The Effect of Isosorbide Content on the Thermal and Compressive Properties of Closed-Cell Rigid Polyurethane Foam
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of RPUFs Based on ISB
2.3. Characterization
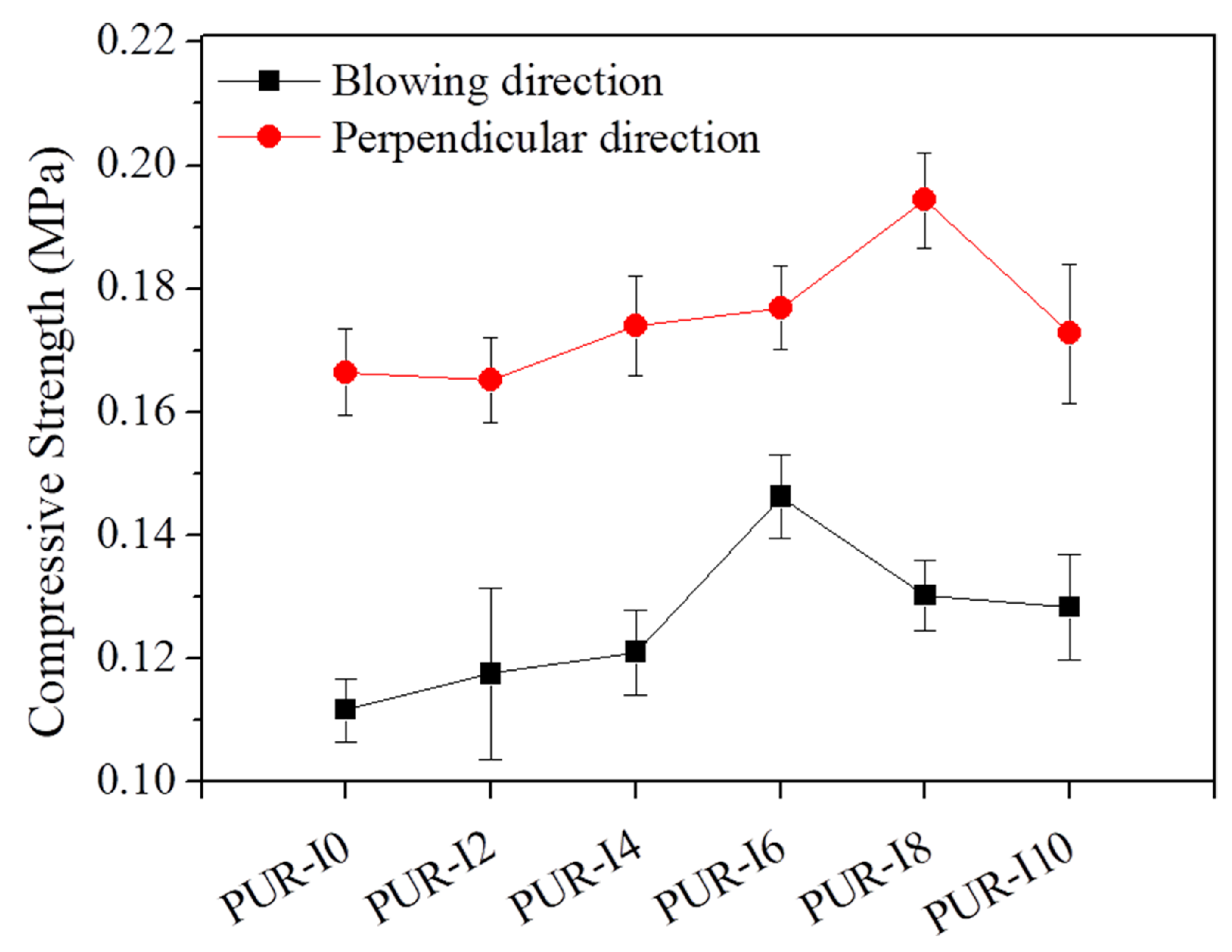
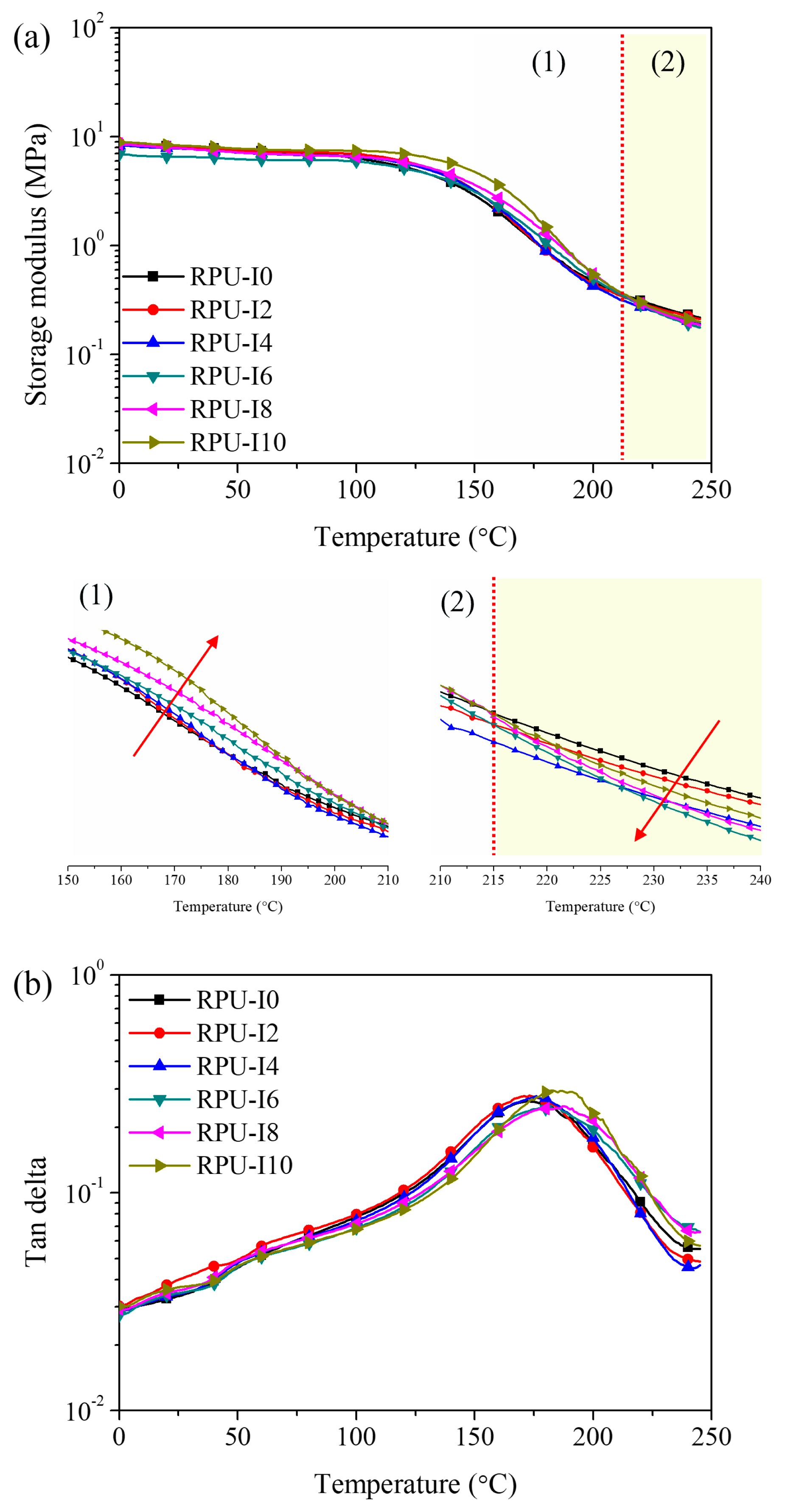
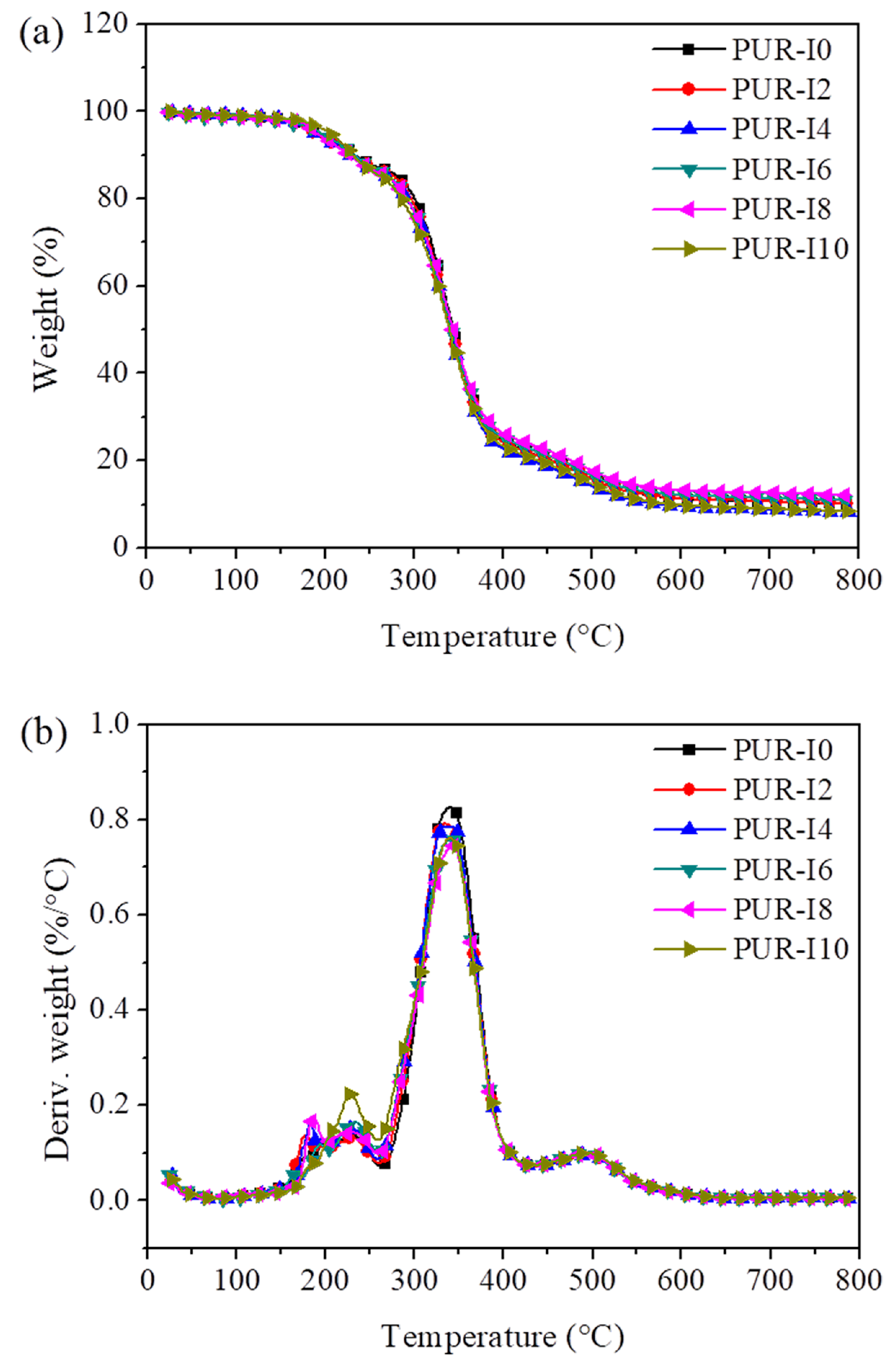
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Szycher, M. Szycher’s Handbook of Polyurethanes; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Mills, N. Polymer Foams Handbook: Engineering and Biomechanics Applications and Design Guide; Elsevier: Oxford, UK, 2007. [Google Scholar]
- Yan, D.; Xu, L.; Chen, C.; Tang, J.; Ji, X.; Li, Z. Enhanced mechanical and thermal properties of rigid polyurethane foam composites containing graphene nanosheets and carbon nanotubes. Polym. Int. 2012, 61, 1107–1114. [Google Scholar] [CrossRef]
- Kang, J.W.; Kim, J.M.; Kim, M.S.; Kim, Y.H.; Kim, W.N.; Jang, W.; Shin, D.S. Effects of nucleating agents on the morphological, mechanical and thermal insulating properties of rigid polyurethane poams. Macromol. Res. 2009, 17, 856–862. [Google Scholar] [CrossRef]
- Lim, H.; Kim, S.; Kim, B. Effects of silicon surfactant in rigid polyurethane foams. Express Polym. Lett 2008, 2, 194–200. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, B.K.; Lim, H. Effect of isocyanate index on the properties of rigid polyurethane foams blown by HFC 365mfc. Macromol. Res. 2008, 16, 467–472. [Google Scholar] [CrossRef]
- Kim, Y.H.; Choi, S.J.; Kim, J.M.; Han, M.S.; Kim, W.N.; Bang, K.T. Effects of organoclay on the thermal insulating properties of rigid polyurethane poams blown by environmentally friendly blowing agents. Macromol. Res. 2007, 15, 676–681. [Google Scholar] [CrossRef]
- Han, M.S.; Choi, S.J.; Kim, J.M.; Kim, Y.H.; Kim, W.N.; Lee, H.S.; Sung, J.Y. Effects of silicone surfactant on the cell size and thermal conductivity of rigid polyurethane foams by environmentally friendly blowing agents. Macromol. Res. 2009, 17, 44–50. [Google Scholar] [CrossRef]
- Biedermann, A.; Kudoke, C.; Merten, A.; Minogue, E.; Rotermund, U.; Ebert, H.P.; Heinemann, U.; Fricke, J.; Seifert, H. Analysis of heat transfer mechanisms in polyurethane rigid foam. J. Cell. Plast. 2001, 37, 467–483. [Google Scholar] [CrossRef]
- Saha, M.; Kabir, M.E.; Jeelani, S. Enhancement in thermal and mechanical properties of polyurethane foam infused with nanoparticles. Mater. Sci. Eng. A 2008, 479, 213–222. [Google Scholar] [CrossRef]
- Lorenzetti, A.; Roso, M.; Bruschetta, A.; Boaretti, C.; Modesti, M. Polyurethane-graphene nanocomposite foams with enhanced thermal insulating properties. Polym. Adv. Technol. 2016, 27, 303–307. [Google Scholar] [CrossRef]
- Ling, J.; Zhai, W.; Feng, W.; Shen, B.; Zhang, J.; Zheng, W.G. Facile preparation of lightweight microcellular polyetherimide/graphene composite foams for electromagnetic interference shielding. ACS Appl. Mater. Interfaces 2013, 5, 2677–2684. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.; Takahashi, J.; Chaussy, D.; Belgacem, M.; Silva, G. Composites of rigid polyurethane foam and cellulose fiber residue. J. Appl. Polym. Sci. 2010, 117, 3665–3672. [Google Scholar] [CrossRef]
- Kim, S.; Lee, M.; Kim, H.; Park, H.; Jeong, H.; Yoon, K.; Kim, B. Nanoclay reinforced rigid polyurethane foams. J. Appl. Polym. Sci. 2010, 117, 1992–1997. [Google Scholar] [CrossRef]
- Acuña, P.; Zhang, J.; Yin, G.Z.; Liu, X.Q.; Wang, D.Y. Bio-based rigid polyurethane foam from castor oil with excellent flame retardancy and high insulation capacity via cooperation with carbon-based materials. J. Mater. Sci. 2021, 56, 2684–2701. [Google Scholar] [CrossRef]
- Borowicz, M.; Paciorek-Sadowska, J.; Lubczak, J.; Czupryński, B. Biodegradable, flame-retardant, and bio-based rigid polyurethane/polyisocyanurate foams for thermal insulation application. Polymers 2019, 11, 1816. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Kessler, M.R. Bio-based polyurethane foam made from compatible blends of vegetable-oil-based polyol and petroleum-based polyol. ACS Sustain. Chem. Eng. 2015, 3, 743–749. [Google Scholar] [CrossRef]
- Prociak, A.; Kucała, M.; Kurańska, M.; Barczewski, M. Effect of Selected Bio-Components on the Cell Structure and Properties of Rigid Polyurethane Foams. Polymers 2023, 15, 3660. [Google Scholar] [CrossRef] [PubMed]
- Marcovich, N.E.; Kurańska, M.; Prociak, A.; Malewska, E.; Kulpa, K. Open cell semi-rigid polyurethane foams synthesized using palm oil-based bio-polyol. Ind. Crop. Prod. 2017, 102, 88–96. [Google Scholar] [CrossRef]
- Olszewski, A.; Kosmela, P.; Vēvere, L.; Kirpluks, M.; Cabulis, U.; Piszczyk, Ł. Effect of bio-polyol molecular weight on the structure and properties of polyurethane-polyisocyanurate (PUR-PIR) foams. Sci. Rep. 2024, 14, 812. [Google Scholar] [CrossRef]
- Leszczyńska, M.; Malewska, E.; Ryszkowska, J.; Kurańska, M.; Gloc, M.; Leszczyński, M.K.; Prociak, A. Vegetable fillers and rapeseed oil-based polyol as natural raw materials for the production of rigid polyurethane foams. Materials 2021, 14, 1772. [Google Scholar] [CrossRef]
- Malewska, E.; Kurańska, M.; Tenczyńska, M.; Prociak, A. Application of modified seed oils of selected fruits in the synthesis of polyurethane thermal insulating materials. Materials 2023, 17, 158. [Google Scholar] [CrossRef]
- Sonnenschein, M.F.; Wendt, B.L. Design and formulation of soybean oil derived flexible polyurethane foams and their underlying polymer structure/property relationships. Polymer 2013, 54, 2511–2520. [Google Scholar] [CrossRef]
- Ugarte, L.; Saralegi, A.; Fernández, R.; Martín, L.; Corcuera, M.A.; Eceiza, A. Flexible polyurethane foams based on 100% renewably sourced polyols. Ind. Crop. Prod. 2014, 62, 545–551. [Google Scholar] [CrossRef]
- Bernardini, J.; Cinelli, P.; Anguillesi, I.; Coltelli, M.B.; Lazzeri, A. Flexible polyurethane foams green production employing lignin or oxypropylated lignin. Eur. Polym. J. 2015, 64, 147–156. [Google Scholar] [CrossRef]
- Prociak, A.; Malewska, E.; Kurańska, M.; Bąk, S.; Budny, P. Flexible polyurethane foams synthesized with palm oil-based bio-polyols obtained with the use of different oxirane ring opener. Ind. Crop. Prod. 2018, 115, 69–77. [Google Scholar] [CrossRef]
- Lan, Z.; Daga, R.; Whitehouse, R.; McCarthy, S.; Schmidt, D. Structure–properties relations in flexible polyurethane foams containing a novel bio-based crosslinker. Polymer 2014, 55, 2635–2644. [Google Scholar] [CrossRef]
- Rashmi, B.J.; Rusu, D.; Prashantha, K.; Lacrampe, M.F.; Krawczak, P. Development of water-blown bio-based thermoplastic polyurethane foams using bio-derived chain extender. J. Appl. Polym. Sci. 2013, 128, 292–303. [Google Scholar] [CrossRef]
- Oltmanns, J.U.; Palkovits, S.; Palkovits, R. Kinetic investigation of sorbitol and xylitol dehydration catalyzed by silicotungstic acid in water. Appl. Catal. A Gen. 2013, 456, 168–173. [Google Scholar] [CrossRef]
- Zou, J.; Cao, D.; Tao, W.; Zhang, S.; Cui, L.; Zeng, F.; Cai, W. Sorbitol dehydration into isosorbide over a cellulose-derived solid acid catalyst. RSC Adv. 2016, 6, 49528–49536. [Google Scholar] [CrossRef]
- Flèche, G.; Huchette, M. Isosorbide. Preparation, properties and chemistry. Starch-Stärke 1986, 38, 26–30. [Google Scholar] [CrossRef]
- Fenouillot, F.; Rousseau, A.; Colomines, G.; Saint-Loup, R.; Pascault, J.P. Polymers from renewable 1, 4: 3, 6-dianhydrohexitols (isosorbide, isomannide and isoidide): A review. Prog. Polym. Sci. 2010, 35, 578–622. [Google Scholar] [CrossRef]
- Dussenne, C.; Delaunay, T.; Wiatz, V.; Wyart, H.; Suisse, I.; Sauthier, M. Synthesis of isosorbide: An overview of challenging reactions. Green Chem. 2017, 19, 5332–5344. [Google Scholar] [CrossRef]
- Kasmi, N.; Roso, M.; Hammami, N.; Majdoub, M.; Boaretti, C.; Sgarbossa, P.; Vianello, C.; Maschio, G.; Modesti, M.; Lorenzetti, A. Microwave-assisted synthesis of isosorbide-derived diols for the preparation of thermally stable thermoplastic polyurethane. Des. Monomers Polym. 2017, 20, 547–563. [Google Scholar] [CrossRef]
- Śmiga-Matuszowicz, M.; Janicki, B.; Jaszcz, K.; Łukaszczyk, J.; Kaczmarek, M.; Lesiak, M.; Sieroń, A.L.; Simka, W.; Mierzwiński, M.; Kusz, D. Novel bioactive polyester scaffolds prepared from unsaturated resins based on isosorbide and succinic acid. Mater. Sci. Eng. C 2014, 45, 64–71. [Google Scholar] [CrossRef]
- Liu, W.; Xie, T.; Qiu, R. Biobased thermosets prepared from rigid isosorbide and flexible soybean oil derivatives. ACS Sustain. Chem. Eng. 2017, 5, 774–783. [Google Scholar] [CrossRef]
- Park, S.A.; Choi, J.; Ju, S.; Jegal, J.; Lee, K.M.; Hwang, S.Y.; Oh, D.X.; Park, J. Copolycarbonates of bio-based rigid isosorbide and flexible 1, 4-cyclohexanedimethanol: Merits over bisphenol-A based polycarbonates. Polymer 2017, 116, 153–159. [Google Scholar] [CrossRef]
- Besse, V.; Auvergne, R.; Carlotti, S.; Boutevin, G.; Otazaghine, B.; Caillol, S.; Pascault, J.P.; Boutevin, B. Synthesis of isosorbide based polyurethanes: An isocyanate free method. React. Funct. Polym. 2013, 73, 588–594. [Google Scholar] [CrossRef]
- Noordover, B.A.; van Staalduinen, V.G.; Duchateau, R.; Koning, C.E.; van Benthem, R.A.; Mak, M.; Heise, A.; Frissen, A.E.; van Haveren, J. Co-and terpolyesters based on isosorbide and succinic acid for coating applications: Synthesis and characterization. Biomacromolecules 2006, 7, 3406–3416. [Google Scholar] [CrossRef] [PubMed]
- Ravey, M.; Pearce, E.M. Flexible polyurethane foam. I. Thermal decomposition of a polyether-based, water-blown commercial type of flexible polyurethane foam. J. Appl. Polym. Sci. 1997, 63, 47–74. [Google Scholar] [CrossRef]
- Delebecq, E.; Pascault, J.P.; Boutevin, B.; Ganachaud, F. On the versatility of urethane/urea bonds: Reversibility, blocked isocyanate, and non-isocyanate polyurethane. Chem. Rev. 2013, 113, 80–118. [Google Scholar] [CrossRef] [PubMed]
- Rolph, M.S.; Markowska, A.L.; Warriner, C.N.; O’Reilly, R.K. Blocked isocyanates: From analytical and experimental considerations to non-polyurethane applications. Polym. Chem. 2016, 7, 7351–7364. [Google Scholar] [CrossRef]
- Nasar, A.S.; Kalaimani, S. Synthesis and studies on forward and reverse reactions of phenol-blocked polyisocyanates: An insight into blocked isocyanates. RSC Adv. 2016, 6, 76802–76812. [Google Scholar] [CrossRef]
- Kalaimani, S.; Nasar, A.S. Catalysis of deblocking and cure reactions of easily cleavable phenol blocked polyisocyanates with poly (polytetrahydrofuran carbonate) diol. Eur. Polym. J. 2017, 91, 221–231. [Google Scholar] [CrossRef]
- D7487-13; Standard Test Method for Determining the Water Solubility of Polymers. ASTM International: West Conshohocken, PA, USA, 2013.
- Kumar, V.; Suh, N.P. A process for making microcellular thermoplastic parts. Polym. Eng. Sci. 1990, 30, 1323–1329. [Google Scholar] [CrossRef]
- D6226; Standard Test Method for Determining the Density of Plastic by the Buoyancy Method. ASTM International: West Conshohocken, PA, USA, 2012.
- C518; Standard Test Method for Steady-State Thermal Transmission Properties by Means of the Heat Flow Meter Apparatus. ASTM International: West Conshohocken, PA, USA, 2017.
- D1621; Standard Test Method for Compressive Properties of Rigid Cellular Plastics. ASTM International: West Conshohocken, PA, USA, 2013.
- D570-98; Standard Test Method for Water Absorption of Plastics. ASTM International: West Conshohocken, PA, USA, 1998.
- Shin, S.R.; Liang, J.Y.; Ryu, H.; Song, G.S.; Lee, D.S. Effects of isosorbide incorporation into flexible polyurethane foams: Reversible urethane linkages and antioxidant activity. Molecules 2019, 24, 1347. [Google Scholar] [CrossRef] [PubMed]
- Bakhshi, H.; Yeganeh, H.; Yari, A.; Nezhad, S.K. Castor oil-based polyurethane coatings containing benzyl triethanol ammonium chloride: Synthesis, characterization, and biological properties. J. Mater. Sci. 2014, 49, 5365–5377. [Google Scholar] [CrossRef]
- Gu, R.; Sain, M.M. Effects of wood fiber and microclay on the performance of soy based polyurethane foams. J. Polym. Environ. 2013, 21, 30–38. [Google Scholar] [CrossRef]
- Pawar, M.S.; Kadam, A.S.; Dawane, B.S.; Yemul, O.S. Synthesis and characterization of rigid polyurethane foams from algae oil using biobased chain extenders. Polym. Bull. 2016, 73, 727–741. [Google Scholar] [CrossRef]
- Kairytė, A.; Vėjelis, S. Evaluation of forming mixture composition impact on properties of water blown rigid polyurethane (PUR) foam from rapeseed oil polyol. Ind. Crop. Prod. 2015, 66, 210–215. [Google Scholar] [CrossRef]
- Thirumal, M.; Khastgir, D.; Singha, N.K.; Manjunath, B.; Naik, Y. Effect of foam density on the properties of water blown rigid polyurethane foam. J. Appl. Polym. Sci. 2008, 108, 1810–1817. [Google Scholar] [CrossRef]
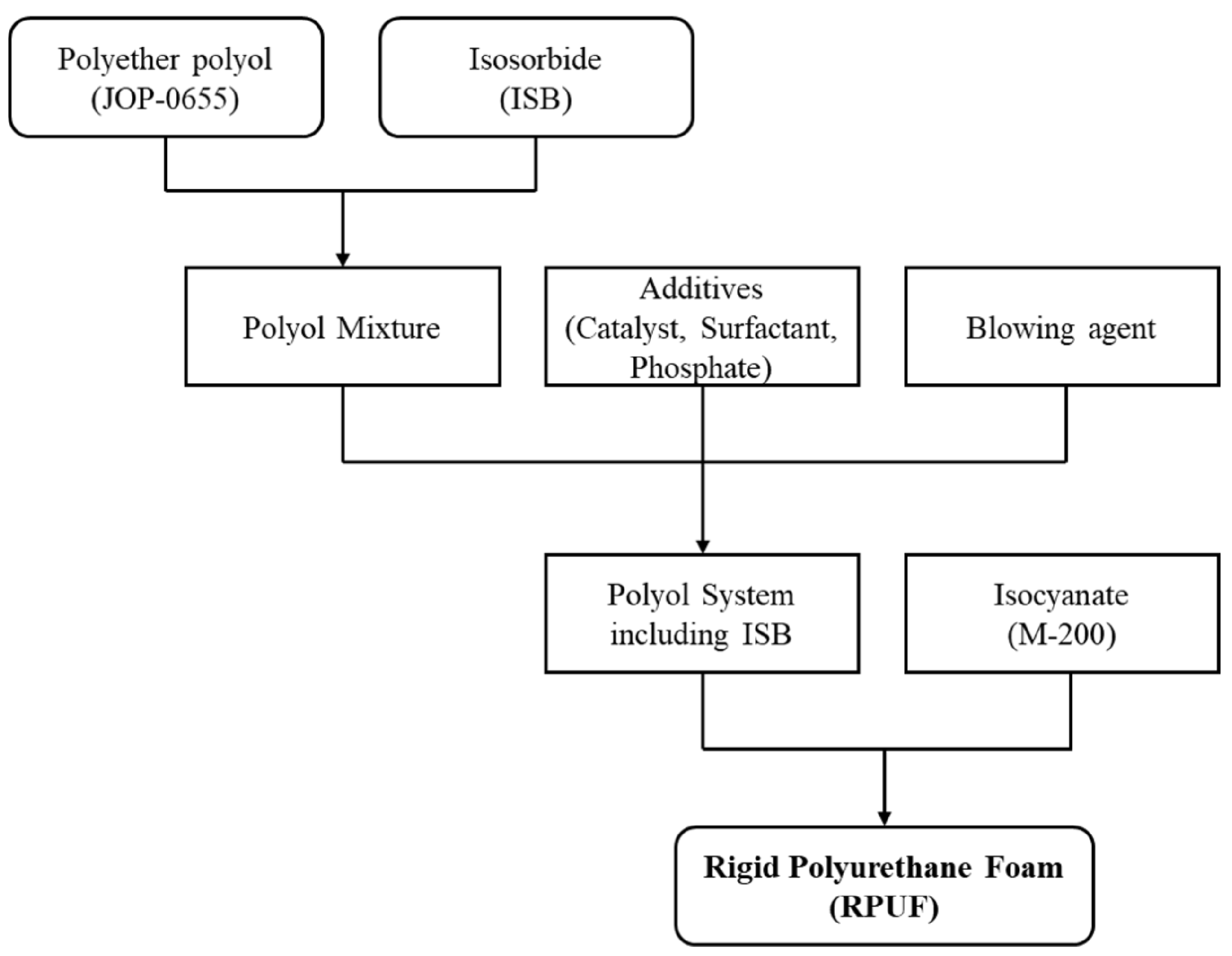


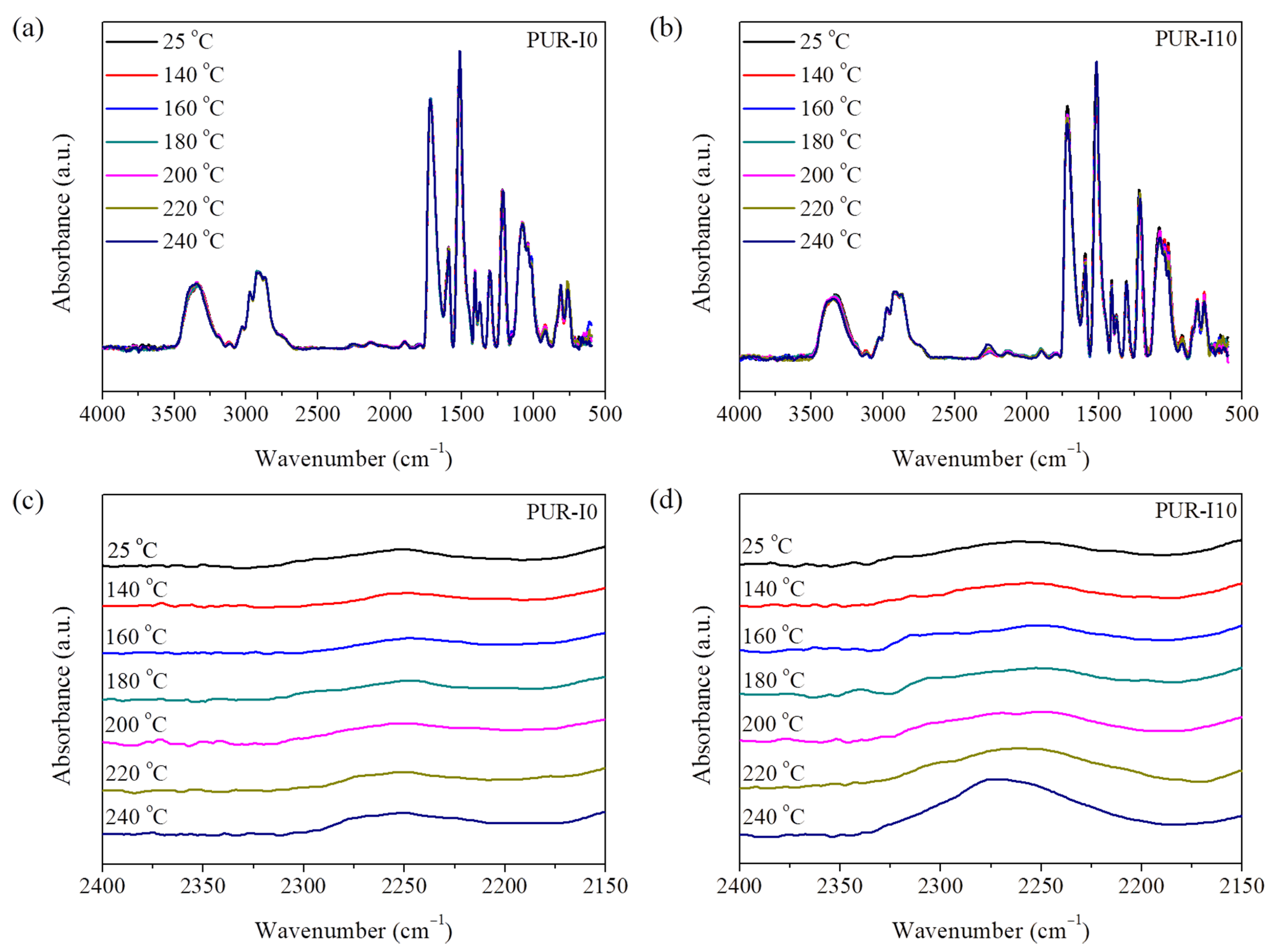
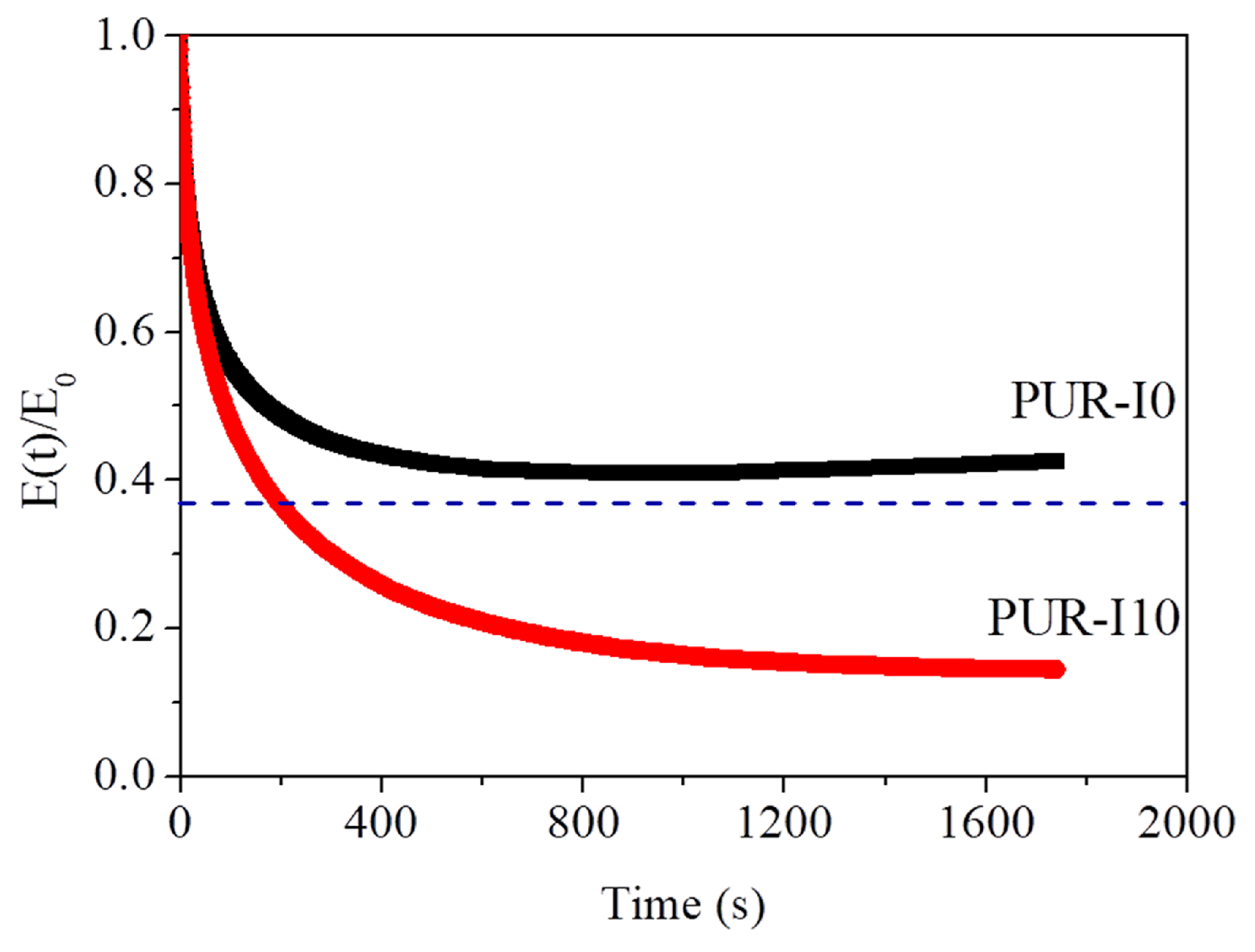

| Components | PUR-I0 | PUR-I2 | PUR-I4 | PUR-I6 | PUR-I8 | PUR-I10 |
|---|---|---|---|---|---|---|
| Composition by wt. | ||||||
| Polyol part | ||||||
| JOP-0655 | 100 | 98.0 | 96.0 | 94.0 | 92.0 | 90.0 |
| ISB | 0.0 | 2.0 | 4.0 | 6.0 | 8.0 | 10.0 |
| TCPP | 15.0 | 15.0 | 15.0 | 15.0 | 15.0 | 15.0 |
| B-8462 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 |
| PC-5 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| PC-8 | 2.4 | 2.4 | 2.4 | 2.4 | 2.4 | 2.4 |
| 365/227 | 30.0 | 30.0 | 30.0 | 30.0 | 30.0 | 30.0 |
| H2O | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Isocyanate part | ||||||
| pMDI | 142.7 | 144.6 | 146.6 | 148.6 | 150.5 | 152.5 |
| NCO index | 120 | 120 | 120 | 120 | 120 | 120 |
| Parameters | PUR-I0 | PUR-I2 | PUR-I4 | PUR-I6 | PUR-I8 | PUR-I10 |
|---|---|---|---|---|---|---|
| CT (s) | 13 | 13 | 13 | 12 | 12 | 12 |
| GT (s) | 129 | 126 | 123 | 120 | 118 | 117 |
| TFT (s) | 187 | 178 | 175 | 170 | 169 | 166 |
| Density (kg/m3) | 46 | 45 | 46 | 45 | 45 | 46 |
| Properties | PUR-I0 | PUR-I2 | PUR-I4 | PUR-I6 | PUR-I8 | PUR-I10 |
|---|---|---|---|---|---|---|
| Average cell size (μm) | 475 ± 142 | 418 ± 84 | 394 ± 78 | 354 ± 63 | 294 ± 54 | 302 ± 49 |
| Average thickness of cell walls (μm) | 33.1 ± 9.8 | 30.9 ± 6.2 | 25.8 ± 7.3 | 26.9 ± 8.5 | 24.8 ± 8.5 | 22.6 ± 6.7 |
| Number of cells ( cells/cm3) | 1.82 | 2.37 | 3.70 | 3.95 | 4.58 | 4.96 |
| Decomposition Temperature () | PUR-I0 | PUR-I2 | PUR-I4 | PUR-I6 | PUR-I8 | PUR-I10 |
|---|---|---|---|---|---|---|
| 1 | 173.0 | 174.0 | 176.9 | 182.6 | 187.9 | 187.0 |
| 2 | 194.4 | 187.3 | 187.3 | 194.4 | 190.5 | 204.0 |
| 3 | 232.9 | 229.7 | 225.2 | 232.9 | 227.1 | 231.0 |
| 4 | 344.8 | 342.6 | 340.0 | 342.6 | 343.9 | 340.0 |
| 5 | 229.7 | 230.3 | 231.6 | 232.2 | 230.3 | 227.7 |
| 6 | 340.7 | 335.5 | 339.4 | 340.0 | 342.6 | 340.0 |
| 7 | 494.6 | 494.0 | 491.4 | 492.7 | 494.6 | 495.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, S.-R.; Lee, D.-S. The Effect of Isosorbide Content on the Thermal and Compressive Properties of Closed-Cell Rigid Polyurethane Foam. Polymers 2025, 17, 495. https://doi.org/10.3390/polym17040495
Shin S-R, Lee D-S. The Effect of Isosorbide Content on the Thermal and Compressive Properties of Closed-Cell Rigid Polyurethane Foam. Polymers. 2025; 17(4):495. https://doi.org/10.3390/polym17040495
Chicago/Turabian StyleShin, Se-Ra, and Dai-Soo Lee. 2025. "The Effect of Isosorbide Content on the Thermal and Compressive Properties of Closed-Cell Rigid Polyurethane Foam" Polymers 17, no. 4: 495. https://doi.org/10.3390/polym17040495
APA StyleShin, S.-R., & Lee, D.-S. (2025). The Effect of Isosorbide Content on the Thermal and Compressive Properties of Closed-Cell Rigid Polyurethane Foam. Polymers, 17(4), 495. https://doi.org/10.3390/polym17040495







