thin Films of Poly(Vinyl Methyl Ether) (PVME) by Nanosized Relaxation Spectroscopy. ACS Appl. Mater. Interfaces 2017, 9, 7535–7546. [Google Scholar] [CrossRef] [PubMed]
- Črešnar, K.P.; Zemljič, L.F.; Papadopoulos, L.; Terzopoulou, Z.; Zamboulis, A.; Klonos, P.A.; Bikiaris, D.N.; Kyritsis, A.; Pissis, P. Effects of Ag, ZnO and TiO2 Nanoparticles at Low Contents on the Crystallization, Semicrystalline Morphology, Interfacial Phenomena and Segmental Dynamics of PLA. Mater. Today Commun. 2021, 27, 102192. [Google Scholar] [CrossRef]
- Böhmer, R.; Ngai, K.; Angell, C.A.; Plazek, D.J. Nonexponential Relaxations in Strong and Fragile Glass Formers. J. Chem. Phys. 1993, 99, 4201–4209. [Google Scholar] [CrossRef]
- Matykiewicz, D. Biochar as an Effective Filler of Carbon Fiber Reinforced Bio-Epoxy Composites. Processes 2020, 8, 724. [Google Scholar] [CrossRef]
- Ho, M.P.; Lau, K.T.; Wang, H.; Hui, D. Improvement on the Properties of Polylactic Acid (PLA) Using Bamboo Charcoal Particles. Compos. Part B Eng. 2015, 81, 14–25. [Google Scholar] [CrossRef]
- Zouari, M.; Mikuljan, M.; Schwarzkopf, M. Impact of Biochar on the Fungal Decay Resistance of Hemp-Polylactic Acid Composites against Wood Basidiomycetes. Front. Mater. 2023, 10, 1–10. [Google Scholar] [CrossRef]
- Zaaba, N.F.; Jaafar, M. A Review on Degradation Mechanisms of Polylactic Acid: Hydrolytic, Photodegradative, Microbial, and Enzymatic Degradation. Polym. Eng. Sci. 2020, 60, 2061–2075. [Google Scholar] [CrossRef]
- Botta, L.; Teresi, R.; Titone, V.; Salvaggio, G.; La Mantia, F.P.; Lopresti, F. Use of Biochar as Filler for Biocomposite Blown Films: Structure-Processing-Properties Relationships. Polymers 2021, 13, 3953. [Google Scholar] [CrossRef]
- McKeown, P.; Jones, M.D. The Chemical Recycling of PLA: A Review. Sustain. Chem. 2020, 1, 1–22. [Google Scholar] [CrossRef]
- Cappello, M.; Rossi, D.; Filippi, S.; Cinelli, P.; Seggiani, M. Wood Residue-Derived Biochar as a Low-Cost, Lubricating Filler in Poly(Butylene Succinate-Co-Adipate) Biocomposites. Materials 2023, 16, 570. [Google Scholar] [CrossRef]
- Haeldermans, T.; Samyn, P.; Cardinaels, R.; Vandamme, D.; Vanreppelen, K.; Cuypers, A.; Schreurs, S. Poly(Lactic Acid) Bio-Composites Containing Biochar Particles: Effects of Fillers and Plasticizer on Crystallization and Thermal Properties. Express Polym. Lett. 2021, 15, 343–360. [Google Scholar] [CrossRef]
- Tarani, E.; Chrissafis, K. Isoconversional Methods: A Powerful Tool for Kinetic Analysis and the Identification of Experimental Data Quality. Thermochim. Acta 2024, 733, 179690. [Google Scholar] [CrossRef]
- Budrugeac, P.; Segal, E.; Pérez-Maqueda, L.A.; Criado, J.M. The Use of the IKP Method for Evaluating the Kinetic Parameters and the Conversion Function of the Thermal Dehydrochlorination of PVC from Non-Isothermal Data. Polym. Degrad. Stab. 2004, 84, 311–320. [Google Scholar] [CrossRef]
- Friedman, H.L. Kinetics of Thermal Degradation of Char-Forming Plastics from Thermogravimetry. Application to a Phenolic Plastic. J. Polym. Sci. Part C Polym. Symp. 2007, 6, 183–195. [Google Scholar] [CrossRef]
- Vyazovkin, S. Evaluation of Activation Energy of Thermally Stimulated Solid-State Reactions under Arbitrary Variation of Temperature. J. Comput. Chem. 1997, 18, 393–402. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar Physicochemical Properties: Pyrolysis Temperature and Feedstock Kind Effects. Rev. Environ. Sci. Biotechnol. 2020, 19, 191–215. [Google Scholar] [CrossRef]






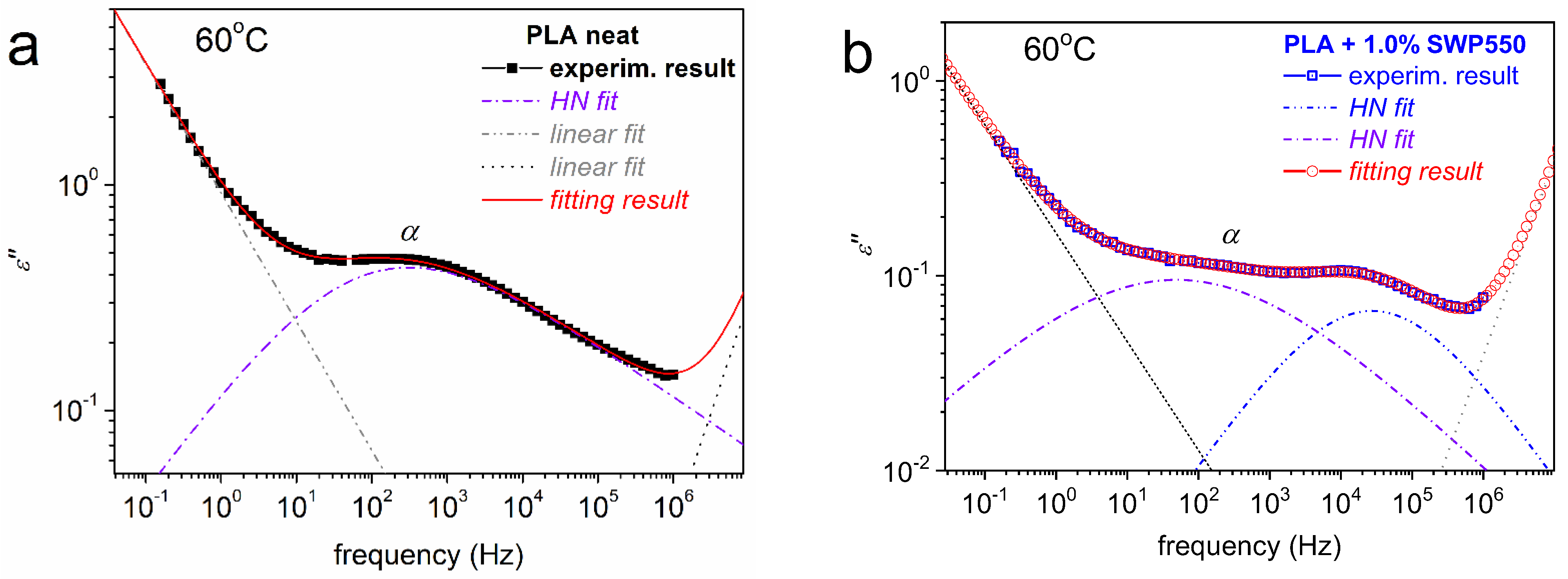
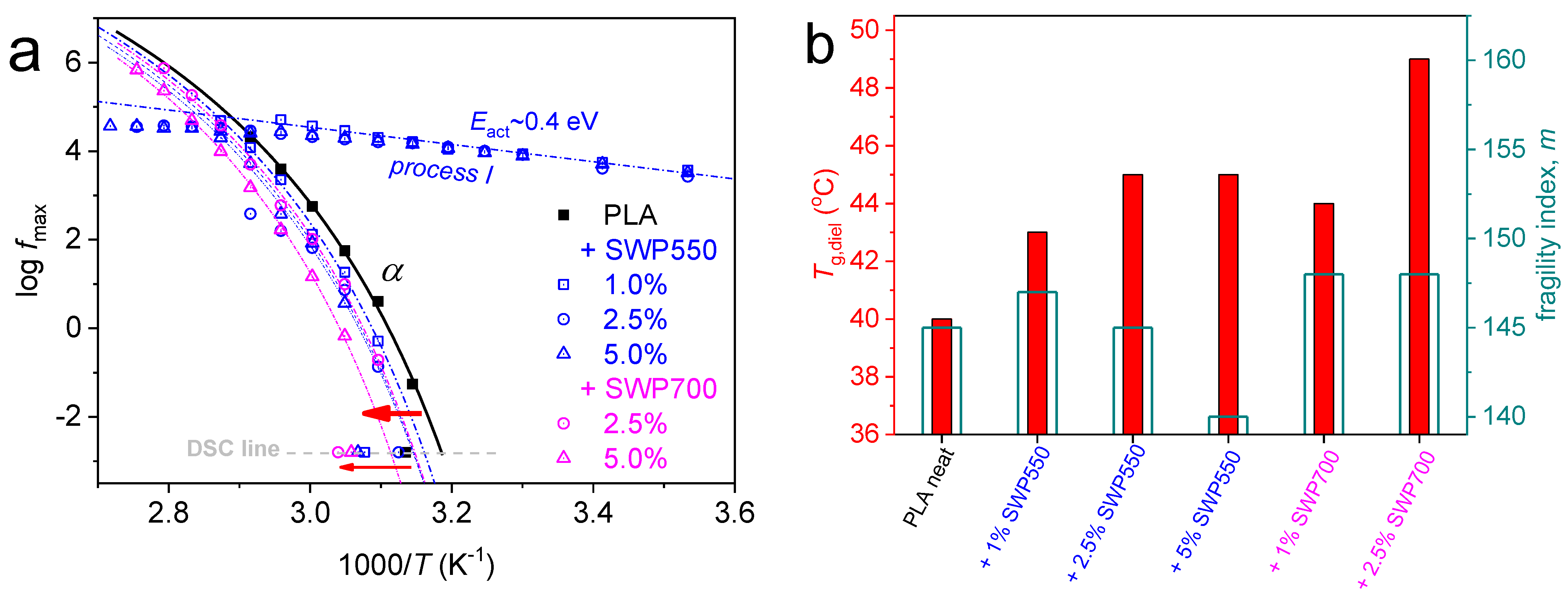
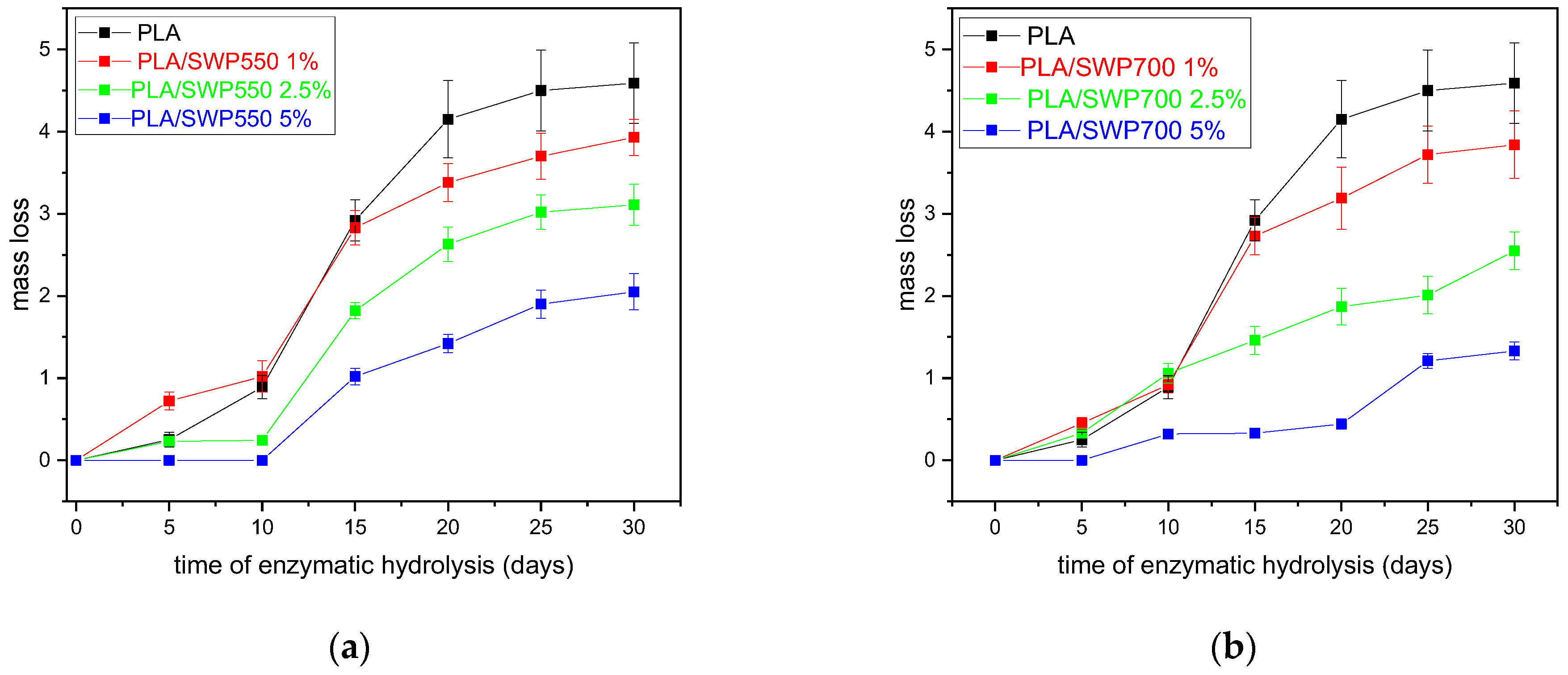
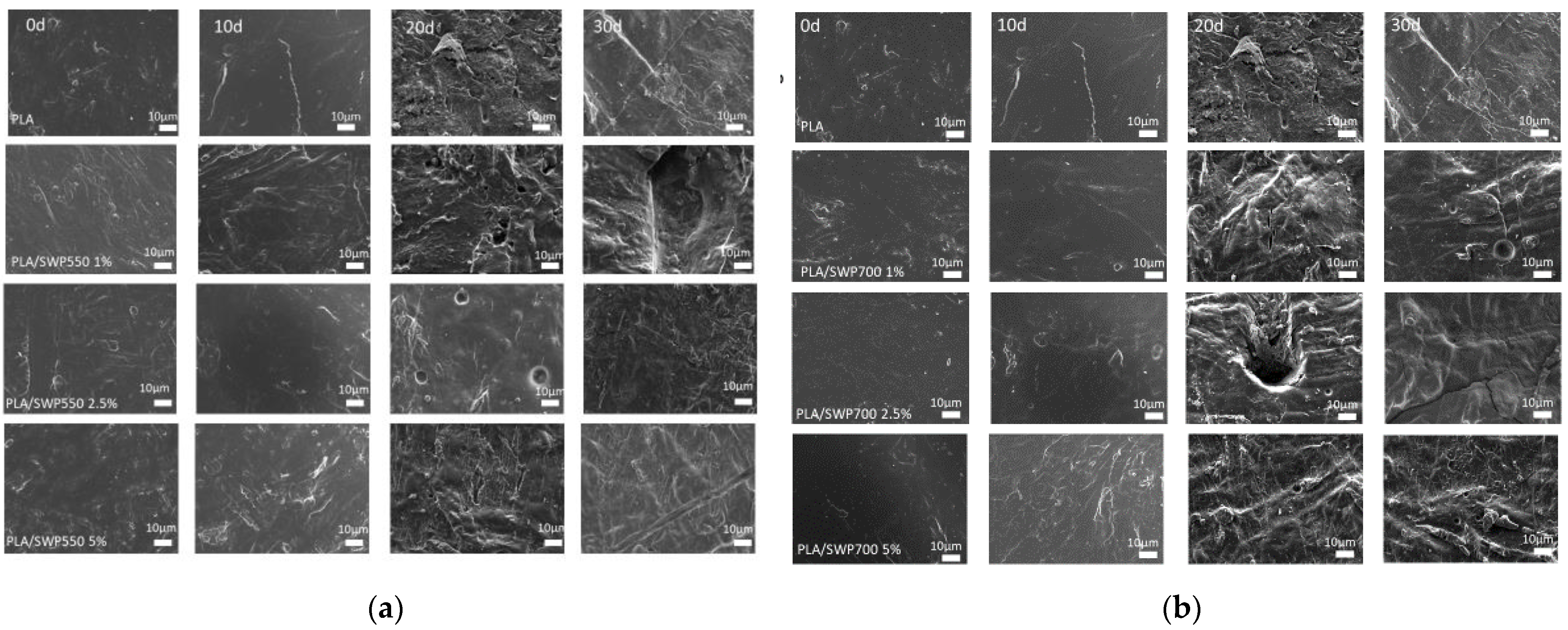



| Sample | η (dL/g) | Mn (g/mol) | PDI |
|---|---|---|---|
| PLA neat | 2.18 | 109,000 | 2.2 |
| PLA/SWP550 1 wt% | 1.26 | 40,200 | 1.53 |
| PLA/SWP550 2.5 wt% | 0.92 | 30,150 | 1.66 |
| PLA/SWP550 5 wt% | 0.8 | 22,500 | 1.71 |
| PLA/SWP700 1 wt% | 1.14 | 40,900 | 1.60 |
| PLA/SWP700 2.5 wt% | 0.99 | 30,800 | 1.81 |
| PLA/SWP700 5 wt% | 0.72 | 20,150 | 1.60 |
| Scan 1 (Fast Cooling ~100 K/min) | Scan 2 (Cooling at 20 K/min) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | Mn (kg/mo) | CFc (wt) | Tg (°C) | Δcp (J/g∙K) | Tcc (°C) | CFcc (wt) | Tc (°C) | CFc (wt) | Tg (°C) | Δcp (J/g∙K) | Tcc (°C) | CFcc (wt) | Tm (°C) |
| neat PLA | 109 | 0 | 46 | 0.50 | 89 | 0.55 | 126/91 | 0.35 | 44 | 0.26 | 87 | 0.09 | 167 |
| PLA/SWP550 1.0% | 40 | 0 | 52 | 0.49 | 103 | 0.47 | 111/97 | 0.08 | 52 | 0.45 | 103 | 0.38 | 175 |
| PLA/SWP550 2.5% | 30 | 0 | 47 | 0.52 | 93 | 0.54 | 92 | 0.25 | 45 | 0.28 | 89 | 0.24 | 167 |
| PLA/SWP550 5.0% | 23 | 0 | 53 | 0.51 | 98 | 0.53 | 94 | 0.19 | 54 | 0.32 | 95 | 0.26 | 156/172 |
| PLA/SWP700 1.0% | 41 | 0 | 47 | 0.50 | 94 | 0.47 | 92 | 0.12 | 49 | 0.39 | 95 | 0.30 | 170 |
| PLA/SWP700 2.5% | 31 | 0 | 56 | 0.47 | 106 | 0.51 | 93 | 0.03 | 56 | 0.47 | 104 | 0.44 | 157 |
| PLA/SWP700 5.0% | 20 | 0 | 54 | 0.53 | 124 | 0.46 | - | 0 | 55 | 0.51 | 124 | 0.46 | 161/164 |
| Sample | Tensile Strength at Break (MPa) | Elongation at Break (%) | Elastic Modulus (MPa) |
|---|---|---|---|
| PLA | 30.08 ± 1.09 | 1.28 ± 0.07 | 3037 ± 231 |
| PLA/SWP550 1 wt% | 32.01 ± 0.34 | 1.51 ± 0.09 | 3143 ± 104 |
| PLA/SWP550 2.5 wt% | 15.25 ± 0.27 | 1.32 ± 0.02 | 2300 ± 111 |
| PLA/SWP700 1 wt% | 31.08 ± 0.72 | 1.12 ± 0.05 | 3187 ± 176 |
| PLA/SWP700 2.5 wt% | 16.82 ± 0.81 | 1.68 ± 0.03 | 1883 ± 59 |
| Samples | L | a | b | C | h | K/S |
|---|---|---|---|---|---|---|
| PLA | 88.98 | −1.12 | 0.82 | 1.39 | 143.77 | 0.13 |
| PLA/SWP550 1 wt% | 30.40 | 0.44 | 1.34 | 1.16 | 64.17 | 9.02 |
| PLA/SWP550 2.5 wt% | 23.50 | 0.31 | 0.62 | 0.39 | 49.17 | 11.93 |
| PLA/SWP550 5 wt% | 19.81 | 0.15 | 0.13 | 0.22 | 321.62 | 16.02 |
| PLA/SWP700 1 wt% | 21.33 | 0.30 | 0 | 0.18 | 41.11 | 13.04 |
| PLA/SWP700 2.5 wt% | 20.34 | 0.36 | −0.13 | 0.16 | 312.63 | 15.46 |
| PLA/SWP700 5 wt% | 19.91 | 0.12 | 0.04 | 0.14 | 347.69 | 16.19 |
| Sample | Contact Angle (Degree) |
|---|---|
| PLA | 73.90 ± 0.40 |
| PLA/SWP550 1 wt% | 73.80 ± 0.39 |
| PLA/SWP550 2.5 wt% | 76.70 ± 0.44 |
| PLA/SWP550 5 wt% | 82.50 ± 0.42 |
| PLA/SWP700 1 wt% | 72.70 ± 0.42 |
| PLA/SWP700 2.5 wt% | 75.20 ± 0.36 |
| PLA/SWP700 5 wt% | 80.00 ± 0.39 |
| Sample | T0.5 (°C) | T2.5 (°C) | T5 (°C) | Td,max1 (°C) | Td,max2 (°C) |
|---|---|---|---|---|---|
| Neat PLA | 162.3 | 250.0 | 286.8 | 343.0 | 374.5 |
| PLA/SWP550 1 wt% | 172.4 | 276.9 | 297.2 | 343.2 | 373.5 |
| PLA/SWP550 2.5 wt% | 166.4 | 262.7 | 296.5 | 343.8 | 377.2 |
| PLA/SWP550 5 wt% | 161.1 | 274.8 | 293.0 | 335.9 | 368.3 |
| PLA/SWP700 1 wt% | 181.5 | 272.8 | 297.0 | 337.2 | 376.1 |
| PLA/SWP700 2.5 wt% | 210.8 | 290.8 | 302.0 | 332.4 | 376.7 |
| PLA/SWP700 5 wt% | 216.2 | 270.2 | 280.8 | - | 326.5 |
| Sample | Model | Activation Energy, E/kJmol−1 | Pre-Exponential Factor, logA1/s−1 | Reaction Order/n | Log Kcat | Contribution | R2 |
|---|---|---|---|---|---|---|---|
| PLA/SWP700 2.5% | Cn | 89.8 | 7.5 | 0.3 | 0.7 | 0.242 | 0.99996 |
| Cn | 109.5 | 10.0 | 0.8 | 0.8 | 0.617 | ||
| Cn | 137.9 | 11.2 | 1.6 | 1.3 | 0.141 | ||
| PLA/SWP700 5% | Cn | 104.9 | 6.9 | 1.2 | 0.9 | 1.000 | 0.99990 |
| PLA/SWP550 2.5% | Cn | 87.2 | 6.4 | 0.4 | 0.3 | 0.266 | 0.99973 |
| Cn | 108.3 | 9.8 | 0.7 | 0.6 | 0.574 | ||
| Cn | 140.4 | 11.0 | 1.7 | 1.0 | 0.160 | ||
| PLA/SWP550 5% | Cn | 79.0 | 5.3 | 0.3 | 0.7 | 0.251 | 0.99954 |
| Cn | 103.6 | 9.2 | 0.6 | 0.9 | 0.501 | ||
| Cn | 133.64 | 10.2 | 1.3 | 1.1 | 0.248 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papadopoulou, K.; Klonos, P.A.; Kyritsis, A.; Tarani, E.; Chrissafis, K.; Mašek, O.; Tsachouridis, K.; Anastasiou, A.D.; Bikiaris, D.N. Synthesis and Characterization of PLA/Biochar Bio-Composites Containing Different Biochar Types and Content. Polymers 2025, 17, 263. https://doi.org/10.3390/polym17030263
Papadopoulou K, Klonos PA, Kyritsis A, Tarani E, Chrissafis K, Mašek O, Tsachouridis K, Anastasiou AD, Bikiaris DN. Synthesis and Characterization of PLA/Biochar Bio-Composites Containing Different Biochar Types and Content. Polymers. 2025; 17(3):263. https://doi.org/10.3390/polym17030263
Chicago/Turabian StylePapadopoulou, Katerina, Panagiotis A. Klonos, Apostolos Kyritsis, Evangelia Tarani, Konstantinos Chrissafis, Ondrej Mašek, Konstantinos Tsachouridis, Antonios D. Anastasiou, and Dimitrios N. Bikiaris. 2025. "Synthesis and Characterization of PLA/Biochar Bio-Composites Containing Different Biochar Types and Content" Polymers 17, no. 3: 263. https://doi.org/10.3390/polym17030263
APA StylePapadopoulou, K., Klonos, P. A., Kyritsis, A., Tarani, E., Chrissafis, K., Mašek, O., Tsachouridis, K., Anastasiou, A. D., & Bikiaris, D. N. (2025). Synthesis and Characterization of PLA/Biochar Bio-Composites Containing Different Biochar Types and Content. Polymers, 17(3), 263. https://doi.org/10.3390/polym17030263












