Real-Time Observation of Polymer Fluctuations During Phase Transition Using Transmission Electron Microscope
Abstract
:1. Introduction
2. Experimental Methods
2.1. Preparation of TEM Samples
2.2. TEM Observation
2.3. Mean Squared Displacement and Probability Density Analysis
2.4. Differential Scanning Calorimetry (DSC)
3. Results
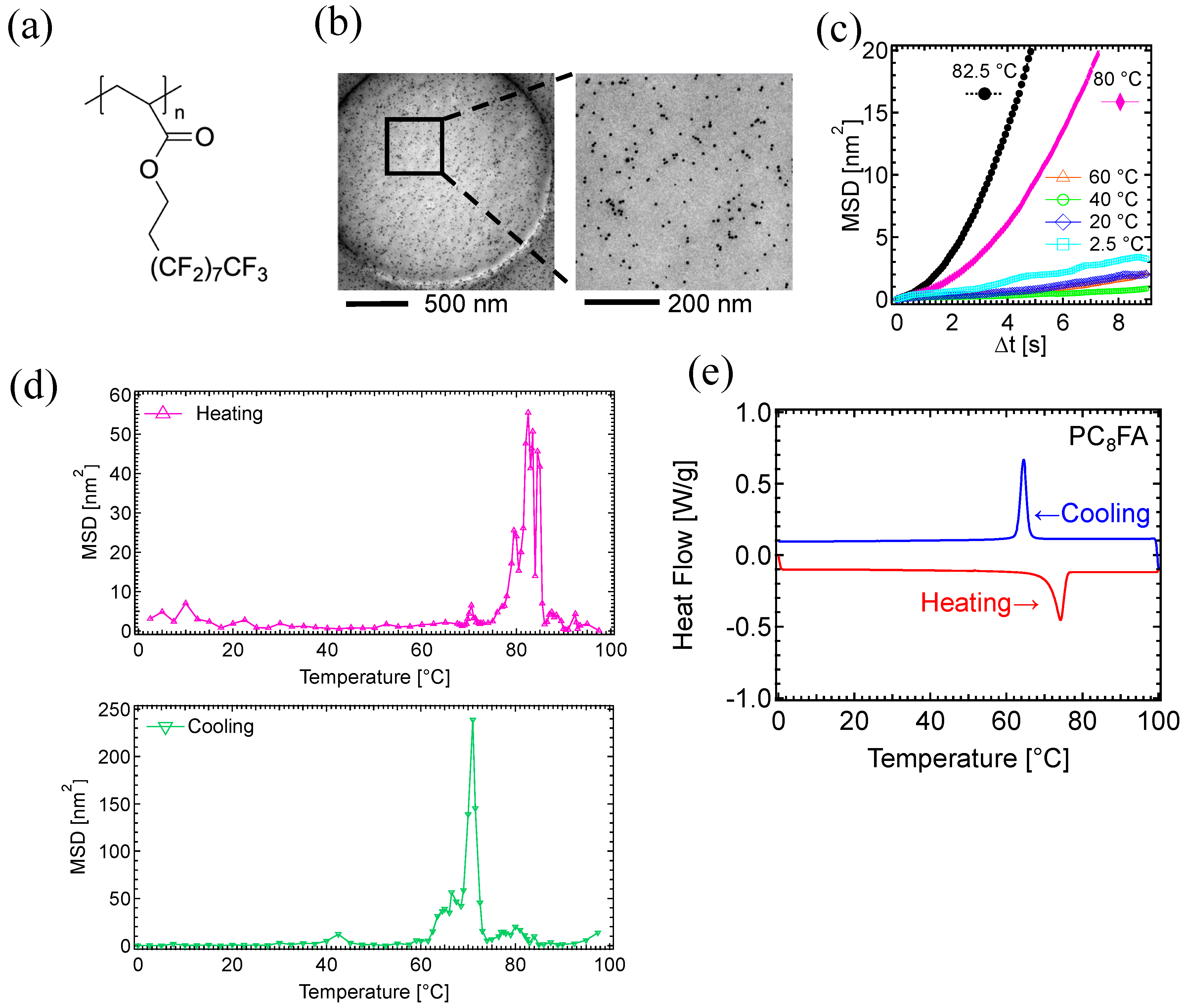
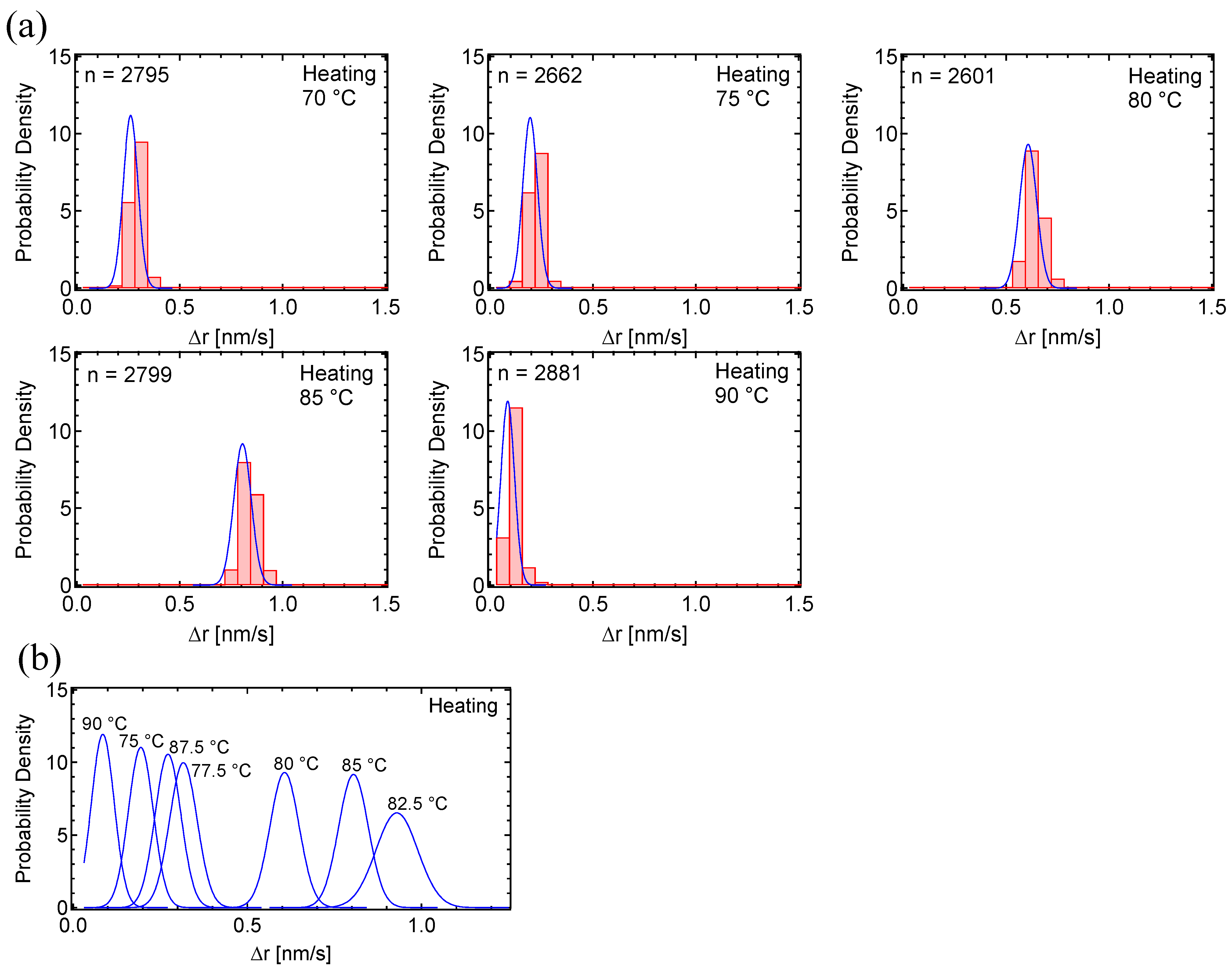
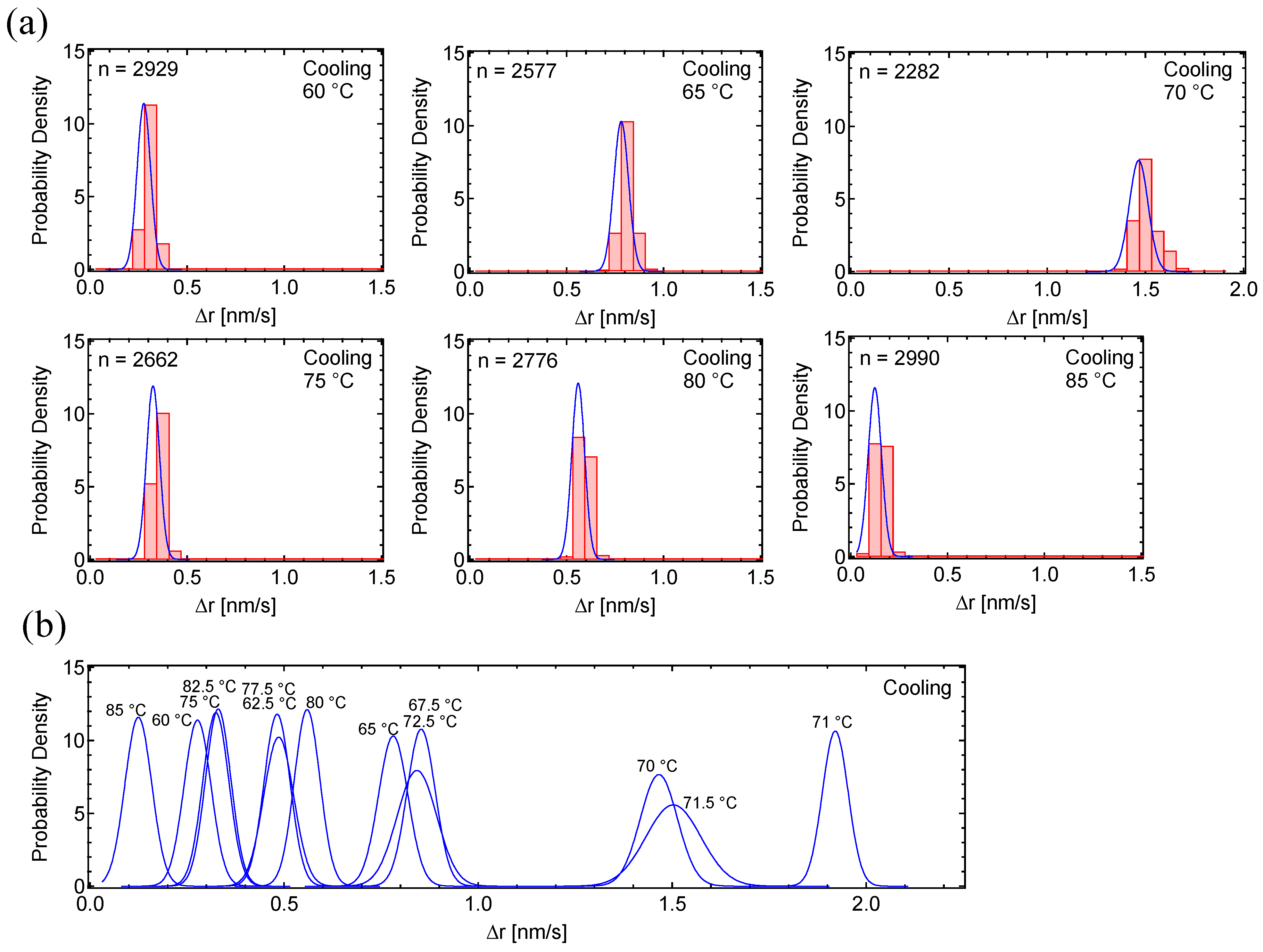
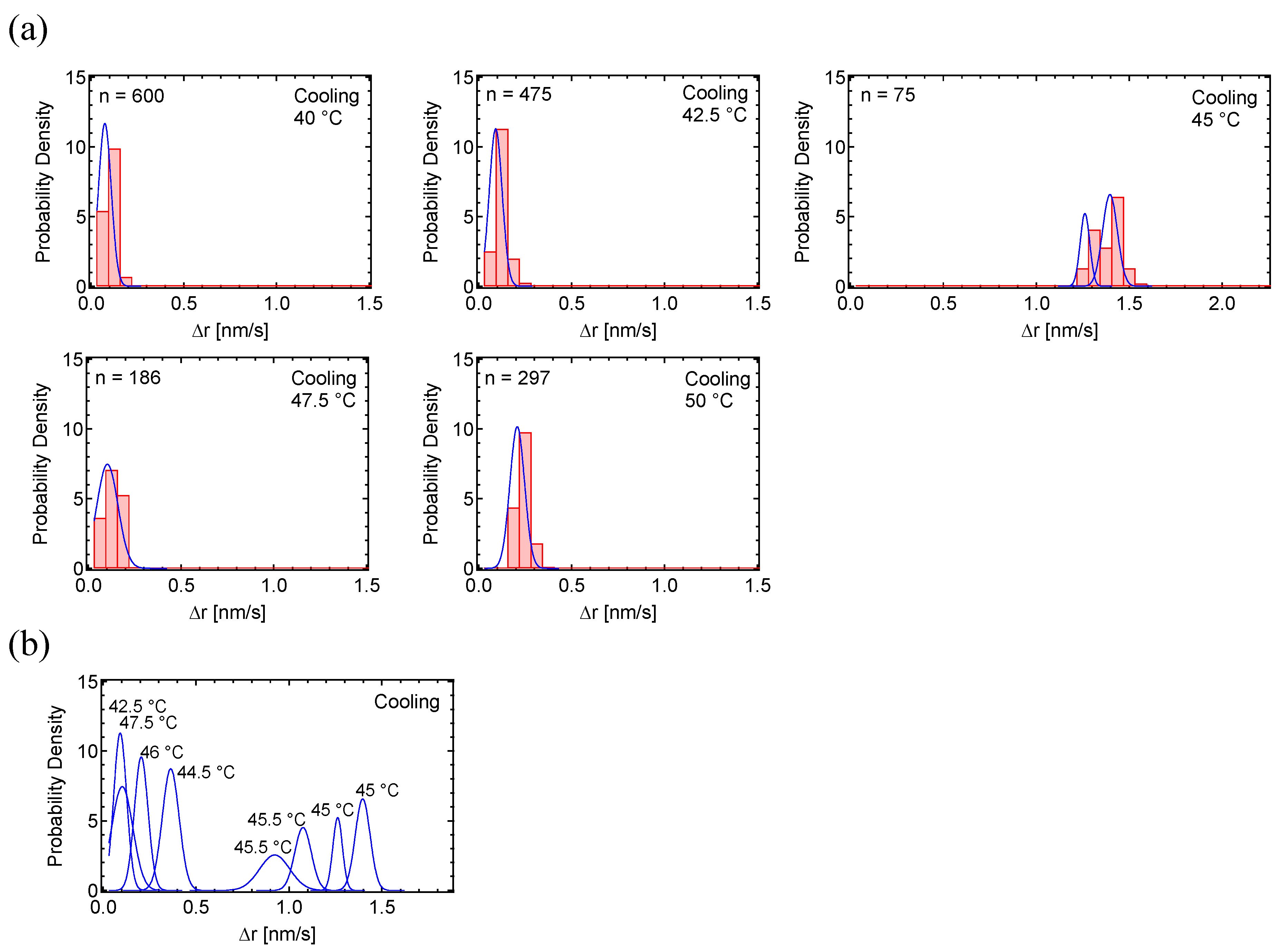
3.1. Motion Analysis of PC8FA
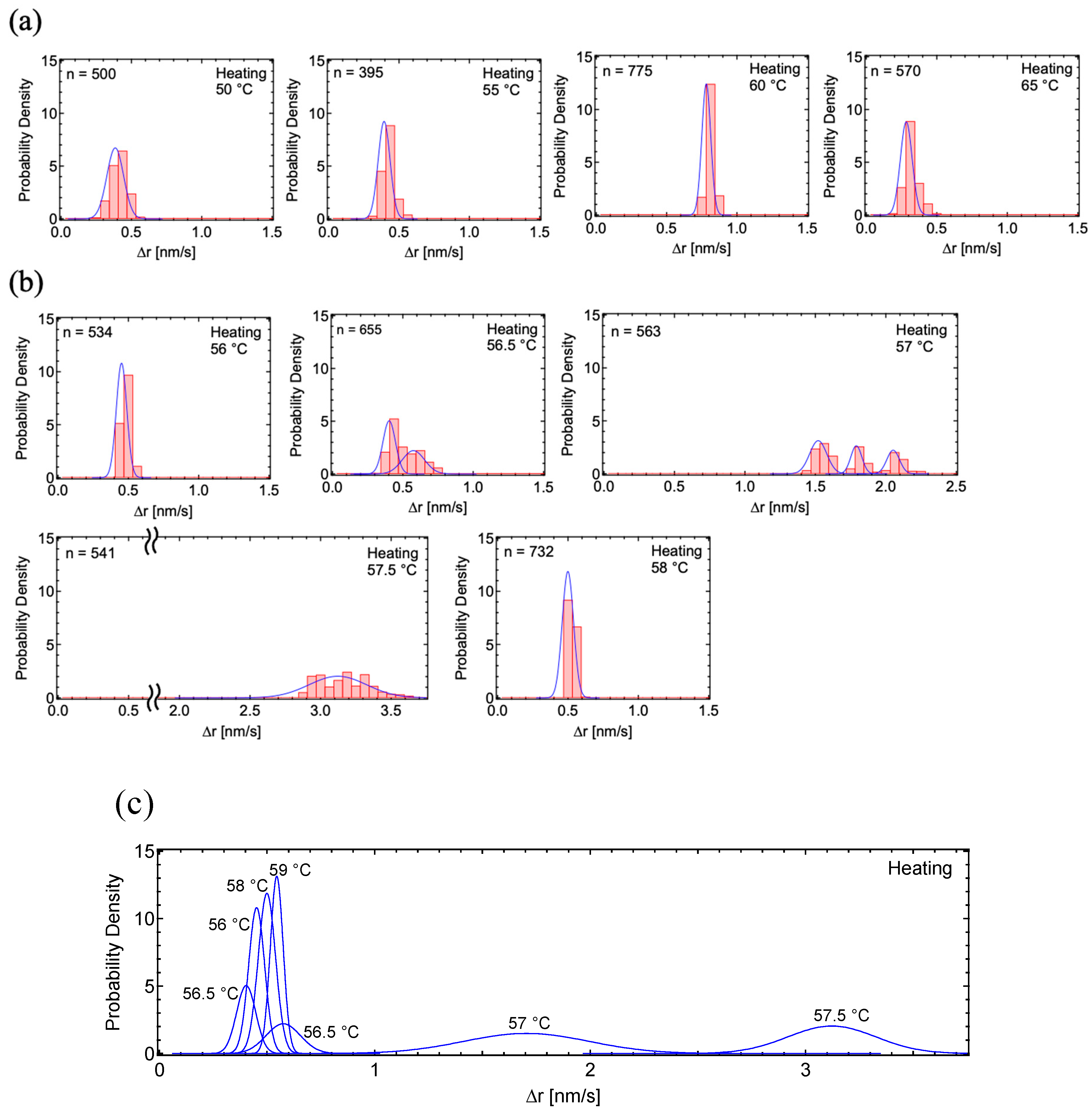
3.2. Motion Analysis of PSA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ohkubo, T.; Shiina, T.; Kawaguchi, K.; Sasaki, D.; Inamasu, R.; Yang, Y.; Li, Z.; Taninaka, K.; Sakaguchi, M.; Fujimura, S.; et al. Visualizing Intramolecular Dynamics of Membrane Proteins. Int. J. Mol. Sci. 2022, 23, 14539. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jinnai, H. Electron microscopy for polymer structures. Microscopy 2022, 71 (Suppl. 1), i148–i164. [Google Scholar] [CrossRef] [PubMed]
- Sabbert, D.; Engelbrecht, S.; Junge, W. Intersubunit rotation in active F-ATPase. Nature 1996, 381, 623–625. [Google Scholar] [CrossRef] [PubMed]
- Noji, H.; Yasuda, R.; Yoshida, M.; Kinosita, K., Jr. Direct observation of the rotation of F1-ATPase. Nature 1997, 386, 299–302. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, X.; Kasimova, M.A.; Cabezas-Bratesco, D.; Galpin, J.D.; Ladron-de-Guevara, E.; Villa, F.; Carnevale, V.; Islas, L.; Ahern, C.A.; Brauchi, S.E. Conformational dynamics in TRPV1 channels reported by an encoded coumarin amino acid. elife 2017, 6, e28626. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kuwashima, Y.; Yanagawa, M.; Abe, M.; Hiroshima, M.; Ueda, M.; Arita, M.; Sako, Y. Comparative Analysis of Single-Molecule Dynamics of TRPV1 and TRPV4 Channels in Living Cells. Int. J. Mol. Sci. 2021, 22, 8473. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cui, Y.; Zhao, Y.; Lu, Y.; Su, X.; Chen, Y.; Shen, Y.; Lin, J.; Li, X. In vivo single-particle tracking of the aquaporin AtPIP2;1 in stomata reveals cell type-specific dynamics. Plant Physiol. 2021, 185, 1666–1681. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nieves, D.J.; Baker, M.A.B. Pushing the super-resolution limit: Recent improvements in microscopy below the diffraction limit. Biochem. Soc. Trans. 2021, 49, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Ando, T.; Kodera, N.; Naito, Y.; Kinoshita, T.; Furuta, K.; Toyoshima, Y.Y. A high-speed atomic force microscope for studying biological macromolecules in action. ChemPhysChem 2003, 4, 1196–1202. [Google Scholar] [CrossRef] [PubMed]
- Matin, T.R.; Heath, G.R.; Huysmans, G.H.M.; Boudker, O.; Scheuring, S. Millisecond dynamics of an unlabeled amino acid transporter. Nat. Commun. 2020, 11, 5016. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Neutze, R.; Wouts, R.; van der Spoel, D.; Weckert, E.; Hajdu, J. Potential for biomolecular imaging with femtosecond X-ray pulses. Nature 2000, 406, 752–757. [Google Scholar] [CrossRef] [PubMed]
- Gaffney, K.J.; Chapman, H.N. Imaging atomic structure and dynamics with ultrafast X-ray scattering. Science 2007, 316, 1444–1448. [Google Scholar] [CrossRef] [PubMed]
- Nango, E.; Iwata, S. Recent progress in membrane protein dynamics revealed by X-ray free electron lasers: Molecular movies of microbial rhodopsins. Curr. Opin. Struct. Biol. 2023, 81, 102629. [Google Scholar] [CrossRef] [PubMed]
- Danev, R.; Yanagisawa, H.; Kikkawa, M. Cryo-Electron Microscopy Methodology: Current Aspects and Future Directions. Trends Biochem. Sci. 2019, 44, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Duong, N.T.; Aoyama, Y.; Kawamoto, K.; Yamazaki, T.; Nishiyama, Y. Structure Solution of Nano-Crystalline Small Molecules Using MicroED and Solid-State NMR Dipolar-Based Experiments. Molecules 2021, 26, 4652. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kakehashi, R.; Tokai, N.; Nakagawa, M.; Kawasaki, K.; Horiuchi, S.; Yamamoto, A. Amidoamine Oxide Surfactants as Low-Molecular-Weight Hydrogelators: Effect of Methylene Chain Length on Aggregate Structure and Rheological Behavior. Gels 2023, 9, 261. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Horiuchi, S.; Liu, Y.; Shigemoto, Y.; Hanada, T.; Shimamoto, K. In-situ TEM investigation of failure processes in metal-plastic joint interfaces. Int. J. Adhes. Adhes. 2022, 117 Pt B, 103003. [Google Scholar] [CrossRef]
- Toom, S.R.; Sato, T.; Milne, Z.; Bernal, R.A.; Jeng, Y.R.; Muratore, C.; Glavin, N.R.; Carpick, R.W.; Schall, J.D. Nanoscale Adhesion and Material Transfer at 2D MoS2-MoS2 Interfaces Elucidated by In Situ Transmission Electron Microscopy and Atomistic Simulations. ACS Appl. Mater. Interfaces 2024, 16, 30506–30520. [Google Scholar] [CrossRef] [PubMed]
- Hunter, R.D.; Takeguchi, M.; Hashimoto, A.; Ridings, K.M.; Hendy, S.C.; Zakharov, D.; Warnken, N.; Isaacs, J.; Fernandez-Muñoz, S.; Ramirez-Rico, J.; et al. Elucidating the Mechanism of Iron-Catalyzed Graphitization: The First Observation of Homogeneous Solid-State Catalysis. Adv. Mater. 2024, 36, e2404170. [Google Scholar] [CrossRef] [PubMed]
- Song, C.K.; Kim, Y.; Park, J. In situ electron microscopy revealing dynamic structure–function relationship of heterogeneous catalyst. MRS Commun. 2024, 14, 248–260. [Google Scholar] [CrossRef]
- Chen, Y.; Yin, K.; Xu, T.; Guo, H.; Sun, L. Characterization of Nanomaterials Using In Situ Liquid-Cell Transmission Electron Microscopy: A Review. ACS Appl. Nano Mater. 2023, 6, 22545–22567. [Google Scholar] [CrossRef]
- Powers, A.S.; Liao, H.G.; Raja, S.N.; Bronstein, N.D.; Alivisatos, A.P.; Zheng, H. Tracking Nanoparticle Diffusion and Interaction during Self-Assembly in a Liquid Cell. Nano Lett. 2017, 17, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Bakalis, E.; Parent, L.R.; Vratsanos, M.; Park, C.; Gianneschi, N.C.; Zerbetto, F. Complex Nanoparticle Diffusional Motion in Liquid-Cell Transmission Electron Microscopy. J. Phys. Chem. C Nanomater. Interfaces 2020, 124, 14881–14890. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Koo, K.; Park, J.; Ji, S.; Toleukhanova, S.; Yuk, J.M. Liquid-Flowing Graphene Chip-Based High-Resolution Electron Microscopy. Adv. Mater. 2021, 33, e2005468. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.W.; Carnevale, L.N.; Das, A.; Chen, Q. Electron videography of a lipid-protein tango. Sci. Adv. 2024, 10, eadk0217. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Unwin, N. Acetylcholine receptor channel imaged in the open state. Nature 1995, 373, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S.; Feng, X.; Maji, S.; Dadhwal, P.; Zhang, Z.; Brown, Z.P.; Frank, J. Time resolution in cryo-EM using a PDMS-based microfluidic chip assembly and its application to the study of HflX-mediated ribosome recycling. Cell 2024, 187, 782–796.e23. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Burke, S.M.; Avstrikova, M.; Noviello, C.M.; Mukhtasimova, N.; Changeux, J.P.; Thakur, G.A.; Sine, S.M.; Cecchini, M.; Hibbs, R.E. Structural mechanisms of α7 nicotinic receptor allosteric modulation and activation. Cell 2024, 187, 1160–1176.e21. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Papasergi-Scott, M.M.; Pérez-Hernández, G.; Batebi, H.; Gao, Y.; Eskici, G.; Seven, A.B.; Panova, O.; Hilger, D.; Casiraghi, M.; He, F.; et al. Time-resolved cryo-EM of G protein activation by a GPCR. Nature 2024, 629, 1182–1191. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Koshino, M.; Tanaka, T.; Solin, N.; Suenaga, K.; Isobe, H.; Nakamura, E. Imaging of single organic molecules in motion. Science 2007, 316, 853. [Google Scholar] [CrossRef] [PubMed]
- Harano, K.; Takenaga, S.; Okada, S.; Niimi, Y.; Yoshikai, N.; Isobe, H.; Suenaga, K.; Kataura, H.; Koshino, M.; Nakamura, E. Conformational analysis of single perfluoroalkyl chains by single-molecule real-time transmission electron microscopic imaging. J. Am. Chem. Soc. 2014, 136, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Nakamuro, T.; Kamei, K.; Sun, K.; Bode, J.W.; Harano, K.; Nakamura, E. Time-Resolved Atomistic Imaging and Statistical Analysis of Daptomycin Oligomers with and without Calcium Ions. J. Am. Chem. Soc. 2022, 144, 13612–13622. [Google Scholar] [CrossRef] [PubMed]
- Baskin, J.S.; Ahmed, H. Zewail. Seeing in 4D with Electrons: Development of Ultrafast Electron Microscopy at Caltech. Comptes Rendus Phys. 2014, 15, 176–189. [Google Scholar] [CrossRef]
- Flannigan, D.J.; Curtis, W.A.; VandenBussche, E.J.; Zhang, Y. Low repetition-rate, high-resolution femtosecond transmission electron microscopy. J. Chem. Phys. 2022, 157, 180903. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y.C.; Suzuki, Y.; Yagi, N.; Adachi, S.; Ishibashi, M.; Suda, H.; Toyota, K.; Yanagihara, M. Tracking of individual nanocrystals using diffracted x rays. Phys. Rev. E Stat. Phys. Plasmas Fluids Relat. Interdiscip. Top. 2000, 62 Pt B, 3843–3847. [Google Scholar] [CrossRef] [PubMed]
- Sekiguchi, H.; Kuramochi, M.; Ikezaki, K.; Okamura, Y.; Yoshimura, K.; Matsubara, K.; Chang, J.W.; Ohta, N.; Kubo, T.; Mio, K.; et al. Diffracted X-ray Blinking Tracks Single Protein Motions. Sci. Rep. 2018, 8, 17090. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sasaki, Y.C. Diffracted X-ray Tracking for Observing the Internal Motions of Individual Protein Molecules and Its Extended Methodologies. Int. J. Mol. Sci. 2023, 24, 14829. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fujimura, S.; Mio, K.; Kuramochi, M.; Sekiguchi, H.; Ikezaki, K.; Mio, M.; Hengphasatporn, K.; Shigeta, Y.; Kubo, T.; Sasaki, Y.C. Agonist and Antagonist-Diverted Twisting Motions of a Single TRPV1 Channel. J. Phys. Chem. B 2020, 124, 11617–11624. [Google Scholar] [CrossRef] [PubMed]
- Mio, K.; Ohkubo, T.; Sasaki, D.; Arai, T.; Sugiura, M.; Fujimura, S.; Nozawa, S.; Sekiguchi, H.; Kuramochi, M.; Sasaki, Y.C. Real-Time Observation of Capsaicin-Induced Intracellular Domain Dynamics of TRPV1 Using the Diffracted X-ray Tracking Method. Membranes 2023, 13, 708. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Saito, K.; Ichiyanagi, K.; Fukaya, R.; Haruki, R.; Nozawa, S.; Sasaki, D.; Arai, T.; Sasaki, Y.C.; McGehee, K.; Saikawa, M.; et al. Visualization of the Dynamics of Photoinduced Crawling Motion of 4-(Methylamino)Azobenzene Crystals via Diffracted X-ray Tracking. Int. J. Mol. Sci. 2023, 24, 17462. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Araki, K.; Watanabe-Nakayama, T.; Sasaki, D.; Sasaki, Y.C.; Mio, K. Molecular Dynamics Mappings of the CCT/TRiC Complex-Mediated Protein Folding Cycle Using Diffracted X-ray Tracking. Int. J. Mol. Sci. 2023, 24, 14850. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, Y.; Arai, T.; Sasaki, D.; Kuramochi, M.; Inagaki, H.; Ohashi, S.; Sekiguchi, H.; Mio, K.; Kubo, T.; Sasaki, Y.C. Real-time tilting and twisting motions of ligand-bound states of α7 nicotinic acetylcholine receptor. Eur. Biophys. J. 2024, 53, 15–25. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kuramochi, M.; Omata, H.; Ishihara, M.; Hanslin, S.Ø.; Mizumaki, M.; Kawamura, N.; Osawa, H.; Suzuki, M.; Mio, K.; Sekiguchi, H.; et al. Tilting and rotational motions of silver halide crystal with diffracted X-ray blinking. Sci. Rep. 2021, 11, 4097. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chang, J.; Arai, T.; Kuramochi, M.; Inamasu, R.; Lee, Z.; Ohkubo, T.; Mio, K.; Sasaki, Y.C. Dynamic observations of various oligomers in amyloid β isoforms using laboratory diffracted X-ray blinking. Biochem. Biophys. Rep. 2022, 31, 101298. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Arai, T.; Inamasu, R.; Yamaguchi, H.; Sasaki, D.; Sato-Tomita, A.; Sekiguchi, H.; Mio, K.; Tsuda, S.; Kuramochi, M.; Sasaki, Y.C. Laboratory diffracted x-ray blinking to monitor picometer motions of protein molecules and application to crystalline materials. Struct. Dyn. 2021, 8, 044302. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Inamasu, R.; Yamaguchi, H.; Arai, T.; Chang, J.; Kuramochi, M.; Mio, K.; Sasaki, Y.C. Observation of molecular motions in polymer thin films by laboratory grazing incidence diffracted X-ray blinking. Polym. J. 2023, 55, 703–709. [Google Scholar] [CrossRef]
- Mio, K.; Ohkubo, T.; Sasaki, D.; Sugiura, M.; Kawaguchi, K.; Araki, K.; Taninaka, K.; Sakaguchi, M.; Nozawa, S.; Arai, T.; et al. Simultaneous Recording of Remote Domain Dynamics in Membrane Proteins Using the Double-Labeled DXB/DXT Technique. Membranes 2024, 14, 75. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ogawa, N.; Hoshisashi, K.; Sekiguchi, H.; Ichiyanagi, K.; Matsushita, Y.; Hirohata, Y.; Suzuki, S.; Ishikawa, A.; Sasaki, Y.C. Tracking 3D picometer-scale motions of single nanoparticles with high-energy electron probes. Sci. Rep. 2013, 3, 2201. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ogawa, N.; Hirohata, Y.; Sasaki, Y.C.; Ishikawa, A. Time-resolved measurement of the three-dimensional motion of gold nanocrystals in water using diffracted electron tracking. Ultramicroscopy 2014, 140, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, N.; Yamamoto, Y.Y.; Abe, K.; Sekiguchi, H.; Sasaki, Y.C.; Ishikawa, A.; Frydman, J.; Yohda, M. Time-Resolved Measurement of the ATP-Dependent Motion of the Group II Chaperonin by Diffracted Electron Tracking. Int. J. Mol. Sci. 2018, 19, 950. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Honda, K.; Morita, M.; Otsuka, H.; Takahara, A. Molecular aggregation structure and surface properties of poly(fluoroalkyl acrylate) thin films. Macromolecules 2005, 38, 5699–5705. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Kikuchi, M.; Kobayashi, M.; Ogawa, H.; Masunaga, H.; Sakata, O.; Takahara, A. Influence of Molecular Weight Dispersity of Poly{2-(perfluorooctyl)ethyl acrylate} Brushes on Their Molecular Aggregation States and Wetting Behavior. Macromolecules 2012, 45, 1509–1516. [Google Scholar] [CrossRef]
- Honda, K.; Yamamoto, I.; Morita, M.; Yamaguchi, H.; Arita, H.; Ishige, R.; Higaki, Y.; Takahara, A. Effect of α-substituents on molecular motion and wetting behaviors of poly(fluoroalkyl acrylate) thin films with short fluoroalkyl side chains. Polymer 2014, 55, 6303–6308. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Q.; Jiang, J.; Zhan, X.; Chen, F. Microphase Structure, Crystallization Behavior, and Wettability Properties of Novel Fluorinated Copolymers Poly(perfluoroalkyl acrylate-co-stearyl acrylate) Containing Short Perfluorohexyl Chains. Langmuir 2015, 31, 4752–4760. [Google Scholar] [CrossRef] [PubMed]
- Parvate, S.; Dixit, P.; Chattopadhyay, S. Superhydrophobic Surfaces: Insights from Theory and Experiment. J. Phys. Chem. B 2020, 124, 1323–1360. [Google Scholar] [CrossRef] [PubMed]
- Milnes-Smith, E.; Stone, C.A.; Willis, C.R.; Perkin, S. Surface Reconstruction of Fluoropolymers in Liquid Media. Langmuir 2022, 38, 4657–4668. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, Y.; Higaki, Y.; Mukai, M.; Ohta, N.; Kabe, T.; Takahara, A. Smectic ordered structure and water repellency of a poly(fluoroalkyl acrylate) with a carbamate linker. Polym. J. 2019, 51, 189–198. [Google Scholar] [CrossRef]
- Yamamoto, A.; Higaki, Y.; Thoma, J.; Kimmle, E.; Ishige, R.; Demé, B.; Takahara, A.; Tanaka, M. Water modulates the lamellar structure and interlayer correlation of poly(perfluorooctyl acrylate) films: A specular and off-specular neutron scattering study. Polym. J. 2022, 54, 57–65. [Google Scholar] [CrossRef]
- Wang, Z.; Zuilhof, H. Self-healing fluoropolymer brushes as highly polymer-repellent coatings. J. Mater. Chem. A 2016, 4, 2408–2412. [Google Scholar] [CrossRef]
- Ma, W.; Ameduri, B.; Takahara, A. Molecular Aggregation Structure and Surface Properties of Biomimetic Catechol-Bearing Poly [2-(perfluorooctyl)ethyl acrylate] and Its Application to Superamphiphobic Coatings. ACS Omega 2020, 5, 8169–8180. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Refaa, Z.; Boutaous, M.; Xin, S.; Siginer, D.A. Thermophysical analysis and modeling of the crystallization and melting behavior of PLA with talc: Kinetics and crystalline structures. J. Therm. Anal. Calorim. 2017, 128, 687–698. [Google Scholar] [CrossRef]
- Somsunan, R.; Mainoiy, N. Isothermal and non-isothermal crystallization kinetics of PLA/PBS blends with talc as nucleating agent. J. Therm. Anal. Calorim. 2019, 139, 1941–1948. [Google Scholar] [CrossRef]
- Fernández-Tena, A.; Pérez-Camargo, R.A.; Coulembier, O.; Sangroniz, L.; Aranburu, N.; Guerrica-Echevarria, G.; Liu, G.; Wang, D.; Cavallo, D.; Müller, A.J. Effect of Molecular Weight on the Crystallization and Melt Memory of Poly(ε-caprolactone) (PCL). Macromolecules 2023, 56, 4602–4620. [Google Scholar] [CrossRef]
- Saalwächter, K.; Thurn-Albrecht, T.; Paul, W. Recent Progress in Understanding Polymer Crystallization. Macromol. Chem. Phys. 2023, 224, 2200424. [Google Scholar] [CrossRef]
- Egerton, R.F. Radiation damage to organic and inorganic specimens in the TEM. Micron 2019, 119, 72–87. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shiina, T.; Ohkubo, T.; McGehee, K.; Inamasu, R.; Arai, T.; Sasaki, D.; Sasaki, Y.C.; Mio, K. Real-Time Observation of Polymer Fluctuations During Phase Transition Using Transmission Electron Microscope. Polymers 2025, 17, 292. https://doi.org/10.3390/polym17030292
Shiina T, Ohkubo T, McGehee K, Inamasu R, Arai T, Sasaki D, Sasaki YC, Mio K. Real-Time Observation of Polymer Fluctuations During Phase Transition Using Transmission Electron Microscope. Polymers. 2025; 17(3):292. https://doi.org/10.3390/polym17030292
Chicago/Turabian StyleShiina, Takaaki, Tatsunari Ohkubo, Keegan McGehee, Rena Inamasu, Tatsuya Arai, Daisuke Sasaki, Yuji C. Sasaki, and Kazuhiro Mio. 2025. "Real-Time Observation of Polymer Fluctuations During Phase Transition Using Transmission Electron Microscope" Polymers 17, no. 3: 292. https://doi.org/10.3390/polym17030292
APA StyleShiina, T., Ohkubo, T., McGehee, K., Inamasu, R., Arai, T., Sasaki, D., Sasaki, Y. C., & Mio, K. (2025). Real-Time Observation of Polymer Fluctuations During Phase Transition Using Transmission Electron Microscope. Polymers, 17(3), 292. https://doi.org/10.3390/polym17030292







