Preparation and Hg0 Removal Performance of MIL-101(Cr)-Derived Carbon Matrix Composites
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Samples
2.1.1. MIL-101(Cr)
2.1.2. Preparation of Modified Biochar (MBC)
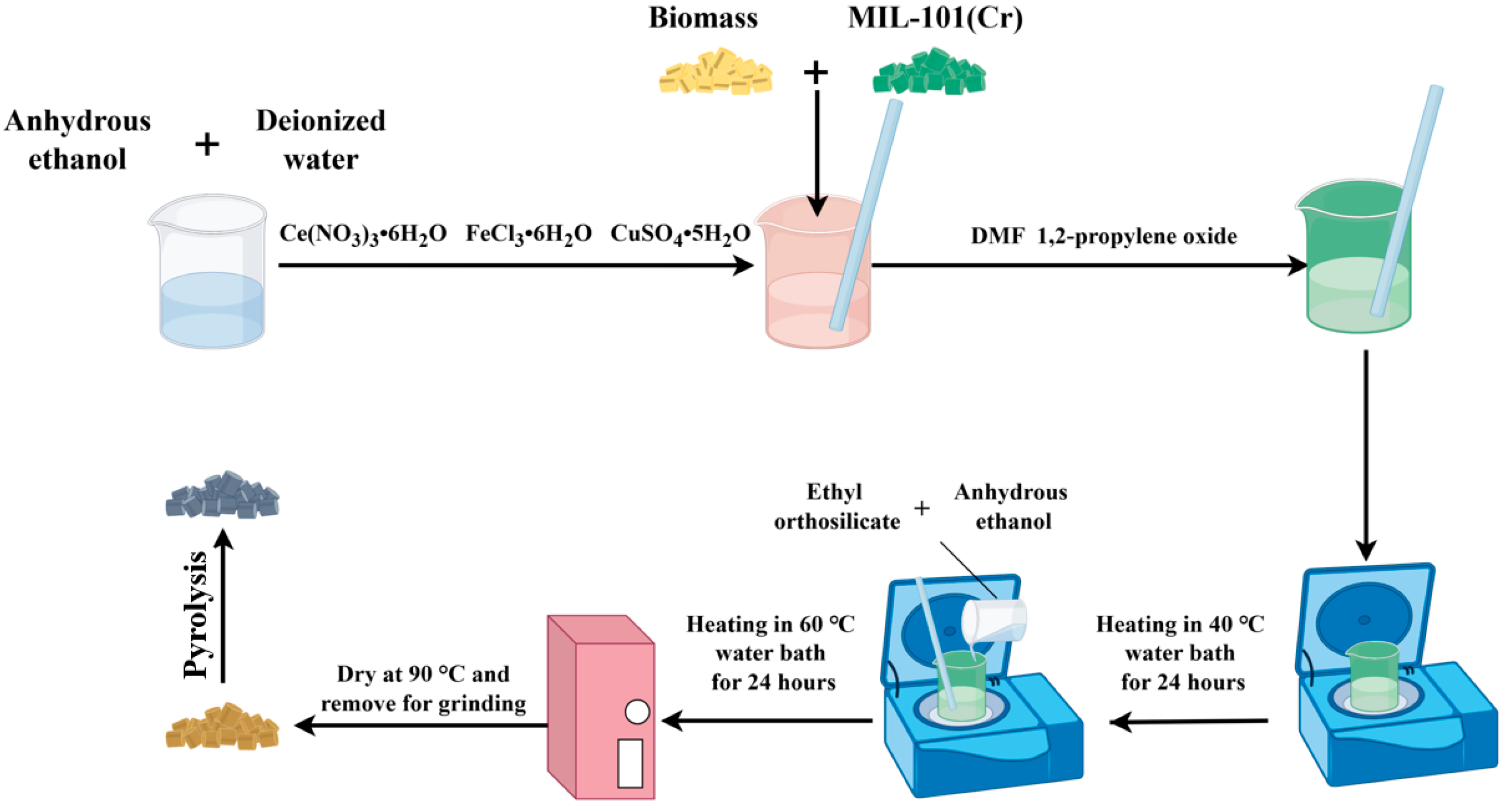
2.1.3. Preparation of MIL-101(Cr)-Derived Carbon Materials (PDC-MIL) and Its Composite Materials (PDC-MIL/BM)
2.2. Experimental System and Characterization Method
3. Results and Discussion
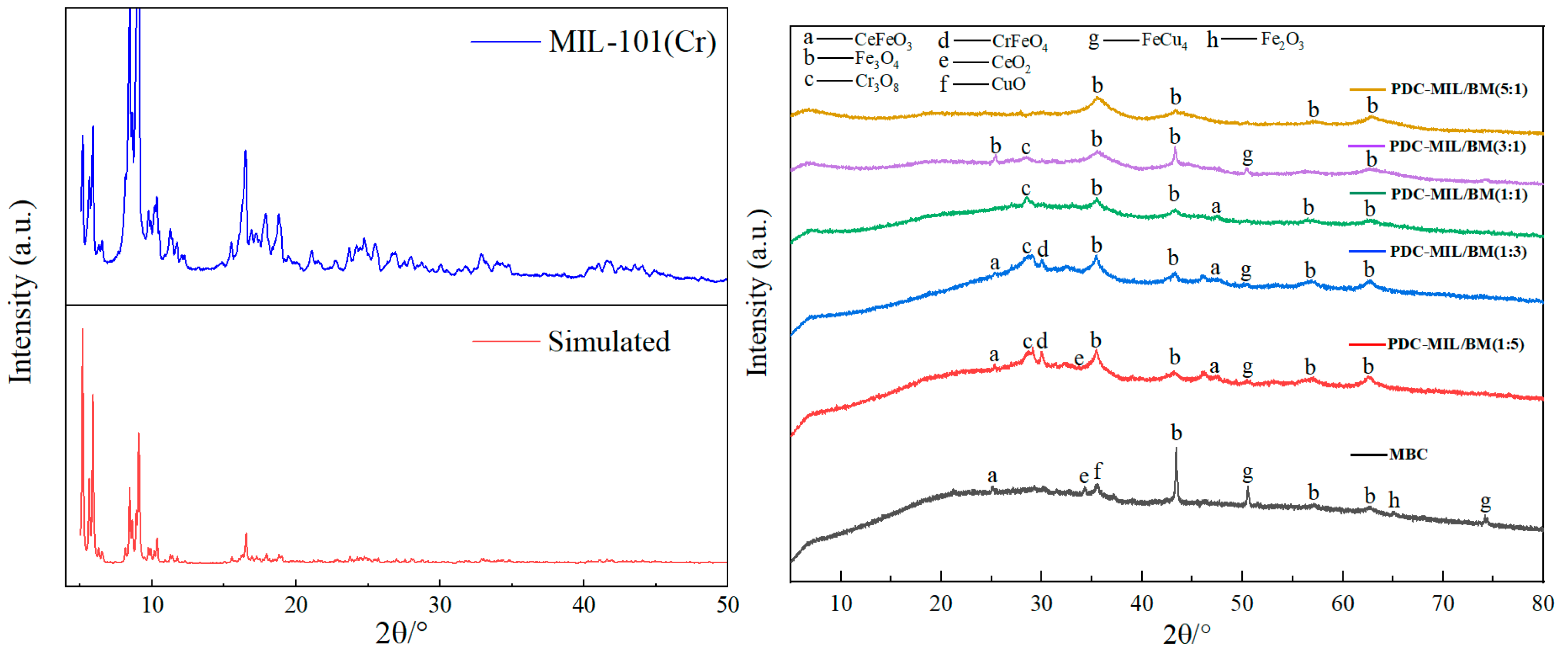
3.1. Crystalline Structure
3.2. Pore Structure
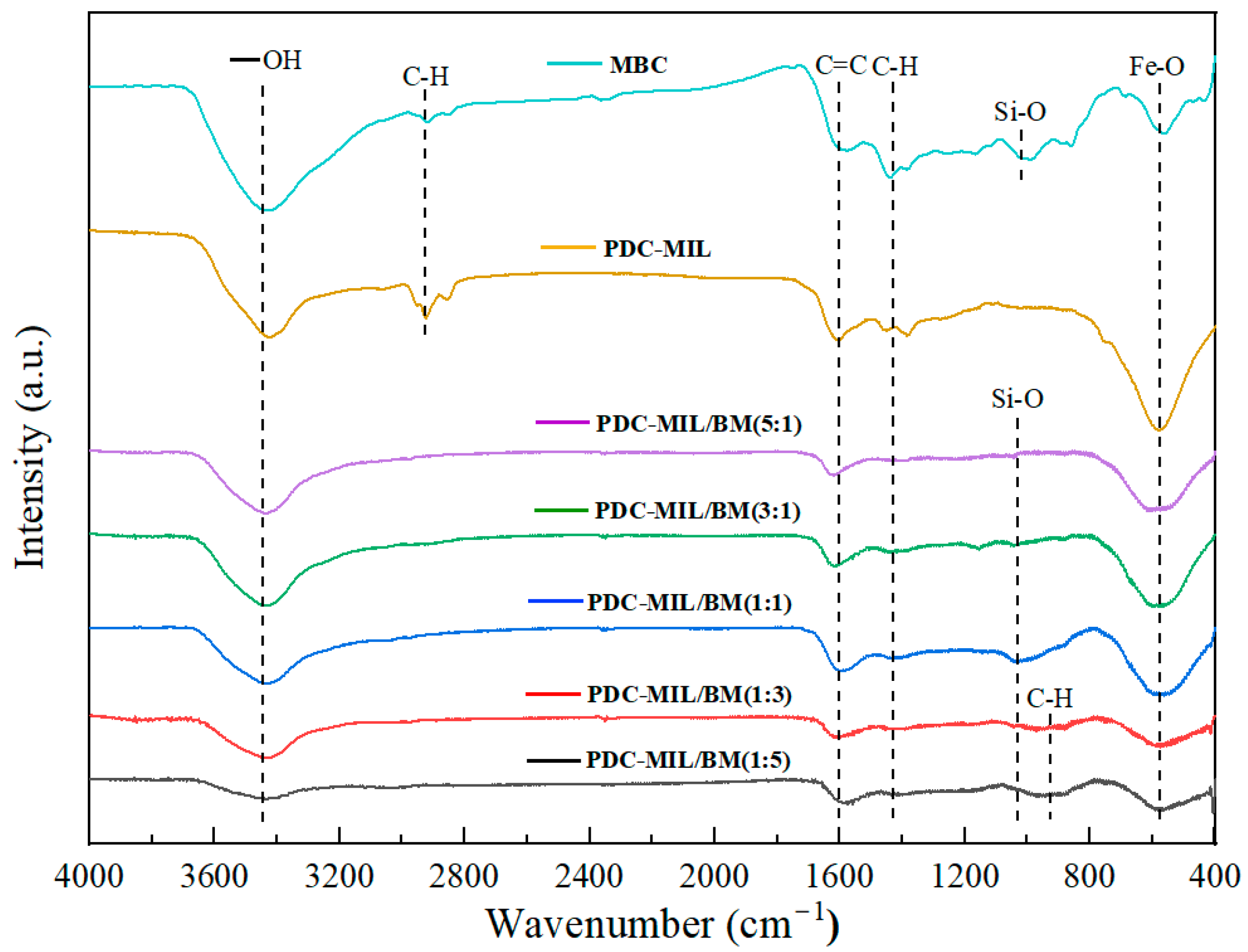
3.3. Surface Chemical Characteristics
3.4. Hg0 Removal Performance
3.4.1. Influence of Adsorption Temperature on Mercury Removal Performance of Materials
3.4.2. Effect of Composite Ratio on Mercury Removal Performance of Composite Materials
4. Conclusions
- By using the sol-gel method, MIL-101(Cr) and biomass were combined in different proportions and doped with multiple metal ions, and high performance Hg0 adsorbent was prepared in a wide temperature range. Among them, PDC-MIL/BM (1:1) has excellent pore structure and good pore homogeneity in the material, which makes the physical adsorption performance of the material good at a low temperature and also reduces the desorption of Hg0 caused by a high temperature, thus enhancing the adsorption performance of the material at high temperatures. The cumulative adsorption capacity of Hg0 reached 192.56 μg/g at 200 °C for 3 h;
- MIL-101(Cr) will act as a catalyst that influences the gel formation process. When the proportion of MOFs is small, the gel formation proceeds slowly, leading to the particles’ aggregation in the sol, which compromises the uniformity and dispersion of the material. However, a high proportion of MOFs accelerates the gel excessively, impacting the growth of the gel’s skeletal structure and resulting in the uneven internal structure of the material. When the ratio of the two is 1:1, the gel skeleton develops effectively, and SiO2 is well dispersed between the MOFs and biochar. On the one hand, the existence of SiO2 plays a role in forming a bridge between the MOFs and biomass; on the other hand, it can facilitate the distribution of the composite material. It mitigates the volume shrinkage and metal agglomeration caused by high temperature pyrolysis, enhances the mechanical properties of the material, and promotes the formation of a three-dimensional interconnected network and a uniformly distributed pore structure;
- Pore structure and active sites are the key factors affecting the adsorption performance of Hg0. Excellent pore structure can reduce the desorption of Hg0 caused by high temperatures on the one hand and increase the contact probability between Hg0 and active sites on the other hand so that the material can exhibit excellent Hg0-removal characteristics at both low and high temperatures. The metal ions introduced in the composite process and the excellent pore structure formed by the coupling of MOFs and biochar together constitute the efficient adsorption performance of the composites in a wide temperature range.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Z.; Sun, P.; Chen, S.; Shi, L. Research on Mercury Emissions Regularity and Adsorbing Mercury by Activated Carbon in Coal-Fired Power Plants. In Proceedings of the Materials and Design, PTS 1–3; Sang, X.M., Wang, P.C., Ai, L., Li, Y.G., Bu, J.L., Eds.; Trans Tech Publications Ltd.: Durnten-Zurich, Switzerland, 2011; Volume 284–286, p. 301. [Google Scholar]
- Li, Y.; Yu, J.; Liu, Y.; Huang, R.; Wang, Z.; Zhao, Y. A Review on Removal of Mercury from Flue Gas Utilizing Existing Air Pollutant Control Devices (APCDs). J. Hazard. Mater. 2022, 427, 128132. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ma, C.; Zhou, J.; Zhu, L.; Cao, L.; Yang, J. Ultra-High Adsorption of Hg0 Using Impregnated Activated Carbon by Selenium. Environ. Sci. Pollut. Res. 2022, 29, 69450–69461. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zeng, X.; Luo, G.; Xu, Y.; Li, X.; Yao, H. Study on the Effects of Carrier and Modifier on Mercury Adsorption Behavior over Halides Modified Sorbents Using Temperature Programmed Desorption Method. Fuel Process. Technol. 2018, 178, 293–300. [Google Scholar] [CrossRef]
- Liu, D.; Xu, K.; Mae, J.; Liu, Q.; Fan, Y.; Wang, C.; Wang, X.; Jin, J.; Shi, H. Advances in Rational Design of Catalysts for Efficient Hg0 Removal. Fuel 2023, 331, 125922. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, B.; Zhu, S.; Xu, H.; Tian, L. UiO-66 and Its Br-Modified Derivates for Elemental Mercury Removal. J. Hazard. Mater. 2016, 320, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.; Dong, M.; Zhao, T. Advances in Metal-Organic Frameworks MIL-101(Cr). Int. J. Mol. Sci. 2022, 23, 9396. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Mei, J.; Xu, H.; Liu, W.; Qu, Z.; Cui, Y.; Yan, N. Research of Mercury Removal from Sintering Flue Gas of Iron and Steel by the Open Metal Site of Mil-101(Cr). J. Hazard. Mater. 2018, 351, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Liu, Y.; Xiao, Y.; Zhong, L.; Wang, T.; Wang, Y.; Pan, W.-P. Hg0 Removal Performance at Low Temperature by Mesoporous MOF-Derived Cr2O3 Nanoparticle with Enriched Metal Sites and Oxygen Vacancy: Mechanism Study on the Role of Oxygen Species. Chem. Eng. J. 2023, 467, 143402. [Google Scholar] [CrossRef]
- Song, X.; Zhou, Y.; Li, L. Synthesis of Core-Shell Metal-Organic Frameworks. Prog. Chem. 2014, 26, 424–435. [Google Scholar]
- Cui, Y.; Huo, Q.; Chen, H.; Chen, S.; Wang, S.; Wang, J.; Chang, L.; Han, L.; Xie, W. Biomass Carbon Magnetic Adsorbent Constructed by One-Step Activation Method for the Removal of Hg0 in Flue Gas. ACS Omega 2022, 7, 9244–9253. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Li, C.; Zhao, L.; Gao, L.; Du, X.; Zhang, J.; Tang, L.; Zeng, G. Removal of Elemental Mercury from Simulated Flue Gas over Peanut Shells Carbon Loaded with Iodine Ions, Manganese Oxides, and Zirconium Dioxide. Energy Fuels 2017, 31, 13909–13920. [Google Scholar] [CrossRef]
- Jia, L.; Yu, Y.; Li, Z.-P.; Qin, S.-N.; Guo, J.-R.; Zhang, Y.-Q.; Wang, J.-C.; Zhang, J.-C.; Fan, B.-G.; Jin, Y. Study on the Hg0 Removal Characteristics and Synergistic Mechanism of Iron-Based Modified Biochar Doped with Multiple Metals. Bioresour. Technol. 2021, 332, 125086. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Hu, Q.; Gao, L.; Hao, Q.; Wang, P.; Qin, D. Adsorption Characteristics of Metal-Organic Framework MIL-101(Cr) towards Sulfamethoxazole and Its Persulfate Oxidation Regeneration. RSC Adv. 2018, 8, 27623–27630. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Li, J.; Cheng, P.; Nie, H.; He, L.; Yan, Q.; Zheng, Y.; Wu, Y.; Jia, L. Preparation and Mercury Removal Performance of Mg-MOF-74 Composites. Atmosphere 2023, 14, 1551. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, B.; Jia, L.; Qiao, X.; Li, Z. Study on Adsorption Mechanism of Mercury on Ce-Cu Modified Iron-Based Biochar. Chem. Eng. J. Adv. 2022, 10, 100259. [Google Scholar] [CrossRef]
- GokirmakSogut, E. Superior Adsorption Efficiency of MIL-101 (Cr) and Nano-MIL-101 (Cr) in Anionic and Cationic Dye Removal from Aqueous Solution. ChemistrySelect 2023, 8, e202205000. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Z.; Feng, Y.; Yu, J.; Zhang, X.; Wen, J.; Nie, H.; Yu, Y.; Jia, L. A Novel Composite Material UiO-66-Br@MBC for Mercury Removal from Flue Gas: Preparation and Mechanism. Polymers 2024, 16, 2508. [Google Scholar] [CrossRef]
- Tarzanagh, Y.J.; Seifzadeh, D.; Rajabalizadeh, Z.; Habibi-Yangjeh, A.; Khodayari, A.; Sohrabnezhad, S. Sol-Gel/MOF Nanocomposite for Effective Protection of 2024 Aluminum Alloy against Corrosion. Surf. Coat. Technol. 2019, 380, 125038. [Google Scholar] [CrossRef]
- Zhang, J. Preparation and Properties of Magnesium Oxide Aerogels and MgO-Al2O3-SiO2 Aerogels. Master’s Thesis, Department of Materials Science and Engineering, Beijing University of Chemical Technology, Beijing, China, 2017. [Google Scholar]


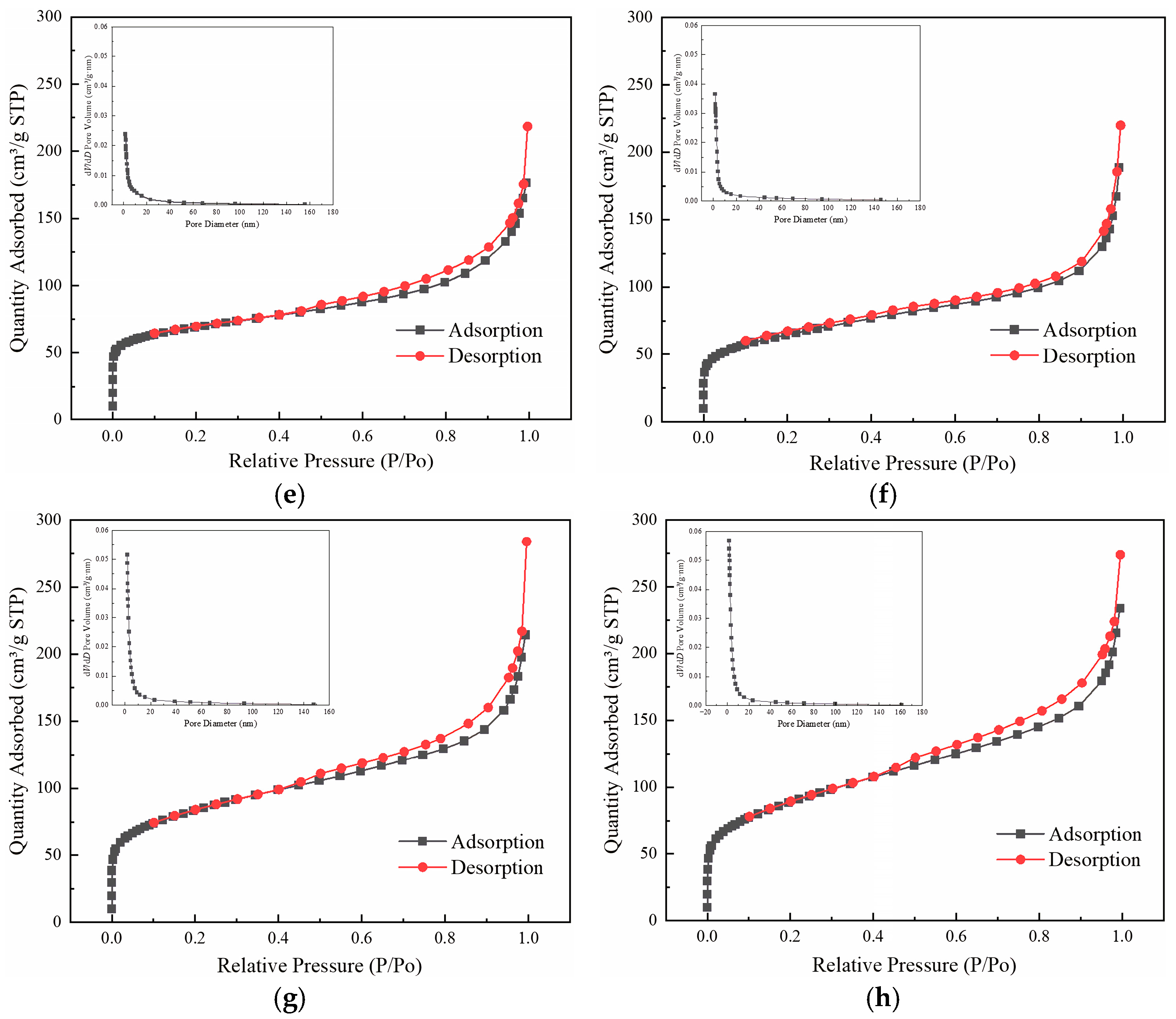


| Sample | BET Specific Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Size (nm) | Relative Specific Pore Volume (%) | ||
|---|---|---|---|---|---|---|
| Microporous | Mesoporous | Macroporous | ||||
| MIL-101(Cr) | 2438.67 | 1.236 | 2.50 | 60.88 | 29.68 | 9.44 |
| MBC | 107.76 | 0.112 | 11.20 | 2.87 | 64.12 | 33.01 |
| PDC-MIL | 333.39 | 0.348 | 5.56 | 4.33 | 84.89 | 10.78 |
| PDC-MIL/BM(1:5) | 192.19 | 0.163 | 8.57 | 3.91 | 63.39 | 32.70 |
| PDC-MIL/BM(1:3) | 225.05 | 0.210 | 8.18 | 4.05 | 66.63 | 29.32 |
| PDC-MIL/BM(1:1) | 268.72 | 0.231 | 7.59 | 5.16 | 68.42 | 26.42 |
| PDC-MIL/BM(3:1) | 284.11 | 0.251 | 6.44 | 8.61 | 67.54 | 27.85 |
| PDC-MIL/BM(5:1) | 306.17 | 0.277 | 6.06 | 9.53 | 64.27 | 26.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nie, H.; Li, Z.; Zhang, X.; Wen, J.; Feng, Y.; Yu, Y.; Jia, L. Preparation and Hg0 Removal Performance of MIL-101(Cr)-Derived Carbon Matrix Composites. Polymers 2025, 17, 413. https://doi.org/10.3390/polym17030413
Nie H, Li Z, Zhang X, Wen J, Feng Y, Yu Y, Jia L. Preparation and Hg0 Removal Performance of MIL-101(Cr)-Derived Carbon Matrix Composites. Polymers. 2025; 17(3):413. https://doi.org/10.3390/polym17030413
Chicago/Turabian StyleNie, Haotian, Zikuo Li, Xikai Zhang, Jinchao Wen, Youxiang Feng, Yue Yu, and Li Jia. 2025. "Preparation and Hg0 Removal Performance of MIL-101(Cr)-Derived Carbon Matrix Composites" Polymers 17, no. 3: 413. https://doi.org/10.3390/polym17030413
APA StyleNie, H., Li, Z., Zhang, X., Wen, J., Feng, Y., Yu, Y., & Jia, L. (2025). Preparation and Hg0 Removal Performance of MIL-101(Cr)-Derived Carbon Matrix Composites. Polymers, 17(3), 413. https://doi.org/10.3390/polym17030413









