Study on the Impact of Diluent Dosages on the Epoxy–Polythiol Self-Healing System
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Determination of Diluent Dosage of AGE
2.3. Preparation of Sample
2.4. Measurement Characterization
2.4.1. Morphology and Size Characteristics of Microcapsules
2.4.2. Chemical Structure and Thermal Properties of Microcapsules
2.4.3. Thermal Properties of the Curing Products of Epoxy–Polythiol Self-Healing Systems
2.4.4. Young’s Modulus of the Curing Products of Epoxy–Polythiol
3. Results and Analysis
3.1. Influence of Different Diluent Concentrations of Microcapsules
3.1.1. The Morphology and Size Distribution of Microcapsules
3.1.2. The Chemical Structure of Self-Healing Microcapsule Using FT-IR
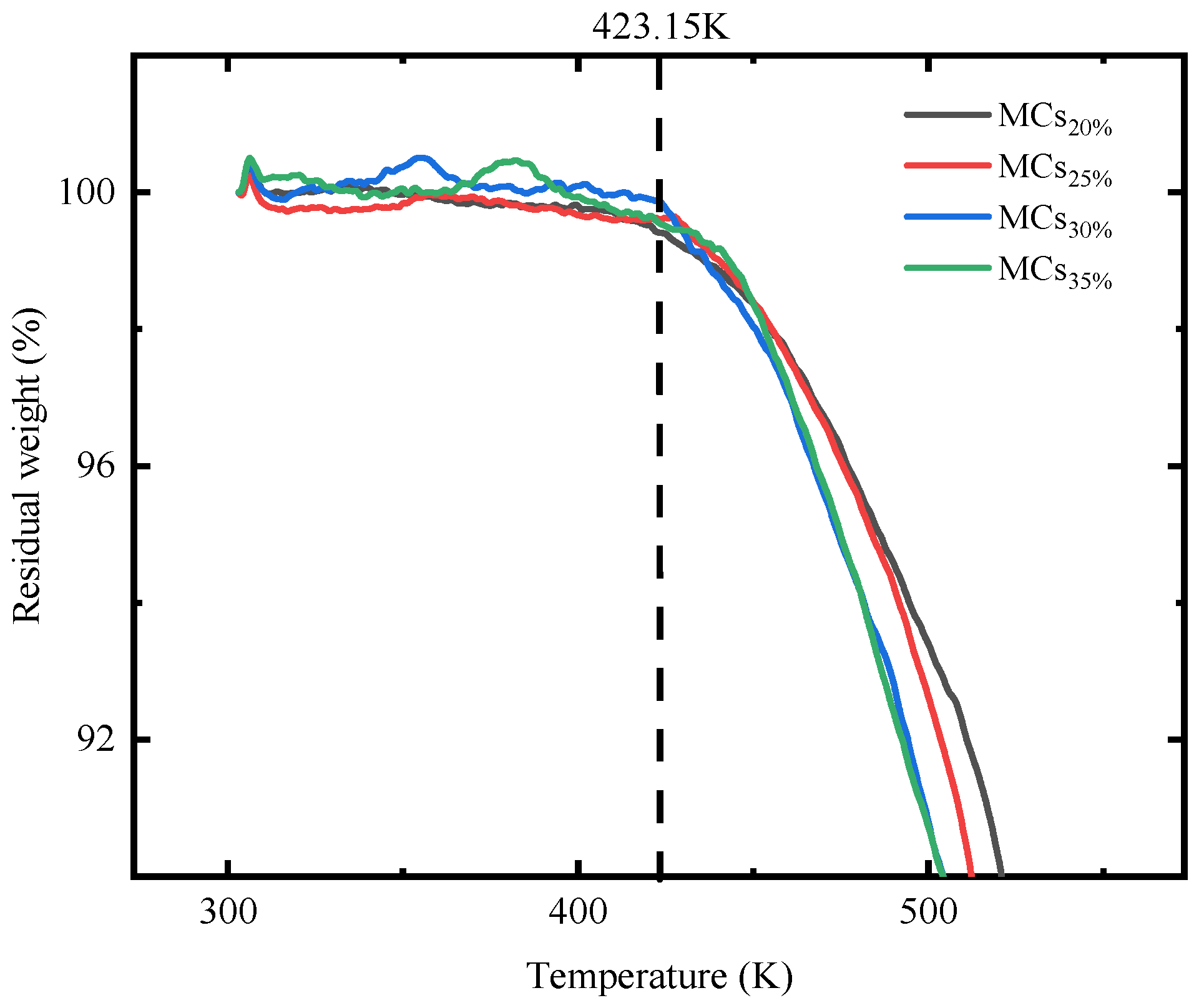
3.1.3. Thermal Stability of Self-Healing Microcapsule Using TGA
3.2. Influence of Different Diluent Concentrations of the Epoxy–Polythiol Self-Healing System
3.2.1. Thermal Stability of Solidified Colloid in the Curing Reaction
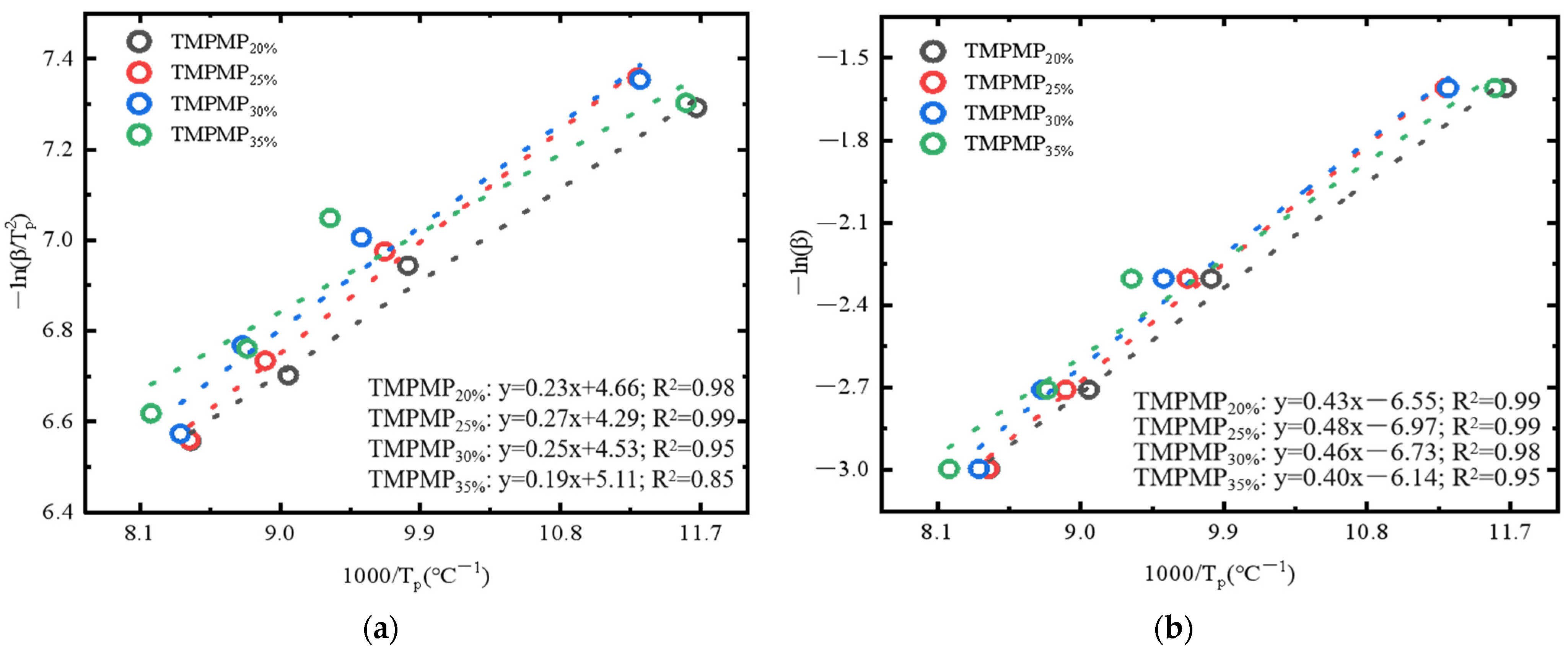
3.2.2. Effect of AGE Diluent Dosage on the Reaction Kinetics in the Curing System
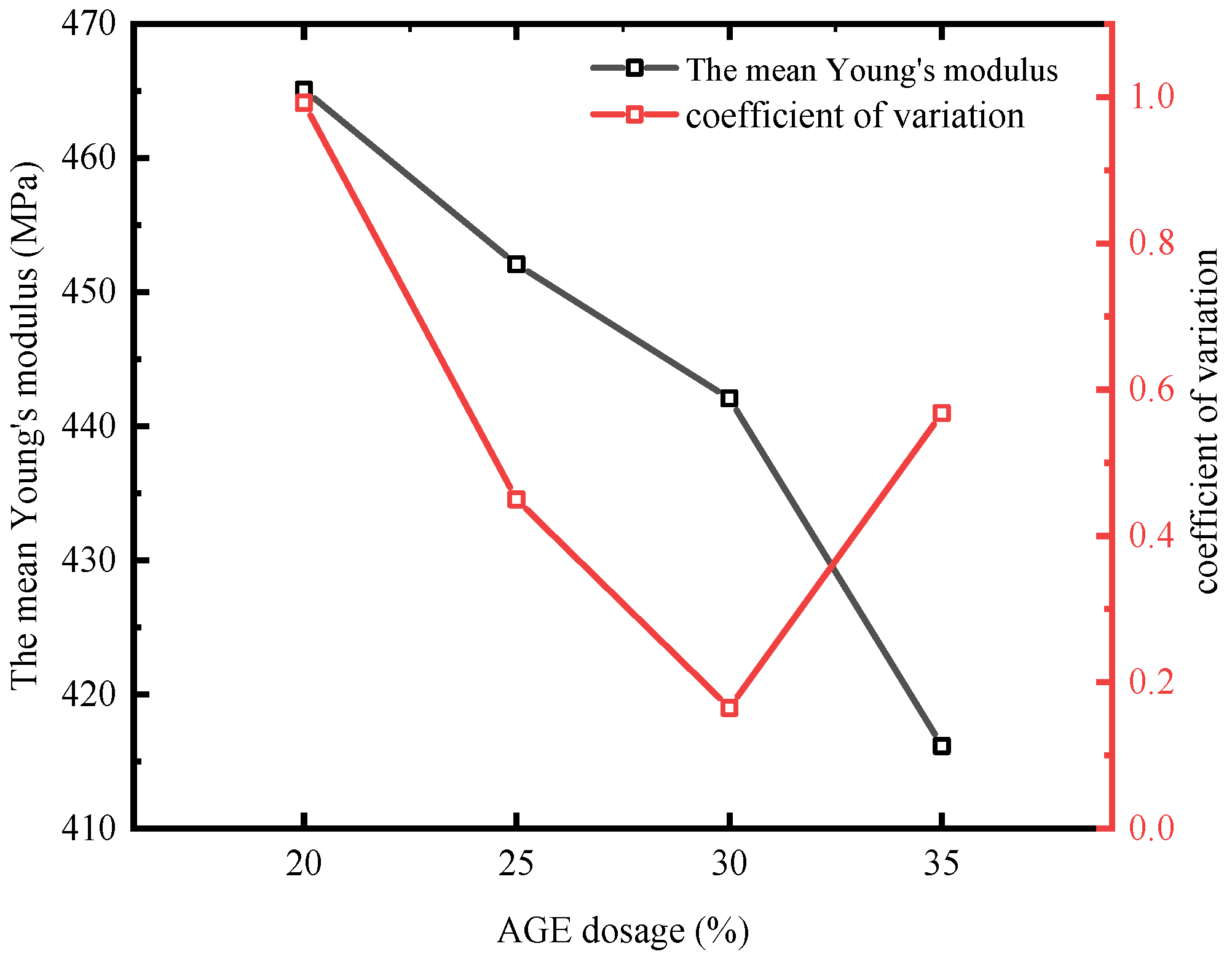
3.2.3. The Effect of Different AGE Additions on the Young’s Modulus of the Self-Healing System
4. Discussion
5. Conclusions
- (1)
- The incorporation of AGE has a noticeable impact on the particle size distribution, chemical bonding, and thermal stability of the microcapsules. With an increase in AGE dosage, the particle size distribution of the microcapsules becomes more concentrated, and the particles become more dispersed. Infrared spectroscopy analysis confirmed that E-51 and AGE were successfully encapsulated. Regarding thermal stability, MCs20%, MCs25%, MCs30%, and MCs35% maintain their structural integrity before 423.15 K. This result is significant for the role of microcapsules in resisting the heat of hydration during the concrete hydration process (≈337.15 K).
- (2)
- The incorporation of AGE significantly affects the thermal stability of the epoxy–polythiol self-healing system. As the temperature increases, the mixture transitions from a liquid to a solid state at 373.15 K, releasing heat that accelerates the thermal degradation of the material. The initial degradation temperature of the mixture shows a decreasing trend with increasing AGE dosage. The initial degradation temperature of TMPMP35% is 370.952 K, while that of TMPMP20% is 584.51 K, representing an increase of 57.57% compared to TMPMP35%. Additionally, the initial and peak temperatures of the curing reaction increase with the amount of AGE incorporated.
- (3)
- The incorporation of AGE significantly affects the thermokinetic properties. The study found a significant linear relationship between the heating rate and the thermokinetic parameters corresponding to the peak curing temperatures. The activation energy (E) exhibited a trend of initially increasing and then decreasing with the addition of AGE, with TMPMP25% having the highest activation energy. Additionally, the frequency factor (A) is positively correlated with the heating rate, indicating that the self-healing system can maintain stable reactive activity at room temperature or lower temperatures, although the changes in the rate constant (k) are small.
- (4)
- The addition of AGE has a certain impact on the rigidity of the self-healing system. The average Young’s modulus shows a significant negative correlation with the amount of diluent added, with the most significant decrease (by 5.88%) occurring when the AGE dosage increases from 30% to 35%. This is because the addition of diluent affects the cross-link density of the self-healing system, which has a negative effect on its mechanical properties.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, K. Effect of composite mineral admixtures on properties of ultra—High performance concrete. J. Munic. Technol. 2023, 41, 256–261. [Google Scholar]
- Yang, Y.Y. Analysis of the workability and durability of recycled aggregate self-compacting concrete. J. Munic. Technol. 2023, 41, 122–128, 133. [Google Scholar]
- Makul, N. Advanced smart concrete—A review of current progress, benefits and challenges. J. Clean. Prod. 2020, 274, 122899. [Google Scholar] [CrossRef]
- Ng, P.L.; Kwan, A.K. Arigorousanalyticalmodel forshrinkagecracking of reinforcedconcrete. Mag. Concr. Res. 2017, 69, 120–133. [Google Scholar] [CrossRef]
- Nair, P.S.; Gupta, R.; Agrawal, V.; Thomas, B.S. A critical review on the effectiveness of microbial concentrations for enhancing self-healing in cement concrete and mortar. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Du, W.; Yu, J.Y.; Gu, S.J.; Wang, R.Y.; Li, J.T.; Han, X.B.; Liu, Q.T. Effect of temperatures on self-healing capabilities of concrete with different shell composition microcapsules containing toluene-di-isocyanate. Constr. Build. Mater. 2020, 247, 118575. [Google Scholar] [CrossRef]
- Li, G.; Meng, H. Overview of Crack Self-Healing; Woodhead Publishing: Baton Rouge, LA, USA, 2015. [Google Scholar]
- Mihashi, H.; Nishiwaki, T. Development of engineered self-Healing and self-repairing concrete—State-of-the-art report. J. Adv. Concr. Technol. 2012, 10, 170–184. [Google Scholar] [CrossRef]
- Sidiq, A.; Gravina, R.; Giustozzi, F. Is concrete healing really efficient? A review. Constr. Build. Mater. 2019, 205, 257–273. [Google Scholar] [CrossRef]
- Huang, H.L.; Ye, G.; Damidot, D. Characterization and quantification of self-healing behaviors of microcracks due to further hydration in cement paste. Cem. Concr. Res. 2013, 52, 71–81. [Google Scholar] [CrossRef]
- Hearn, N. Autogenous healing and continued hydration: What is the difference. Mater. Struct. 1998, 31, 563–567. [Google Scholar] [CrossRef]
- Wang, X.F.; Zhang, J.H.; Zhao, W.; Han, R.; Han, N.X.; Xing, F. Permeability and pore structure of microcapsule-based self-healing cementitious composite. Constr. Build. Mater. 2018, 165, 149–162. [Google Scholar] [CrossRef]
- Beglarigale, A.; Eyice, D.; Seki, Y.; Yalçınkaya, Ç. Sodium silicate/polyurethane microcapsules synthesized for enhancing self-healing ability of cementitious materials: Optimization of stirring speeds and evaluation of self-healing efficiency. J. Build. Eng. 2021, 39, 102279. [Google Scholar] [CrossRef]
- Re, Y.M.; Abbas, N.; Zhu, G.M.; Tang, J.N. Synthesis and mechanical properties of large size silica shell microcapsules for self-healing cementitious materials. Colloids Surf. A Physicochem. Eng. Asp. 2020, 587, 124347. [Google Scholar]
- Sidiq, A.; Setunge, S.; Gravina, R.J.; Giustozzi, F. Self-repairing cement mortars with microcapsules: A microstructural evaluation approach. Constr. Build. Mater. 2020, 232, 117239. [Google Scholar] [CrossRef]
- Li, Y.; Yu, J.Y.; Cao, Z.L.; Du, W.; Zhang, Y.C.; Zou, Y. Preparation and characterization of nano-Fe3O4/paraffin encapsulated isocyanate microcapsule by electromagnetic controlled rupture for self-healing cementitious materials. Constr. Build. Mater. 2020, 265, 120703. [Google Scholar] [CrossRef]
- Hia, I.L.; Chan, E.S.; Chan, S.P.; Pasbakhsh, P. Novel repeated self-healing epoxy composite with alginate Multicore Microcapsules. J. Mater. Chem. A 2018, 6, 8470–8478. [Google Scholar]
- Wang, X.G.; Zhu, J.L.; Zou, F.B.; Zhou, N.G.; Li, Y.J.; Lei, W.Y. Ca-Al LDH hybrid self-healing microcapsules for corrosion protection. Chem. Eng. J. 2022, 447, 137125. [Google Scholar] [CrossRef]
- Wang, X.F.; Zhang, M.; Xing, F.; Han, N.X. Effect of a healing agent on the curing reaction kinetics and its mechanism in a self-Healing system. Appl. Sci. 2018, 8, 2241. [Google Scholar] [CrossRef]
- Dong, B.Q.; Wang, Y.S.; Fang, G.H.; Han, N.X.; Xing, F.; Lu, Y.Y. Smart releasing behavior of a chemical self-healing microcapsule in the stimulated concrete pore solution. Cem. Concr. Compos. 2015, 56, 46–50. [Google Scholar] [CrossRef]
- Song, Y.K.; Jo, Y.H.; Lim, Y.J.; Cho, S.Y.; Yu, H.C.; Ryu, B.C. Sunlight-Induced Self-Healing of a Microcapsule-Type Protective Coating. ACS Appl. Mater. Interfaces 2013, 5, 1378–1384. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yu, J.Y.; Cao, Z.L.; He, P.; Liu, Q.T.; Han, X.B.; Wan, Y. Preparation and application of novel microcapsules ruptured by microwave for self-healing concrete. Constr. Build. Mater. 2021, 304, 124616. [Google Scholar] [CrossRef]
- Xu, N.; Song, Z.J.; Guo, M.Z.; Jiang, L.H.; Chu, H.Q.; Pei, C.; Yu, P.P.; Liu, Q.Y.; Li, Z.M. Employing ultrasonic wave as a novel trigger of microcapsule self-healing cementitious materials. Cem. Concr. Compos. 2021, 118, 103951. [Google Scholar] [CrossRef]
- Du, W.; Yu, J.Y.; Gu, Y.; Li, Y.; Han, X.B.; Liu, Q.T. Preparation and application of microcapsules containing toluene-di-isocyanate for self-healing of concrete. Constr. Build. Mater. 2019, 202, 762–769. [Google Scholar] [CrossRef]
- Su, J.F.; Han, S.; Wang, Y.Y.; Schlangen, E.; Han, N.X.; Liu, B.; Zhang, X.L.; Yang, P.; Li, W. Experimental observation of the self-healing microcapsules containing rejuvenator states in asphalt binder. Constr. Build. Mater. 2017, 147, 533–542. [Google Scholar] [CrossRef]
- Ahangaran, F.; Navarchian, A.H.; Hayaty, M.; Esmailpour, K. Effect of mixing mode and emulsifying agents on micro/nanoencapsulation of low viscosity self-healing agents in polymethyl methacrylate shell. Smart Mater. Struct. 2016, 25, 95035–95048. [Google Scholar] [CrossRef]
- Ahangaran, F.; Hayaty, M.; Navarchian, A.H.; Picchioni, F. Micromechanical assessment of PMMA microcapsules containing epoxy and mercaptan as self-healing agents. Polym. Test. 2017, 64, 330–336. [Google Scholar] [CrossRef]
- Alizadeh, M.; Sarabi, A.A. pH-Responsive MFPTT microcapsules containing dimethyl sulfoxide: Preparation, characterization and tuning the release behavior of microcapsule contents. Prog. Org. Coat. 2019, 134, 78–90. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Su, J.F.; Schlangen, E.; Han, N.X.; Han, S.; Li, W. Fabrication and characterization of self-healing microcapsules containing bituminous rejuvenator by a nano-inorganic/organic hybrid method. Constr. Build. Mater. 2016, 121, 471–482. [Google Scholar] [CrossRef]
- Li, W.T.; Jiang, Z.W.; Yang, Z.H. Acoustic characterization of damage and healing of microencapsulation-based self-healing cement matrices. Cem. Concr. Compos. 2017, 84, 48–61. [Google Scholar] [CrossRef]
- Han, T.L.; Wang, X.F.; Li, D.W.; Li, D.F.; Xing, F.; Han, N.X.; Li, Z.H. Uniaxial deformation characteristics and mechanical model of microcapsule-based self-healing cementitious composite. Constr. Build. Mater. 2017, 274, 121227. [Google Scholar] [CrossRef]
- Ahangaran, F.; Hayaty, M.; Navarchian, A.H.; Pei, Y.T. Development of self-healing epoxy composites via incorporation of microencapsulated epoxy and mercaptan in poly (methyl methacrylate) shell. Polym. Test. 2019, 73, 395–403. [Google Scholar] [CrossRef]
- Dong, B.Q.; Ding, W.J.; Qin, S.F.; Han, N.X.; Fang, G.H.; Liu, Y.Q.; Xing, F.; Hong, S.X. Chemical self-healing system with novel microcapsules for corrosion inhibition of rebar in concrete. Cem. Concr. Compos. 2018, 85, 83–91. [Google Scholar] [CrossRef]
- Wang, X.F.; Huang, Y.J.; Huang, Y.X.; Zhang, J.H.; Fang, C.; Yu, K.; Chen, Q.; Li, T.R.; Han, R.; Yang, Z.H.; et al. Laboratory and field study on the performance of microcapsule-based self-healing concrete in tunnel engineering. Constr. Build. Mater. 2019, 220, 90–101. [Google Scholar] [CrossRef]
- Yao, J.L.; Yang, C.P.; Zhu, C.F.; Hou, B.Q. Preparation process of epoxy resin microcapsules for self—Healing coatings. Prog. Org. Coat. 2019, 132, 440–444. [Google Scholar]
- Ollier, R.P.; Alvarez, V.A. Synthesis of epoxy-loaded poly (melamine-formaldehyde) microcapsules: Effect of pH regulation method and emulsifier selection. Colloids Surf. A Physicochem. Eng. Asp. 2017, 520, 872–882. [Google Scholar] [CrossRef]
- Wang, X.Q.; Ma, B.; Wei, K.; Zhang, W.J. Thermal stability and mechanical properties of epoxy resin/microcapsule composite phase change materials. Constr. Build. Mater. 2021, 312, 125392. [Google Scholar] [CrossRef]
- Wang, W.Z.; Zhao, W.Q.; Zhang, J.J.; Zhou, J.H. Epoxy-based grouting materials with super-low viscosities and improved toughness. Constr. Build. Mater. 2021, 267, 121104. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Pang, H.; Wei, D.D.; Li, J.L.; Li, S.M.; Liu, X.J.; Wang, F.; Liao, B. Preparation and characterization of chemical grouting derived from lignin epoxy resin. Eur. Polym. J. 2019, 118, 290–305. [Google Scholar] [CrossRef]
- Wang, C.H.; Niu, L.L.; Zhang, H.J.; Xiao, X.D.; Liu, Z.S. Working performance and composition optimization of low viscosity epoxy grouting material for cast-in-place cement concrete. Am. Soc. Civ. Eng. 2022, 34, 4022196. [Google Scholar] [CrossRef]
- Yuan, Q.; Wang, Z.; Yao, H.; Huang, J.; Zuo, S.H.; Huang, H. Comparative study of reactive diluents with different molecular structures on the curing properties of epoxy adhesives and the interface bonding properties with mortar. Int. J. Adhes. Adhes. 2023, 126, 103473. [Google Scholar] [CrossRef]
- Mostavi, E.; Asadi, S.; Hassan, M.M.; Alansari, M. Evaluation of self-healing mechanisms in concrete with double-walled sodium silicate microcapsules. J. Mater. Civ. Eng. 2015, 27, 04015035. [Google Scholar] [CrossRef]
- Dong, B.Q.; Fang, G.H.; Wang, Y.S.; Liu, Y.Q.; Hong, S.X.; Zhang, J.C.; Lin, S.M.; Xing, F. Performance recovery concerning the permeability of concrete by means of a microcapsule based self-healing system. Cem. Concr. Compos. 2017, 78, 84–96. [Google Scholar] [CrossRef]
- Mookhoek, S.D.; Blaiszik, B.J.; Fischer, H.R.; Sottos, N.R.; White, S.R.; Zwaag, S.V.D. Peripherally decorated binary microcapsules containing two liquids. J. Mater. Chem. 2008, 18, 5390. [Google Scholar] [CrossRef]
- Lv, L.; Yang, Z.; Chen, G.; Han, N.; Schlangen, E.; Xin, F. Synthesis and characterization of a new polymeric microcapsule and feasibility investigation in self-healing cementitious materials. Constr. Build. Mater. 2016, 105, 487–495. [Google Scholar] [CrossRef]
- Zhang, X.; Jin, Z.; Li, M.; Qian, C.X. Effects of carrier on the performance of bacteria-based self-healing concrete. Constr. Build. Mater. 2021, 305, 124771. [Google Scholar] [CrossRef]
- Zhu, D.Y.; Rong, M.Z.; Zhang, M.Q. Self-healing polymeric materials based on microencapsulated healing agents: From design to preparation. Prog. Polym. Sci. 2015, 49–50, 175–220. [Google Scholar] [CrossRef]
- Carvalho, A.C.M.; Fereira, E.P.A.; Bomio, M.; Melo, J.D.; Barbosa, A.P.C.; Costa, M.C.B. Influence of synthesis parameters on properties and characteristics of poly (urea-formaldehyde) microcapsules for self-healing applications. J. Microencapsul. 2019, 36, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Han, N.X.; Xing, F. A Comprehensive Review of the Study and Development of Microcapsule Based Self-Resilience Systems for Concrete Structures at Shenzhen University. Materials 2017, 10, 2. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimnezhad-Khaljiri, H.; Eslami-Farsani, R. The tensile properties and interlaminar shear strength of microcapsules-glass fibers/epoxy self-healable composites. Eng. Fract. Mech. 2020, 230, 106937. [Google Scholar] [CrossRef]
- Qiao, H.Y.; Qi, P.F.; Zhang, X.H.; Wang, L.N.; Tan, Y.Q.; Luan, Z.H.; Xia, Y.Z.; Li, Y.H.; Sui, K.Y. Multiple Weak H-Bonds Lead to Highly Sensitive, Stretchable, Self-Adhesive, and Self-Healing Ionic Sensors. ACS Appl. Mater. Interfaces 2019, 11, 7755–7763. [Google Scholar] [CrossRef] [PubMed]
- Ellis, B. Chemistry and Technology of Epoxy Resins; Chapman and Hali: London, UK, 1993. [Google Scholar]
- Ma, X.F. Study on the Synthesis and Application of the Rapid Curing Agent for Epoxy Resin. Master’s Thesis, School of Materials Science and Engineering, Beijing University of Chemical Technology, Beijing, China, 2023. [Google Scholar]
- Jin, F.L.; Li, X.; Park, S.J. Synthesis and application of epoxy resins: A review. J. Ind. Eng. Chem. 2015, 29, 1–11. [Google Scholar] [CrossRef]
- Ren, J.; Zhang, J.H.; Wang, X.F.; Li, D.F.; Han, N.X.; Xing, F. Electrochemical Impedance Spectroscopy: A potential approach for detecting the breakage rate of microcapsules for self-healing cementitious materials. Cem. Concr. Compos. 2020, 114, 103776. [Google Scholar] [CrossRef]
- Zhu, J.L.; Wang, X.G.; Wang, X.J.; Lei, Y.X.; Li, Y.J. Carbonyl iron powder/ethyl cellulose hybrid wall microcapsules encapsulating epoxy resin for wave absorption and self-healing. Compos. Sci. Technol. 2021, 214, 108960. [Google Scholar] [CrossRef]
- Dong, B.Q.; Fang, G.H.; Ding, W.J.; Liu, Y.Q.; Zhang, J.C.; Han, N.X.; Xing, F. Self-healing features in cementitious material with urea–formaldehyde/epoxy microcapsules. Constr. Build. Mater. 2016, 106, 608–617. [Google Scholar] [CrossRef]
- Thakur, T.; Gaur, B.; Singha, A.S. Bio-based epoxy/imidoamine encapsulated microcapsules and their application for high performance self-healing coatings. Prog. Org. Coat. 2021, 159, 106436. [Google Scholar] [CrossRef]
- Liu, Y.; Bridgette, B. Self-healing nanocomposites comprised of poly(urea formaldehyde) nanocapsules in a thermosetting polyurea. Eur. Polym. J. 2020, 126, 109545. [Google Scholar] [CrossRef]
- Fang, Y.F.; Ma, B.; Wei, K.; Wang, L.; Kang, X.X.; Chang, X.G. Orthogonal experimental analysis of the material ratio and preparation technology of single-component epoxy resin for asphalt pavement crack repair. Constr. Build. Mater. 2021, 288, 123074. [Google Scholar] [CrossRef]
- Pang, X.; Yang, H.L.; Sheng, J.J.; Wang, L.B. Characterization of epoxy resin microcapsulated by one-step method. In Proceedings of the 9th International Conference on Geology Resources Management and Sustainable Development, Beijing, China, 19 December 2021. [Google Scholar]
- Mamat, M.; Liu, C.H.; Abdukerem, D.; Abdukader, A. A visible-light-induced thiol addition/aerobic oxidation cascade reaction of epoxides and thiols for the synthesis of beta-hydroxylsulfoxides. Org. Biomol. Chem. 2021, 19, 9855–9856. [Google Scholar] [CrossRef]
- Zhu, D.Y.; Cao, G.S.; Qiu, W.L.; Rong, M.Z.; Zhang, M.Q. Self-healing polyvinyl chloride (PVC) based on microencapsulated nucleophilic thiol-click chemistry. Polymer 2015, 69, 1–9. [Google Scholar] [CrossRef]
- Guzmán, D.; Ramis, X.; Fernández-Francos, X.; Serra, A. Enhancement in the Glass Transition Temperature in Latent Thiol-Epoxy Click Cured Thermosets. Polymers 2015, 7, 680–694. [Google Scholar] [CrossRef]
- Li, D.L.; Wei, Z.; Li, L.F.; Deng, W.X.; Xiong, S.F.; Hu, Y.H.; Chen, X.H.; Yu, P. High-pressure cure kinetics and unexpected cure separation of peroxide-cured silicone rubber under compressed CO2. Thermochim. Acta 2024, 737, 179772. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Ye, L.D.; Wei, Q.Y.; Li, D.C.; Wu, Z.J.; Zhang, L.L.; Yu, C.H.; Li, Z.W.; Lu, S.R. Molecular dynamics simulation and curing kinetics of recycled PET/PEGc toughened epoxy resin. React. Funct. Polym. 2025, 207, 106142. [Google Scholar] [CrossRef]
- Zeng, Z.Y.; Zhao, T.Q.; Yu, Z.Q. Synthesis, curing kinetics and properties of novel aromatic multifunctional epoxy resin. Eur. Polym. J. 2024, 219, 113375. [Google Scholar] [CrossRef]
- Liu, Z.H.; Chen, X.S.; Shan, L.Z. Polymer Calorimetry; Chemical Industry Press: Beijing, China, 2002. [Google Scholar]
- Kissinger, H.E. Reaction knetics in differential thermal analysis. Anal. Chem. 1957, 9, 1702–1706. [Google Scholar] [CrossRef]
- Crane, L.W.; Dynes, P.J.; Kaelble, D.H. Analysis of curing kinetics in polymer composites. J. Polym. Sci. Polym. Lett. Ed. 1973, 11, 533–540. [Google Scholar] [CrossRef]
- Derjaguin, B.V.; Muller, V.M.; Toporov, Y.P. Effect of contact deformations on the adhesion of particles. J. Colloid Interface Sci. 1975, 53, 314–326. [Google Scholar] [CrossRef]
- Dong, B.Q.; Diao, H.Q.; Ren, H.B.; Hong, S.X.; Wang, Y.S.; Fang, G.H.; Zhang, Y.Y. Chloride-ion-triggered microcapsule for self-suppression of capillary suction in cement paste. Cem. Concr. Compos. 2023, 141, 105144. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, T. Developments and thermal properties of thermochromic microcapsule and thermochromic asphalt-based composite coatings. Constr. Build. Mater. 2024, 438, 137184. [Google Scholar] [CrossRef]
- Tao, M.Q.; Xu, T. Development and properties of bio-based rice oil microcapsule and its dispersibility in emulsified asphalt. J. Ind. Eng. Chem. 2023, 131, 503–513. [Google Scholar] [CrossRef]
- Klemczak, B.; Batog, M.; Pilch, M.; Żmij, A. Analysis of cracking risk in early age mass concrete with different aggregate types. Procedia Eng. 2017, 193, 234–241. [Google Scholar] [CrossRef]
- Jung, S.H.; Choi, Y.C.; Choi, S. Use of ternary blended concrete to mitigate thermal cracking in massive concrete structures—A field feasibility and monitoring case study. Constr. Build. Mater. 2017, 137, 208–215. [Google Scholar] [CrossRef]
- Ha, J.H.; Jung, Y.S.; Cho, Y.G. Thermal crack control in mass concrete structure using an automated curing system. Autom. Constr. 2015, 45, 16–24. [Google Scholar] [CrossRef]
- Mao, Q.J.; Feng, X.J.; Liang, P.; Wang, R.; Wang, Z.M.; Cui, S.P.; Lan, M.Z. Characteristics of Self-Healing Microcapsules for Cementitious Composites. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2018, 33, 1108–1112. [Google Scholar] [CrossRef]
- Zubkiewicz, A.; Szymczyk, A.; Franciszczak, P.; Kochmanska, A.; Janowska, I.; Paszkiewicz, S. Comparing Multi-Walled Carbon Nanotubes and Halloysite Nanotubes as Reinforcements in EVA Nanocomposites. Materials 2020, 13, 3809. [Google Scholar] [CrossRef] [PubMed]
- Su, S.P.; Xu, Y.H.; China, P.R.; Wilkie, C.A. Thermal degradation of polymer—Carbon nanotube composites. Polym.–Carbon Nanotub. Compos. 2011, 482–510. [Google Scholar] [CrossRef]
- Fang, A.M.; Wu, X.W.; Zhu, X.T.; Luo, C.; Li, D.; Liu, Q.F.; Wang, K. Curing kinetics study of thermosetting resin material with ultra-low dielectric loss for advanced electronic packaging. Polym. Test. 2024, 130, 108312. [Google Scholar] [CrossRef]
- Khalina, M.; Beheshty, M.H.; Salimi, A. The effect of reactive diluent on mechanical properties and microstructure of epoxy resins. Polym. Bull. 2019, 76, 3905–3927. [Google Scholar] [CrossRef]
- Tong, J.D.; Bai, R.K.; Zou, Y.F.; Pan, C.Y.; Ichimura, S. Flexibility improvement of epoxy resin by using polysiloxanes and their derivatives. J. Appl. Polym. Sci. 1994, 52, 1373–1381. [Google Scholar] [CrossRef]
- Mahnam, N.; Beheshty, M.H.; Barmar, M.; Shervin, M. Modification of dicyandiamide-cured epoxy resin with different molecular weights of polyethylene glycol and its effect on epoxy/glass prepreg characteristics. High Perform. Polym. 2013, 25, 705–713. [Google Scholar] [CrossRef]
- Zhao, F.; Sun, Q.C.; Fang, D.P.; Yao, K.D. Preparation and properties of polydimethylsiloxane-modified epoxy resins. Appl. Polym. 2000, 76, 1383–1390. [Google Scholar] [CrossRef]
- Sun, D.Q.; Lu, T.; Zhu, X.Y.; Li, B.; Tian, Y. Optimization of synthesis technology to improve the design of asphalt self-healing microcapsules. Constr. Build. Mater. 2018, 175, 88–103. [Google Scholar] [CrossRef]
- Su, J.F.; Schlangen, E.; Wang, Y.Y. Investigation the self-healing mechanism of aged bitumen using microcapsules containing rejuvenator. Constr. Build. Mater. 2015, 85, 49–56. [Google Scholar] [CrossRef]











| Materials | Component |
|---|---|
| Sodium dodecyl benzene sulfonate 0.75 wt% (AR) | 150 mL |
| Urea (AR, 99%) | 10 g |
| Ammonium chloride (AR, 99.5) | 1 g |
| Resorcinol (AR, 99%) | 1 g |
| Formaldehyde (AR, 37% in H2O) | 27.1 g |
| Epoxy resin (E-51) | 50 g |
| C12-14 alkyl glycidyl ether (AR, epoxy value: 0.3–0.34) | 15 g |
| n-Octanol (AR, 99%) | Quantum satis |
| Citric acid 10 wt% | Quantum satis |
| Ethanol (AR, 75% in H2O) | Quantum satis |
| Sample | Td5% (K) |
|---|---|
| AGE | 420.65 |
| E-51 | 517.15 |
| TMPMP | 515.14 |
| TMPMP20% | 584.51 |
| TMPMP25% | 548.23 |
| TMPMP30% | 530.17 |
| TMPMP35% | 370.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheng, J.; Guo, Y.; Pang, X.; Ma, W.; Yang, H.; Liu, Y.; Wang, L.; Song, S. Study on the Impact of Diluent Dosages on the Epoxy–Polythiol Self-Healing System. Polymers 2025, 17, 538. https://doi.org/10.3390/polym17040538
Sheng J, Guo Y, Pang X, Ma W, Yang H, Liu Y, Wang L, Song S. Study on the Impact of Diluent Dosages on the Epoxy–Polythiol Self-Healing System. Polymers. 2025; 17(4):538. https://doi.org/10.3390/polym17040538
Chicago/Turabian StyleSheng, Jiajia, Yang Guo, Xin Pang, Wenjing Ma, Hailu Yang, Yalin Liu, Linbing Wang, and Shanglin Song. 2025. "Study on the Impact of Diluent Dosages on the Epoxy–Polythiol Self-Healing System" Polymers 17, no. 4: 538. https://doi.org/10.3390/polym17040538
APA StyleSheng, J., Guo, Y., Pang, X., Ma, W., Yang, H., Liu, Y., Wang, L., & Song, S. (2025). Study on the Impact of Diluent Dosages on the Epoxy–Polythiol Self-Healing System. Polymers, 17(4), 538. https://doi.org/10.3390/polym17040538







