Modification of Polyamide Resins by Addition of Polyvinylpyrrolidone
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Measurements
3. Results and Discussion
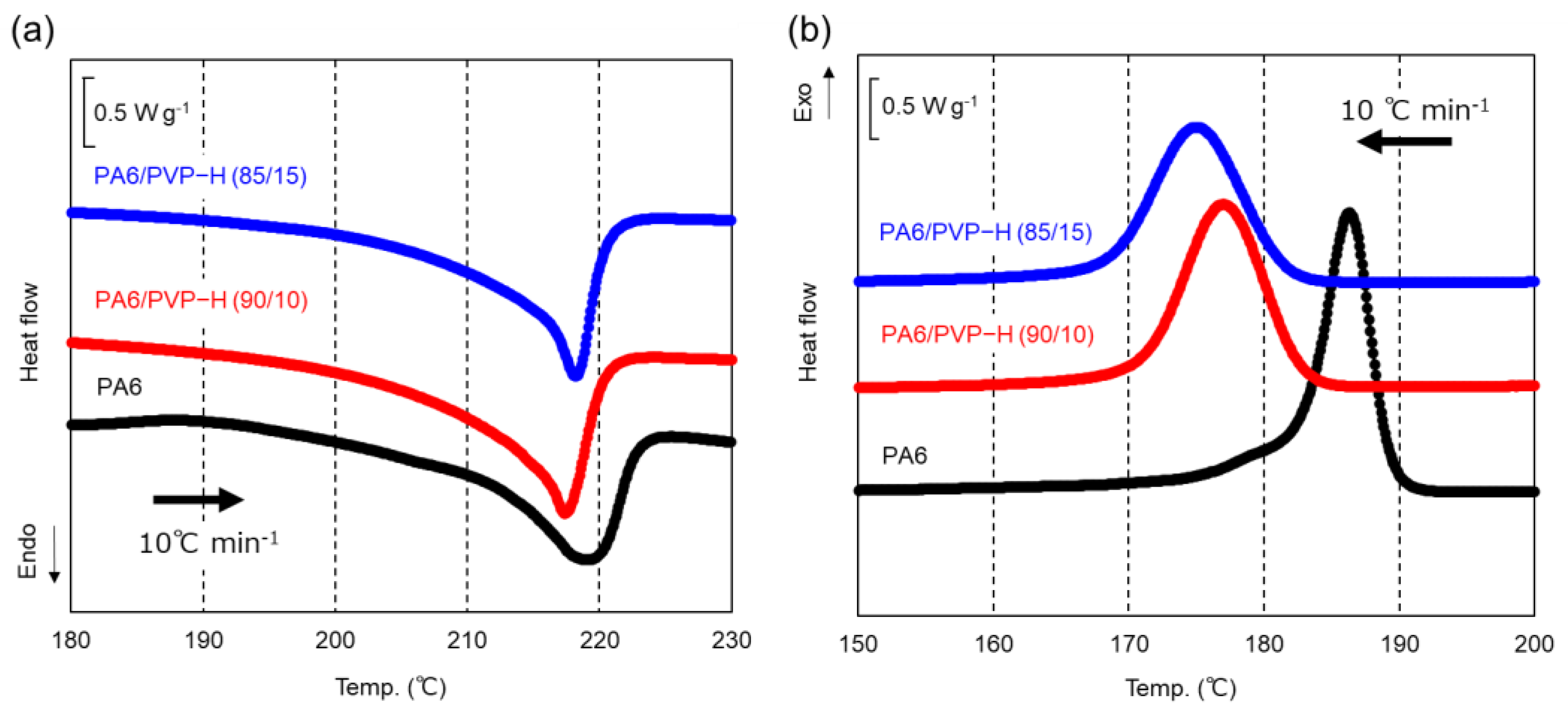
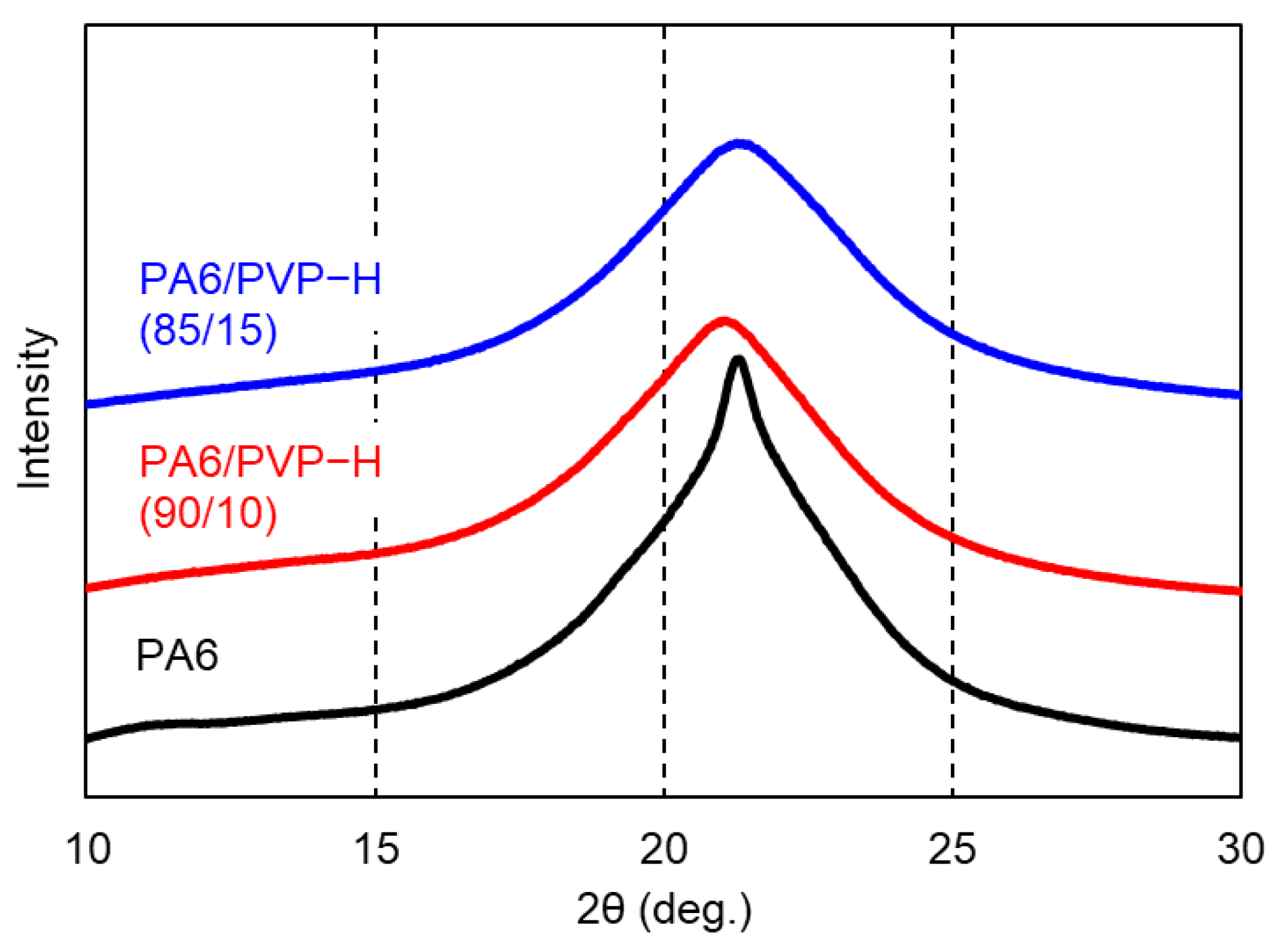
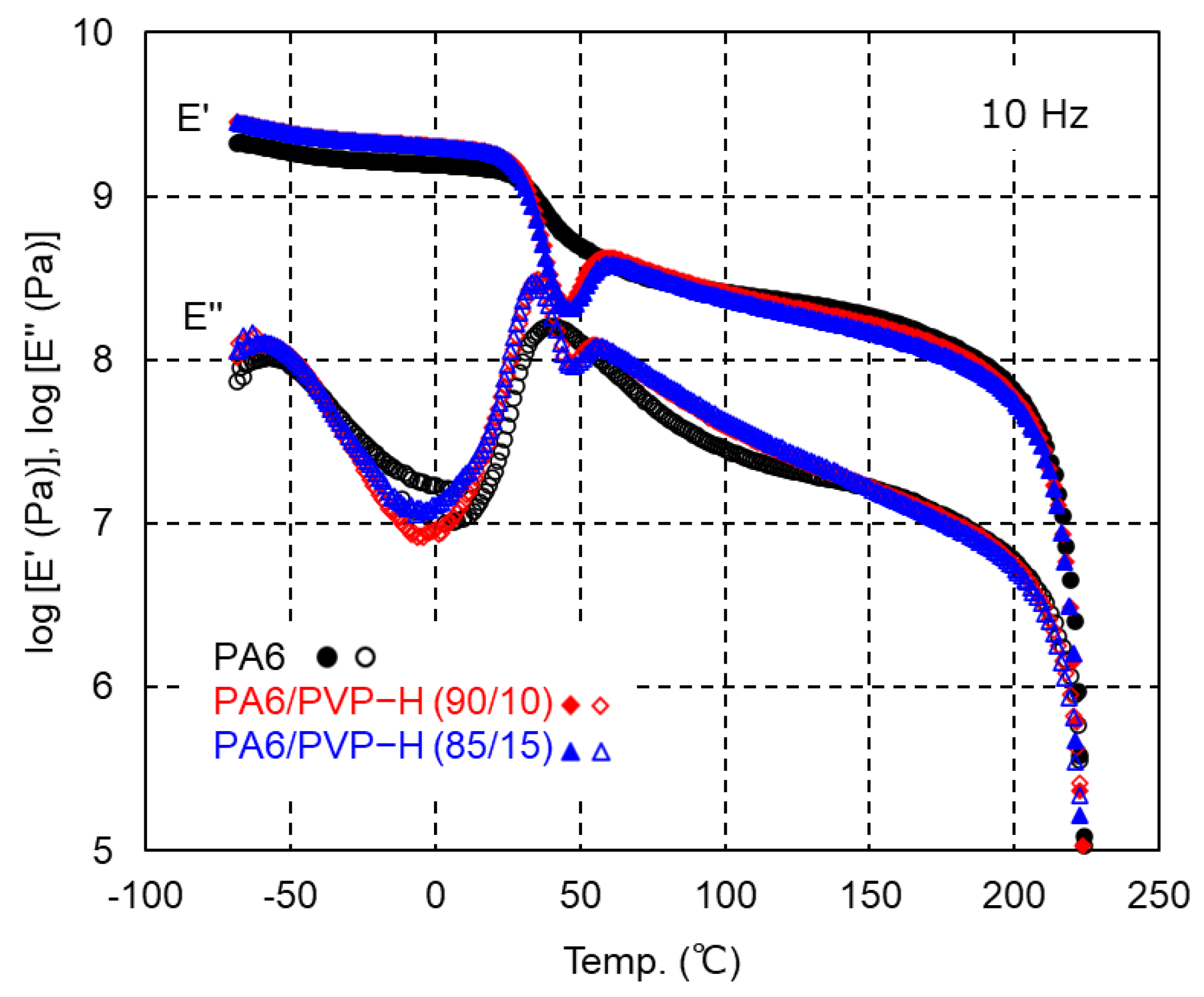
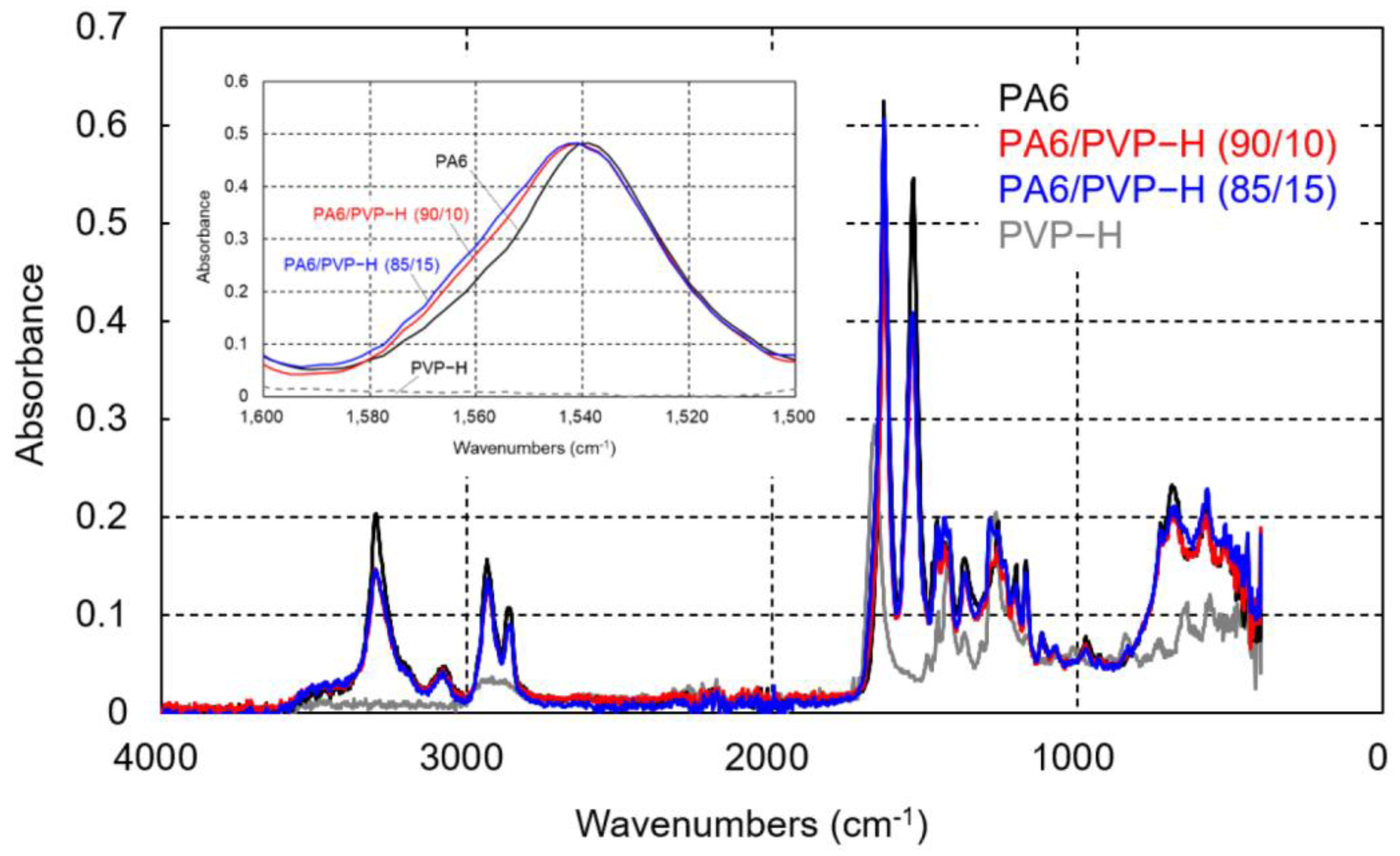
3.1. PA6/PVP-H Blends
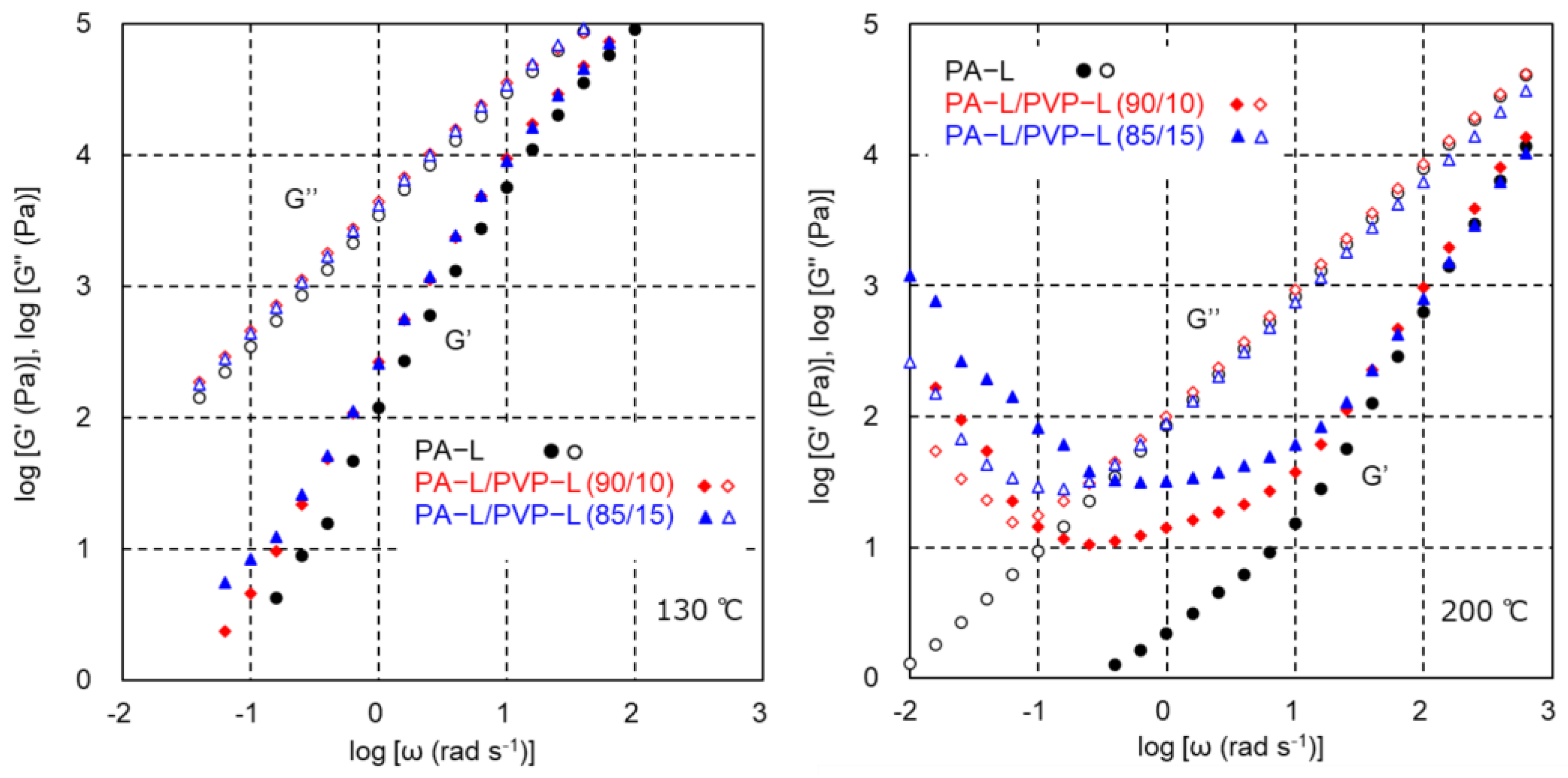
3.2. PA-L/PVP-L Blends
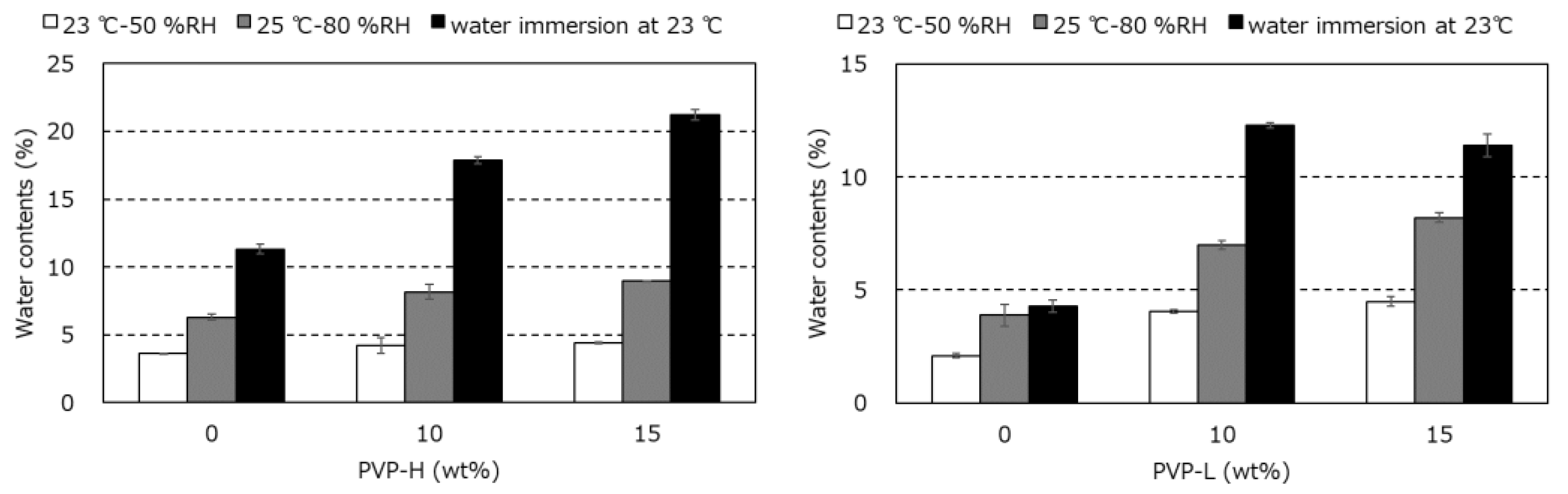
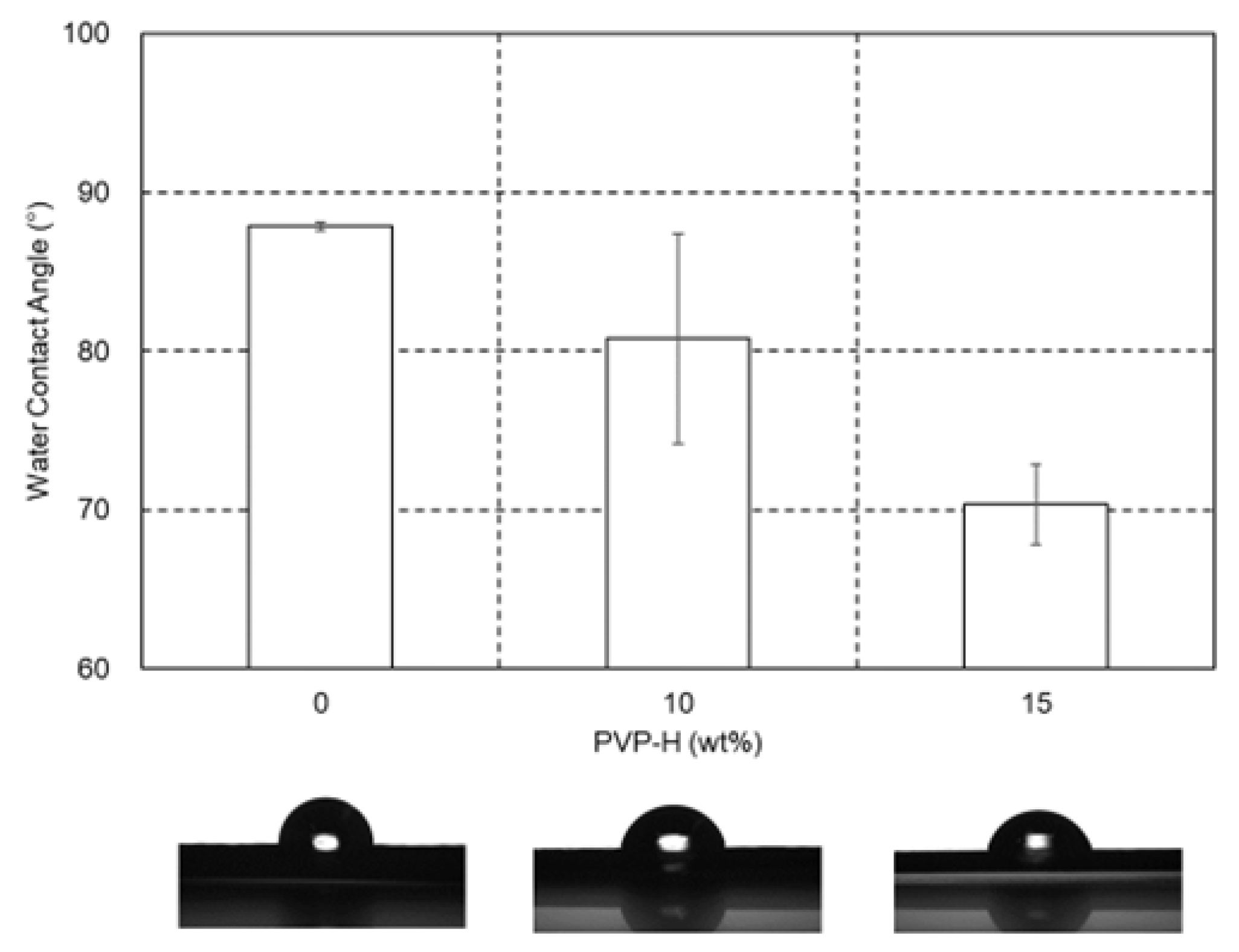
3.3. Water Contents and Surface Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Page, I.B. Polyamides as Engineering Thermoplastic Materials; Repra Review Reports; Report 121; Rapra Technology: Hyderabad, India, 2000; Volume 11, No. 1. [Google Scholar]
- Deopure, B.L.; Alagirusamy, R.; Joshi, M.; Gupta, B. Polyesters and Polyamides; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Gong, Y.; Yang, G. Manufacturing and physical properties of all-polyamide composites. J. Mater. Sci. 2009, 44, 4639–4644. [Google Scholar] [CrossRef]
- Ramazani, S.; Morshed, M.; Ghane, M. Effect of service temperature on structure and mechanical properties of polyamide 6 & 66 tyre cords. J. Polym. Res. 2011, 18, 781–792. [Google Scholar]
- Ma, Y.; Zhou, T.; Su, G.; Li, Y.; Zhang, A. Understanding the crystallization behavior of polyamide 6/polyamide 66 alloys from the perspective of hydrogen bonds: Projection moving-window 2D correlation FTIR spectroscopy and the enthalpy. RSC Adv. 2016, 6, 87405–87415. [Google Scholar] [CrossRef]
- Liu, Y.; Peng, L.; Lin, J.; Zhou, Y.; Wang, D.; Han, C.C.; Ye, N.; Huang, X.; Dong, X. Nucleation retardation of polyamide 6,6 by tuning hydrogen bonding reorganization via nigrosine for high-speed injection molding applications. ACS Appl. Polym. Mater. 2023, 5, 4127–4135. [Google Scholar] [CrossRef]
- Yang, S.H.; Cha, S.H.; Kim, J.I.; Lee, M.H.; Kim, S.G. Change of thermal properties of polyamide-6,6/glass fiber composite by the addition of nigrosine. Polym. Bull. 2017, 74, 5083–5094. [Google Scholar] [CrossRef]
- Sakata, K.; Takeuchi, H.; Shimada, H.; Agari, Y. Influence of the nigrosine dye on the thermal behavior of polyamide 66. J. Appl. Polym. Sci. 2006, 101, 3270–3274. [Google Scholar] [CrossRef]
- Sato, Y.; Ito, A.; Maeda, S.; Yamaguchi, M. Structure and optical properties of transparent polyamide 6 containing lithium bromide. J. Polym. Sci. Part B Polym. Phys. Ed. 2018, 56, 1513–1520. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Takatani, R.; Sato, Y.; Maeda, S. Smart hot-melt adhesive from polyamide 6. SN Appl. Sci. 2020, 2, 1567. [Google Scholar] [CrossRef]
- Tominaga, Y.; Izumi, Y.; Kwak, G.H.; Asai, S.; Sumita, M. Effect of supercritical carbon dioxide processing on ionic association and conduction in a crystalline poly(ethylene oxide)−LiCF3SO3 complex. Macromolecules 2003, 36, 8766–8772. [Google Scholar] [CrossRef]
- Miyagawa, A.; Ayerdurai, V.; Nobukawa, S.; Yamaguchi, M. Viscoelastic properties of poly(methyl methacrylate) with high glass transition temperature by lithium salt addition. J. Polym. Sci. Part B Polym. Phys. Ed. 2016, 54, 2388–2394. [Google Scholar] [CrossRef]
- Hao, C.W.; Ma, Q.F.; Wang, Z.; Jian, J.X.; Lai, G.Q. Study on the thermal behaviors and the morphology in PVP/PA66 blends. Adv. Mater. Res. 2011, 335, 916–920. [Google Scholar] [CrossRef]
- Liu, Y.; Peng, L.; Lin, J.L.; Zhou, Y.; Wang, D.J.; Han, C.C.; Huang, X.B.; Dong, X. The crystallization behavior regulating nature of hydrogen bonds interaction on polyamide 6,6 by poly(vinyl pyrrolidone). Chin. J. Polym. Sci. 2023, 41, 394–404. [Google Scholar] [CrossRef]
- Yu, D.G.; Shen, X.X.; Branford-White, C.; White, K.; Zhu, L.M.; Bligh, S.W.A. Oral fast-dissolving drug delivery membranes prepared from electrospun polyvinylpyrrolidone ultrafine fibers. Nanotechnology 2009, 20, 055104. [Google Scholar] [CrossRef] [PubMed]
- Amith, V.; Sridhar, R. Development of electrospinning system for synthesis of polyvinylpyrrolidone thin films for sensor applications. Mater. Today Proc. 2018, 5, 20920–20926. [Google Scholar] [CrossRef]
- Kim, W.T.; Park, D.C.; Yang, W.H.; Cho, C.H.; Choi, W.Y. Effects of electrospinning parameters on the microstructure of PVP/TiO2 nanofibers. Nanomaterials 2021, 11, 1616. [Google Scholar] [CrossRef]
- Rosiak, J.; Olejniczak, J.; Pekala, W. Fast reaction of irradiated polymers—I. Crosslinking and degradation of polyvinylpyrrolidone. Int. J. Radiat. Appl. Instrum. C Radiat. Phys. Chem. 1990, 36, 747–755. [Google Scholar] [CrossRef]
- Scheirs, J.; Bigger, S.W.; Then, E.T.H.; Billingham, N.C. The application of simultaneous chemiluminescence and thermal analysis for studying the glass transition and oxidative stability of poly 1N-vinyl-2-pyrrolidone. J. Polym. Sci. Part B Polym. Phys. Ed. 1993, 31, 287–297. [Google Scholar] [CrossRef]
- Herrera, M.; Matuschek, G.; Kettrup, A. Main products and kinetics of the thermal degradation of polyamides. Chemosphere 2001, 42, 601–607. [Google Scholar] [CrossRef]
- Pliquet, M.; Rapeaux, M.; Delange, F.; Bussiere, P.O.; Therias, S.; Gardette, J.L. Multiscale analysis of the thermal degradation of polyamide 6,6: Correlating chemical structure to mechanical properties. Polym. Degrad. Stab. 2021, 185, 109496. [Google Scholar] [CrossRef]
- Venoor, V.; Park, J.H.; Kazmer, D.O.; Sobkowicz, M.J. Understanding the effect of water in polyamides: A review. Polym. Rev. 2021, 61, 598–645. [Google Scholar] [CrossRef]
- Mei, X.; Do, Q.-V.; Narita, T.; Yamaguchi, M.; Yamaguchi, M. Modification of the rheological properties of a polyamide by adding a copolymer comprising an alpha-olefin and maleic anhydride. Molecules 2024, 29, 3730. [Google Scholar] [CrossRef] [PubMed]
- Fox, T.G. Influence of diluent and of copolymer composition on the glass temperature of a polymer system. Bull. Am. Phys. Soc. 1956, 1, 123. [Google Scholar]
- Ferry, J.D. Viscoelastic Properties of Polymers; Wiley: New York, NY, USA, 1980. [Google Scholar]
- Doi, M.; Edwards, S.F. The Theory of Polymer Dynamics; Oxford Science Publications Clarendon Press: Oxford, UK, 1986. [Google Scholar]
- Murthy, N.S.; Curran, S.A.; Aharoni, S.M.; Minor, H. Premelting crystalline relaxations and phase transitions in Nylon 6 and 6,6. Macromolecules 1991, 24, 3215–3220. [Google Scholar] [CrossRef]
- Vasanthan, N. Orientation and structure development in polyamide 6 fibers upon drawing. J. Polym. Sci. Part B Polym. Phys. Ed. 2003, 41, 2870–2877. [Google Scholar] [CrossRef]
- Tol, R.T.; Mathot, V.B.F.; Reynaers, H.; Goderis, B.; Groeninckx, G. Confined crystallization phenomena in immiscible polymer blends with dispersed micro-and nanometer sized PA6 droplets part 4: Polymorphous structure and (meta)-stability of PA6 crystals formed in different temperature regions. Polymer 2005, 46, 2966–2977. [Google Scholar] [CrossRef]
- Songsurang, K.; Mohd Edeerozey, A.M.; Miyagawa, A.; Phulkerd, P.; Nobukawa, S.; Yamaguchi, M. Optical anisotropy in solution-cast film of cellulose triacetate. Cellulose 2013, 20, 83–96. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Shu, W.; Kimura, T.; Vo, H.G.D.; Kida, T.; Mori, T.; Kitani, M.; Aridome, N.; Miyamoto, A. Anomalous post-processing dimensional change of injection-molded products composed of poly(lactic acid) and poly(vinyl alcohol). ACS Appl. Polym. Mater. 2023, 5, 2136–2143. [Google Scholar] [CrossRef]
- Tazima, K.; Kanemoto, T.; Ito, M. Structure and deformation of high molecular weight nylon 6 above the static melting point. Kobunshi Ronbunshu 2005, 62, 533–540. [Google Scholar]
- Vasanthan, N.; Salem, D.R. FTIR spectroscopic characterization of structural changes in polyamide-6 fibers during annealing and drawing. J. Polym. Sci. Part B Polym. Phys. Ed. 2001, 39, 536–547. [Google Scholar] [CrossRef]
- van Krevelen, D.W. Properties of Polymers; Elsevier: New York, NY, USA, 1976. [Google Scholar]
- Tomova, D.; Kressler, J.; Radusch, H.J. Phase behaviour in ternary polyamide 6/polyamide 66/elastomer blends. Polymer 2000, 41, 7773–7783. [Google Scholar] [CrossRef]
- Gebru, K.A.; Das, C. Effects of solubility parameter differences among PEG, PVP and CA on the preparation of ultrafiltration membranes: Impacts of solvents and additives on morphology, permeability and fouling performances. Chin. J. Chem. Eng. 2017, 25, 911. [Google Scholar] [CrossRef]
- Li, L.; Jiang, Z.; Fang, T. Predicting poly(vinyl pyrrolidone)’s solubility parameter and systematic investigation of the parameters of electrospinning with response surface methodology. J. Appl. Polym. Sci. 2014, 131, 40304. [Google Scholar] [CrossRef]
- Puffr, R.; Šebenda, J. On the structure and properties of polyamides. XXVII. The mechanism of water sorption in polyamides. J. Polym. Sci. Part C Polym. Symp. 1967, 16, 79–93. [Google Scholar] [CrossRef]
- Pai, C.C.; Jeng, R.J.; Grossman, S.J.; Huang, J.C. Effects of moisture on thermal and mechanical properties of nylon-6,6. Adv. Polym. Technol. 1989, 9, 157–163. [Google Scholar] [CrossRef]
- Langer, B.; Seidler, S.; Grellmann, W. Influence of temperature and moisture on toughness behaviour of polyamide. In Deformation and Fracture Behaviour of Polymers; Grellmann, W., Seidler, S., Eds.; Engineering Materials; Springer: Berlin/Heidelberg, Germany, 2001. [Google Scholar]
- Mohd Edeerozey, A.M.; Tsuji, M.; Nobukawa, S.; Yamaguchi, M. Effect of moisture on the orientation birefringence of cellulose esters. Polymers 2011, 3, 955–966. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikemoto, Y.; Yamaguchi, M. Modification of Polyamide Resins by Addition of Polyvinylpyrrolidone. Polymers 2025, 17, 360. https://doi.org/10.3390/polym17030360
Ikemoto Y, Yamaguchi M. Modification of Polyamide Resins by Addition of Polyvinylpyrrolidone. Polymers. 2025; 17(3):360. https://doi.org/10.3390/polym17030360
Chicago/Turabian StyleIkemoto, Yui, and Masayuki Yamaguchi. 2025. "Modification of Polyamide Resins by Addition of Polyvinylpyrrolidone" Polymers 17, no. 3: 360. https://doi.org/10.3390/polym17030360
APA StyleIkemoto, Y., & Yamaguchi, M. (2025). Modification of Polyamide Resins by Addition of Polyvinylpyrrolidone. Polymers, 17(3), 360. https://doi.org/10.3390/polym17030360







