Polysaccharides: The Sweet and Bitter Impacts on Cardiovascular Risk
Abstract
:1. Introduction
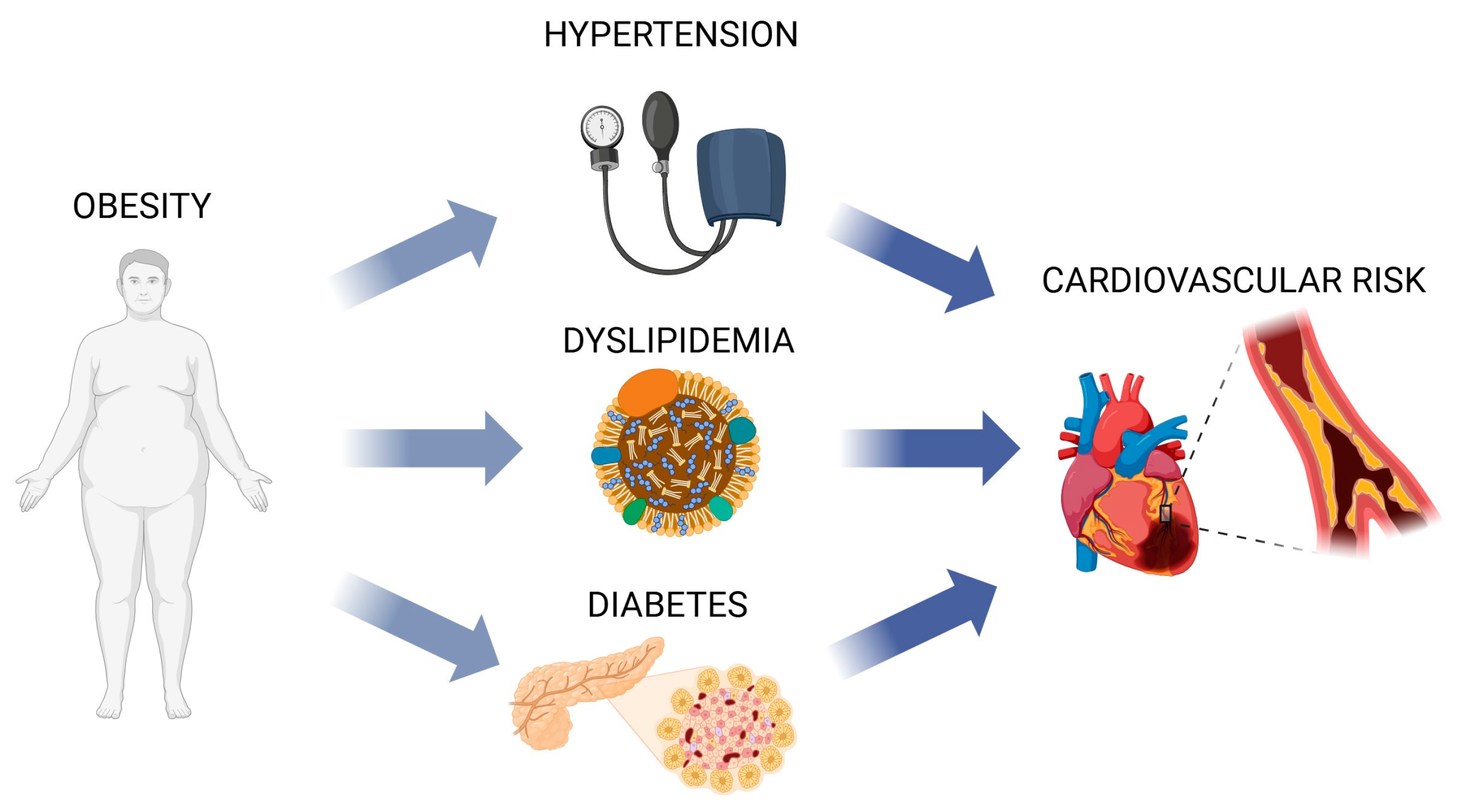
2. Dyslipidemia
3. Hypertension
4. Obesity
5. Diabetes
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. 2021 ESC Guidelines on Cardiovascular Disease Prevention in Clinical Practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef] [PubMed]
- Pagidipati, N.J.; Gaziano, T.A. Estimating Deaths From Cardiovascular Disease: A Review of Global Methodologies of Mortality Measurement. Circulation 2013, 127, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Popiolek-Kalisz, J.; Blaszczak, P.; Fornal, E. Dietary Isorhamnetin Intake Is Associated with Lower Blood Pressure in Coronary Artery Disease Patients. Nutrients 2022, 14, 4586. [Google Scholar] [CrossRef] [PubMed]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the Management of Dyslipidaemias: Lipid Modification to Reduce Cardiovascular Risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Trautwein, E.A.; McKay, S. The Role of Specific Components of a Plant-Based Diet in Management of Dyslipidemia and the Impact on Cardiovascular Risk. Nutrients 2020, 12, 2671. [Google Scholar] [CrossRef]
- Ma, J.; Karlsen, M.C.; Chung, M.; Jacques, P.F.; Saltzman, E.; Smith, C.E.; Fox, C.S.; McKeown, N.M. Potential Link between Excess Added Sugar Intake and Ectopic Fat: A Systematic Review of Randomized Controlled Trials. Nutr. Rev. 2016, 74, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, J.; Wang, Z.; Duan, F.; Jia, Z.; Chen, X.; Li, S. The Promising Role of Probiotics/Prebiotics/Synbiotics in Energy Metabolism Biomarkers in Patients with NAFLD: A Systematic Review and Meta-Analysis. Front. Public Health 2022, 10, 862266. [Google Scholar] [CrossRef] [PubMed]
- Froidurot, A.; Julliand, V. Cellulolytic Bacteria in the Large Intestine of Mammals. Gut Microbes 2022, 14, 2031694. [Google Scholar] [CrossRef]
- Llewellyn, S.R.; Britton, G.J.; Contijoch, E.J.; Vennaro, O.H.; Mortha, A.; Colombel, J.-F.; Grinspan, A.; Clemente, J.C.; Merad, M.; Faith, J.J. Interactions Between Diet and the Intestinal Microbiota Alter Intestinal Permeability and Colitis Severity in Mice. Gastroenterology 2018, 154, 1037–1046.e2. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xia, Y.; Liu, H.-Y.; Guo, H.; He, X.-Q.; Liu, Y.; Wu, D.-T.; Mai, Y.-H.; Li, H.-B.; Zou, L.; et al. Nutritional Values, Beneficial Effects, and Food Applications of Broccoli (Brassica Oleracea Var. Italica Plenck). Trends Food Sci. Technol. 2022, 119, 288–308. [Google Scholar] [CrossRef]
- Güzel, M.; Akpınar, Ö. Preparation and Characterization of Bacterial Cellulose Produced from Fruit and Vegetable Peels by Komagataeibacter hansenii GA2016. Int. J. Biol. Macromol. 2020, 162, 1597–1604. [Google Scholar] [CrossRef]
- Saldívar, S.O.S.; Hernández, D.S. Dietary Fiber in Cereals, Legumes, Pseudocereals and Other Seeds. In Science and Technology of Fibers in Food Systems; Springer: Cham, Switzerland, 2020; pp. 87–122. [Google Scholar]
- Qiu, S.; Yadav, M.P.; Yin, L. Characterization and Functionalities Study of Hemicellulose and Cellulose Components Isolated from Sorghum Bran, Bagasse and Biomass. Food Chem. 2017, 230, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Surampudi, P.; Enkhmaa, B.; Anuurad, E.; Berglund, L. Lipid Lowering with Soluble Dietary Fiber. Curr. Atheroscler. Rep. 2016, 18, 75. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zeng, Y.; Xu, J.; Zheng, H.; Liu, J.; Fan, R.; Zhu, W.; Yuan, L.; Qin, Y.; Chen, S.; et al. Therapeutic Effects of Soluble Dietary Fiber Consumption on Type 2 Diabetes Mellitus. Exp. Ther. Med. 2016, 12, 1232–1242. [Google Scholar] [CrossRef] [PubMed]
- Giuntini, E.B.; Sardá, F.A.H.; de Menezes, E.W. The Effects of Soluble Dietary Fibers on Glycemic Response: An Overview and Futures Perspectives. Foods 2022, 11, 3934. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.W.; Baird, P.; Davis Jr, R.H.; Ferreri, S.; Knudtson, M.; Koraym, A.; Waters, V.; Williams, C.L. Health Benefits of Dietary Fiber. Nutr. Rev. 2009, 67, 188–205. [Google Scholar] [CrossRef]
- Naude, C.E.; Brand, A.; Schoonees, A.; Nguyen, K.A.; Chaplin, M.; Volmink, J. Low-Carbohydrate versus Balanced-Carbohydrate Diets for Reducing Weight and Cardiovascular Risk. Cochrane Database Syst. Rev. 2022, CD013334. [Google Scholar] [CrossRef]
- Xie, C.; Lee, Z.J.; Ye, S.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. A Review on Seaweeds and Seaweed-Derived Polysaccharides: Nutrition, Chemistry, Bioactivities, and Applications. Food Rev. Int. 2024, 40, 1312–1347. [Google Scholar] [CrossRef]
- Castro-Acosta, M.L.; Lenihan-Geels, G.N.; Corpe, C.P.; Hall, W.L. Berries and Anthocyanins: Promising Functional Food Ingredients with Postprandial Glycaemia-Lowering Effects. Proc. Nutr. Soc. 2016, 75, 342–355. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.; Mann, J.; Cummings, J.; Winter, N.; Mete, E.; Morenga, L.T. Carbohydrate Quality and Human Health: A Series of Systematic Reviews and Meta-Analyses. The Lancet 2019, 393, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.N.; Akerman, A.P.; Mann, J. Dietary Fibre and Whole Grains in Diabetes Management: Systematic Review and Meta-Analyses. PLoS Med. 2020, 17, e1003053. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.; Rosner, B.; Willett, W.W.; Sacks, F.M. Cholesterol-Lowering Effects of Dietary Fiber: A Meta-Analysis. Am. J. Clin. Nutr. 1999, 69, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Xia, J.; Yang, C.; Pan, D.; Xu, D.; Sun, G.; Xia, H. Effects of Oat Beta-Glucan Intake on Lipid Profiles in Hypercholesterolemic Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients. 2022, 14, 2043. [Google Scholar] [CrossRef]
- Pagidipati, N.J.; Taub, P.R.; Ostfeld, R.J.; Kirkpatrick, C.F. Dietary Patterns to Promote Cardiometabolic Health. Nat. Rev. Cardiol. 2025, 22, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Marx, N.; Federici, M.; Schütt, K.; Müller-Wieland, D.; Ajjan, R.A.; Antunes, M.J.; Christodorescu, R.M.; Crawford, C.; Di Angelantonio, E.; Eliasson, B.; et al. 2023 ESC Guidelines for the Management of Cardiovascular Disease in Patients with Diabetes: Developed by the Task Force on the Management of Cardiovascular Disease in Patients with Diabetes of the European Society of Cardiology (ESC). Eur. Heart J. 2023, 44, 4043–4140. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Prabhakar, M.; Ju, J.; Long, H.; Zhou, H.-W. Effect of Inulin-Type Fructans on Blood Lipid Profile and Glucose Level: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Eur. J. Clin. Nutr. 2017, 71, 9–20. [Google Scholar] [CrossRef]
- Scientific Opinion on the Substantiation of Health Claims Related to Chitosan and Reduction in Body Weight (ID 679, 1499), Maintenance of Normal Blood LDL-Cholesterol Concentrations (ID 4663), Reduction of Intestinal Transit Time (ID 4664) and Reduction of Inflammation (ID 1985) Pursuant to Article 13(1) of Regulation (EC) No 1924/2006. Eur. Heart J. 2011, 9, 2214. [CrossRef]
- Soliman, G.A. Dietary Fiber, Atherosclerosis, and Cardiovascular Disease. Nutrients 2019, 11, 1155. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Gao, J.; Li, Y.; Qi, S.; Meng, T.; Yu, S.; Zhang, Y.; He, Q. Efficacy of Oats in Dyslipidemia: A Systematic Review and Meta-Analysis. Food Funct. 2024, 15, 3232–3245. [Google Scholar] [CrossRef]
- Ghavami, A.; Ziaei, R.; Talebi, S.; Barghchi, H.; Nattagh-Eshtivani, E.; Moradi, S.; Rahbarinejad, P.; Mohammadi, H.; Ghasemi-Tehrani, H.; Marx, W.; et al. Soluble Fiber Supplementation and Serum Lipid Profile: A Systematic Review and Dose-Response Meta-Analysis of Randomized Controlled Trials. Adv. Nutr. 2023, 14, 465–474. [Google Scholar] [CrossRef]
- Ho, H.V.T.; Sievenpiper, J.L.; Zurbau, A.; Blanco Mejia, S.; Jovanovski, E.; Au-Yeung, F.; Jenkins, A.L.; Vuksan, V. The Effect of Oat β -Glucan on LDL-Cholesterol, Non-HDL-Cholesterol and apoB for CVD Risk Reduction: A Systematic Review and Meta-Analysis of Randomised-Controlled Trials. Br. J. Nutr. 2016, 116, 1369–1382. [Google Scholar] [CrossRef]
- Zhu, X.; Sun, X.; Wang, M.; Zhang, C.; Cao, Y.; Mo, G.; Liang, J.; Zhu, S. Quantitative Assessment of the Effects of Beta-Glucan Consumption on Serum Lipid Profile and Glucose Level in Hypercholesterolemic Subjects. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 714–723. [Google Scholar] [CrossRef]
- de Morais Junior, A.C.; Schincaglia, R.M.; Viana, R.B.; Armet, A.M.; Prado, C.M.; Walter, J.; Mota, J.F. The Separate Effects of Whole Oats and Isolated Beta-Glucan on Lipid Profile: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Clin. Nutr. ESPEN 2023, 53, 224–237. [Google Scholar] [CrossRef]
- Talati, R.; Baker, W.L.; Pabilonia, M.S.; White, C.M.; Coleman, C.I. The Effects of Barley-Derived Soluble Fiber on Serum Lipids. Ann. Fam. Med. 2009, 7, 157–163. [Google Scholar] [CrossRef]
- AbuMweis, S.S.; Jew, S.; Ames, N.P. β-Glucan from Barley and Its Lipid-Lowering Capacity: A Meta-Analysis of Randomized, Controlled Trials. Eur. J. Clin. Nutr. 2010, 64, 1472–1480. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Liu, H.; Yang, C.; Xia, H.; Pan, D.; Yang, X.; Yang, L.; Wang, S.; Sun, G. Effects of Different Delivering Matrices of β-Glucan on Lipids in Mildly Hypercholesterolaemic Individuals: A Meta-Analysis of Randomised Controlled Trials. Br. J. Nutr. 2021, 125, 294–307. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.d.A.; Verneque, B.J.F.; Mota, A.P.L.; Duarte, C.K. Chia Seed (Salvia hispanica L.) Consumption and Lipid Profile: A Systematic Review and Meta-Analysis. Food Funct. 2021, 12, 8835–8849. [Google Scholar] [CrossRef] [PubMed]
- Jovanovski, E.; Yashpal, S.; Komishon, A.; Zurbau, A.; Blanco Mejia, S.; Ho, H.V.T.; Li, D.; Sievenpiper, J.; Duvnjak, L.; Vuksan, V. Effect of Psyllium (Plantago ovata) Fiber on LDL Cholesterol and Alternative Lipid Targets, Non-HDL Cholesterol and Apolipoprotein B: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Am. J. Clin. Nutr. 2018, 108, 922–932. [Google Scholar] [CrossRef] [PubMed]
- Brum, J.; Ramsey, D.; McRorie, J.; Bauer, B.; Kopecky, S.L. Meta-Analysis of Usefulness of Psyllium Fiber as Adjuvant Antilipid Therapy to Enhance Cholesterol Lowering Efficacy of Statins. Am. J. Cardiol. 2018, 122, 1169–1174. [Google Scholar] [CrossRef]
- Wei, Z.; Wang, H.; Chen, X.; Wang, B.; Rong, Z.; Wang, B.; Su, B.; Chen, H. Time- and Dose-Dependent Effect of Psyllium on Serum Lipids in Mild-to-Moderate Hypercholesterolemia: A Meta-Analysis of Controlled Clinical Trials. Eur. J. Clin. Nutr. 2009, 63, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.W.; Allgood, L.D.; Lawrence, A.; Altringer, L.A.; Jerdack, G.R.; Hengehold, D.A.; Morel, J.G. Cholesterol-Lowering Effects of Psyllium Intake Adjunctive to Diet Therapy in Men and Women with Hypercholesterolemia: Meta-Analysis of 8 Controlled Trials. Am. J. Clin. Nutr. 2000, 71, 472–479. [Google Scholar] [CrossRef]
- Olson, B.H.; Anderson, S.M.; Becker, M.P.; Anderson, J.W.; Hunninghake, D.B.; Jenkins, D.J.A.; LaRosa, J.C.; Rippe, J.M.; Roberts, D.C.K.; Stoy, D.B.; et al. Psyllium-Enriched Cereals Lower Blood Total Cholesterol and LDL Cholesterol, but Not HDL Cholesterol, in Hypercholesterolemic Adults: Results of a Meta-Analysis. J. Nutr. 1997, 127, 1973–1980. [Google Scholar] [CrossRef] [PubMed]
- Bazzano, L.A.; Thompson, A.M.; Tees, M.T.; Nguyen, C.H.; Winham, D.M. Non-Soy Legume Consumption Lowers Cholesterol Levels: A Meta-Analysis of Randomized Controlled Trials. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.; Shim, S.R.; Wu, Y.; Hong, G.; Jeon, H.; Kim, C.-G.; Lee, K.J. How Do Brown Seaweeds Work on Biomarkers of Dyslipidemia? A Systematic Review with Meta-Analysis and Meta-Regression. Mar. Drugs 2023, 21, 220. [Google Scholar] [CrossRef]
- Malhotra, A.; Shafiq, N.; Arora, A.; Singh, M.; Kumar, R.; Malhotra, S. Dietary Interventions (Plant Sterols, Stanols, Omega-3 Fatty Acids, Soy Protein and Dietary Fibers) for Familial Hypercholesterolaemia. Cochrane Database Syst. Rev. 2014, 2014, CD001918. [Google Scholar] [CrossRef]
- Huang, H.; Zou, Y.; Chi, H.; Liao, D. Lipid-Modifying Effects of Chitosan Supplementation in Humans: A Pooled Analysis with Trial Sequential Analysis. Mol. Nutr. Food Res. 2018, 62, 1700842. [Google Scholar] [CrossRef]
- Ghavami, A.; Banpouri, S.; Ziaei, R.; Talebi, S.; Vajdi, M.; Nattagh-Eshtivani, E.; Barghchi, H.; Mohammadi, H.; Askari, G. Effect of Soluble Fiber on Blood Pressure in Adults: A Systematic Review and Dose–Response Meta-Analysis of Randomized Controlled Trials. Nutr. J. 2023, 22, 51. [Google Scholar] [CrossRef]
- Reynolds, A.N.; Akerman, A.; Kumar, S.; Diep Pham, H.T.; Coffey, S.; Mann, J. Dietary Fibre in Hypertension and Cardiovascular Disease Management: Systematic Review and Meta-Analyses. BMC Med. 2022, 20, 139. [Google Scholar] [CrossRef]
- Clark, C.C.T.; Salek, M.; Aghabagheri, E.; Jafarnejad, S. The Effect of Psyllium Supplementation on Blood Pressure: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Korean J. Intern. Med. 2020, 35, 1385–1399. [Google Scholar] [CrossRef]
- Khan, K.; Jovanovski, E.; Ho, H.V.T.; Marques, A.C.R.; Zurbau, A.; Mejia, S.B.; Sievenpiper, J.L.; Vuksan, V. The Effect of Viscous Soluble Fiber on Blood Pressure: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 3–13. [Google Scholar] [CrossRef]
- Khalesi, S.; Irwin, C.; Schubert, M. Flaxseed Consumption May Reduce Blood Pressure: A Systematic Review and Meta-Analysis of Controlled Trials. J. Nutr. 2015, 145, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Xi, H.; Zhou, W.; Niu, Y.; Zhu, R.; Wang, S.; Guo, Y.; Liu, W.; Xiong, X.; Guo, L. Effect of Oat Consumption on Blood Pressure: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Acad. Nutr. Diet. 2023, 123, 809–823. [Google Scholar] [CrossRef]
- Huang, H.; Zou, Y.; Chi, H. Quantitative Assessment of the Effects of Chitosan Intervention on Blood Pressure Control. Drug Des. Dev. Ther. 2018, 12, 67–75. [Google Scholar] [CrossRef]
- Man, A.W.C.; Li, H.; Xia, N. Impact of Lifestyles (Diet and Exercise) on Vascular Health: Oxidative Stress and Endothelial Function. Oxidative Med. Cell. Longev. 2020, 2020, 1496462. [Google Scholar] [CrossRef]
- Virani, S.S.; Newby, L.K.; Arnold, S.V.; Bittner, V.; Brewer, L.C.; Demeter, S.H.; Dixon, D.L.; Fearon, W.F.; Hess, B.; Johnson, H.M.; et al. 2023 AHA/ACC/ACCP/ASPC/NLA/PCNA Guideline for the Management of Patients with Chronic Coronary Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2023, 148, e9–e119. [Google Scholar] [CrossRef] [PubMed]
- Griel, A.E.; Ruder, E.H.; Kris-Etherton, P.M. The Changing Roles of Dietary Carbohydrates: From Simple to Complex. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1958–1965. [Google Scholar] [CrossRef] [PubMed]
- Dreher, M. Fiber and Hypertension. In Dietary Fiber in Health and Disease; Springer: Cham, Switzerland, 2018; pp. 291–303. [Google Scholar]
- Khalid, W.; Arshad, M.S.; Jabeen, A.; Muhammad Anjum, F.; Qaisrani, T.B.; Suleria, H.A.R. Fiber-Enriched Botanicals: A Therapeutic Tool against Certain Metabolic Ailments. Food Sci. Nutr. 2022, 10, 3203–3218. [Google Scholar] [CrossRef]
- Hu, T.; Wu, Q.; Yao, Q.; Jiang, K.; Yu, J.; Tang, Q. Short-Chain Fatty Acid Metabolism and Multiple Effects on Cardiovascular Diseases. Ageing Res. Rev. 2022, 81, 101706. [Google Scholar] [CrossRef] [PubMed]
- Havlik, J.; Marinello, V.; Gardyne, A.; Hou, M.; Mullen, W.; Morrison, D.J.; Preston, T.; Combet, E.; Edwards, C.A. Dietary Fibres Differentially Impact on the Production of Phenolic Acids from Rutin in an In Vitro Fermentation Model of the Human Gut Microbiota. Nutrients 2020, 12, 1577. [Google Scholar] [CrossRef] [PubMed]
- Shahinfar, H.; Jayedi, A.; Torabynasab, K.; Payandeh, N.; Martami, F.; Moosavi, H.; Bazshahi, E.; Shab-Bidar, S. Comparative Effects of Nutraceuticals on Body Weight in Adults with Overweight or Obesity: A Systematic Review and Network Meta-Analysis of 111 Randomized Clinical Trials. Pharmacol. Res. 2023, 196, 106944. [Google Scholar] [CrossRef]
- Rajestary, R.; Landi, L.; Romanazzi, G. Chitosan and Postharvest Decay of Fresh Fruit: Meta-Analysis of Disease Control and Antimicrobial and Eliciting Activities. Compr. Rev. Food Sci. Food Saf. 2021, 20, 563–582. [Google Scholar] [CrossRef]
- Zhang, P.; Jia, J.; Jiang, P.; Zheng, W.; Li, X.; Song, S.; Ai, C. Polysaccharides from Edible Brown Seaweed Undaria Pinnatifida Are Effective against High-Fat Diet-Induced Obesity in Mice through the Modulation of Intestinal Microecology. Food Funct. 2022, 13, 2581–2593. [Google Scholar] [CrossRef]
- Murakami, S.; Hirazawa, C.; Ohya, T.; Yoshikawa, R.; Mizutani, T.; Ma, N.; Moriyama, M.; Ito, T.; Matsuzaki, C. The Edible Brown Seaweed Sargassum horneri (Turner) C. Agardh Ameliorates High-Fat Diet-Induced Obesity, Diabetes, and Hepatic Steatosis in Mice. Nutrients 2021, 13, 551. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Z.; Dai, T.; Zhang, Z.; Zhang, Q.; Yao, J.; Wang, L.; He, N.; Li, S. The Therapeutic Effect and Possible Mechanisms of Alginate Oligosaccharide on Metabolic Syndrome by Regulating Gut Microbiota. Food Funct. 2024, 15, 9632–9661. [Google Scholar] [CrossRef]
- Patil, N.P.; Le, V.; Sligar, A.D.; Mei, L.; Chavarria, D.; Yang, E.Y.; Baker, A.B. Algal Polysaccharides as Therapeutic Agents for Atherosclerosis. Front. Cardiovasc. Med. 2018, 5, 153. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, R.; Chen, Y.; Zhu, D.; Wu, Z.; Chen, F.; Huang, X.; Ali Khan, B.; Al Hennawi, H.E.; Albazee, E.; et al. The Effect of Guar Gum Consumption on the Lipid Profile in Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Crit. Rev. Food Sci. Nutr. 2023, 63, 2886–2895. [Google Scholar] [CrossRef]
- Zou, Y.; Liao, D.; Huang, H.; Li, T.; Chi, H. A Systematic Review and Meta-Analysis of Beta-Glucan Consumption on Glycemic Control in Hypercholesterolemic Individuals. Int. J. Food Sci. Nutr. 2015, 66, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Chambers, E.S.; Byrne, C.S.; Morrison, D.J.; Murphy, K.G.; Preston, T.; Tedford, C.; Garcia-Perez, I.; Fountana, S.; Serrano-Contreras, J.I.; Holmes, E.; et al. Dietary Supplementation with Inulin-Propionate Ester or Inulin Improves Insulin Sensitivity in Adults with Overweight and Obesity with Distinct Effects on the Gut Microbiota, Plasma Metabolome and Systemic Inflammatory Responses: A Randomised Cross-over Trial. Gut 2019, 68, 1430–1438. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes-2024. Diabetes Care 2024, 47, S20–S42. [Google Scholar] [CrossRef]
- World Health Organization. Guideline: Sugars Intake for Adults and Children; WHO Guidelines Approved by the Guidelines Review Committee; World Health Organization: Geneva, Switzerland, 2015; ISBN 978-92-4-154902-8. [Google Scholar]
- Vaz, E.C.; Porfírio, G.J.M.; Nunes, H.R.D.C.; Nunes-Nogueira, V.D.S. Effectiveness and Safety of Carbohydrate Counting in the Management of Adult Patients with Type 1 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Arch. Endocrinol. Metab. 2018, 62, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Diabetes and Nutrition Study Group (DNSG) of the European Association for the Study of Diabetes (EASD) Evidence-Based European Recommendations for the Dietary Management of Diabetes. Diabetologia 2023, 66, 965–985. [CrossRef] [PubMed]
- Tavares, J.O.; Cotas, J.; Valado, A.; Pereira, L. Algae Food Products as a Healthcare Solution. Mar. Drugs 2023, 21, 578. [Google Scholar] [CrossRef] [PubMed]
- Fizpatrick, F.W.; DiCarlo, F.J. Zymosan. Ann. N. Y. Acad. Sci. 1964, 118, 235–261. [Google Scholar] [CrossRef]
- Nakashima, A.; Yamada, K.; Iwata, O.; Sugimoto, R.; Atsuji, K.; Ogawa, T.; Ishibashi-Ohgo, N.; Suzuki, K. β-Glucan in Foods and Its Physiological Functions. J. Nutr. Sci. Vitaminol. 2018, 64, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Zurbau, A.; Noronha, J.C.; Khan, T.A.; Sievenpiper, J.L.; Wolever, T.M.S. The Effect of Oat β-Glucan on Postprandial Blood Glucose and Insulin Responses: A Systematic Review and Meta-Analysis. Eur. J. Clin. Nutr. 2021, 75, 1540–1554. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhao, J.; Huang, Y.; Li, Y. The Difference between Oats and Beta-Glucan Extract Intake in the Management of HbA1c, Fasting Glucose and Insulin Sensitivity: A Meta-Analysis of Randomized Controlled Trials. Food Funct. 2016, 7, 1413–1428. [Google Scholar] [CrossRef]
- Francelino Andrade, E.; Vieira Lobato, R.; Vasques Araújo, T.; Gilberto Zangerônimo, M.; Vicente Sousa, R.; José Pereira, L. Effect of Beta-Glucans in the Control of Blood Glucose Levels of Diabetic Patients: A Systematic Review. Nutr. Hosp. 2014, 31, 170–177. [Google Scholar] [CrossRef]
- Mozaffarian, D. Dietary and Policy Priorities for Cardiovascular Disease, Diabetes, and Obesity: A Comprehensive Review. Circulation 2016, 133, 187–225. [Google Scholar] [CrossRef]
- Guo, W.; Yi, L.; Zhou, B.; Li, M. Chitosan Modifies Glycemic Levels in People with Metabolic Syndrome and Related Disorders: Meta-Analysis with Trial Sequential Analysis. Nutr. J. 2020, 19, 130. [Google Scholar] [CrossRef]
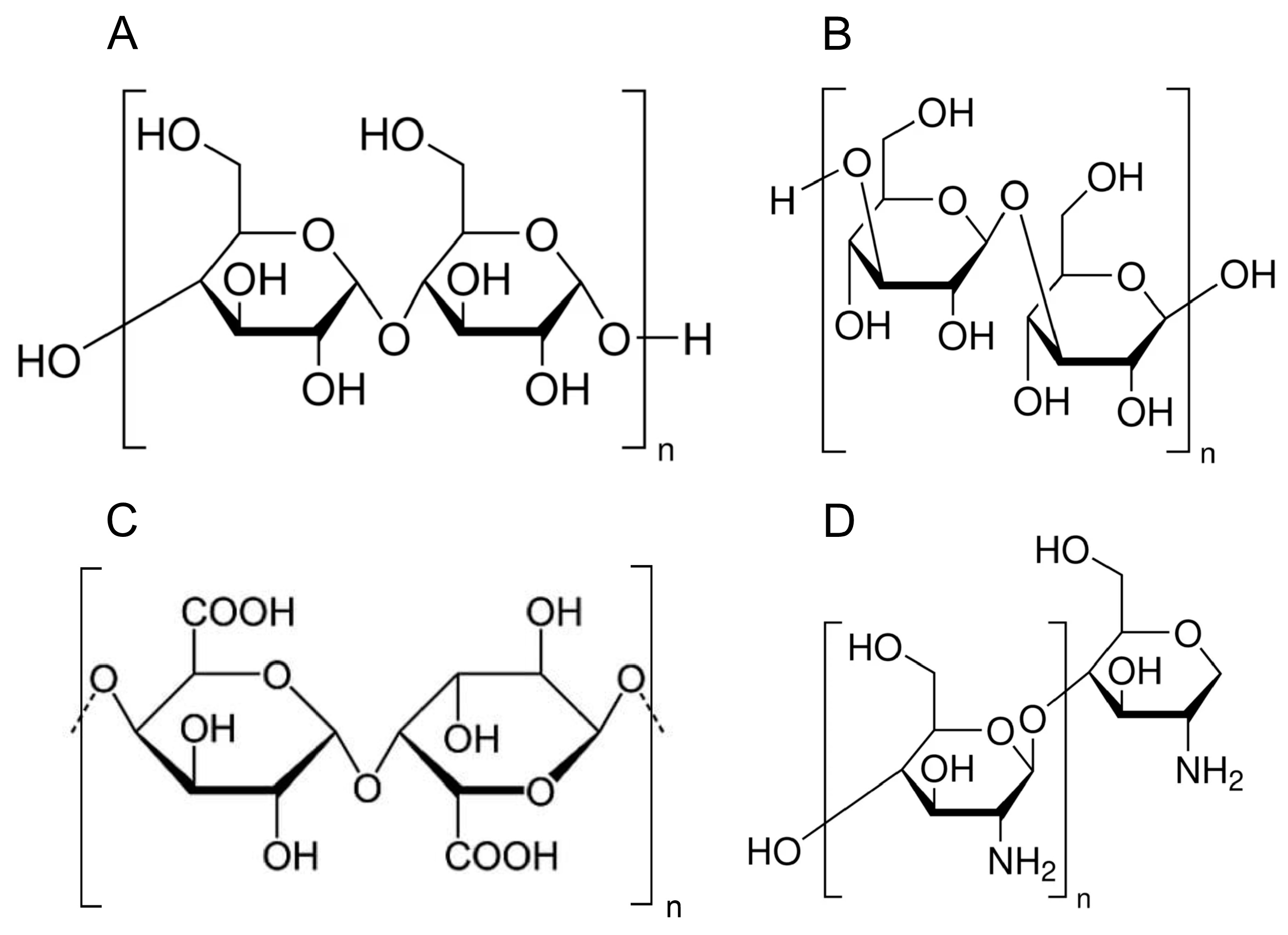
| Polysaccharides | Sources | Recommendation | Result |
|---|---|---|---|
| Soluble Fiber | Oats, barley, beans, lentils, peas, apples, citrus fruits, carrots, psyllium husk | intake of 4–10 g/day [21,22,23] | protective in cardiometabolic complications decrease of mortality lowering glucose lowering TC, LDL, TG |
| Insoluble fiber | Whole grains (wheat bran, brown rice), nuts, seeds, vegetables (cauliflower, green beans, potatoes) | 70–75% of total fiber [21,22,23] | |
| Beta-glucans | Oats, barley, mushrooms (e.g., shiitake, maitake), yeast | 3–6 g/day [24] | lowers LDL and non-HDL |
| Refined starches | White bread, white rice, pastries, many processed foods, crackers, cakes, and some breakfast cereals | should be minimized 1–2 servings/day [25] | worsening of cardiometabolic health and an increased risk of CVD events |
| Starch | Potatoes, corn, peas, pasta, bread, and other grains | No recommendation | high glycemic index, glycemia spikes |
| Resistant starch | Green bananas, legumes, cooked and cooled potatoes, rice, pasta, high-amylose cornstarch | 15–60 g/day [26] | decrease of available energy lower blood glucose increase of TG |
| Inulin | Chicory root, onions, garlic, leeks, asparagus, artichokes, bananas | 5–10 g/day [27] | lowering TC and LDL Modulation of inflammation enhance the growth of beneficial gut bacteria |
| Chitosan | Exoskeleton of crustaceans (crab, shrimp, lobster) | no recommendation, considered 1–6 g/day [28] | Decreasing body weight Has prebiotic and antimicrobial properties |
| Dietary Component | Key Effect in Dyslipidemia |
|---|---|
| Soluble fibers | ↓TC, ↓LDL |
| Beta-glucans | ↓LDL, ↓non-HDL, ↓apoB |
| Guar gum | ↓TC, ↓LDL (with therapy of familial hypercholesterolemia) |
| Inulin-type fructans | ↓LDL |
| Chitosan | ↓TC, ↓LDL |
| Psyllium | ↓LDL, ↓non-HDL, ↓apoB |
| Dietary Component | Key Effect in Hypertension |
|---|---|
| Soluble fibers | ↓SBP, ↓DBP, improves endothelial function, ↓Inflammation |
| Beta-glucans | ↓SBP |
| Chitosan | ↓DBP (in high doses) |
| Psyllium | ↓SBP |
| Dietary Component | Key Effects in Obesity |
|---|---|
| Dietary fiber | ↑satiety, ↓gastric emptying, ↓caloric intake, ↑body weight loss, ↓CV risk, ↓LDL-C, ↓inflammation |
| Inulin | ↓LDL-C, improves lipid profile |
| Pectins | improve endothelial function, ↓inflammation, ↓CV risk |
| Chitosan | ↓body weight in overweight patients, can disrupt gut microbiota balance in prolonged use |
| Psyllium and Glucomannan | ↓body weight |
| Alginates | ↓cholesterol and glucose absorption |
| Dietary Component | Key Effects in Diabetes |
|---|---|
| Low carbohydrate diets (High fiber) | ↓CVD risk, ↓hypertension, ↓insulin resistance |
| Beta-glucans | ↓postprandial glycemic spikes, ↓fasting glucose, ↓insulin, ↓HbA1c |
| Chitosan | ↓fasting glucose, ↓HbA1c |
| Resistant starches | ↑insulin sensitivity, ↓caloric intake, ↓CVD risk |
| Guar gum | ↓TC, ↓LDL-C, ↓TG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalisz, G.; Popiolek-Kalisz, J. Polysaccharides: The Sweet and Bitter Impacts on Cardiovascular Risk. Polymers 2025, 17, 405. https://doi.org/10.3390/polym17030405
Kalisz G, Popiolek-Kalisz J. Polysaccharides: The Sweet and Bitter Impacts on Cardiovascular Risk. Polymers. 2025; 17(3):405. https://doi.org/10.3390/polym17030405
Chicago/Turabian StyleKalisz, Grzegorz, and Joanna Popiolek-Kalisz. 2025. "Polysaccharides: The Sweet and Bitter Impacts on Cardiovascular Risk" Polymers 17, no. 3: 405. https://doi.org/10.3390/polym17030405
APA StyleKalisz, G., & Popiolek-Kalisz, J. (2025). Polysaccharides: The Sweet and Bitter Impacts on Cardiovascular Risk. Polymers, 17(3), 405. https://doi.org/10.3390/polym17030405







