Thermal Insulating and Mechanically Strong Polyimide Aerogel Composites Reinforced by Polyhedral Oligomeric Silsesquioxane-Grafted Carbon Nanotubes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
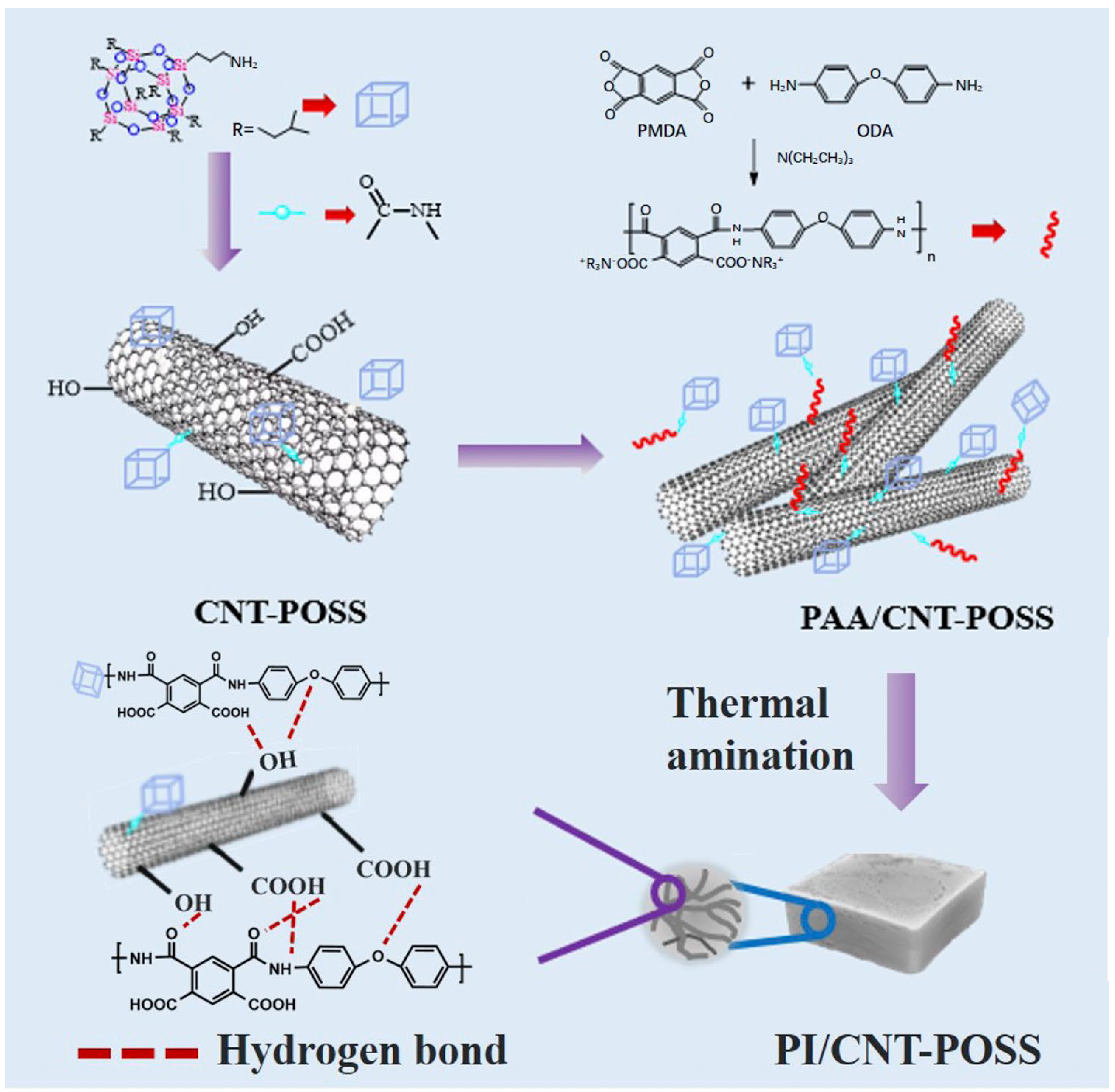
2.2. Preparation of POSS-Functionalized CNTs
2.3. Preparation of Polyamide Acid (PAA)
2.4. Preparation of PI Composite Aerogels
2.5. Characterization
3. Results and Discussion
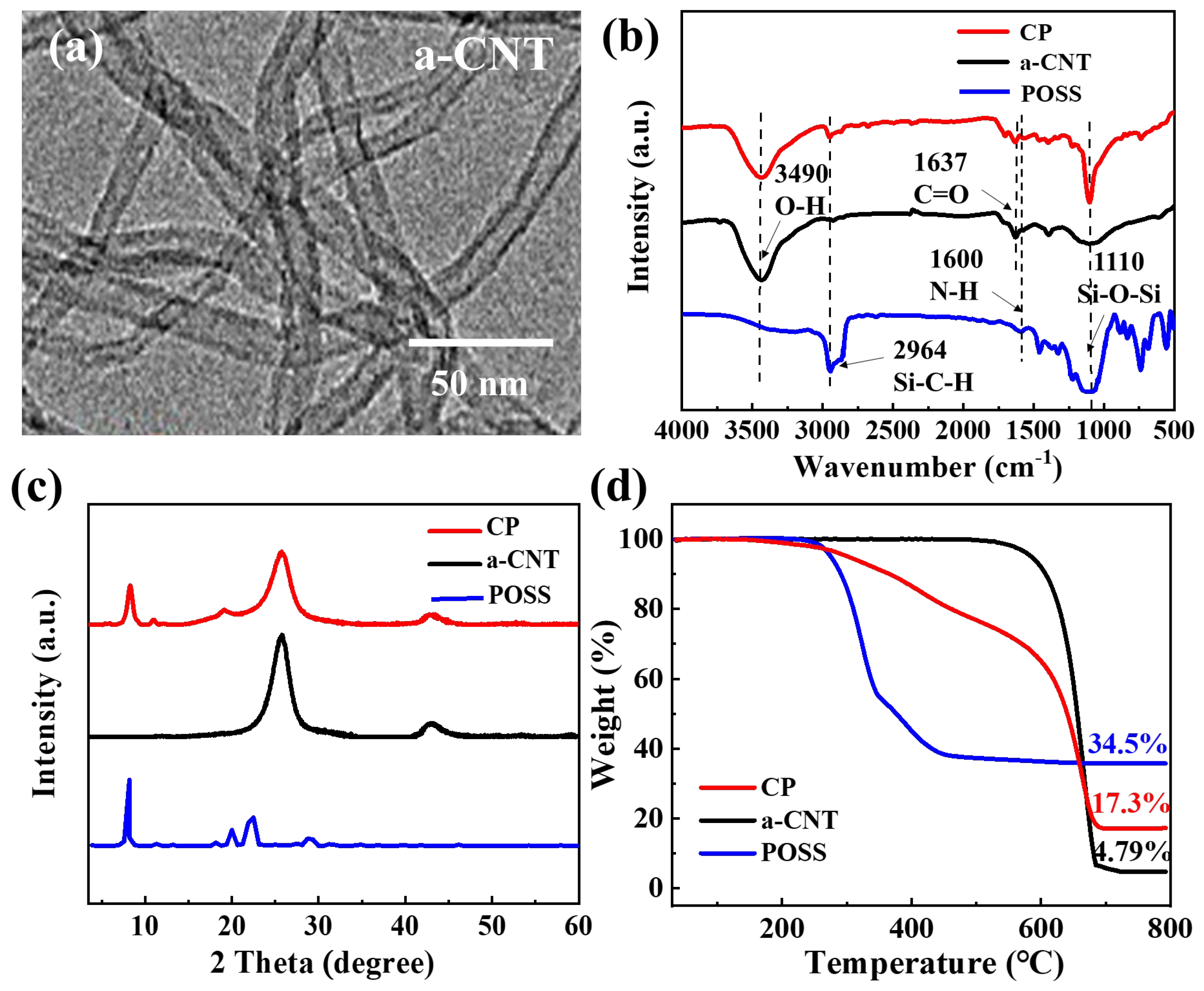
3.1. Composition and Structure of PI Composite Aerogels
3.2. Mechanical Performance of PI Composite Aerogels
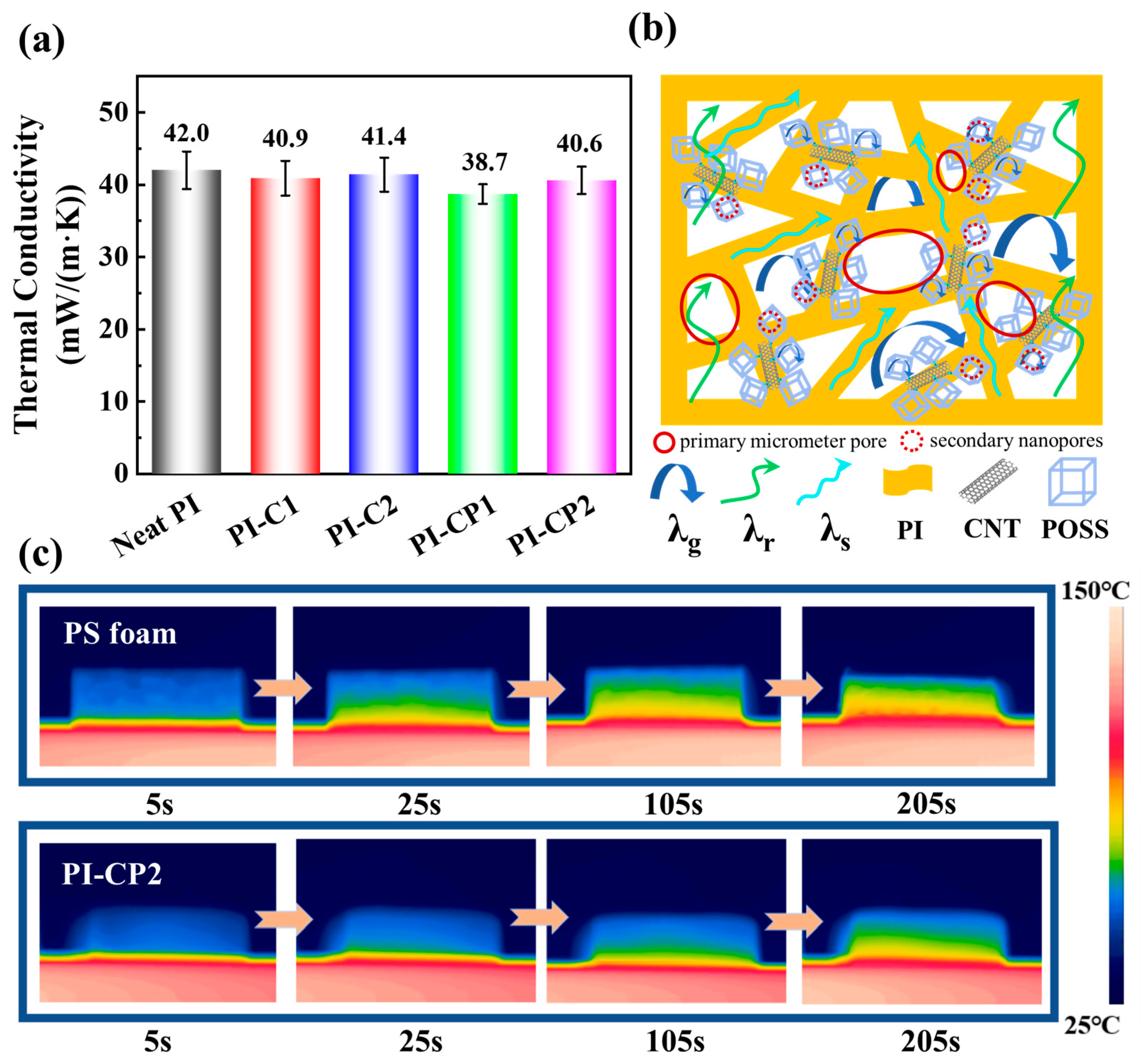
3.3. Thermal Conductivity and Insulation Mechanism of PI Aerogels
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, R.Z.; Sun, J.C.; Zhang, R.B. Super-flexible and low-shrinkage polyimide aerogel composites with excellent thermal insulation: Boosted by SiO2 micron aerogel powder. Mater. Lett. 2024, 371, 136882. [Google Scholar] [CrossRef]
- Ghaffari-Mosanenzadeh, S.; Tafreshi, O.A.; Karamikamkar, S.; Saadatnia, Z.; Rad, E.; Meysami, M.; Naguib, H.E. Recent advances in tailoring and improving the properties of polyimide aerogels and their application. Adv. Colloid Interface Sci. 2022, 304, 102646. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-Y.; Cao, J.-H.; Wang, Y.; Shen, S.-G.; Liang, W.-H.; Wu, D.-Y. Colorless Polyamide–Imide Films with Enhanced Thermal and Dimensional Stability and Their Application in Flexible OLED Devices. ACS Appl. Polym. Mater. J. 2022, 4, 7664–7673. [Google Scholar] [CrossRef]
- Zhang, T.Y.; Zhao, Y.; Muhetaer, M.; Wang, K. Silver nanoparticles cross-linked polyimide aerogels with improved high temperature microstructure stabilities and high mechanical performances. Microporous Mesoporous Mater. 2020, 297, 110035. [Google Scholar] [CrossRef]
- Yu, D.Y.; Xue, T.X.; Ma, Z.C.; Hu, Z.Y.; Long, L.J.; Miao, Y.-E.; Fan, W.; Liu, T.X. 3D Printed Polyimide/Silica Composite Aerogels for Customizable Thermal Insulation from −50 °C to 1300 °C. Chin. J. Polym. Sci. 2024, 42, 936–945. [Google Scholar] [CrossRef]
- Revin, V.V.; Pestov, N.A.; Shchankin, M.V.; Mishkin, V.P.; Platonov, V.I.; Uglanov, D.A. A Study of the Physical and Mechanical Properties of Aerogels Obtained from Bacterial Cellulose. Biomacromolecules 2019, 20, 1401–1411. [Google Scholar] [CrossRef]
- Qian, H.R.; Li, Z.Q.; He, S. Cross-Linked Polyimide Aerogels with Excellent Thermal and Mechanical Properties. Gels 2024, 10, 667. [Google Scholar] [CrossRef]
- Wu, S.; Du, A.; Xiang, Y.L.; Liu, M.F.; Li, T.M.; Shen, J.; Zhang, Z.H.; Li, C.H.; Zhou, B. Silica-aerogel-powders “jammed” polyimide aerogels with excellent hydrophobicity and conversion to ultra-light polyimide aerogel. RSC Adv. 2016, 6, 58268–58278. [Google Scholar] [CrossRef]
- Li, J.Q.; Zhang, L.; Pang, Y.C.; Wang, X.; Bai, B.; Zhang, W.T.; Luo, C.Y.; Liu, W.S.; Zhang, L.Y. Polyimide composite aerogels towards highly efficient microwave absorption and thermal insulation. Compos. Part A 2022, 161, 107112. [Google Scholar] [CrossRef]
- Zhu, Z.X.; Yao, H.J.; Dong, J.X.; Qian, Z.; Dong, W.; Long, D.H. High-mechanical-strength polyimide aerogels crosslinked with 4, 4′-oxydianiline-functionalized carbon nanotubes. Carbon 2019, 144, 24–31. [Google Scholar] [CrossRef]
- Wu, Y.S.; Zhang, X.; Guo, Y.; Wang, X.B. Reduced Graphene Oxide (rGO)/ZrO2 Reinforced Polyimide Nanocomposite Aerogels with Enhanced Properties: A Synergistic Effect of the Nanofillers. ChemistrySelect 2019, 4, 10868–10875. [Google Scholar] [CrossRef]
- Zhan, H.-J.; Wu, K.-J.; Hu, Y.-L.; Liu, J.-W.; Li, H.; Guo, X.; Xu, J.; Yang, Y.; Yu, Z.-L.; Gao, H.-L.; et al. Biomimetic Carbon Tube Aerogel Enables Super-Elasticity and Thermal Insulation. Chem 2019, 5, 1871–1882. [Google Scholar] [CrossRef]
- Sun, M.; Guo, Z.Y.; Zhang, W.T.; Ding, E.J.; Li, X.; Yin, M.; Luo, C.Y.; Zhang, L.Y. A strategy to fabricate hierarchical microporous architecture of polyimide nanofibrous aerogels with efficient electromagnetic wave absorption and thermal insulation. Compos. Part A 2024, 177, 107940. [Google Scholar] [CrossRef]
- Xue, T.T.; Zhu, C.Y.; Feng, X.L.; Wali, Q.; Fan, W.; Liu, T.X. Polyimide Aerogel Fibers with Controllable Porous Microstructure for Super-Thermal Insulation Under Extreme Environments. Adv. Fiber Mater. 2022, 4, 1118–1128. [Google Scholar] [CrossRef]
- Yang, H.K.; He, C.F.; Russell, T.P.; Wang, D. Epoxy-polyhedral oligomeric silsesquioxanes (POSS) nanocomposite vitrimers with high strength, toughness, and efficient relaxation. Giant 2020, 4, 100035. [Google Scholar] [CrossRef]
- Guo, H.; Meador, M.A.B.; McCorkle, L.; Quade, D.J.; Guo, J.; Hamilton, B.; Cakmak, M.; Sprowl, G. Polyimide aerogels cross-linked through amine functionalized polyoligomeric silsesquioxane. ACS Appl. Mater. Interfaces 2011, 3, 546–552. [Google Scholar] [CrossRef]
- Wu, Y.-W.; Ye, M.-F.; Zhang, W.-C.; Yang, R.-J. Polyimide aerogels crosslinked through cyclic ladder-like and cage polyamine functionalized polysilsesquioxanes. J. Appl. Polym. 2017, 134, 45296. [Google Scholar] [CrossRef]
- Wu, Y.-w.; Zhang, W.-C.; Yang, R.-j. Ultralight and Low Thermal Conductivity Polyimide–Polyhedral Oligomeric Silsesquioxanes Aerogels. Macromol. Mater. Eng. 2017, 303, 1700403. [Google Scholar] [CrossRef]
- Xi, S.; Wang, X.D.; Liu, T.; Zhang, Z.; Zhang, X.X.; Shen, J. Moisture-Resistant and Mechanically Strong Polyimide-Polymethylsilsesquioxane Hybrid Aerogels with Tunable Microstructure. Macromol. Mater. Eng. 2021, 306, 2000612. [Google Scholar] [CrossRef]
- Wang, Y.; Ge, Q.; Chen, X.; Qi, S.; Tian, G.; Wu, D. Ultralight and flexible mwnts/polyimide hybrid aerogels for elastic conductors. Macromol. Mater. Eng. 2017, 302, 1700082. [Google Scholar] [CrossRef]
- Xiao, S.D.; Cui, X.M.; Iroh, J.O. A Study of the Degradation Mechanism of Ladder-like Polyhedral Oligomeric Silsesquioxane via Fourier Transform Infrared Spectroscopy. Fire 2023, 6, 429. [Google Scholar] [CrossRef]
- Farghali, A.A.; Abdel Tawab, H.A.; Abdel Moaty, S.A.; Khaled, R. Functionalization of acidified multi-walled carbon nanotubes for removal of heavy metals in aqueous solutions. J. Nanostruct. Chem. 2017, 7, 101–111. [Google Scholar] [CrossRef]
- Song, S.S.; Cao, M.; Shan, H.T.; Du, C.Y.; Li, B.A. Polyhedral oligomeric silsesquioxane functionalized carbon nanotubes for high thermal conductive poly(vinylidene fluoride) composite membrane. Mater. Des. 2018, 156, 242–251. [Google Scholar] [CrossRef]
- Zhu, Y.J.; Wu, P.X.; Yang, S.S.; Lu, Y.H.; Li, W.; Zhu, N.W.; Dang, Z.; Huang, Z.Y. Synergetic effect of functionalized carbon nanotubes on ZnCr–mixed metal oxides for enhanced solar light-driven photocatalytic performance. RSC Adv. 2016, 6, 37689–37700. [Google Scholar] [CrossRef]
- Mansourian-Tabaei, M.; Majdoub, M.; Sengottuvelu, D.; Stoddard, D.L.; Thirumalai, R.V.K.G.; Ucak-Astarlioglu, M.G.; Al-Ostaz, A.; Nouranian, S. Polyurea/Aminopropyl Isobutyl Polyhedral Oligomeric Silsesquioxane-Functionalized Graphene Nanoplatelet Nanocomposites for Force Protection Applications. ACS Appl. Mater. Interfaces 2024, 16, 19625–19641. [Google Scholar] [CrossRef]
- Gakis, G.P.; Termine, S.; Trompeta, A.-F.A.; Aviziotis, I.G.; Charitidis, C.A. Unraveling the mechanisms of carbon nanotube growth by chemical vapor deposition. Chem. Eng. J. 2022, 445, 136807. [Google Scholar] [CrossRef]
- Guerrero, G.; Hägg, M.-B.; Kignelman, G.; Simon, C.; Peters, T.; Rival, N.; Denonville, C. Investigation of amino and amidino functionalized Polyhedral Oligomeric SilSesquioxanes (POSS®) nanoparticles in PVA-based hybrid membranes for CO2/N2 separation. J. Membr. Sci. 2017, 544, 161–173. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, X.Y.; Xue, T.T.; Yang, F.; Fan, W.; Liu, T.X. Bidirectional anisotropic polyimide/bacterial cellulose aerogels by freeze-drying for super-thermal insulation. Chem. Eng. J. 2020, 385, 123963. [Google Scholar] [CrossRef]
- Zhang, D.; Lin, Y.; Wang, W.; Li, Y.; Wu, G. Mechanically strong polyimide aerogels cross-linked with dopamine-functionalized carbon nanotubes for oil absorption. Appl. Surf. Sci. 2021, 543, 148833. [Google Scholar] [CrossRef]
- Meador, M.A.B.; Malow, E.J.; Silva, R.; Wright, S.; Quade, D.; Vivod, S.L.; Guo, H.; Guo, J.; Cakmak, M. Mechanically Strong, Flexible Polyimide Aerogels Cross-Linked with Aromatic Triamine. ACS Appl. Mater. Interfaces. 2012, 4, 536–544. [Google Scholar] [CrossRef]
- Chen, W.W.; Zhou, X.M.; Wan, M.M.; Tang, Y.T. Recent progress on polyimide aerogels against shrinkage: A review. J. Mater. Sci. 2022, 57, 13233–13263. [Google Scholar] [CrossRef]
- Lin, X.; Deng, Y.-Y.; Zhang, Q.; Han, D.; Fu, Q. Effect of POSS Size on the Porosity and Adsorption Performance of Hybrid Porous Polymers. Macromolecules 2023, 56, 1243–1252. [Google Scholar] [CrossRef]
- Hasani Baferani, A.; Ohadi, A.; Katbab, A.A. Toward mechanistic understanding the effect of aspect ratio of carbon nanotubes upon different properties of polyurethane/carbon nanotube nanocomposite foam. Polym. Eng. Sci. 2021, 61, 3037–3049. [Google Scholar] [CrossRef]
- Fan, W.; Zuo, L.Z.; Zhang, Y.F.; Chen, Y.; Liu, T.X. Mechanically strong polyimide / carbon nanotube composite aerogels with controllable porous structure. Compos. Sci. Technol. 2018, 156, 186–191. [Google Scholar] [CrossRef]
- Wang, L.B.; Song, G.M.; Guo, R.L.; Qiao, X.X.; Chen, G.X.; Zhou, Z.; Li, Q.F. Enhancing aerogel mechanical properties with incorporation of POSS. Ceram. Int. 2019, 45, 14586–14593. [Google Scholar] [CrossRef]
- Yu, X.H.; Zhang, Y.X.; Fan, X.S.; Lv, Y.; Wang, Z.C.; Zhang, X.; Liu, T.X. Macromolecular PEI-modified carbon nanotubes as multifunctional additives in controlling crystallization and enhancing comprehensive performance of polyamide 6 nanocomposites. Compos. Commun. 2024, 51, 102057. [Google Scholar] [CrossRef]
- Xie, Y.S.; Xu, S.; Xu, Z.L.; Wu, H.C.; Deng, C.; Wang, X.W. Interface-mediated extremely low thermal conductivity of graphene aerogel. Carbon 2016, 98, 381–390. [Google Scholar] [CrossRef]
- Peng, J.J.; Wang, G.; Zhang, Y. Synergistic effect of multifunctional POSS and carbon nanotubes on mechanical properties and thermal stability of silicone rubber composites. Polym. Compos. 2024, 45, 14358–14370. [Google Scholar] [CrossRef]
- Ren, J.F.; Feng, J.Y.; Wang, L.B.; Chen, G.X.; Zhou, Z.; Li, Q.F. High specific surface area hybrid silica aerogel containing POSS. Micropor. Mesopor. Mat. 2021, 310, 110456. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Li, X.; Ding, E.J.; Guo, Z.Y.; Luo, C.Y.; Zhang, H.; Yu, J.Y. Shape memory polyimide/carbon nanotube composite aerogels with physical and chemical crosslinking architectures for thermal insulating applications. Compos. Sci. Technol. 2024, 251, 110588. [Google Scholar] [CrossRef]
- Wang, M.X.; Miao, X.R.; Hou, C.; Xu, K.; Ke, Z.; Dai, F.N.; Liu, M.Y.; Li, H.; Chen, C.H. Devisable pore structures and tunable thermal management properties of aerogels composed of carbon nanotubes and cellulose nanofibers with various aspect ratios. Carbohyd. Polym. 2024, 323, 121437. [Google Scholar] [CrossRef]


| Samples | Density (mg/cm3) | Volume Shrinkage (%) | Porosity (%) |
|---|---|---|---|
| Neat PI | 89.0 | 47.5 | 94.2 |
| PI-C1 | 85.0 | 45.6 | 95.1 |
| PI-C2 | 83.0 | 43.8 | 95.8 |
| PI-CP1 | 78.0 | 41.7 | 96.7 |
| PI-CP2 | 74.0 | 38.6 | 97.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Yang, R.; Zhang, Z.; Shan, Z.; Zhang, L. Thermal Insulating and Mechanically Strong Polyimide Aerogel Composites Reinforced by Polyhedral Oligomeric Silsesquioxane-Grafted Carbon Nanotubes. Polymers 2025, 17, 332. https://doi.org/10.3390/polym17030332
Wang Y, Yang R, Zhang Z, Shan Z, Zhang L. Thermal Insulating and Mechanically Strong Polyimide Aerogel Composites Reinforced by Polyhedral Oligomeric Silsesquioxane-Grafted Carbon Nanotubes. Polymers. 2025; 17(3):332. https://doi.org/10.3390/polym17030332
Chicago/Turabian StyleWang, Yating, Ruirui Yang, Zhe Zhang, Zicheng Shan, and Liying Zhang. 2025. "Thermal Insulating and Mechanically Strong Polyimide Aerogel Composites Reinforced by Polyhedral Oligomeric Silsesquioxane-Grafted Carbon Nanotubes" Polymers 17, no. 3: 332. https://doi.org/10.3390/polym17030332
APA StyleWang, Y., Yang, R., Zhang, Z., Shan, Z., & Zhang, L. (2025). Thermal Insulating and Mechanically Strong Polyimide Aerogel Composites Reinforced by Polyhedral Oligomeric Silsesquioxane-Grafted Carbon Nanotubes. Polymers, 17(3), 332. https://doi.org/10.3390/polym17030332







