Surface Modification of Intumescent Flame Retardant and Its Application in Polypropylene with Excellent Fire Performance and Water Resistance
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
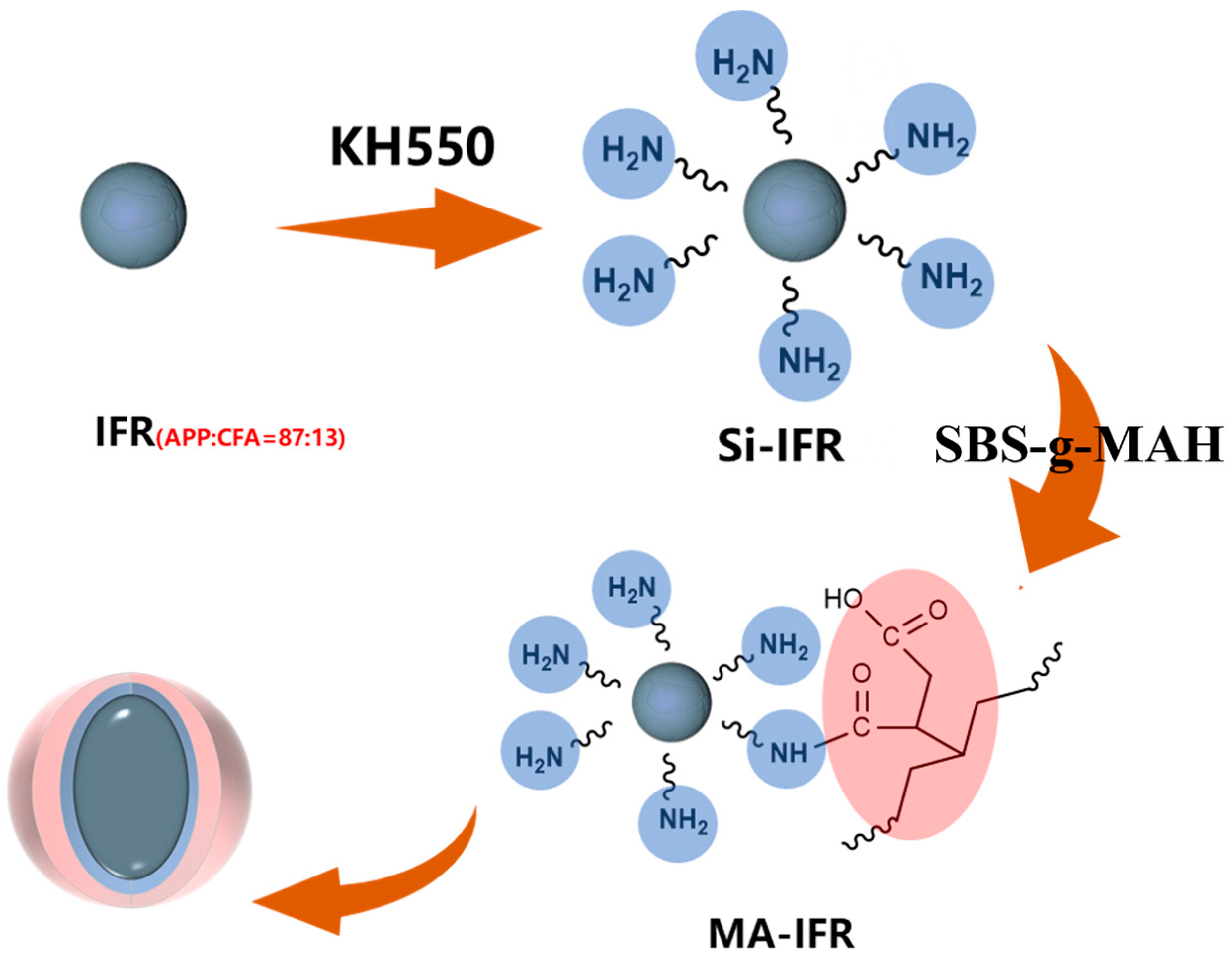
2.2. Preparation of Si-IFR
2.3. Preparation of MA-IFR
2.4. Preparation of MA/IFR
2.5. Preparation of PP Composites
2.6. Characterization
3. Results and Discussion
3.1. Characterizations of IFR, Si-IFR, MA-IFR, and MA/IFR
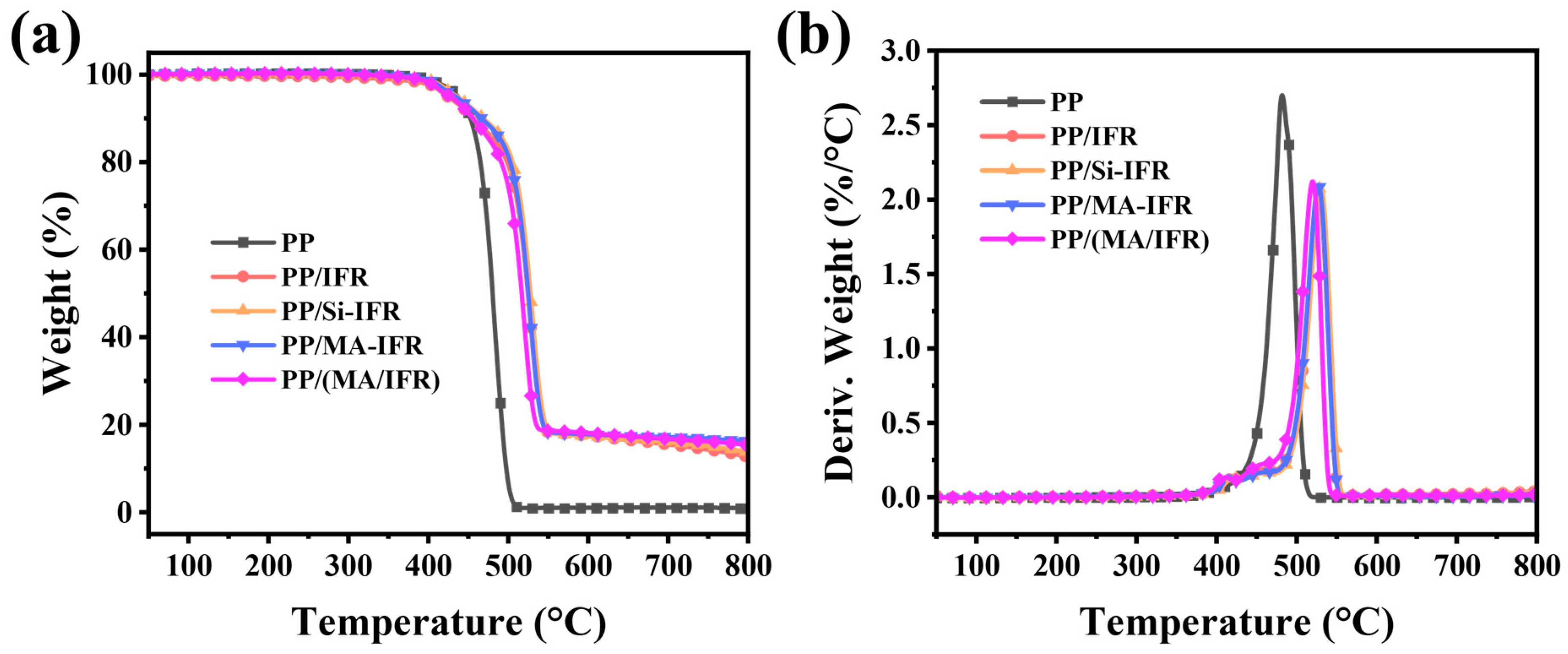
3.2. Thermal Stability of PP and PP Composites
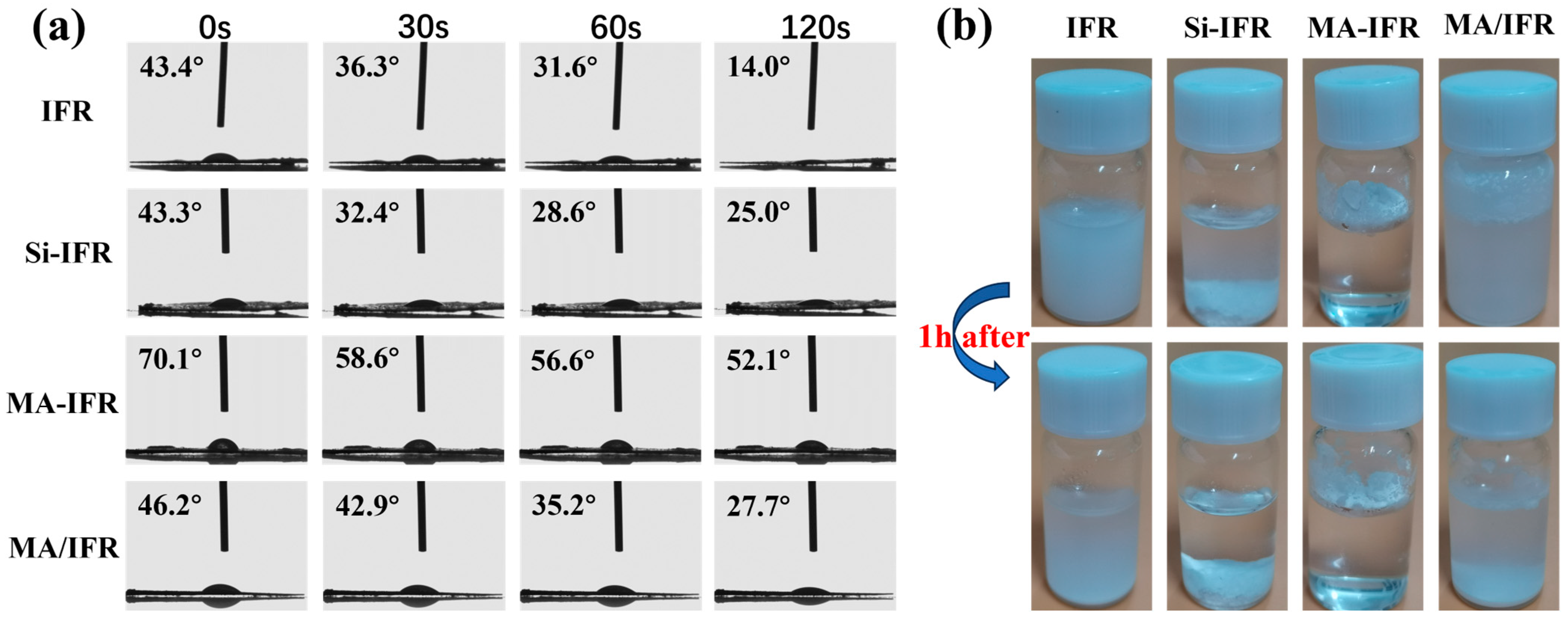
3.3. Water Resistance and Flame Retardancy of PP and PP Composites
3.4. Flame Performance of PP and PP Composites
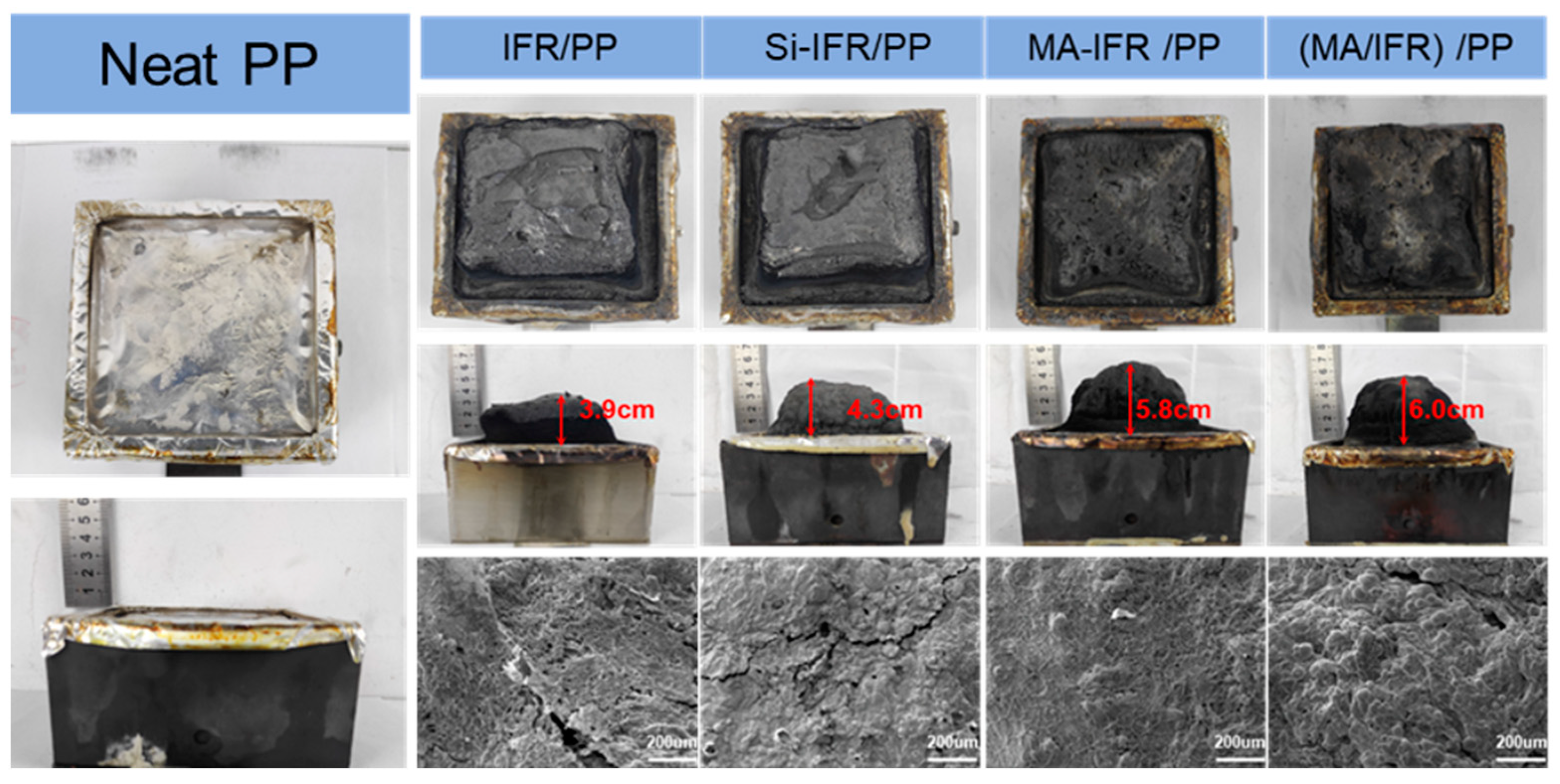
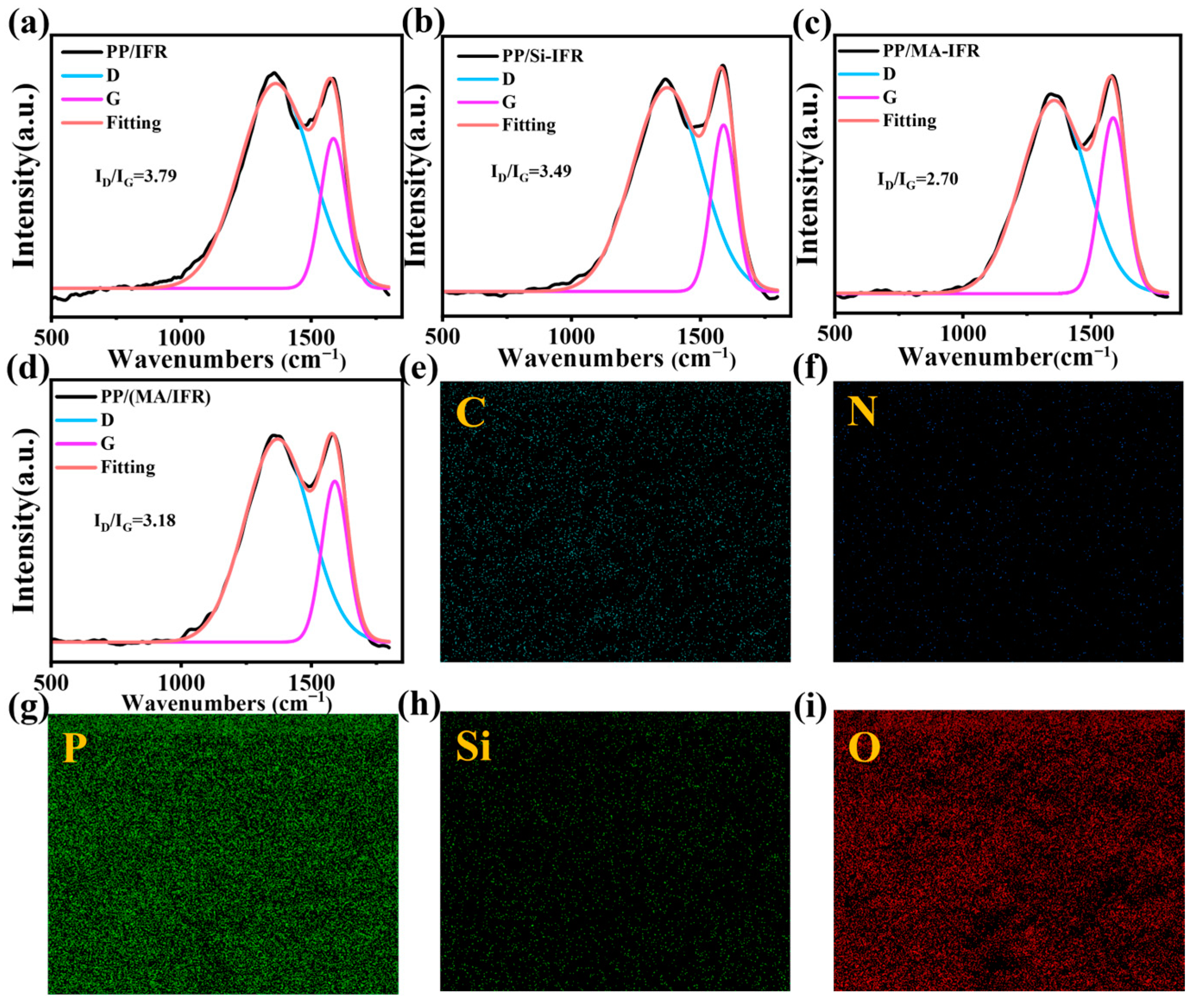
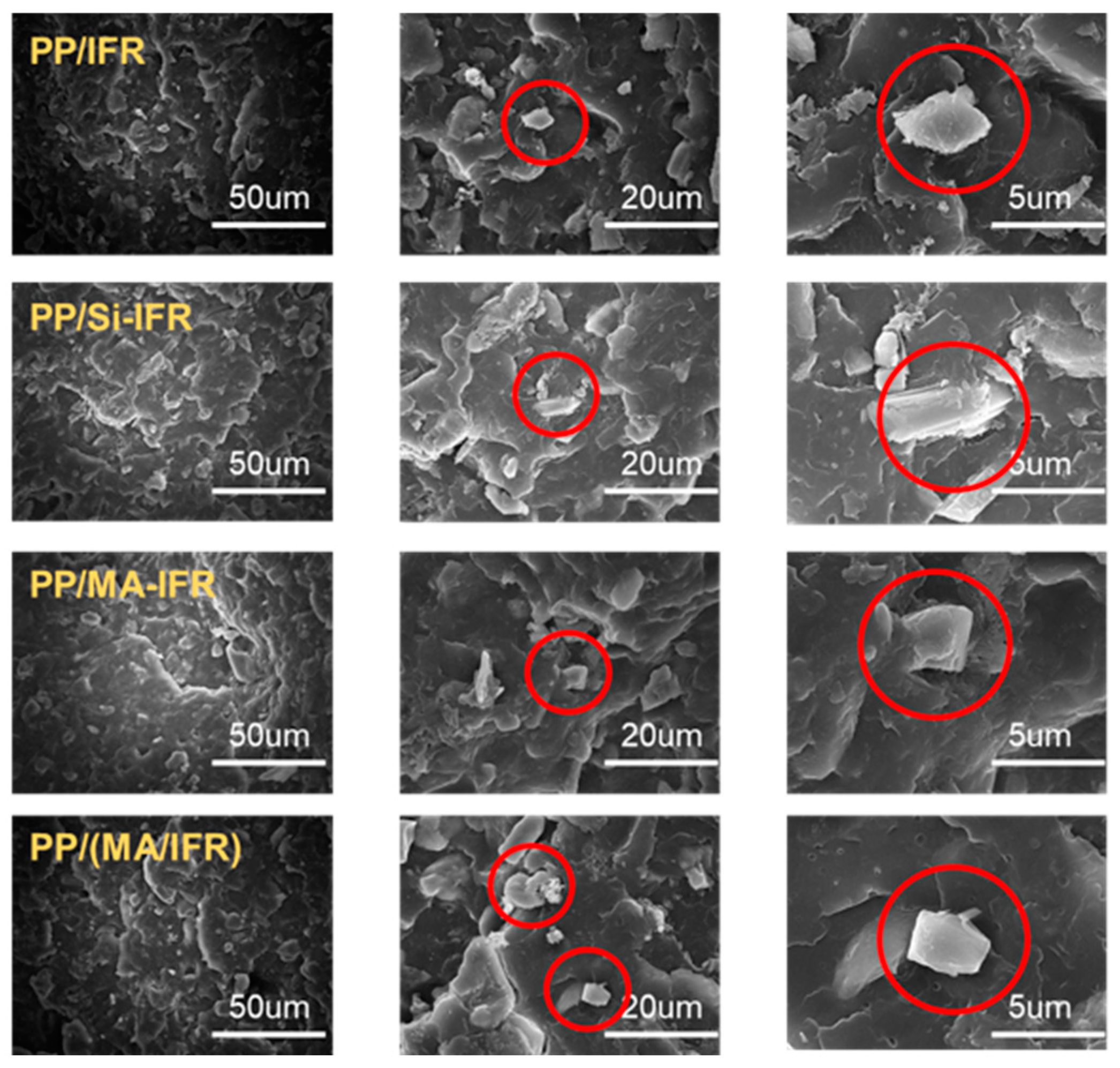
3.5. Char Residue Analysis
3.6. Flame Retardant Mechanism
3.7. Mechanical Properties of PP and PP Composites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Quan, Y.; Shen, R.; Schweizer, C.; Parajuli, P.; Zhang, Z.; Kulatilaka, W.; Wang, Q. Synergistic effects of zeolitic imidazolate frameworks (ZIFs) with different transition metals on intumescent flame-retarded polypropylene composites: A comparative study. J. Mater. Sci. Technol. 2023, 155, 102–110. [Google Scholar] [CrossRef]
- Zhu, C.; He, M.; Liu, Y.; Cui, J.; Tai, Q.; Song, L.; Hu, Y. Synthesis and application of a mono-component intumescent flame retardant for polypropylene. Polym. Degrad. Stab. 2018, 151, 144–151. [Google Scholar] [CrossRef]
- Zhao, W.; Kundu, C.K.; Li, Z.; Li, X.; Zhang, Z. Flame retardant treatments for polypropylene: Strategies and recent advances. Compos. Part A Appl. Sci. Manuf. 2021, 145, 106382. [Google Scholar] [CrossRef]
- Liu, Z.; Xing, S.; Li, Y.; Sun, J.; Li, H.; Gu, X.; Zhang, S. Surface modification of zinc oxide and its application in polypropylene with excellent fire performance and ultra-violet resistance. J. Colloid Interface Sci. 2024, 661, 307–316. [Google Scholar] [CrossRef]
- Deng, M.; Zhang, Z.; Sun, J.; Liu, X.; Li, H.; Gu, X.; Zhang, S. Improving the flame retardancy and water resistance of polypropylene by introducing microcapsule flame retardant system and modified zinc oxide. Polym. Degrad. Stab. 2024, 221, 110668. [Google Scholar] [CrossRef]
- Huang, Z.; Li, S.; Tsai, L.-C.; Jiang, T.; Ma, N.; Tsai, F.-C. Flame retardant polypropylene with a single molecule intumescent flame retardant based on chitosan. Mater. Today Commun. 2022, 33, 104689. [Google Scholar] [CrossRef]
- Gramatica, P.; Cassani, S.; Sangion, A. Are some “safer alternatives” hazardous as PBTs? The case study of new flame retardants. J. Hazard. Mater. 2016, 306, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Mapossa, A.B.; Anjos, E.G.R.D.; Sundararaj, U. Boosting Flame Retardancy of Polypropylene/Calcium Carbonate Composites with Inorganic Flame Retardants. Materials 2024, 17, 4553. [Google Scholar] [CrossRef]
- Tu, Z.; Ou, H.; Ran, Y.; Xue, H.; Zhu, F. Chitosan-based biopolyelectrolyte complexes intercalated montmorillonite: A strategy for green flame retardant and mechanical reinforcement of polypropylene composites. Int. J. Biol. Macromol. 2024, 277, 134316. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Xiao, K.; Tian, T.; Liao, W. Thermochemical Foaming of Mixed Single-Use Plastics Toward Flame Retardant Potential Foams. ACS Sustain. Chem. Eng. 2024, 12, 1151–1160. [Google Scholar] [CrossRef]
- Xie, H.; Lai, X.; Li, H.; Zeng, X. Synthesis of a novel macromolecular charring agent with free-radical quenching capability and its synergism in flame retardant polypropylene. Polym. Degrad. Stab. 2016, 130, 68–77. [Google Scholar] [CrossRef]
- Li, X.; Zhao, Z.; Wang, Y.; Yan, H.; Zhang, X.; Xu, B. Highly efficient flame retardant, flexible, and strong adhesive intumescent coating on polypropylene using hyperbranched polyamide. Chem. Eng. J. 2017, 324, 237–250. [Google Scholar] [CrossRef]
- Yan, H.; Zhao, Z.; Ge, W.; Zhang, N.; Jin, Q. A novel hyperbranched polyurea as charring agent for simultaneously improving flame retardancy and mechanical properties of ammonium polyphosphate/polypropylene composites. Ind. Eng. Chem. Res. 2017, 56, 8408–8415. [Google Scholar] [CrossRef]
- Sun, Q.; Ding, Y.; Stoliarov, S.I.; Sun, J.; Fontaine, G.; Bourbigot, S. Development of a pyrolysis model for an intumescent flame retardant system: Poly(lactic acid) blended with melamine and ammonium polyphosphate. Compos. Part B Eng. 2020, 194, 108055. [Google Scholar] [CrossRef]
- Lecouvet, B.; Sclavons, M.; Bailly, C.; Bourbigot, S. A comprehensive study of the synergistic flame retardant mechanisms of halloysite in intumescent polypropylene. Polym. Degrad. Stab. 2013, 98, 2268–2281. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, J.; Yan, H.; Guo, X.; Sun, Q.; Guo, R. A novel organic-inorganic hybrid K-HBPE@APP performing excellent flame retardancy and smoke suppression for polypropylene. J. Hazard. Mater. 2019, 373, 856–865. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Liu, P.; Zhang, S.; Ding, Y.; Wang, F.; Gao, C.; Yang, M. Preparation and characterization of cyclodextrin microencapsulated ammonium polyphosphate and its application in flame retardant polypropylene. J. Appl. Polym. Sci. 2020, 137, 49001. [Google Scholar] [CrossRef]
- Sun, Y.; Yuan, B.; Shang, S.; Zhang, H.; Shi, Y.; Yu, B.; Qi, C.; Dong, H.; Chen, X.; Yang, X. Surface modification of ammonium polyphosphate by supramolecular assembly for enhancing fire safety properties of polypropylene. Compos. Part B Eng. 2019, 181, 107588. [Google Scholar] [CrossRef]
- Shao, Z.-B.; Deng, C.; Tan, Y.; Chen, M.-J.; Chen, L.; Wang, Y.-Z. Flame retardation of polypropylene via a novel intumescent flame retardant: Ethylenediamine-modified ammonium polyphosphate. Polym. Degrad. Stab. 2013, 106, 88–96. [Google Scholar] [CrossRef]
- Yang, W.; Dong, X.; Nie, S.-B.; Yang, J.-N.; Zhang, X.-F.; Wu, X.-Q.; Fang, C.-Y.; Su, H.-L. Flame-retardant and thermal properties of highly efficient water-resistant intumescent flame-retardant polypropylene composites. J. Therm. Anal. Calorim. 2021, 147, 7323–7336. [Google Scholar] [CrossRef]
- Liu, J.-C.; Xu, M.-J.; Lai, T.; Li, B. Effect of Surface-Modified Ammonium Polyphosphate with KH550 and Silicon Resin on the Flame Retardancy, Water Resistance, Mechanical and Thermal Properties of Intumescent Flame Retardant Polypropylene. Ind. Eng. Chem. Res. 2015, 54, 9733–9741. [Google Scholar] [CrossRef]
- Tao, M.; Qian, L.; Wang, J.; Zhu, H.; Tang, W.; Chen, Y.; Xi, W.; Qiu, Y. From physical mixtures to block copolymer: Impose outstandingly toughening and flame retardant effect to polypropylene. Compos. Part B Eng. 2023, 253, 110538. [Google Scholar] [CrossRef]
- Tao, M.; Tang, W.; Qian, L.; Wang, J.; Xi, W.; Xu, B.; Qiu, Y. Toughening behavior and synergistic flame retardant effect of a ternary block copolymer with amorphous morphology and thermal softening property in polypropylene. Chem. Eng. J. 2023, 461, 141963. [Google Scholar] [CrossRef]
- Li, Y.; Chen, X.; Liu, Q.; Sun, J.; Li, H.; Gu, X.; Jiang, S.; Zhang, S. Constructing cross-functional intumescent flame retardants with UV resistance for polypropylene composites. Mater. Today Chem. 2022, 26, 101048. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, J.; Li, J.; Sun, J.; Xu, B.; Li, H.; Gu, X.; Zhang, S. Synthesis of a novel triazine-based intumescent flame retardant and its effects on the fire performance of expanded polystyrene foams. Polym. Degrad. Stab. 2022, 203, 110079. [Google Scholar] [CrossRef]
- Wang, S.; Jiang, Y.; Meng, Y.; Li, Y.; Han, Z.; Liu, X.; Li, H.; Sun, J.; Fei, B.; Gu, X.; et al. The encapsulation of intumescent flame retardants by poly-siloxane for thermoplastic polyolefin: Fire safety and water resistance. Polym. Degrad. Stab. 2021, 188, 109561. [Google Scholar] [CrossRef]
- Liu, X.; Sun, J.; Zhang, S.; Guo, J.; Tang, W.; Li, H.; Gu, X. Effects of carboxymethyl chitosan microencapsulated melamine polyphosphate on the flame retardancy and water resistance of thermoplastic polyurethane. Polym. Degrad. Stab. 2018, 160, 168–176. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, M.-J.; Li, B. Synthesis of N-methyl triazine-ethylenediamine copolymer charring foaming agent and its enhancement on flame retardancy and water resistance for polypropylene composites. Polym. Degrad. Stab. 2016, 131, 20–29. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, L.; Wu, X.; Xu, B. The influence of nano ZnO coated by phosphazene/triazine bi-group molecular on the flame retardant property and mechanical property of intumescent flame retardant poly (lactic acid) composites. Thermochim. Acta 2019, 679, 178336. [Google Scholar] [CrossRef]
- Shi, C.; Wan, M.; Duan, J.; Qian, X.; Che, H.; Li, J.; Ren, F.; Li, J.; Yang, L. Core-shell flame retardants based on Chitosan@MMT coated ammonia polyphosphate for enhancing flame retardancy of polyurethane. Compos. Part A Appl. Sci. Manuf. 2023, 176, 107831. [Google Scholar] [CrossRef]
- ISO4589; Plastics—Determination of Burning Behaviour by Oxygen Index. International Organization for Standardization: Geneva, Switzerland, 2017.
- ISO9773; Plastics—Determination of Burning Behaviour of Thin Flexible Vertical Specimens in Contact with a Small Flame Ignition Source. International Organization for Standardization: Geneva, Switzerland, 2024.
- ISO5660; Reaction-to-Fire Tests Heat Release, Smoke Production and Mass Loss Rate. International Organization for Standardization: Geneva, Switzerland, 2015.
- ASTMD256; Standard Test Methods for Determining the Izod Pendulum Impact Resistance of Plastics. ASTM International: West Conshohocken, PA, USA, 2010.
- ISO527; Plastics—Determination of Tensile Properties. International Organization for Standardization: Geneva, Switzerland, 2019.
- Ren, Y.; Yuan, D.; Li, W.; Cai, X. Flame retardant efficiency of KH-550 modified urea-formaldehyde resin cooperating with ammonium polyphosphate on polypropylene. Polym. Degrad. Stab. 2018, 151, 160–171. [Google Scholar] [CrossRef]
- Tang, Q.; Wang, K.; Ren, X.; Zhang, Q.; Lei, W.; Jiang, T.; Shi, D. Preparation of porous antibacterial polyamide 6 (PA6) membrane with zinc oxide (ZnO) nanoparticles selectively localized at the pore walls via reactive extrusion. Sci. Total Environ. 2020, 715, 137018. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Mu, X.; Wang, B.; Hu, W.; Wang, J.; Zhou, F.; Ma, C.; Hu, Y.; Song, L. A novel phosphorous-containing polymeric compatibilizer: Effective reinforcement and flame retardancy in glass fiber reinforced polyamide 6 composites. Compos. Part B Eng. 2020, 205, 108536. [Google Scholar] [CrossRef]
- Kalali, E.N.; Montes, A.; Wang, X.; Zhang, L.; Shabestari, M.E.; Li, Z.; Wang, D.-Y. Effect of phytic acid–modified layered double hydroxide on flammability and mechanical properties of intumescent flame retardant polypropylene system. Fire Mater. 2017, 42, 213–220. [Google Scholar] [CrossRef]
- Ronkay, F.; Molnár, B.; Szalay, F.; Nagy, D.; Bodzay, B.; Sajó, I.E.; Bocz, K. Development of Flame-Retarded Nanocomposites from Recycled PET Bottles for the Electronics Industry. Polymers 2019, 11, 233. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Liu, J.; Zhang, J.; Liu, Z.; Hu, G.; Yang, Y.; Sui, Y.; Sun, J.; Gu, X.; Zhang, S. A compound with boron and phosphorus towards epoxy resin with excellent flame retardancy, smoke suppression, transparency, and dielectric properties. Chem. Eng. J. 2024, 483, 149212. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, J.; Xiaojing, M. Effect of expandable graphite on polyester resin-based intumescent flame retardant coating. Prog. Org. Coat. 2019, 132, 178–183. [Google Scholar] [CrossRef]
- Zhu, M.; Jia, P.; Yang, G.; Song, L.; Hu, Y.; Wang, B. Synergistic effects of core-shell structured piperazine pyrophosphate microcapsules on fire safety and mechanical property in styrenic thermoplastic elastomer. J. Colloid Interface Sci. 2023, 653, 1112–1122. [Google Scholar] [CrossRef]



| Samples | PP (wt.%) | IFR (wt.%) | Si-IFR (wt.%) | MA-IFR (wt.%) | MA/IFR (wt.%) |
|---|---|---|---|---|---|
| PP | 100 | 0 | 0 | 0 | 0 |
| PP/IFR | 73 | 27 | 0 | 0 | 0 |
| PP/Si-IFR | 73 | 0 | 27 | 0 | 0 |
| PP/MA-IFR | 73 | 0 | 0 | 27 | 0 |
| PP/(MA/IFR) | 73 | 0 | 0 | 0 | 27 |
| Samples | T5% (°C) | Tmax (°C) | Residual Char (wt.%) |
|---|---|---|---|
| IFR | 347 | 456 | 43.2 |
| Si-IFR | 344 | 453 | 46.6 |
| MA-IFR | 337 | 456 | 54.3 |
| MA/IFR | 338 | 453 | 38.3 |
| Samples | T5% (°C) | TMax1 (°C) | T Max2 (°C) | Residual Char (wt.%) | |
|---|---|---|---|---|---|
| Calculated | Experimental | ||||
| PP | 437.0 | 482.0 | - | - | 0.0 |
| PP/IFR | 424.1 | 412.4 | 526.7 | 11.7 | 12.8 |
| PP/Si-IFR | 435.5 | 421.3 | 530.1 | 12.6 | 13.8 |
| PP/MA-IFR | 433.7 | 413.9 | 525.5 | 14.7 | 16.3 |
| PP/(MA/IFR) | 426.8 | 412.6 | 519.7 | 10.3 | 15.5 |
| Sample | UL-94Before boiling | UL-94After boiling | LOIBefore boiling (%) | LOIAfter boiling (%) | ΔLOI (%) | ||
|---|---|---|---|---|---|---|---|
| (t1 + t2)/s | Rating | (t1 + t2)/s | Rating | ||||
| PP/IFR | 0.2 | V-0 | 4.3 | V-2 | 37.5 ± 0.3 | 31.5 ± 0.3 | 6.0 |
| PP/Si-IFR | 0.1 | V-0 | 2.2 | V-0 | 37.5 ± 0.3 | 33.2 ± 0.3 | 4.3 |
| PP/MA-IFR | 0.2 | V-0 | 1.8 | V-0 | 39.7 ± 0.3 | 35.5 ± 0.3 | 4.2 |
| PP/(MA/IFR) | 0.2 | V-0 | 3.3 | V-2 | 38.4 ± 0.3 | 32.5 ± 0.3 | 5.9 |
| Sample | TTI (s) | pHRR (kW/m2) | TpHRR (s) | THR (MJ/m2) | TSP (m2) | pSPR (m2/s) | Residual Mass (wt.%) |
|---|---|---|---|---|---|---|---|
| PP | 28 | 1325 ± 41 | 129 ± 4 | 138 ± 5 | 10.6 ± 0.3 | 0.097 ± 0.004 | 0 |
| PP/IFR | 31 | 266 ± 8 | 755 ± 22 | 110 ± 4 | 7.1 ± 0.2 | 0.034 ± 0.001 | 14.2 ± 0.4 |
| PP/Si-IFR | 35 | 227 ± 8 | 691 ± 21 | 104 ± 4 | 10.1 ± 0.3 | 0.049 ± 0.002 | 14.7 ± 0.4 |
| PP/MA-IFR | 38 | 198 ± 6 | 1392 ± 42 | 87 ± 3 | 3.4 ± 0.1 | 0.017 ± 0.001 | 25.9 ± 1 |
| PP/(MA/IFR) | 34 | 231 ± 7 | 1315 ± 40 | 108 ± 4 | 4.7 ± 0.1 | 0.021 ± 0.001 | 19.4 ± 0.8 |
| Sample | Tensile Strength (MPa) | Elongation at Break (%) | Impact Strength (KJ/m2) |
|---|---|---|---|
| PP/IFR | 21.4 ± 1.4 | 6.3 ± 1.0 | 1.92 ± 0.06 |
| PP/Si-IFR | 23.1 ± 1.5 | 11.6 ± 0.7 | 2.05 ± 0.04 |
| PP/MA-IFR | 27.7 ± 1.4 | 15.4 ± 0.4 | 2.27 ± 0.02 |
| PP/(MA/IFR) | 18.3 ± 3.1 | 6.3 ± 1.0 | 2.15 ± 0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, X.; Deng, M.; Jia, H.; Chen, X.; Wang, R.; Sun, J.; Li, H.; Gu, X.; Zhang, S. Surface Modification of Intumescent Flame Retardant and Its Application in Polypropylene with Excellent Fire Performance and Water Resistance. Polymers 2025, 17, 399. https://doi.org/10.3390/polym17030399
Zheng X, Deng M, Jia H, Chen X, Wang R, Sun J, Li H, Gu X, Zhang S. Surface Modification of Intumescent Flame Retardant and Its Application in Polypropylene with Excellent Fire Performance and Water Resistance. Polymers. 2025; 17(3):399. https://doi.org/10.3390/polym17030399
Chicago/Turabian StyleZheng, Xuqiang, Mike Deng, Hao Jia, Xinyu Chen, Ruicheng Wang, Jun Sun, Hongfei Li, Xiaoyu Gu, and Sheng Zhang. 2025. "Surface Modification of Intumescent Flame Retardant and Its Application in Polypropylene with Excellent Fire Performance and Water Resistance" Polymers 17, no. 3: 399. https://doi.org/10.3390/polym17030399
APA StyleZheng, X., Deng, M., Jia, H., Chen, X., Wang, R., Sun, J., Li, H., Gu, X., & Zhang, S. (2025). Surface Modification of Intumescent Flame Retardant and Its Application in Polypropylene with Excellent Fire Performance and Water Resistance. Polymers, 17(3), 399. https://doi.org/10.3390/polym17030399







