Multifunctional Flexible Hard Coatings with Weathering Resistance and Heat-Shielding Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Functionalized Nanoparticles
2.3. Fabrication of Multifunctional Coatings
2.4. Characterization
3. Results and Discussion
3.1. Macro-Mechanical Properties of Multifunctional Coatings
3.2. Weathering Resistance of Multifunctional Coatings
3.3. Heat-Shielding Property
3.4. Optical Properties
3.5. Mechanism of Multifunctional Coatings
3.5.1. FT-IR and UV-Vis Analysis
3.5.2. XPSAnalysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Vinodhini, J.; Harish, S.; Ikeda, H.; Navaneethan, M. Flexible Ag2Se/Ag2S nanocomposite on carbon fabric optimized via interfaced engineered energy filtering effect for a textile based wearable thermoelectric generator. Surf. Interfaces 2024, 54, 105105. [Google Scholar] [CrossRef]
- Stroea, L.; Chibac-Scutaru, A.L.; Melinte, V. Aliphatic Polyurethane Elastomers Quaternized with Silane-Functionalized TiO2 Nanoparticles with UV-Shielding Features. Polymers 2021, 13, 1318. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-G.; Jung, B.; Kim, K.-H.; Cho, K.Y.; Jeong, Y.-C. Highly robust and transparent flexible cover window films based on UV-curable polysilsesquioxane nano sol. J. Appl. Polym. Sci. 2020, 137, e49012. [Google Scholar] [CrossRef]
- Hou, C.M.; Fan, Z.; Yang, J.Q. Preparation and properties of super hydrophobic cotton fabric constructed by modified nano-SiO2 hybrid fluoro-epoxy copolymer. Surf. Interfaces 2024, 45, 103814. [Google Scholar] [CrossRef]
- Jia, Z.; Hong, R. Anticorrosive and photocatalytic properties research of epoxy-silica organic-inorganic coating. Colloids Surf. A Physicochem. Eng. Asp. 2021, 622, 126647. [Google Scholar] [CrossRef]
- Al-Otaibi, W.; Alandis, N.M.; Al-Mohammad, Y.M.; Alam, M. Advanced Anticorrosive Graphene Oxide-Doped Organic-Inorganic Hybrid Nanocomposite Coating Derived from Leucaena leucocephala Oil. Polymers 2023, 15, 4390. [Google Scholar] [CrossRef]
- Choi, G.M.; Jin, J.; Shin, D.; Kim, Y.H.; Ko, J.H.; Im, H.G.; Jang, J.; Jang, D.; Bae, B.S. Flexible Hard Coating: Glass-Like Wear Resistant, Yet Plastic-Like Compliant, Transparent Protective Coating for Foldable Displays. Adv. Mater. 2017, 29, 1700205. [Google Scholar] [CrossRef]
- Li, Y.W.; Dong, X.H.; Zou, Y.; Wang, Z.; Yue, K.; Huang, M.J.; Liu, H.; Feng, X.Y.; Lin, Z.W.; Zhang, W.; et al. Polyhedral oligomeric silsesquioxane meets “click” chemistry: Rational design and facile preparation of functional hybrid materials. Polymer 2017, 125, 303–329. [Google Scholar] [CrossRef]
- Deng, Y.Y.; Han, D.; Zhou, D.L.; Liu, Z.Q.; Zhang, Q.; Li, Y.; Fu, Q. Monodispersed hybrid microparticles based on polyhedral oligomeric silsesquioxane with good UV resistance and high thermal stability: From organic to inorganic. Polymer 2019, 178, 121609. [Google Scholar] [CrossRef]
- Zhang, K.K.; Huang, S.S.; Wang, J.D.; Liu, G.J. Transparent Omniphobic Coating with Glass-Like Wear Resistance and Polymer-Like Bendability. Angew. Chem.-Int. Ed. 2019, 58, 12004–12009. [Google Scholar] [CrossRef]
- Chen, R.Z.; Zhang, Y.S.; Xie, Q.Y.; Chen, Z.X.; Ma, C.F.; Zhang, G.Z. Transparent Polymer-Ceramic Hybrid Antifouling Coating with Superior Mechanical Properties. Adv. Funct. Mater. 2021, 31, 2011145. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Chen, Z.X.; Zheng, H.; Chen, R.Z.; Ma, C.F.; Zhang, G.Z. Multifunctional Hard Yet Flexible Coatings Fabricated Using a Universal Step-by-Step Strategy. Adv. Sci. 2022, 9, 2200268. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Xu, Q.; Li, H.; Liu, M.; Li, Z.; Yang, K.; Zhao, J.; Qian, L.; Peng, Z.; Zhang, G. Fabrication of High-Performance Silver Mesh for Transparent Glass Heaters via Electric-Field-Driven Microscale 3D Printing and UV-Assisted Microtransfer. Adv. Mater. 2019, 31, 1902479. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Yu, Q.; Wang, J.; Wu, T. Design and Fabrication of a Renewable and Highly Transparent Multilayer Coating on Poly(lactic acid) Film Capable of UV-Shielding and Antifogging. Ind. Eng. Chem. Process Des. Dev. 2018, 57, 4577–4584. [Google Scholar] [CrossRef]
- Miao, Y.A.; Tang, Z.W.; Zhang, Q.W.; Reheman, A.; Xiao, H.; Zhang, M.; Liu, K.; Huang, L.L.; Chen, L.H.; Wu, H. Biocompatible Lignin-Containing Hydrogels with Self-Adhesion, Conductivity, UV Shielding, and Antioxidant Activity as Wearable Sensors. ACS Appl. Polym. Mater. 2022, 4, 1448–1456. [Google Scholar] [CrossRef]
- Bergamonti, L.; Bondioli, F.; Alfieri, I.; Alinovi, S.; Lorenzi, A.; Predieri, G.; Lottici, P.P. Weathering resistance of PMMA/SiO2/ZrO2 hybrid coatings for sandstone conservation. Polym. Degrad. Stab. 2018, 147, 274–283. [Google Scholar] [CrossRef]
- Lowe, N.J. An overview of ultraviolet radiation, sunscreens, and photo-induced dermatoses. Dermatol. Clin. 2006, 24, 9–17. [Google Scholar] [CrossRef]
- Chae, D.; Lim, H.; So, S.; Son, S.; Ju, S.; Kim, W.; Rho, J.; Lee, H. Spectrally Selective Nanoparticle Mixture Coating for Passive Daytime Radiative Cooling. ACS Appl. Mater. Interfaces 2021, 13, 21119–21126. [Google Scholar] [CrossRef]
- Kim, T.H.; Park, S.; Lee, S.; Bharadwaj, A.; Lee, Y.S.; Yoo, C.; Kim, T.H. A Review of Biomass-Derived UV-Shielding Materials for Bio-Composites. Energies 2023, 16, 2231. [Google Scholar] [CrossRef]
- Shanghai Furniture Research Institute. Furniture--Assessment of Adhesion of Surface Coatings--Cross Cut; General Administration of Quality Supervision: Beijing, China, 1985. [Google Scholar]
- CNOOC; Changzhou Coatings and Chemical Research Institute. Paints and Varnishes--Artificial Weathering and Exposure to Artificial Radiatio--Exposure to Filtered Xenon-Arc Radiation; General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China; China National Standardization Administration: Beijing, China, 2009. [Google Scholar]
- Sc, T.C. Plastics--Determination of Thermal Conductivity and Thermal Diffusivity--Part 2: Transient Plane Heat Source (hot disc) Method; International Organization for Standardization: Geneva, Switzerland, 2015. [Google Scholar]
- Arruda, L.M.; del Menezzi, C.H. Effect of thermomechanical treatment on physical properties of wood veneers. Int. Wood Prod. J. 2013, 4, 217–224. [Google Scholar] [CrossRef]
- Atari, M.; Labbaf, S.; Javanmard, S.H. Fabrication and characterization of a 3D scaffold based on elastomeric poly-glycerol Sebacate polymer for heart valve applications. J. Manuf. Process. 2023, 102, 350–364. [Google Scholar] [CrossRef]
- Zhang, X.X.; Cai, S.; You, D.; Yan, L.H.; Lv, H.B.; Yuan, X.D.; Jiang, B. Template-Free Sol-Gel Preparation of Superhydrophobic ORMOSIL Films for Double-Wavelength Broadband Antireflective Coatings. Adv. Funct. Mater. 2013, 23, 4361–4365. [Google Scholar] [CrossRef]
- Hao, S.A.; Qi, Y.H.; Zhang, Z.P. Settlement behavior and mechanism of Navicula sp. on WLAP/PDMS composite coating under simulative diurnal alternation, constant light and dark conditions. Surf. Interfaces 2023, 42, 103527. [Google Scholar] [CrossRef]
- Jeevajothi, K.; Crossiya, D.; Subasri, R. Non-fluorinated, room temperature curable hydrophobic coatings by sol–gel process—ScienceDirect. Ceram. Int. 2012, 38, 2971–2976. [Google Scholar] [CrossRef]
- Muzata, T.S.; Gebrekrstos, A.; Orasugh, J.T.; Ray, S.S. An overview of recent advances in polymer composites with improved UV-shielding properties. J. Appl. Polym. Sci. 2023, 140, e53693. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Dao, P.H.; Duong, K.L.; Duongc, Q.H.; Vu, Q.T.; Nguyen, A.H.; Mac, V.P.; Le, T.L. Effect of R-TiO2 and ZnO nanoparticles on the UV-shielding efficiency of water-borne acrylic coating. Prog. Org. Coat. 2017, 110, 114–121. [Google Scholar] [CrossRef]
- Qiao, M.Y.; Xu, W.C.; Ji, G.J.; Zhang, B.B. Anti-corrosion, anti-bacterial and durable atmospheric weatherability of polydimethylsiloxane coating with intrinsic hydrophobicity. Surf. Topogr.-Metrol. Prop. 2022, 10, 025009. [Google Scholar] [CrossRef]
- Abshirini, M.; Saha, M.C.; Altan, M.C.; Liu, Y.T. Synthesis and characterization of hierarchical porous structure of polydimethylsiloxane (PDMS) sheets via two-step phase separation method. Mater. Des. 2021, 212, 110194. [Google Scholar] [CrossRef]
- Tao, S.; Xu, X.; Chen, M.; Xu, W.; Xu, Z. Construction of efficient passive radiative cooling emitter with selective emission in the whole atmospheric window and durable anti-contamination performance. Sol. Energy Mater. Sol. Cells 2021, 224, 110998. [Google Scholar] [CrossRef]
- Jeon, S.; Son, S.; Lee, S.Y.; Chae, D.; Oh, S.J. Multifunctional Daytime Radiative Cooling Devices with Simultaneous Light-Emitting and Radiative Cooling Functional Layers. ACS Appl. Mater. Interfaces 2020, 12, 54763–54772. [Google Scholar] [CrossRef]
- Sun, Y.; Javed, M.; Ji, Y.; Nawaz, M.Z.; Wang, Y.; Cai, Z.; Xu, B. Design and preparation of flexible double-layered daytime radiative cooling composite film with antifouling property. Sol. Energy Mater. Sol. Cells 2022, 245, 111836. [Google Scholar] [CrossRef]
- Pu, J.H.; Yu, X.Y.; Zhao, Y.W.; Tang, G.; Ren, X.J.; Du, M. Dynamic aerogel window with switchable solar transmittance and low haze. Energy 2023, 285, 129437. [Google Scholar] [CrossRef]
- Liu, J.; Cheng, Y.; Xu, K.; An, L.L.; Su, Y.H.; Li, X.H.; Zhang, Z.J. Effect of nano-silica filler on-microstructure and mechanical properties of polydimethylsiloxane-based nanocomposites prepared by “inhibition-grafting” method. Compos. Sci. Technol. 2018, 167, 355–363. [Google Scholar] [CrossRef]
- Aili, A.; Wei, Z.Y.; Chen, Y.Z.; Zhao, D.L.; Yang, R.G.; Yin, X.B. Selection of polymers with functional groups for daytime radiative cooling. Mater. Today Phys. 2019, 10, 100127. [Google Scholar] [CrossRef]
- Wang, H.D.; Xue, C.H.; Guo, X.J.; Liu, B.Y.; Ji, Z.Y.; Huang, M.C.; Jia, S.T. Superhydrophobic porous film for daytime radiative cooling. Appl. Mater. Today 2021, 24, 101100. [Google Scholar] [CrossRef]
- Nie, S.J.; Tan, X.Y.; Li, X.Y.; Wei, K.; Xiao, T.; Jiang, L.H.; Geng, J.L.; Liu, Y.; Hu, W.W.; Chen, X.B. Facile and environmentally-friendly fabrication of robust composite film with superhydrophobicity and radiative cooling property. Compos. Sci. Technol. 2022, 230, 104613. [Google Scholar] [CrossRef]
- Kockott, D. Natural and artificial weathering of polymers. Polym. Degrad. Stab. 1989, 25, 181–208. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, R.; Luo, J. Improvement of anti-aging property of UV-curable coatings with silica-coated TiO2. Prog. Org. Coat. 2023, 179, 107479. [Google Scholar] [CrossRef]
- Saengkaew, J.; Le, D.; Samart, C.; Sawada, H.; Nishida, M.; Chanlek, N.; Kongparakul, S.; Kiatkamjornwong, S. Superhydrophobic coating from fluoroalkylsilane modified natural rubber encapsulated SiO2 composites for self-driven oil/water separation. Appl. Surf. Sci. 2018, 462, 164–174. [Google Scholar] [CrossRef]
- Hong, H.; Zhang, J.; Zhu, Y.; Tse, S.D.; Guo, H.; Lai, Y.; Xi, Y.; He, L.; Zhu, Z.; Yin, K. In Situ Polymer-Solution-Processed Graphene–PDMS Nanocomposites for Application in Intracranial Pressure Sensors. Nanomaterials 2024, 14, 399. [Google Scholar] [CrossRef]
- Cordoba, A.; Vargascoronado, R.F.; Esquivel, K. A Novel In Situ Sol-Gel Synthesis Method for PDMS Composites Reinforced with Silica Nanoparticles. Polymers 2024, 16, 1125. [Google Scholar] [CrossRef]


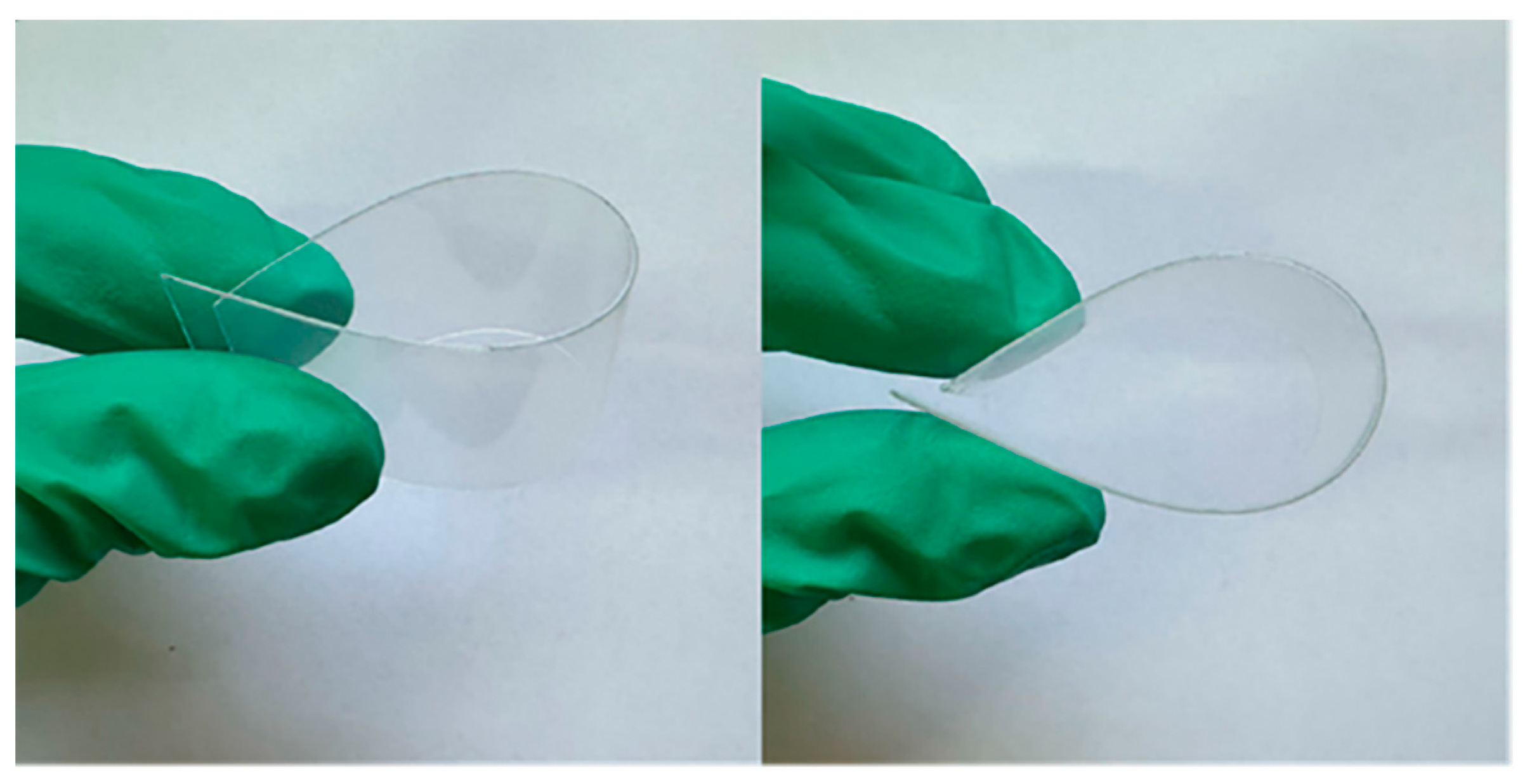



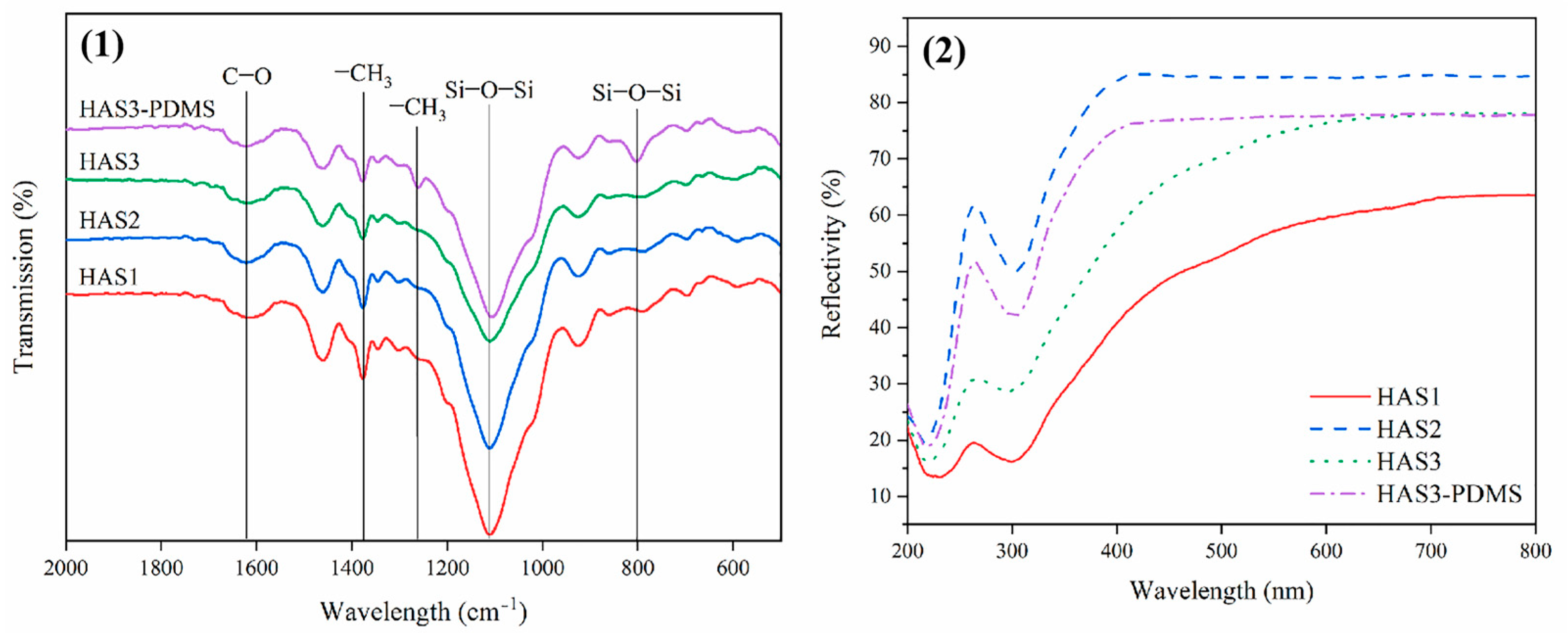
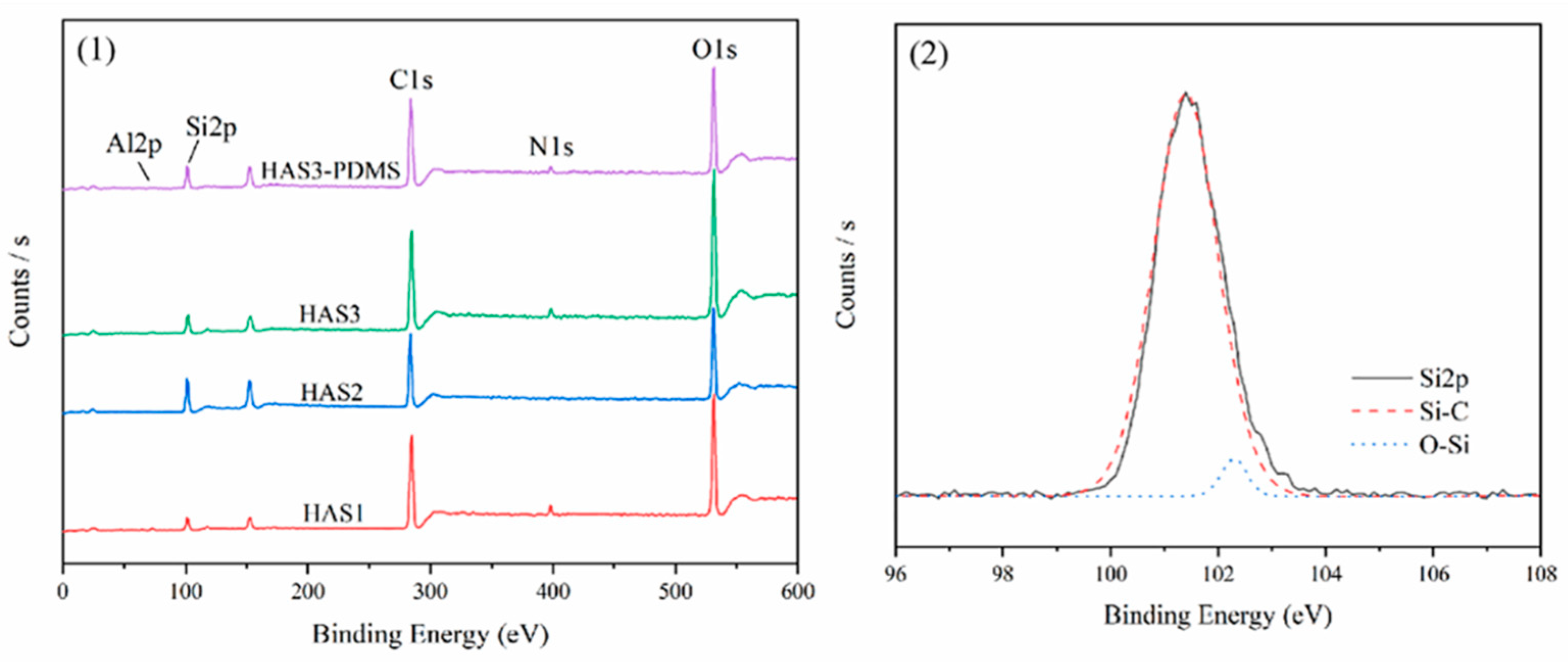
| Samples | Formulation (g) | ||||
|---|---|---|---|---|---|
| HMDS-SiO2 | KH560-SiO2 | KH560-Al2O3 | D400 | PDMS | |
| HAS1 | 0.5 | 0.2 | 0.3 | 10 | / |
| HAS2 | 0.6 | 0.4 | 1.0 | 10 | / |
| HAS3 | 1.0 | 0.4 | 0.6 | 10 | / |
| HAS3-PDMS | 1.0 | 0.4 | 0.6 | 7 | 3 |
| Samples | Hardness | Adhesion | Ra (µm) | Flexibility | |
|---|---|---|---|---|---|
| Glass | PET | ||||
| HAS1 | 2H | 1 | 2 | 2.417 | 10 mm |
| HAS2 | 4H | 0 | 1 | 5.733 | 10 mm |
| HAS3 | 4H | 0 | 1 | 5.207 | 10 mm |
| HAS3-PDMS | 3H | 1 | 2 | 4.610 | 10 mm |
| Samples | Transmittance (%) | Haze (%) |
|---|---|---|
| HAS1 | 99.6 | 1.57 |
| HAS2 | 98.8 | 8.50 |
| HAS3 | 98.8 | 5.03 |
| HAS3-PDMS | 97.5 | 10.24 |
| Samples | Atomic (%) | Atomic Ratio | ||||
|---|---|---|---|---|---|---|
| Al2p | Si2p | C1s | N1s | O1s | C/(Si + Al) | |
| HAS1 | 2.23 | 6.95 | 58.45 | 4.16 | 28.21 | 6.37 |
| HAS2 | 1.24 | 21.34 | 48.72 | 1.33 | 27.38 | 2.16 |
| HAS3 | 1.99 | 8.78 | 54.70 | 3.88 | 30.65 | 5.08 |
| HAS3-PDMS | 1.34 | 12.10 | 56.83 | 2.61 | 27.12 | 4.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Tian, S.; Ruan, J.; Chen, R.; Qu, L.; Li, L. Multifunctional Flexible Hard Coatings with Weathering Resistance and Heat-Shielding Properties. Polymers 2025, 17, 519. https://doi.org/10.3390/polym17040519
Chen Y, Tian S, Ruan J, Chen R, Qu L, Li L. Multifunctional Flexible Hard Coatings with Weathering Resistance and Heat-Shielding Properties. Polymers. 2025; 17(4):519. https://doi.org/10.3390/polym17040519
Chicago/Turabian StyleChen, Yuxi, Shenglan Tian, Jincheng Ruan, Ruyu Chen, Lijie Qu, and Luming Li. 2025. "Multifunctional Flexible Hard Coatings with Weathering Resistance and Heat-Shielding Properties" Polymers 17, no. 4: 519. https://doi.org/10.3390/polym17040519
APA StyleChen, Y., Tian, S., Ruan, J., Chen, R., Qu, L., & Li, L. (2025). Multifunctional Flexible Hard Coatings with Weathering Resistance and Heat-Shielding Properties. Polymers, 17(4), 519. https://doi.org/10.3390/polym17040519






