Preparation and Properties of Epoxy Modified Acrylic Polymer
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Viscosity Reducer
2.3. Preparation of Heavy Oil Emulsion
2.4. Polymer Characterization
2.5. Thermal Properties’ Analysis
2.6. Evaluation of the Viscosity Reduction Effect
3. Results and Discussion
3.1. Reaction Mechanism of Epoxy Acrylate Viscosity Reducer
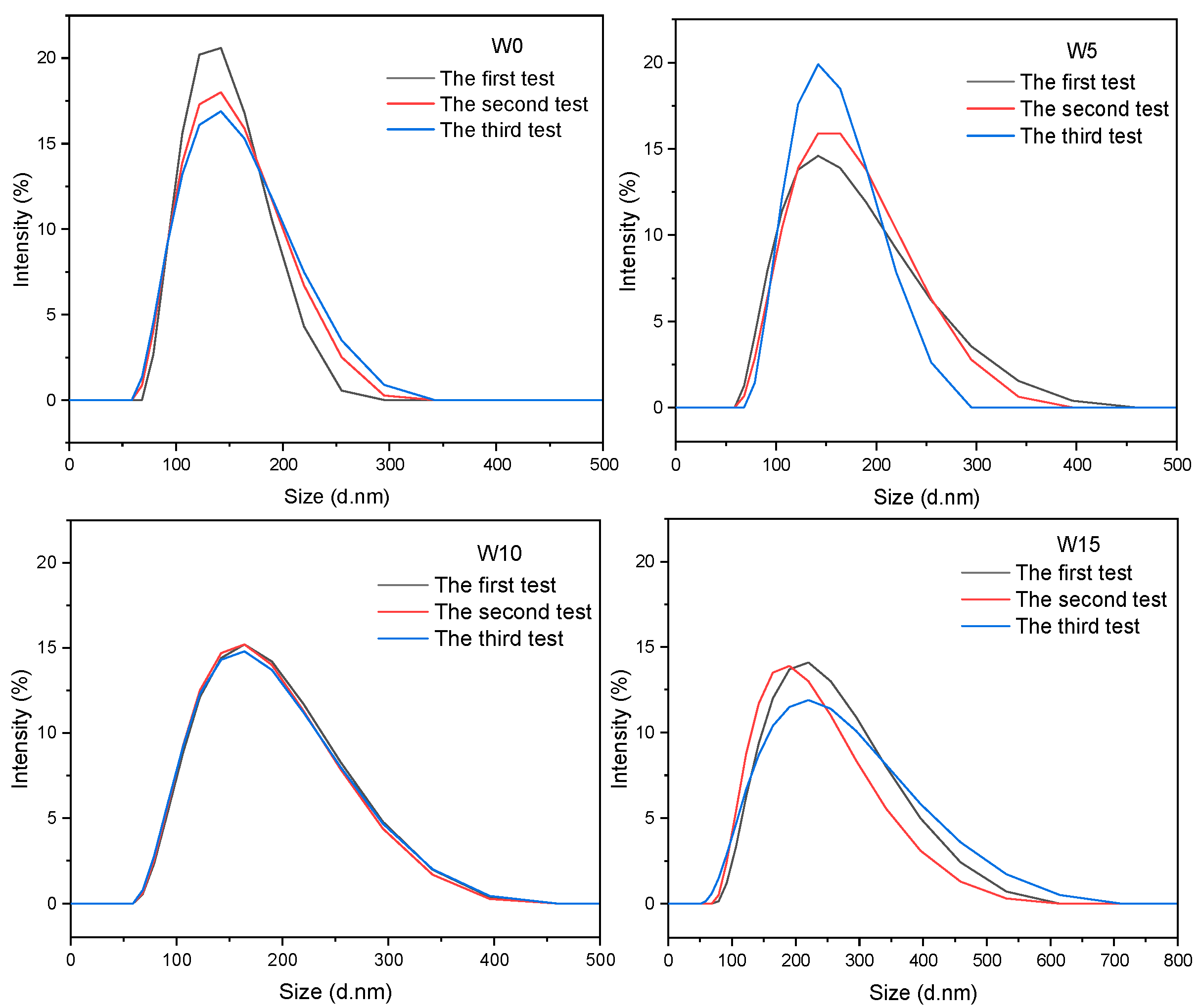
3.2. Effect of GMA Dosage on the Stability of Viscosity Reducer
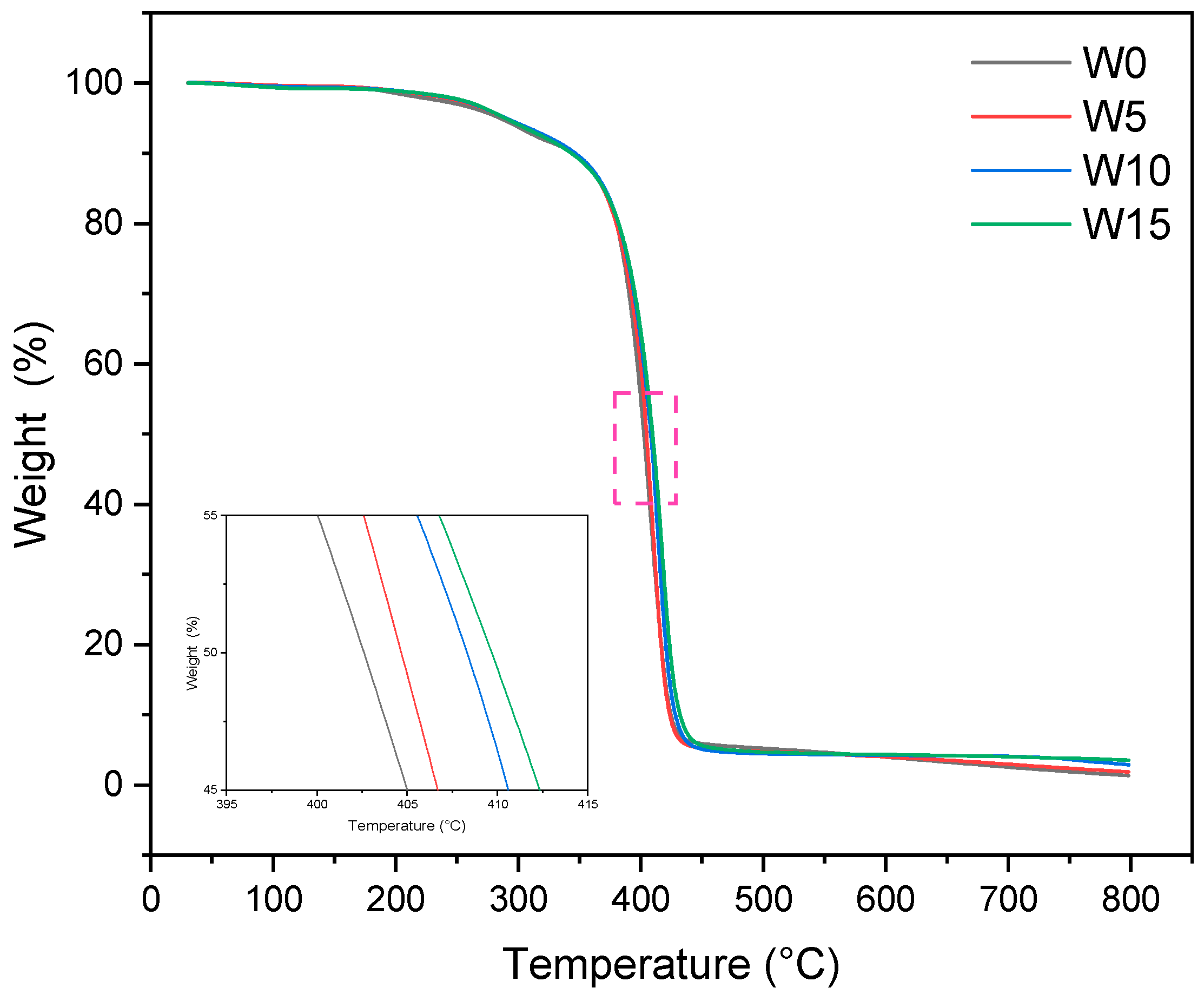
3.3. Thermal Stability Measurement
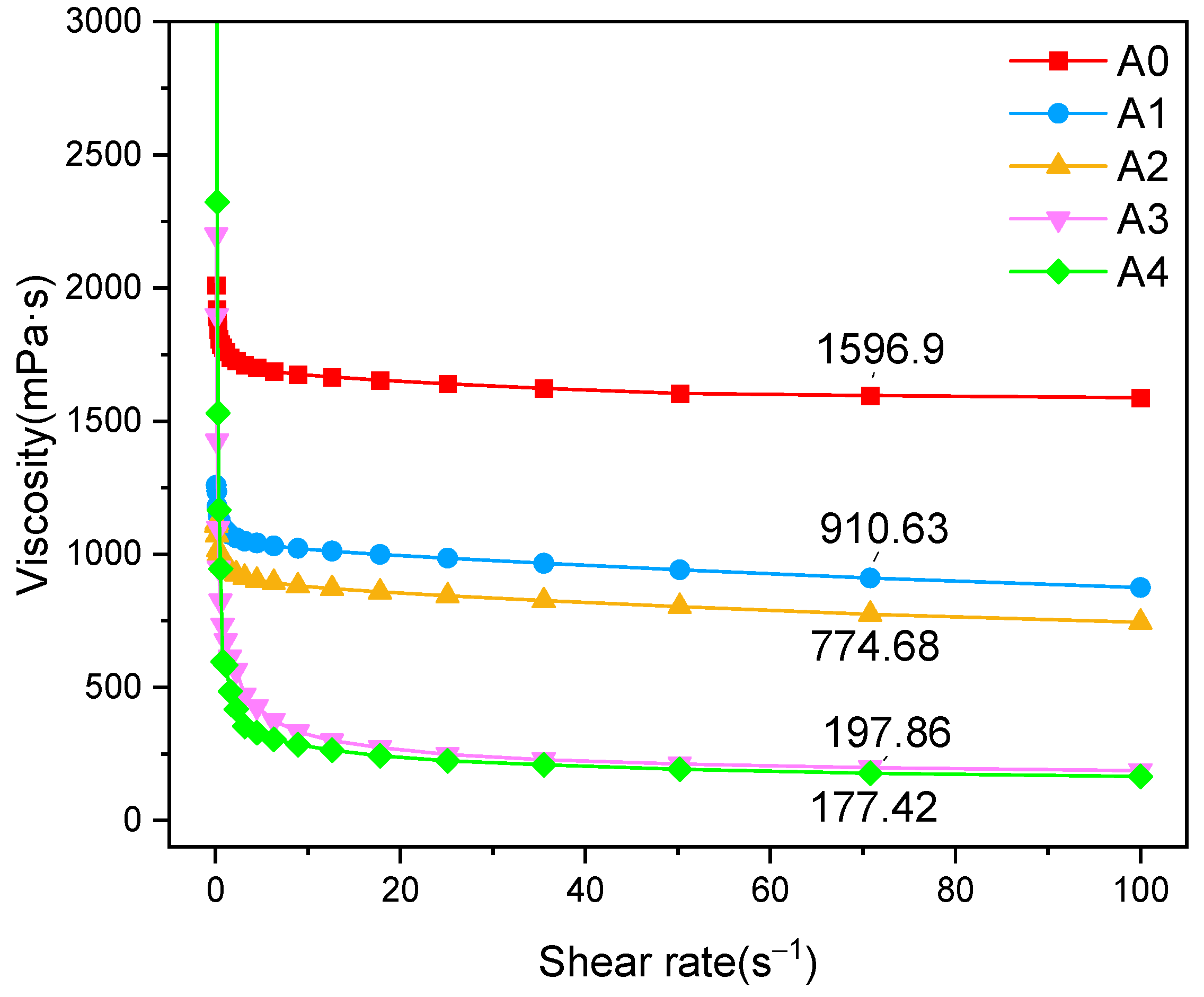
3.4. Heavy Oil Viscosity Reduction Performance of Polymer
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BA | butyl acrylate |
| EMA | ethyl methacrylate |
| AA | acrylic acid |
| N-MAM | N-hydroxymethylacrylamide |
| GMA | glycidyl methacrylate |
| FTIR | Fourier transform infrared spectroscopy |
| TG | thermogravimetric |
| O/W | oil-in-water |
| VRR | viscosity reduction rate |
References
- Shah, A.; Fishwick, R.; Wood, J.; Leeke, G.; Rigby, S.; Greaves, M. A review of novel techniques for heavy oil and bitumen extraction and upgrading. Energy Environ. Sci. 2010, 3, 700–714. [Google Scholar] [CrossRef]
- Dong, X.; Liu, H.; Chen, Z.; Wu, K.; Lu, N.; Zhang, Q. Enhanced oil recovery techniques for heavy oil and oilsands reservoirs after steam injection. Appl. Energy 2019, 239, 1190–1211. [Google Scholar] [CrossRef]
- Wu, Z.; Huiqing, L.; Cao, P.; Yang, R. Experimental investigation on improved vertical sweep efficiency by steam-air injection for heavy oil reservoirs. Fuel 2021, 285, 119138. [Google Scholar] [CrossRef]
- Guo, K.; Li, H.; Yu, Z. In-Situ heavy and extra-heavy oil recovery: A review. Fuel 2016, 185, 886–902. [Google Scholar] [CrossRef]
- Kumar, S.; Mahto, V. Use of a Novel Surfactant to Prepare Oil-in-Water Emulsion of an Indian Heavy Crude Oil for Pipeline Transportation. Energy Fuels 2017, 31, 12010–12020. [Google Scholar] [CrossRef]
- Mironova, M.V.; Ilyin, S.O. Effect of silica and clay minerals on rheology of heavy crude oil emulsions. Fuel 2018, 232, 290–298. [Google Scholar] [CrossRef]
- Yu, L.; Dong, M.; Ding, B.; Yuan, Y. Emulsification of heavy crude oil in brine and its plugging performance in porous media. Chem. Eng. Sci. 2018, 178, 335–347. [Google Scholar] [CrossRef]
- Kunieda, M.; Nakaoka, K.; Liang, Y.; Miranda, C.R.; Ueda, A.; Takahashi, S.; Okabe, H.; Matsuoka, T. Self-accumulation of aromatics at the oil−water interface through weak hydrogen bonding. J. Am. Chem. Soc. 2010, 132, 18281–18286. [Google Scholar] [CrossRef]
- Shigemoto, N.; Al-Maamari, R.S.; Jibril, B.Y.; Hirayama, A.; Sueyoshi, M. Effect of water content and surfactant type on viscosity and stability of emulsified heavy Mukhaizna crude oil. Energy Fuels 2007, 21, 1014–1018. [Google Scholar] [CrossRef]
- Lim, J.; Wong, S.; Law, M.; Samyudia, Y.; Dol, S. A review on the effects of emulsions on flow behaviours and common factors affecting the stability of emulsions. J. Appl. Sci. 2015, 15, 167–172. [Google Scholar] [CrossRef]
- Malkin, A.Y.; Zuev, K.V.; Arinina, M.P.; Kulichikhin, V.G. Modifying the Viscosity of Heavy Crude Oil Using Surfactants and Polymer Additives. Energy Fuels 2018, 32, 11991–11999. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Wang, Q.; Han, Y.; Wang, M.; Tan, Y. Heavy Oil Viscosity Reduction Performance of Novel Water-Soluble Terpolymers. Energy Fuels 2019, 33, 9736–9746. [Google Scholar] [CrossRef]
- Lv, W.; Bazin, B.; Ma, D.; Liu, Q.; Han, D.; Wu, K. Static and dynamic adsorption of anionic and amphoteric surfactants with and without the presence of alkali. J. Pet. Sci. Eng. 2011, 77, 209–218. [Google Scholar] [CrossRef]
- Wang, X.; Wang, F.; Taleb, M.A.M.; Wen, Z.; Chen, X. A Review of the Seepage Mechanisms of Heavy Oil Emulsions during Chemical Flooding. Energies 2022, 15, 8397. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y.; Gao, W.; Chen, C. Experimental investigation on transport property and emulsification mechanism of polymeric surfactants in porous media. J. Pet. Sci. Eng. 2020, 186, 106687. [Google Scholar] [CrossRef]
- Wang, H.; Wei, B.; Hou, J.; Liu, Y.; Du, Q. Heavy oil recovery in blind-ends during chemical flooding: Pore scale study using microfluidic experiments. J. Mol. Liq. 2022, 368, 120724. [Google Scholar] [CrossRef]
- Zhang, F.; Lei, Q.; Liu, G.; Liao, G.; Xu, X.; Meng, W. Synthesis and laboratory evaluation ofiso-tridecyloxypolyethylene glycol acrylate copolymers as potential viscosity reducers for heavy oil. Energy Sci. Eng. 2020, 8, 3303–3313. [Google Scholar] [CrossRef]
- Liu, Z.; Cheng, H.; Li, Y.; Li, Y.; Chen, X.; Zhuang, Y. Experimental Investigation of Synergy of Components in Surfactant/Polymer Flooding Using Three-Dimensional Core Model. Transp. Porous Media 2018, 126, 317–335. [Google Scholar] [CrossRef]
- Li, W.; Dai, C.; Ouyang, J.; Aziz, H.; Tao, J.; He, X.; Zhao, G. Adsorption and retention behaviors of heterogeneous combination flooding system composed of dispersed particle gel and surfactant. Colloids Surf. A Physicochem. Eng. Asp. 2018, 538, 250–261. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, T.; Lin, X.; Wang, X.; Su, L. Chemicals loss and the effect on formation damage in reservoirs with ASP flooding enhanced oil recovery. J. Nat. Gas Sci. Eng. 2016, 33, 1381–1389. [Google Scholar] [CrossRef]
- Wang, J.; Yuan, S.; Shi, F.; Jia, X. Experimental study of chemical concentration variation of ASP flooding. Sci. China Ser. E Technol. Sci. 2009, 52, 1882–1890. [Google Scholar] [CrossRef]
- Xiao, H.M.; Sun, L.H.; Kou, H.H. Mass Transfer Mechanisms of ASP Flooding in Porous Media. Adv. Mater. Res. 2012, 550–553, 2738–2744. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, J.; Lv, J.; Huang, B.; Yang, G.; Tang, E.; Chen, M.; Xu, C.; Ge, Y. Development of Novel Star-Like Branched-Chain Acrylamide (AM)-Sodium Styrene Sulfonate (SSS) Copolymers for Heavy Oil Emulsion Viscosity Reduction and Its Potential Application in Enhanced Oil Recovery. ACS Omega 2023, 9, 422–436. [Google Scholar] [CrossRef]
- Li, P.; Zhang, F.; Gong, Y.; Tang, J.; Zhang, C.; Sun, Z.; Liu, G.; Li, X. Synthesis and properties of functional polymer for heavy oil viscosity reduction. J. Mol. Liq. 2021, 330, 115635. [Google Scholar] [CrossRef]
- Tian, S.; Gao, W.; Liu, Y.; Kang, W. Study on the stability of heavy crude oil-in-water emulsions stabilized by two different hydrophobic amphiphilic polymers. Colloids Surf. A Physicochem. Eng. Asp. 2019, 572, 299–306. [Google Scholar] [CrossRef]
- Li, J.; Wang, Q.; Liu, Y.; Wang, M.; Tan, Y. Long Branched-Chain Amphiphilic Copolymers: Synthesis, Properties, and Application in Heavy Oil Recovery. Energy Fuels 2018, 32, 7002–7010. [Google Scholar] [CrossRef]
- Fei, D.; Guo, J.; Xiong, R.; Zhang, X.; Kang, C.; Kiyingi, W. Preparation and Performance Evaluation of Amphiphilic Polymers for Enhanced Heavy Oil Recovery. Polymers 2023, 15, 4606. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, Y.; Wang, Q.; Han, Y.; Yan, Z.; Chen, H.; Tan, Y. Fabricating a heavy oil viscosity reducer with weak interaction effect: Synthesis and viscosity reduction mechanism. Colloid Interface Sci. Commun. 2021, 42, 100426. [Google Scholar] [CrossRef]
- Miadonye, A.; Evans, L. The Solubility of Asphaltenes in Different Hydrocarbon Liquids. Pet. Sci. Technol. 2010, 28, 1407–1414. [Google Scholar] [CrossRef]
- Castro, L.V.; Flores, E.A.; Vazquez, F. Terpolymers as Flow Improvers for Mexican Crude Oils. Energy Fuels 2011, 25, 539–544. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Z.; Zhao, S.; Hong, K.H.; Zhang, M.-Y.; Song, L.; Yu, F.; Luo, G.; He, Y.-P. Pyromellitic-based low molecular weight gelators and computational studies of intermolecular interactions: A potential additive for lubricant. Langmuir 2021, 37, 2954–2962. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, K.; Wang, C.; Zhao, S.; Zhang, Z.; Yu, F.; He, Y.-P. Enhanced oil recovery: QM/MM based descriptors for anionic surfactant salt-resistance. Colloids Surf. A Physicochem. Eng. Asp. 2022, 641, 128422. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, W.; Liu, N.; Hu, D.; Yu, F.; He, Y.-P. Methionine-derived Organogels as lubricant additives enhance the continuity of the oil film through dynamic self-healing assembly. Langmuir 2022, 38, 11492–11501. [Google Scholar] [CrossRef]
- Zhang, W.; Luo, F.; Gao, Z.; Chi, H.; Wang, J.; Yu, F.; He, Y.-P. Study of Interfacial Properties of Anionic–Nonionic Surfactants Based on Succinic Acid Derivatives via Molecular Dynamics Simulations and the IGMH Method. Colloids Interfaces 2024, 8, 41. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, Z.; Guo, J.; Wang, H.; Ma, Y.; Kong, X.; Fan, H.; Yu, Q. Layer-by-layer assembly of polystyrene/Ag for a highly reproducible SERS substrate and its use for the detection of food contaminants. Polymers 2021, 13, 3270. [Google Scholar] [CrossRef]
- Geng, R.; Wang, J.; Zhang, Z.; Dong, Q.; Wu, F.; Chen, S.; Su, T.; Qi, X. Adsorption of antibiotics by polydopamine-modified salecan hydrogel: Performance, kinetics and mechanism studies. Chem. Eng. J. 2023, 454, 140446. [Google Scholar] [CrossRef]
- Yang, K.; Zhao, Y.; Liu, X. Enhanced Breakdown Strength of Epoxy Composites by Constructing Dual-interface Charge Barriers at the Micron Filler/Epoxy Matrix Interface. Compos. Part B Eng. 2024, 111602. [Google Scholar] [CrossRef]
- Zuo, R.; Kong, X.; Wang, Y.; He, Y.; Deng, S.; Zhuang, X.; Qiu, D. Isolation and characterization of natural nano starch from amaranth starch. Int. J. Biol. Macromol. 2024, 260, 129525. [Google Scholar] [CrossRef]
- Qi, X.; Ge, X.; Chen, X.; Cai, E.; Xiang, Y.; Xu, H.; Li, Y.; Lan, Y.; Shi, Y.; Deng, H.; et al. An Immunoregulation Hydrogel with Controlled Hyperthermia-Augmented Oxygenation and ROS Scavenging for Treating Diabetic Foot Ulcers. Adv. Funct. Mater. 2024, 34, 2400489. [Google Scholar] [CrossRef]
- Ma, J.; Zhou, S.; Wang, Y.; Chen, R.; Wang, S.; Zhang, F.; He, Y.-P. Preparation and properties of vinyltriethoxysilane-modified waterborne acrylate resins. Polymer 2024, 314, 127781. [Google Scholar] [CrossRef]




| Component | Amount (wt%) a | Component | Amount (wt%) a |
|---|---|---|---|
| EMA | 15 | SDS, OP-10 | 1.8 |
| BA | 13.5 | APS | 0.6 |
| AA | 0.6 | SBS | 0.19 |
| N-MAM | 0.9 | Deionized water | 67.41 |
| Sample | Appearance of Latex | Z-Average Particle Size (d. nm) | Polydispersity Index (PDI) | Zeta Potential (mV) |
|---|---|---|---|---|
| W0 | milky white, intense blue light | 132 | 0.066 | −43 |
| W5 | milky white, intense blue light | 142 | 0.09 | −42 |
| W10 | milky white, intense blue light | 150 | 0.119 | −42 |
| W15 | milky white, low blue light | 187 | 0.173 | −40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, S.; Ma, J.; Yu, J.-W.; Gao, Z.; Li, F.; Zhang, F.; He, Y.-P. Preparation and Properties of Epoxy Modified Acrylic Polymer. Polymers 2025, 17, 380. https://doi.org/10.3390/polym17030380
Zhou S, Ma J, Yu J-W, Gao Z, Li F, Zhang F, He Y-P. Preparation and Properties of Epoxy Modified Acrylic Polymer. Polymers. 2025; 17(3):380. https://doi.org/10.3390/polym17030380
Chicago/Turabian StyleZhou, Shiyan, Jinmei Ma, Jun-Wen Yu, Zhigang Gao, Fei Li, Fenghua Zhang, and Yu-Peng He. 2025. "Preparation and Properties of Epoxy Modified Acrylic Polymer" Polymers 17, no. 3: 380. https://doi.org/10.3390/polym17030380
APA StyleZhou, S., Ma, J., Yu, J.-W., Gao, Z., Li, F., Zhang, F., & He, Y.-P. (2025). Preparation and Properties of Epoxy Modified Acrylic Polymer. Polymers, 17(3), 380. https://doi.org/10.3390/polym17030380






