Copper(II)-Complexed Polyethylenimine-Entrapped Gold Nanoparticles Enable Targeted CT/MR Imaging and Chemodynamic Therapy of Tumors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of PEI.NH2-FI-(PEG-FA)-PA
2.2. Formation of FA-Au/Cu(II) PENPs
2.3. Cu(II) Release Property In Vitro
2.4. FA-Au/Cu(II) PENPs-Mediated GSH Depletion
2.5. Generation of ·OH by Fenton-like Reaction
2.6. Targeted CT/MR Imaging In Vitro
2.7. Cytotoxicity Assay and Cell Morphology Analysis
2.8. Cell Apoptosis Assay
2.9. Determination of Intracellular ROS and LPO Levels
2.10. Targeted CT/MR Imaging In Vivo
2.11. CDT of Tumor In Vivo
3. Results and Discussion
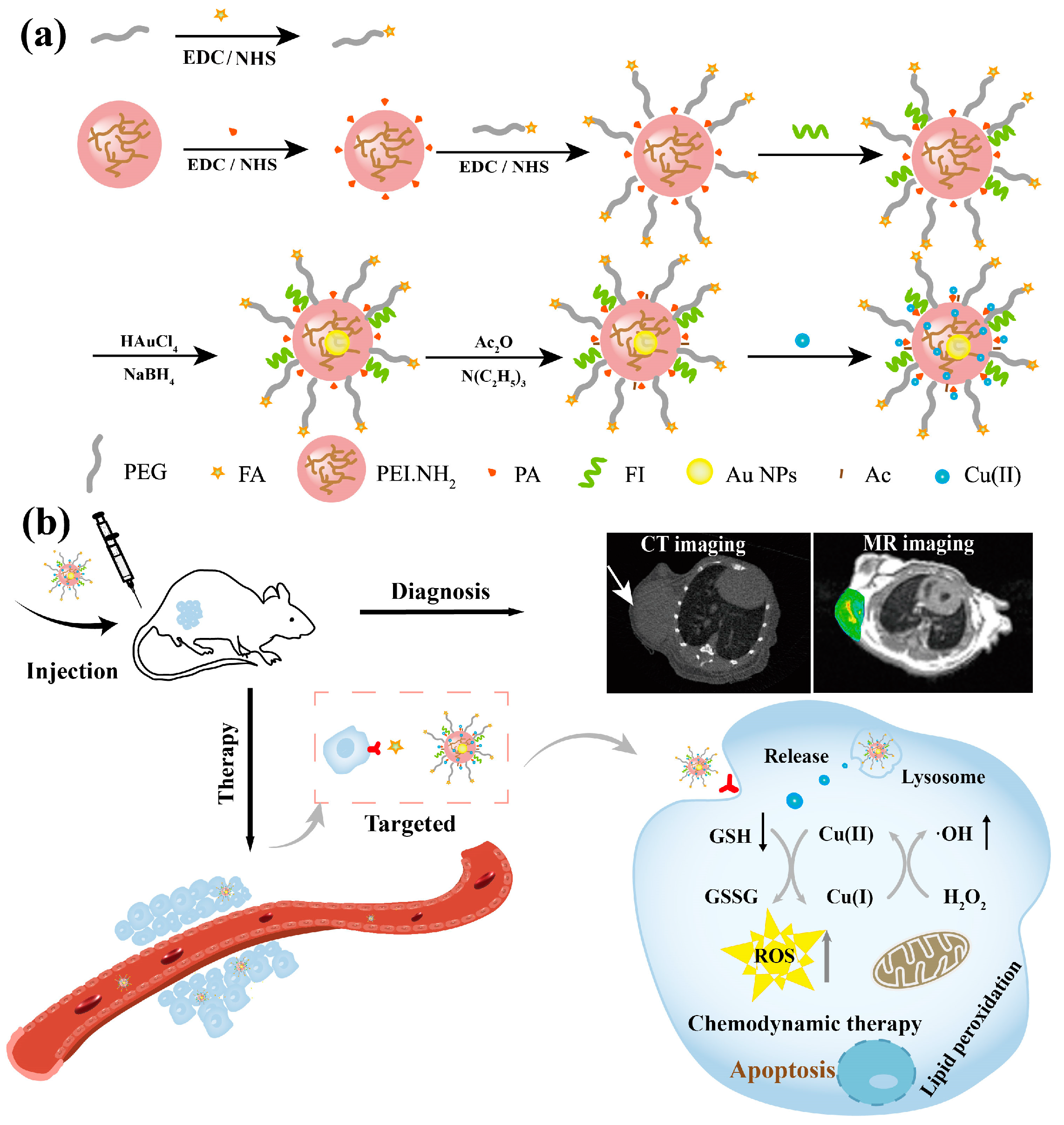
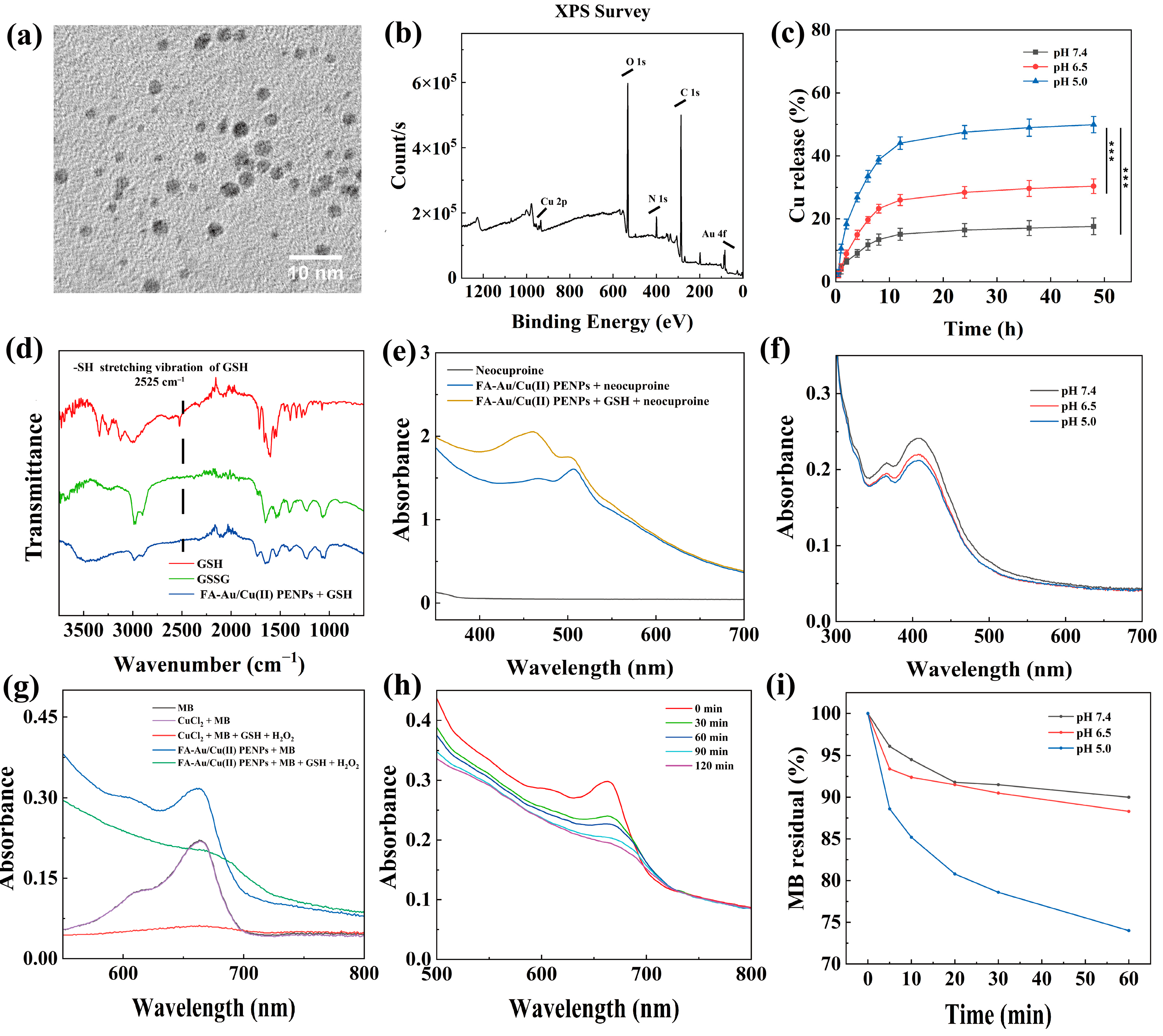
3.1. Preparation and Characterization of FA-Au/Cu(II) PENPs
3.2. In Vitro Stability and Hemocompatibility Assessments
3.3. Cu(II) Release Study In Vitro
3.4. FA-Au/Cu(II) PENPs-Mediated GSH Depletion and ·OH Generation
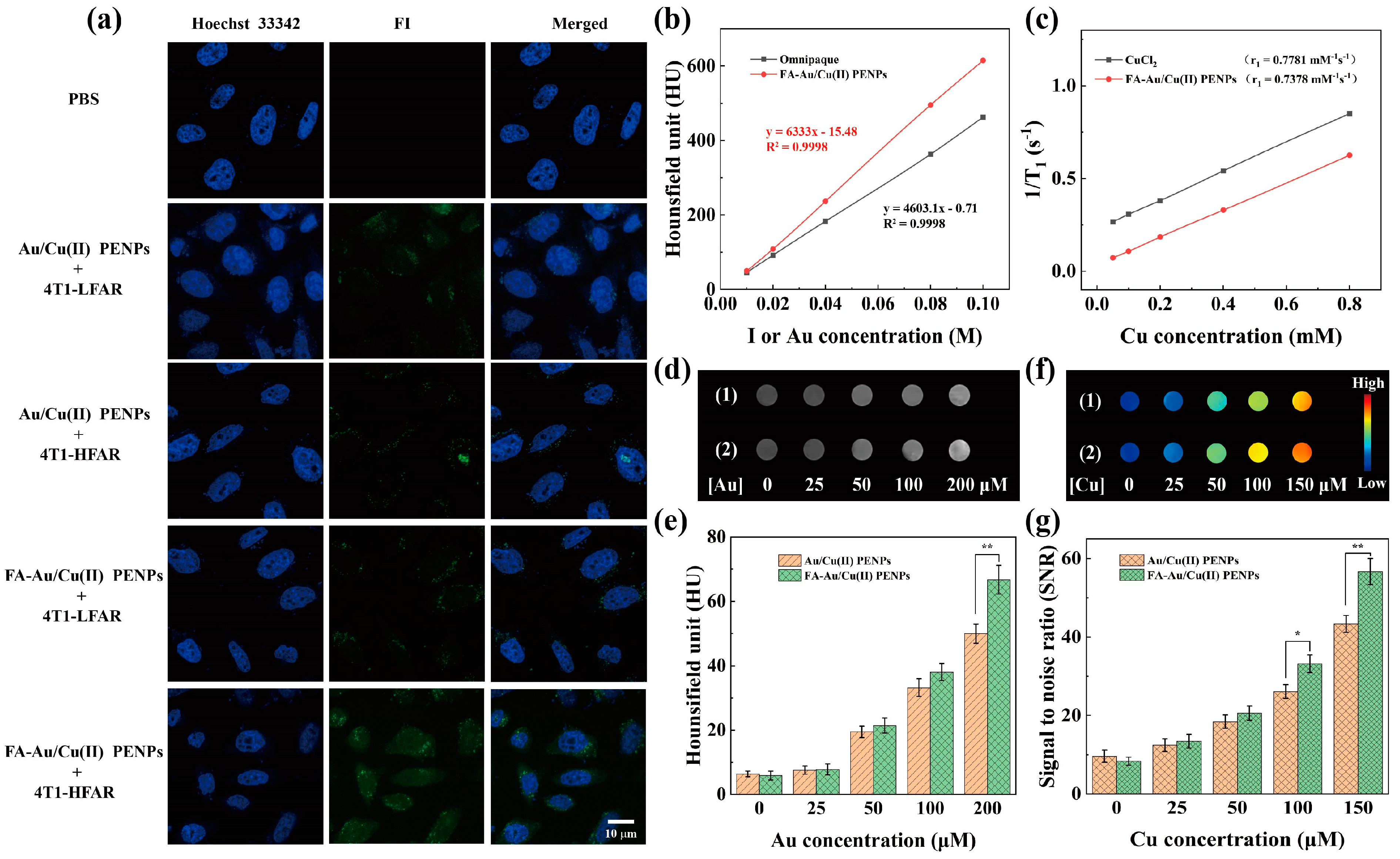
3.5. Targeting Specificity and CT/MR Imaging In Vitro
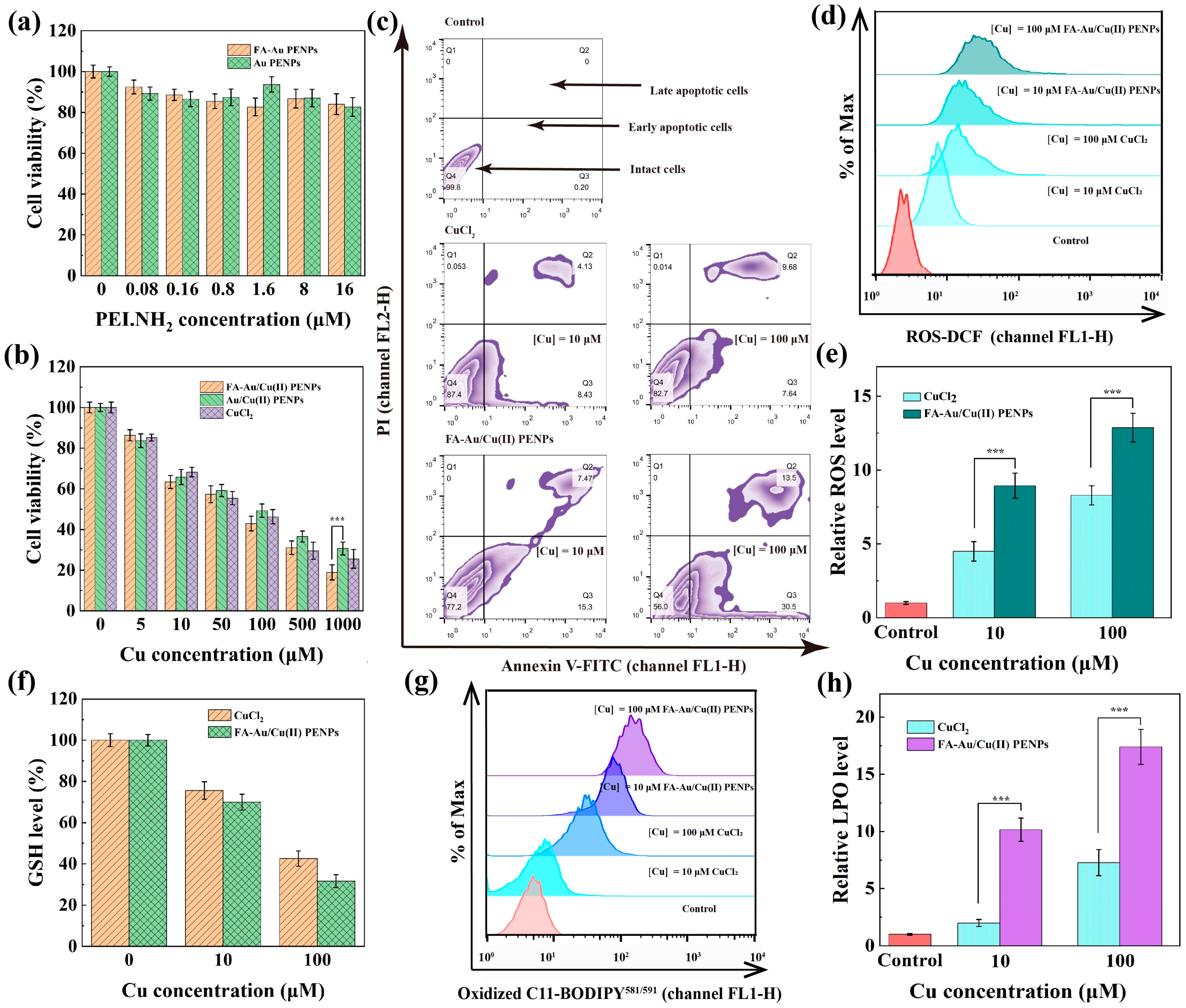
3.6. FA-Au/Cu(II) PENPs-Mediated CDT In Vitro
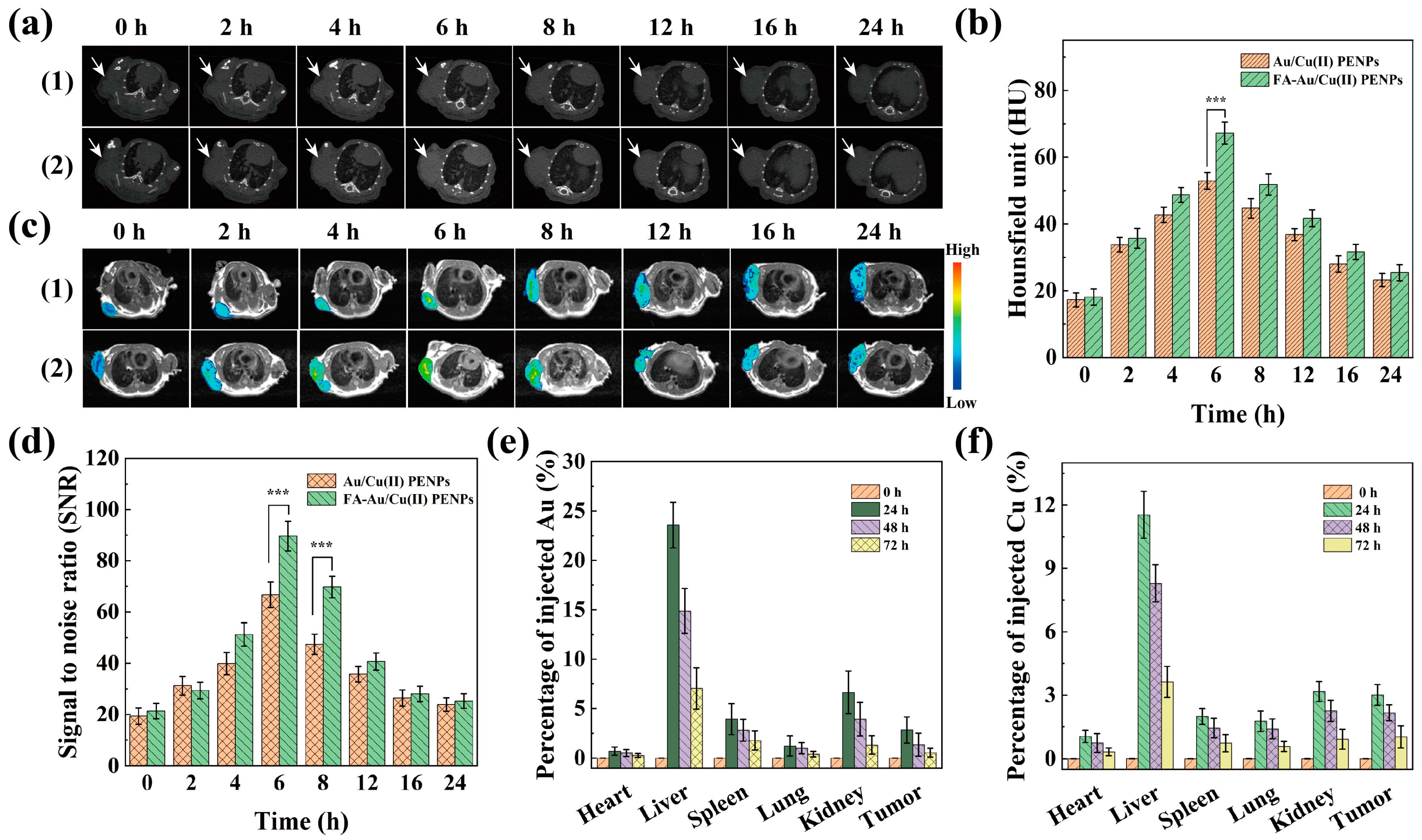
3.7. Targeted CT/MR Imaging In Vivo and Biodistribution
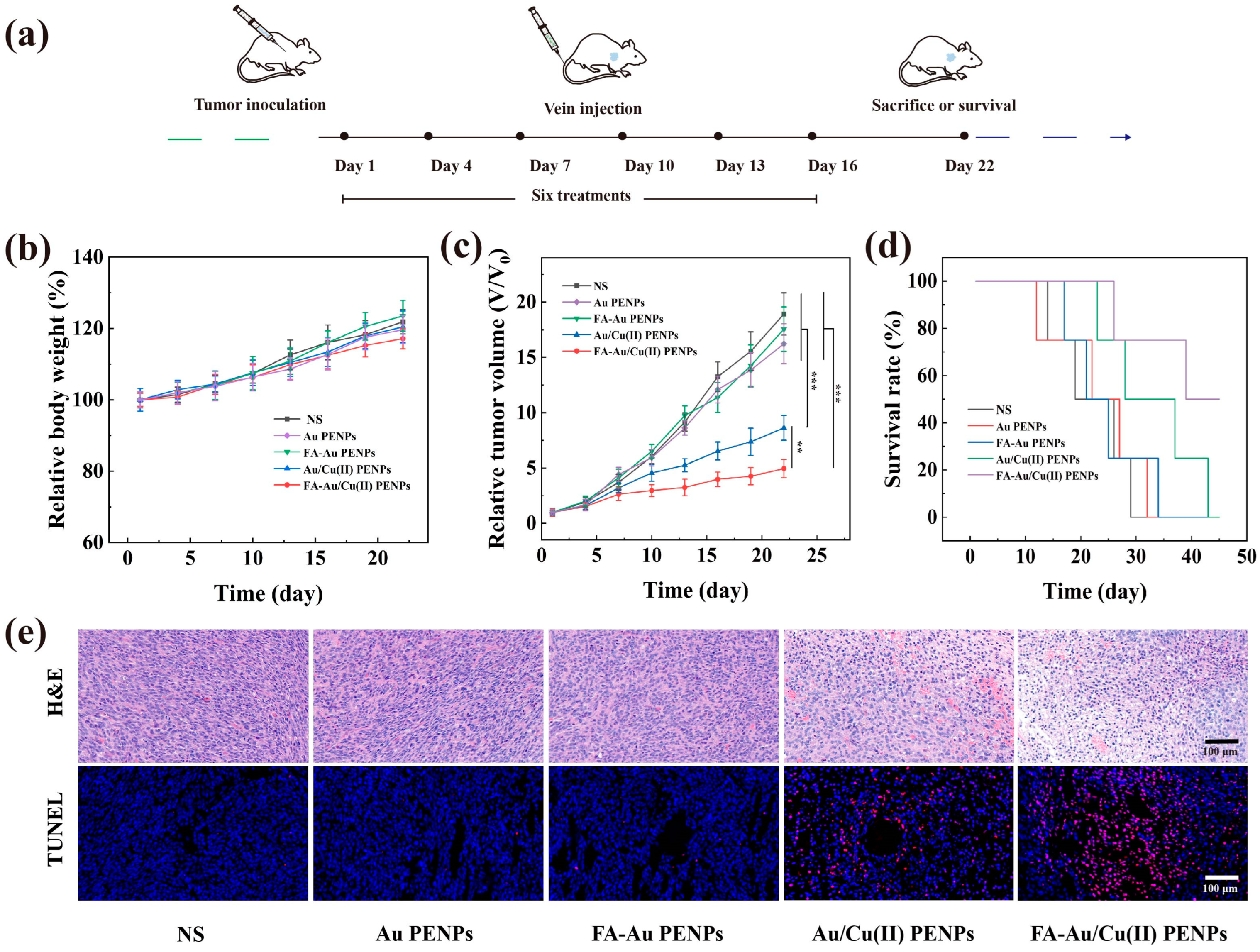
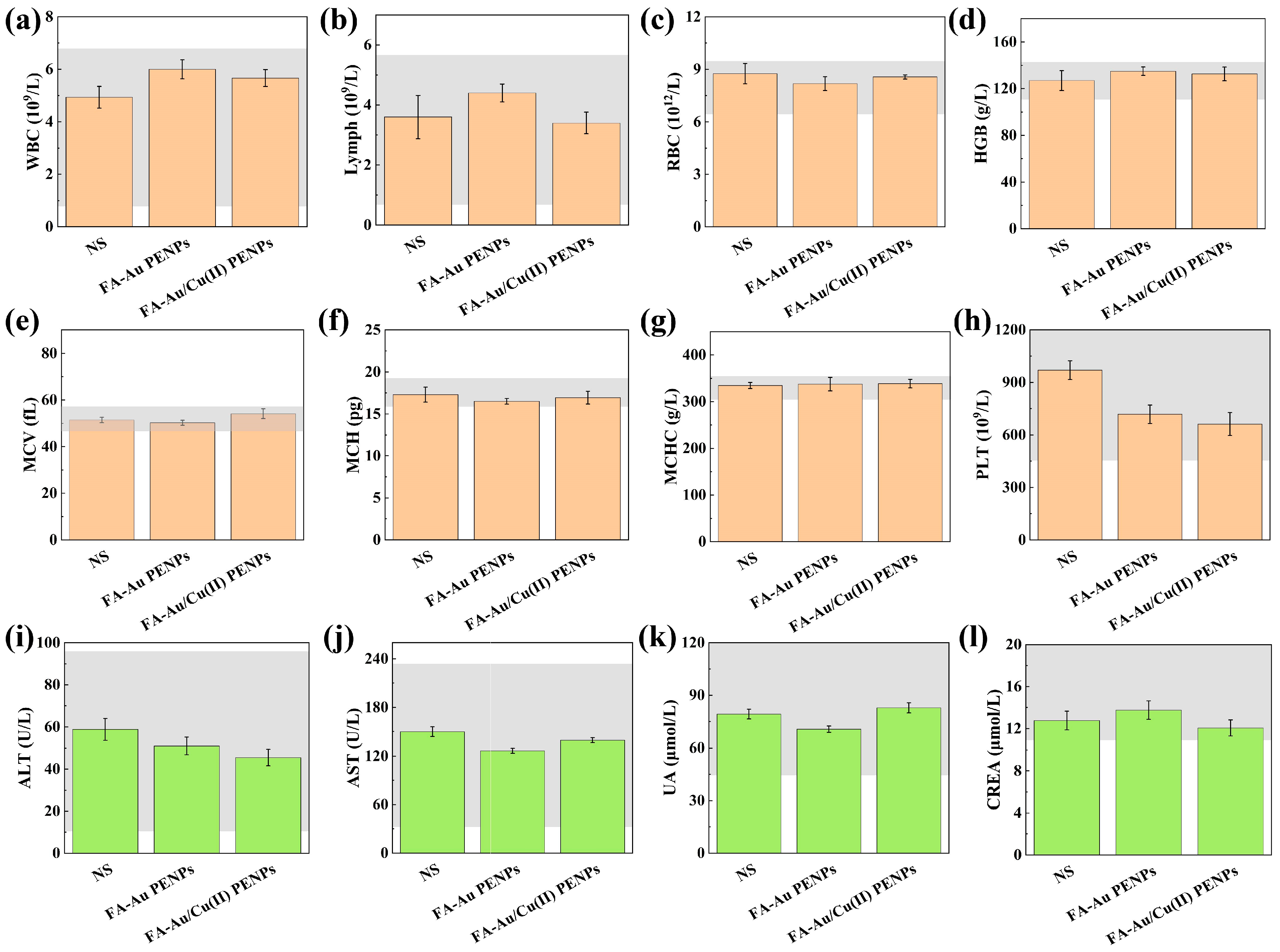
3.8. CDT of Tumor In Vivo and Biosafety Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, J.C.; Wang, S.Q.; Fontana, F.; Tapeinos, C.; Shahbazi, M.A.; Han, H.J.; Santos, H.A. Nanoparticles-based phototherapy systems for cancer treatment: Current status and clinical potential. Bioact. Mater. 2023, 23, 471–507. [Google Scholar] [CrossRef]
- Hegde, M.; Naliyadhara, N.; Unnikrishnan, J.; Alqahtani, M.S.; Abbas, M.; Girisa, S.; Sethi, G.; Kunnumakkara, A.B. Nanoparticles in the diagnosis and treatment of cancer metastases: Current and future perspectives. Cancer Lett. 2023, 556, 216066. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Muhammad, K.; Waheed, Y. Emerging applications of nanotechnology in healthcare and medicine. Molecules 2023, 28, 6624. [Google Scholar] [CrossRef] [PubMed]
- Escutia-Gutiérrez, R.; Sandoval-Rodríguez, A.; Zamudio-Ojeda, A.; Guevara-Martínez, S.J.; Armendáriz-Borunda, J. Advances of nanotechnology in the diagnosis and treatment of hepatocellular carcinoma. J. Clin. Med. 2023, 12, 6867. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.M.; Mohammadnejad, J.; Salamat, S.; Zadeh, Z.B.; Tanhaei, M.; Ramakrishna, S. Theranostic polymeric nanoparticles as a new approach in cancer therapy and diagnosis: A review. Mater. Today Chem. 2023, 29, 101400. [Google Scholar] [CrossRef]
- Lee, H.; Park, B.; Lee, J.; Kang, Y.; Han, M.; Lee, J.; Kim, C.; Kim, W.J. Transcytosis-inducing multifunctional albumin nanomedicines with deep penetration ability for image-guided solid tumor treatment. Small 2023, 19, 2303668. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.; Rocchi, P.; Carmès, L.; Guthier, R.; Iyer, M.; Seban, L.; Morris, T.; Bennett, S.; Lavelle, M.; Penailillo, J.; et al. Tuning ultrasmall theranostic nanoparticles for MRI contrast and radiation dose amplification. Theranostics 2023, 13, 4711–4729. [Google Scholar] [CrossRef]
- Li, Y.; Pan, X.; Hai, P.; Zheng, Y.; Shan, Y.; Zhang, J. All-in-one nanotheranostic platform based on tumor microenvironment: New strategies in multimodal imaging and therapeutic protocol. Drug Discov. Today 2024, 29, 104029. [Google Scholar] [CrossRef] [PubMed]
- Pranav; Laskar, P.; Jaggi, M.; Chauhan, S.C.; Yallapu, M.M. Biomolecule-functionalized nanoformulations for prostate cancer theranostics. J. Adv. Res. 2023, 51, 197–217. [Google Scholar] [CrossRef]
- Scialla, S.; Hanafy, M.S.; Wang, J.L.; Genicio, N.; Da Silva, M.C.; Costa, M.; Oliveira-Pinto, S.; Baltazar, F.; Gallo, J.; Cui, Z.R.; et al. Targeted treatment of triple-negative-breast cancer through pH-triggered tumour associated macrophages using smart theranostic nanoformulations. Int. J. Pharm. 2023, 632, 122575. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, Y.; He, C.X.; Zhang, X.M.; Li, X.; Li, Y.Y.; Tang, Y.F.; Lu, X.M.; Fan, Q.L. Small-molecule phototheranostic agent with extended π-conjugation for efficient NIR-II photoacoustic-imaging-guided photothermal therapy. Small 2024, 20, 2307829. [Google Scholar] [CrossRef]
- Chavda, V.P.; Balar, P.C.; Nalla, L.V.; Bezbaruah, R.; Gogoi, N.R.; Gajula, S.N.R.; Peng, B.R.; Meena, A.S.; Conde, J.; Prasad, R. Conjugated nanoparticles for solid tumor theranostics: Unraveling the interplay of known and unknown factors. ACS Omega 2023, 8, 37654–37684. [Google Scholar] [CrossRef] [PubMed]
- Caballero, D.; Abreu, C.M.; Lima, A.C.; Neves, N.N.; Reis, R.L.; Kundu, S.C. Precision biomaterials in cancer theranostics and modelling. Biomaterials 2022, 280, 121299. [Google Scholar] [CrossRef] [PubMed]
- Guidolin, K.; Zheng, G. Nanomedicines lost in translation. ACS Nano 2019, 13, 13620–13626. [Google Scholar] [CrossRef] [PubMed]
- Gawne, P.J.; Ferreira, M.; Papaluca, M.; Grimm, J.; Decuzzi, P. New opportunities and old challenges in the clinical translation of nanotheranostics. Nat. Rev. Mater. 2023, 8, 783–798. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.Y.; Song, Y.N.; Hu, Y.L.; Chen, H.J.; Yang, D.L.; Song, X.J. Multifaceted roles of copper ions in anticancer nanomedicine. Adv. Healthc. Mater. 2023, 12, 2300410. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.F.; Xie, N.L.; Zhou, W.H. Copper coordination-based nanomedicine for tumor theranostics. Adv. Ther. 2024, 7, 2300305. [Google Scholar] [CrossRef]
- Zhou, Y.; Fan, S.; Feng, L.; Huang, X.; Chen, X. Manipulating intratumoral fenton chemistry for enhanced chemodynamic and chemodynamic-synergized multimodal therapy. Adv. Mater. 2021, 33, 2104223. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.B.; Zhao, Y.C.; Yao, S.C.; Wang, Z.; Zhang, Z.Y.; Wen, K.K.; Ma, B.J.; Li, L.L. Chirality of copper-amino acid nanoparticles determines chemodynamic cancer therapeutic outcome. Small 2024, 20, 2309328. [Google Scholar] [CrossRef]
- He, T.; Tang, Q.A.; Ren, Q.J.; Liu, Y.R.; He, G.; Pan, Y.T.; Wang, Z.G.; Huang, P.; Lin, J. Different valence states of copper ion delivery against triple-negative breast cancer. ACS Nano 2024, 18, 5434–5445. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, D.; Zhang, S.; Cheng, Y.; Yang, F.; Xing, Y.; Xu, T.; Dong, H.; Zhang, X. Biodegradable biomimic copper/manganese silicate nanospheres for chemodynamic/photodynamic synergistic therapy with simultaneous glutathione depletion and hypoxia relief. ACS Nano 2019, 13, 4267–4277. [Google Scholar] [CrossRef]
- Zhao, P.; Li, H.; Bu, W. A forward vision for chemodynamic therapy: Issues and opportunities. Angew. Chem. Int. Ed. 2023, 62, e202210415. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, L.; Zhang, R.; Xu, Z.P. The adjacent effect between Gd(III) and Cu(II) in layered double hydroxide nanoparticles synergistically enhances T1-weighted magnetic resonance imaging contrast. Nanoscale Horiz. 2023, 8, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Tian, M.; Li, C. Copper-based nanomaterials for cancer imaging and therapy. Bioconjugate Chem. 2016, 27, 1188–1199. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.B.; Li, J.; Wang, Q.C.; Yang, K. A review: Research on MR-compatible alloys in MRI. Acta Metall. Sin. 2017, 53, 1323–1330. [Google Scholar]
- Chang, J.; Yin, W.M.; Zhi, H.; Chen, S.Y.; Sun, J.Y.; Zhao, Y.G.; Huang, L.; Xue, L.Y.; Zhang, X.Y.; Zhang, T.T.; et al. Copper deposition in polydopamine nanostructure to promote cuproptosis by catalytically inhibiting copper exporters of tumor cells for cancer immunotherapy. Small 2024, 20, 2308565. [Google Scholar] [CrossRef]
- Xiong, Z.J.; Shen, M.W.; Shi, X.Y. Zwitterionic modification of nanomaterials for improved diagnosis of cancer cells. Bioconjugate Chem. 2019, 30, 2519–2527. [Google Scholar] [CrossRef]
- Mekuria, S.L.; Li, G.M.; Wang, Z.Q.; Girma, W.M.; Li, A.Y.; He, M.J.; Wang, H.; Hameed, M.M.A.; El-Newehy, M.; Shi, X.Y.; et al. Dendrimer nanoclusters loaded with gold nanoparticles for enhanced tumor CT imaging and chemotherapy via an amplified EPR effect. J. Mater. Chem. B 2024, 12, 9524–9532. [Google Scholar] [CrossRef]
- Jin, Y.; Adams, F.; Moeller, J.; Isert, L.; Zimmermann, C.M.M.; Keul, D.; Merkel, O.M.M. Synthesis and application of low molecular weight PEI-based copolymers for siRNA delivery with smart polymer blends. Macromol. Biosci. 2023, 23, 2200409. [Google Scholar] [CrossRef] [PubMed]
- Li, J.C.; Yu, X.R.; Shi, X.Y.; Shen, M.W. Cancer nanomedicine based on polyethylenimine-mediated multifunctional nanosystems. Prog. Mater. Sci. 2022, 124, 100871. [Google Scholar] [CrossRef]
- Chen, Z.; Lv, Z.; Sun, Y.; Chi, Z.; Qing, G. Recent advancements in polyethyleneimine-based materials and their biomedical, biotechnology, and biomaterial applications. J. Mater. Chem. B 2020, 8, 2951–2973. [Google Scholar] [CrossRef] [PubMed]
- Han, T.Y.; Hou, L.S.; Li, J.X.; Huan, M.L.; Zhou, S.Y.; Zhang, B.L. Bone targeted miRNA delivery system for miR-34a with enhanced anti-tumor efficacy to bone-associated metastatic breast cancer. Int. J. Pharm. 2023, 635, 122755. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Zhao, L.Z.; Yang, J.X.; Chen, L.; Shi, J.H.; Zhao, J.H.; Shi, X.Y. 99mTc-labeled polyethylenimine-entrapped gold nanoparticles with pH-responsive charge conversion property for enhanced dual mode SPECT/CT imaging of cancer cells. Langmuir 2019, 35, 13405–13412. [Google Scholar] [CrossRef]
- Li, L.L.; Gao, Y.; Zhang, Y.M.; Yang, R.; Ouyang, Z.J.; Guo, R.; Yu, H.W.; Shi, X.Y.; Cao, X.Y. A biomimetic nanogel system restores macrophage phagocytosis for magnetic resonance imaging-guided synergistic chemoimmunotherapy of breast cancer. Adv. Healthc. Mater. 2023, 12, 2300967. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Zheng, L.F.; Wen, S.H.; Tang, Y.Q.; Shen, M.W.; Zhang, G.X.; Shi, X.Y. Targeted cancer theranostics using alpha-tocopheryl succinate-conjugated multifunctional dendrimer-entrapped gold nanoparticles. Biomaterials 2014, 35, 7635–7646. [Google Scholar] [CrossRef]
- Zhu, J.; Zhao, L.; Zhao, P.; Yang, J.; Shi, J.; Zhao, J. Charge-conversional polyethylenimine-entrapped gold nanoparticles with 131I-labeling for enhanced dual mode SPECT/CT imaging and radiotherapy of tumors. Biomater. Sci. 2020, 8, 3956–3965. [Google Scholar] [CrossRef]
- Song, C.; Ouyang, Z.; Gao, Y.; Guo, H.; Wang, S.; Wang, D.; Xia, J.; Shen, M.; Shi, X. Modular design of multifunctional core-shell tecto dendrimers complexed with copper(II) for MR imaging-guided chemodynamic therapy of orthotopic glioma. Nano Today 2021, 41, 101325. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, J.; Shi, M.; Li, D.; Lu, C.; Cao, X.; Peng, C.; Mignani, S.; Majoral, J.-P.; Shi, X. Poly(amidoamine) dendrimer-coordinated copper(II) complexes as a theranostic nanoplatform for the radiotherapy-enhanced magnetic resonance imaging and chemotherapy of tumors and tumor metastasis. Nano Lett. 2019, 19, 1216–1226. [Google Scholar] [CrossRef]
- Wang, L.; Xu, Y.; Liu, C.; Si, W.; Wang, W.; Zhang, Y.; Zhong, L.; Dong, X.; Zhao, Y. Copper-doped MOF-based nanocomposite for GSH depleted chemo/photothermal/chemodynamic combination therapy. Chem. Eng. J. 2022, 438, 135567. [Google Scholar] [CrossRef]
- Cai, X.Y.; Cai, D.; Wang, X.Z.; Zhang, D.; Qiu, L.; Diao, Z.Y.; Liu, Y.; Sun, J.B.; Cui, D.X.; Liu, Y.L.; et al. Manganese self-boosting hollow nanoenzymes with glutathione depletion for synergistic cancer chemo-chemodynamic therapy. Biomater. Sci. 2024, 12, 3622–3632. [Google Scholar] [CrossRef]
- Fan, Y.; Tu, W.Z.; Shen, M.W.; Chen, X.M.; Ning, Y.S.; Li, J.J.; Chen, T.F.; Wang, H.; Yin, F.F.; Liu, Y.; et al. Targeted tumor hypoxia dual-mode CT/MR imaging and enhanced radiation therapy using dendrimer-based nanosensitizers. Adv. Funct. Mater. 2020, 30, 1909285. [Google Scholar] [CrossRef]
- Diallo, M.S.; Christie, S.; Swaminathan, P.; Balogh, L.; Shi, X.Y.; Um, W.; Papelis, C.; Goddard, W.A.; Johnson, J.H. Dendritic chelating agents. 1. Cu(II) binding to ethylene diamine core poly(amidoamine) dendrimers in aqueous solutions. Langmuir 2004, 20, 2640–2651. [Google Scholar] [CrossRef]
- Ni, C.; Ouyang, Z.J.; Li, G.M.; Liu, J.J.; Cao, X.Y.; Zheng, L.F.; Shi, X.Y.; Guo, R. A tumor microenvironment-responsive core-shell tecto dendrimer nanoplatform for magnetic resonance imaging-guided and cuproptosis-promoted chemo-chemodynamic therapy. Acta Biomater. 2023, 164, 474–486. [Google Scholar] [CrossRef] [PubMed]
- Mo, Y.Y.; Zhang, Y.; Vincent, T.; Faur, C.; Guibal, E. Investigation of mercury(II) and copper(II) sorption in single and binary systems by alginate/polyethylenimine membranes. Carbohydr. Polym. 2021, 257, 117588. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.Y.; Wang, G.; Alves, C.S.; Tomas, H.; Long, Z.J.; Shen, M.W.; Rodrigues, J.; Shi, X.Y. Multifunctional dendrimer-entrapped gold nanoparticles conjugated with doxorubicin for pH-responsive drug delivery and targeted computed tomography imaging. Langmuir 2018, 34, 12428–12435. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-H.; Altukhov, O.; Cheng, C.-C.; Chang, F.-C.; Kuo, S.-W. Supramolecular structures of uracil-functionalized PEG with multi-diamidopyridine POSS through complementary hydrogen bonding interactions. Soft Matter 2013, 9, 5196–5206. [Google Scholar] [CrossRef]
- Hao, Y.; Gao, Y.; Fan, Y.; Zhang, C.; Zhan, M.; Cao, X.; Shi, X.; Guo, R. A tumor microenvironment-responsive poly(amidoamine) dendrimer nanoplatform for hypoxia-responsive chemo/chemodynamic therapy. J. Nanobiotechnol. 2022, 20, 43. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Qu, C.; Shu, Y.; Wang, J.; Chen, W. Construction of novel nanocomposites (Cu-MOF/GOD@HA) for chemodynamic therapy. Nanomaterials 2021, 11, 1843. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.X.; Song, X.N.; Yuan, Y.; Yao, X.T.; Chen, X.J.; Li, G.; Li, S.N. Designed synthesis of prussian blue@Cu-doped zinc phosphate nanocomposites for chemo/chemodynamic/photothermal combined cancer therapy. J. Mater. Chem. B 2023, 11, 6404–6411. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Wang, S.; Meshinchi, S.; Van Antwerp, M.E.; Bi, X.; Lee, I.; Baker, J.R., Jr. Dendrimer-entrapped gold nanoparticles as a platform for cancer-cell targeting and imaging. Small 2007, 3, 1245–1252. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, L.; Liu, N.; Pan, R.; Zhu, J. Copper(II)-Complexed Polyethylenimine-Entrapped Gold Nanoparticles Enable Targeted CT/MR Imaging and Chemodynamic Therapy of Tumors. Polymers 2025, 17, 423. https://doi.org/10.3390/polym17030423
He L, Liu N, Pan R, Zhu J. Copper(II)-Complexed Polyethylenimine-Entrapped Gold Nanoparticles Enable Targeted CT/MR Imaging and Chemodynamic Therapy of Tumors. Polymers. 2025; 17(3):423. https://doi.org/10.3390/polym17030423
Chicago/Turabian StyleHe, Lingxiu, Na Liu, Risong Pan, and Jingyi Zhu. 2025. "Copper(II)-Complexed Polyethylenimine-Entrapped Gold Nanoparticles Enable Targeted CT/MR Imaging and Chemodynamic Therapy of Tumors" Polymers 17, no. 3: 423. https://doi.org/10.3390/polym17030423
APA StyleHe, L., Liu, N., Pan, R., & Zhu, J. (2025). Copper(II)-Complexed Polyethylenimine-Entrapped Gold Nanoparticles Enable Targeted CT/MR Imaging and Chemodynamic Therapy of Tumors. Polymers, 17(3), 423. https://doi.org/10.3390/polym17030423





