Current Trends for Cementation in Prosthodontics: Part 1—The Substrate
Abstract
1. Introduction
2. Substrates for Adhesion in Prosthodontics
2.1. Enamel
2.2. Dentin
2.3. Differences Between Enamel and Dentin with Regard to Adhesion
2.4. Pretreated Dentin as a Substrate for Adhesive Cementation
2.5. Immediate Dentin Sealing
2.6. Substrate Decontamination Before Cementation
2.7. Build-Ups as Substrates for Cementation
2.8. Pulp Protection and Material Toxicity
3. Discussion and Future Perspectives
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Petrauskas, A.; Novaes Olivieri, K.; Pupo, Y.; Berger, G.; Gonçalves Betiol, E. Influence of Different Resin Cements and Surface Treatments on Microshear Bond Strength of Zirconia-Based Ceramics. J. Conserv. Dent. 2018, 21, 198. [Google Scholar] [CrossRef] [PubMed]
- Ge, C.; Green, C.C.; Sederstrom, D.A.; McLaren, E.A.; Chalfant, J.A.; White, S.N. Effect of Tooth Substrate and Porcelain Thickness on Porcelain Veneer Failure Loads in Vitro. J. Prosthet. Dent. 2018, 120, 85–91. [Google Scholar] [CrossRef]
- Walcher, J.G.; Leitune, V.C.B.; Collares, F.M.; de Souza Balbinot, G.; Samuel, S.M.W. Physical and Mechanical Properties of Dual Functional Cements—An in Vitro Study. Clin. Oral Investig. 2018, 23, 1715–1721. [Google Scholar] [CrossRef] [PubMed]
- Manso, A.P.; Silva, N.R.F.A.; Bonfante, E.A.; Pegoraro, T.A.; Dias, R.A.; Carvalho, R.M. Cements and Adhesives for All-Ceramic Restorations. Dent. Clin. N. Am. 2011, 55, 311–332. [Google Scholar] [CrossRef]
- Heboyan, A.; Vardanyan, A.; Karobari, M.I.; Marya, A.; Avagyan, T.; Tebyaniyan, H.; Mustafa, M.; Rokaya, D.; Avetisyan, A. Dental Luting Cements: An Updated Comprehensive Review. Molecules 2023, 28, 1619. [Google Scholar] [CrossRef]
- Rohr, N.; Fischer, J. Tooth Surface Treatment Strategies for Adhesive Cementation. J. Adv. Prosthodont. 2017, 9, 85–92. [Google Scholar] [CrossRef]
- Ghodsi, S.; Shekarian, M.; Aghamohseni, M.M.; Rasaeipour, S.; Arzani, S. Resin Cement Selection for Different Types of Fixed Partial Coverage Restorations: A Narrative Systematic Review. Clin. Exp. Dent. Res. 2023, 9, 1096–1111. [Google Scholar] [CrossRef]
- Cadenaro, M.; Josic, U.; Maravić, T.; Mazzitelli, C.; Marchesi, G.; Mancuso, E.; Breschi, L.; Mazzoni, A. Progress in Dental Adhesive Materials. J. Dent. Res. 2023, 102, 254–262. [Google Scholar]
- Han, F.; Jin, X.; Yuan, X.; Bai, Z.; Wang, Q.; Xie, H. Interactions of Two Phosphate Ester Monomers with Hydroxyapatite and Collagen Fibers and Their Contributions to Dentine Bond Performance. J. Dent. 2022, 122, 104159. [Google Scholar] [CrossRef]
- Maravić, T.; Mazzitelli, C.; Mancuso, E.; Del Bianco, F.; Josić, U.; Cadenaro, M.; Breschi, L.; Mazzoni, A. Resin Composite Cements: Current Status and a Novel Classification Proposal. J. Esthet. Restor. Dent. 2023, 35, 1085–1097. [Google Scholar]
- Cui, F.-Z.; Ge, J. New Observations of the Hierarchical Structure of Human Enamel, from Nanoscale to Microscale. J. Tissue Eng. Regen. Med. 2007, 1, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Risnes, S. Growth Tracks in Dental Enamel. J. Hum. Evol. 1998, 35, 331–350. [Google Scholar] [CrossRef] [PubMed]
- Peumans, M.; Van Meerbeek, B.; Lambrechts, P.; Vanherle, G. Porcelain Veneers: A Review of the Literature. J. Dent. 2000, 28, 163–177. [Google Scholar] [CrossRef]
- Wang, C.; Ou, Y.; Zhang, L.; Zhou, Z.; Li, M.; Xu, J.; Fan, J.; Fu, B.; Hannig, M. Effects of Regional Enamel and Prism Orientations on Bovine Enamel Bond Strength and Cohesive Strength. Eur. J. Oral Sci. 2018, 126, 334–342. [Google Scholar] [CrossRef]
- Comba, A.; Baldi, A.; Garavelli, M.; Maravic, T.; Breschi, L.; Mazzoni, A.; Mazzitelli, C.; Scotti, N. Effects of surface pretreatments on bond strength and morphology of aprismatic enamel. J. Adhes. Dent. 2022, 24, 367–374. [Google Scholar]
- Öztürk, E.; Bolay, Ş.; Hickel, R.; Ilie, N. Shear Bond Strength of Porcelain Laminate Veneers to Enamel, Dentine and Enamel–Dentine Complex Bonded with Different Adhesive Luting Systems. J. Dent. 2013, 41, 97–105. [Google Scholar] [CrossRef]
- Javaheri, D. Considerations for Planning Esthetic Treatment with Veneers Involving No or Minimal Preparation. J. Am. Dent. Assoc. 2007, 138, 331–337. [Google Scholar] [CrossRef]
- Giannini, M.; Soares, C.J.; de Carvalho, R.M. Ultimate Tensile Strength of Tooth Structures. Dent. Mater. 2004, 20, 322–329. [Google Scholar] [CrossRef]
- Al-Harbi, F.; Kaisarly, D.; Michna, A.; ArRejaie, A.; Bader, D.; El Gezawi, M. Cervical Interfacial Bonding Effectiveness of Class II Bulk versus Incremental Fill Resin Composite Restorations. Oper. Dent. 2015, 40, 622–635. [Google Scholar] [CrossRef]
- Lynch, C.D.; O’Sullivan, V.R.; Dockery, P.; McGillycuddy, C.T.; Rees, J.S.; Sloan, A.J. Hunter-Schreger Band Patterns and Their Implications for Clinical Dentistry. J. Oral Rehabil. 2011, 38, 359–365. [Google Scholar] [CrossRef]
- Ikeda, T.; Uno, S.; Tanaka, T.; Kawakami, S.; Komatsu, H.; Sano, H. Relation of Enamel Prism Orientation to Microtensile Bond Strength. Am. J. Dent. 2002, 15, 109–113. [Google Scholar] [PubMed]
- Carvalho, R.M.; Santiago, S.L.; Fernandes, C.A.; Suh, B.I.; Pashley, D.H. Effects of Prism Orientation on Tensile Strength of Enamel. J. Adhes. Dent. 2000, 2, 251–257. [Google Scholar] [PubMed]
- Veneziani, M. Posterior Indirect Adhesive Restorations: Updated Indications and the Morphology Driven Preparation Technique. Int. J. Esthet. Dent. 2017, 12, 204–230. [Google Scholar]
- Tjäderhane, L.; Carrilho, M.R.; Breschi, L.; Tay, F.R.; Pashley, D.H. Dentin Basic Structure and Composition-an Overview. Endod. Top. 2009, 20, 3–29. [Google Scholar] [CrossRef]
- Nanci, A. Ten Cate’s Oral Histology: Development, Structure, and Function, 8th ed.; Elsevier: Amsterdam, The Netherlands; Mosby: St. Louis, MI, USA, 2008. [Google Scholar]
- Carvalho, R.M.; Tjäderhane, L.; Manso, A.P.; Carrilho, M.R.; Carvalho, C.A.R. Dentin as a Bonding Substrate. Endod. Top. 2012, 21, 62–88. [Google Scholar] [CrossRef]
- Pashley, D.H.; Ciucchi, B.; Sano, H.; Carvalho, R.M.; Russell, C.M. Bond Strength versus Dentine Structure: A Modelling Approach. Arch. Oral Biol. 1995, 40, 1109–1118. [Google Scholar] [CrossRef]
- Tay, F.R.; Pashley, D.H. Resin Bonding to Cervical Sclerotic Dentin: A Review. J. Dent. 2004, 32, 173–196. [Google Scholar] [CrossRef]
- Özcan, M.; Volpato, C.A.M. Current Perspectives on Dental Adhesion: (3) Adhesion to Intraradicular Dentin: Concepts and Applications. Jpn. Dent. Sci. Rev. 2020, 56, 216–223. [Google Scholar] [CrossRef]
- Prati, C.; Pashley, D.H. Dentin Wetness, Permeability and Thickness and Bond Strength of Adhesive Systems. Am. J. Dent. 1992, 5, 33–38. [Google Scholar]
- Tay, F.R.; Loushine, R.J.; Lambrechts, P.; Weller, R.N.; Pashley, D.H. Geometric Factors Affecting Dentin Bonding in Root Canals: A Theoretical Modeling Approach. J. Endod. 2005, 31, 584–589. [Google Scholar] [CrossRef]
- Josic, U.; Mazzitelli, C.; Maravic, T.; Comba, A.; Mayer-Santos, E.; Florenzano, F.; Breschi, L.; Mazzoni, A. Evaluation of Fiber Post Adhesion to Root Dentin Achieved with Different Composite Cements: 1-Year In Vitro Results. J. Adhes. Dent. 2022, 24, 95–104. [Google Scholar] [PubMed]
- Braga, R.R.; Ferracane, J.L. Alternatives in Polymerization Contraction Stress Management. Crit. Rev. Oral Biol. Med. 2004, 15, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.; Carvalho, C.A.; Goracci, C.; Antoniolli, F.; Mazzoni, A.; Mazzotti, G.; Cadenaro, M.; Breschi, L. Influence of Luting Material Filler Content on Post Cementation. J. Dent. Res. 2009, 88, 951–956. [Google Scholar] [CrossRef] [PubMed]
- de Almeida Neves, A.; Coutinho, E.; Cardoso, M.V.; Lambrechts, P.; Van Meerbeek, B. Current Concepts and Techniques for Caries Excavation and Adhesion to Residual Dentin. J. Adhes. Dent. 2011, 13, 7–22. [Google Scholar] [CrossRef]
- Banerjee, A.; Splieth, C.; Breschi, L.; Fontana, M.; Paris, S.; Burrow, M.F.; Crombie, F.; Page, L.F.; Gatón-Hernández, P.; Giacaman, R.; et al. When to Intervene in the Caries Process? An Expert Delphi Consensus Statement. Br. Dent. J. 2020, 229, 474–482. [Google Scholar] [CrossRef]
- Wang, Y.; Spencer, P.; Walker, M.P. Chemical Profile of Adhesive/Caries-Affected Dentin Interfaces Using Raman Microspectroscopy. J. Biomed. Mater. Res. A 2007, 81, 279–286. [Google Scholar] [CrossRef]
- Ito, S.; Saito, T.; Tay, F.R.; Carvalho, R.M.; Yoshiyama, M.; Pashley, D.H. Water Content and Apparent Stiffness of Non-Caries versus Caries-Affected Human Dentin. J. Biomed. Mater. Res. 2005, 72, 109–116. [Google Scholar] [CrossRef]
- Hsu, K.-W.; Marshall, S.J.; Pinzon, L.M.; Watanabe, L.; Saiz, E.; Marshall, G.W. SEM Evaluation of Resin-Carious Dentin Interfaces Formed by Two Dentin Adhesive Systems. Dent. Mater. 2008, 24, 880–887. [Google Scholar] [CrossRef][Green Version]
- Spencer, P.; Wang, Y.; Katz, J.L.; Misra, A. Physicochemical Interactions at the Dentin/Adhesive Interface Using FTIR Chemical Imaging. J. Biomed. Opt. 2005, 10, 031104. [Google Scholar] [CrossRef]
- Ceballos, L.; Camejo, D.G.; Victoria Fuentes, M.; Osorio, R.; Toledano, M.; Carvalho, R.M.; Pashley, D.H. Microtensile Bond Strength of Total-Etch and Self-Etching Adhesives to Caries-Affected Dentine. J. Dent. 2003, 31, 469–477. [Google Scholar] [CrossRef]
- Perdigão, J. Dentin Bonding-Variables Related to the Clinical Situation and the Substrate Treatment. Dent. Mater. 2010, 26, e24–e37. [Google Scholar] [CrossRef] [PubMed]
- Yoshiyama, M.; Urayama, A.; Kimochi, T.; Matsuo, T.; Pashley, D.H. Comparison of Conventional vs Self-Etching Adhesive Bonds to Caries-Affected Dentin. Oper. Dent. 2000, 25, 163–169. [Google Scholar] [PubMed]
- Erhardt, M.C.G.; Toledano, M.; Osorio, R.; Pimenta, L.A. Histomorphologic Characterization and Bond Strength Evaluation of Caries-Affected Dentin/Resin Interfaces: Effects of Long-Term Water Exposure. Dent. Mater. 2008, 24, 786–798. [Google Scholar] [CrossRef] [PubMed]
- Opdam, N.J.M.; Bronkhorst, E.M.; Loomans, B.A.C.; Huysmans, M.-C.D.N.J.M. 12-Year Survival of Composite vs Amalgam Restorations. J. Dent. Res. 2010, 89, 1063–1067. [Google Scholar] [CrossRef]
- Da Rosa Rodolpho, P.A.; Donassollo, T.A.; Cenci, M.S.; Loguércio, A.D.; Moraes, R.R.; Bronkhorst, E.M.; Opdam, N.J.M.; Demarco, F.F. 22-Year Clinical Evaluation of the Performance of Two Posterior Composites with Different Filler Characteristics. Dent. Mater. 2011, 27, 955–963. [Google Scholar] [CrossRef]
- Gwinnett, A.J. Quantitative Contribution of Resin Infiltration/Hybridization to Dentin Bonding. Am. J. Dent. 1993, 6, 7–9. [Google Scholar]
- Kwong, S.M.; Cheung, G.S.P.; Kei, L.H.; Itthagarun, A.; Smales, R.J.; Tay, F.R.; Pashley, D.H. Micro-Tensile Bond Strengths to Sclerotic Dentin Using a Self-Etching and a Total-Etching Technique. Dent. Mater. 2002, 18, 359–369. [Google Scholar] [CrossRef]
- Xie, C.; Han, Y.; Zhao, X.Y.; Wang, Z.Y.; He, H.M. Microtensile Bond Strength of One- and Two-Step Self-Etching Adhesives on Sclerotic Dentin: The Effects of Thermocycling. Oper. Dent. 2010, 35, 547–555. [Google Scholar] [CrossRef]
- Lopes, G.C.; Baratieri, C.M.; Baratieri, L.N.; Monteiro, S.; Cardoso Vieira, L.C. Bonding to Cervical Sclerotic Dentin: Effect of Acid Etching Time. J. Adhes. Dent. 2004, 6, 19–23. [Google Scholar]
- Luque-Martinez, I.V.; Muñoz, M.A.; Hass, V.; Sutil, E.; Reis, A.; Loguercio, A.D. EDTA Conditioning Increases the Long-Term Microtensile Bond Strength to Sclerotic Dentin Mediated by Self-Etch Adhesives. J. Adhes. Dent. 2018, 20, 397–403. [Google Scholar] [CrossRef]
- Zhang, G.; He, W.; Ding, N.; Su, Y.; Yu, G. Sandblasting Increases the Microtensile Bond Strength between Resin and Sclerotic Dentin in Noncarious Cervical Lesions. Am. J. Dent. 2024, 37, 121–125. [Google Scholar] [PubMed]
- Rodrigues, R.F.; Ramos, C.M.; Francisconi, P.A.S.; Borges, A.F.S. The Shear Bond Strength of Self-Adhesive Resin Cements to Dentin and Enamel: An in Vitro Study. J. Prosthet. Dent. 2015, 113, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Temel, U.B.; Van Ende, A.; Van Meerbeek, B.; Ermis, R.B. Bond Strength and Cement-Tooth Interfacial Characterization of Self-Adhesive Composite Cements. Am. J. Dent. 2017, 30, 205–211. [Google Scholar] [PubMed]
- Scholz, K.J.; Tabenski, I.M.; Vogl, V.; Cieplik, F.; Schmalz, G.; Buchalla, W.; Hiller, K.; Federlin, M.; Cam, C.A.D. Randomized Clinical Split-Mouth Study on the Performance of CAD/CAM-Partial Ceramic Crowns Luted with a Self-Adhesive Resin Cement or a Universal Adhesive and a Conventional Resin Cement after 39 Months. J. Dent. 2021, 115, 103837. [Google Scholar] [CrossRef]
- Van Meerbeek, B.; Yoshihara, K.; Yoshida, Y.; Mine, A.; De Munck, J.; Van Landuyt, K.L. State of the Art of Self-Etch Adhesives. Dent. Mater. 2011, 27, 17–28. [Google Scholar] [CrossRef]
- Mazzitelli, C.; Maravic, T.; Sebold, M.; Checchi, V.; Josic, U.; Breschi, L.; Mazzoni, A. Effect of Shelf-Life of a Universal Adhesive to Dentin. Int. J. Adhes. Adhes. 2020, 102, 102673. [Google Scholar] [CrossRef]
- Glasspoole, E.A.; Erickson, R.L.; Davidson, C.L. Effect of Surface Treatments on the Bond Strength of Glass Ionomers to Enamel. Dent. Mater. 2002, 18, 454–462. [Google Scholar] [CrossRef]
- Yiu, C.K.Y.; Tay, F.R.; King, N.M.; Pashley, D.H.; Sidhu, S.K.; Neo, J.C.L.; Toledano, M.; Wong, S.L. Interaction of Glass-Ionomer Cements with Moist Dentin. J. Dent. Res. 2004, 83, 283–289. [Google Scholar] [CrossRef]
- Xu, X.; Burgess, J.O. Compressive Strength, Fluoride Release and Recharge of Fluoride-Releasing Materials. Biomaterials 2003, 24, 2451–2461. [Google Scholar] [CrossRef]
- Somani, R.; Jaidka, S.; Singh, D.J.; Sibal, G.K. Comparative Evaluation of Shear Bond Strength of Various Glass Ionomer Cements to Dentin of Primary Teeth: An in Vitro Study. Int. J. Clin. Pediatr. Dent. 2016, 9, 192–196. [Google Scholar] [CrossRef]
- Parisay, I.; Khazaei, Y. Evaluation of Retentive Strength of Four Luting Cements with Stainless Steel Crowns in Primary Molars: An in Vitro Study. Dent. Res. J. 2018, 15, 201–207. [Google Scholar]
- Münchow, E.A.; Bottino, M.C. Recent Advances in Adhesive Bonding: The Role of Biomolecules, Nanocompounds, and Bonding Strategies in Enhancing Resin Bonding to Dental Substrates. Curr. Oral Health Rep. 2017, 4, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Parameswari, B.D.; Rajakumar, M.; Lambodaran, G.; Sundar, S. Comparative Study on the Tensile Bond Strength and Marginal Fit of Complete Veneer Cast Metal Crowns Using Various Luting Agents: An in Vitro Study. J. Pharm. Bioallied Sci. 2016, 8, S138–S143. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, M.; de Almeida Neves, A.; Mine, A.; Coutinho, E.; Van Landuyt, K.; De Munck, J.; Van Meerbeek, B. Current Aspects on Bonding Effectiveness and Stability in Adhesive Dentistry. Aust. Dent. J. 2011, 56, 31–44. [Google Scholar] [CrossRef]
- Maravić, T.; Comba, A.; Cunha, S.R.; Angeloni, V.; Cadenaro, M.; Visinitini, E.; Navarra, C.O.; Salgarello, S.; Breschi, L.; Mazzoni, A. Long-Term Bond Strength and Endogenous Enzymatic Activity of a Chlorhexidine-Containing Commercially Available Adhesive. J. Dent. 2019, 84, 60–66. [Google Scholar] [CrossRef]
- Comba, A.; Scotti, N.; Mazzoni, A.; Maravic, T.; Ribeiro Cunha, S.; Michelotto Tempesta, R.; Carossa, M.; Pashley, D.H.; Tay, F.R.; Breschi, L. Carbodiimide Inactivation of Matrix Metalloproteinases in Radicular Dentine. J. Dent. 2019, 82, 56–62. [Google Scholar] [CrossRef]
- Maravic, T.; Breschi, L.; Comba, A.; Cunha, S.R.; Angeloni, V.; Nucci, C.; Hebling, J.; Pashley, D.; Tay, F.; Mazzoni, A. Experimental Use of an Acrolein-Based Primer as Collagen Cross-Linker for Dentine Bonding. J. Dent. 2017, 68, 85–90. [Google Scholar] [CrossRef]
- Checchi, V.; Maravic, T.; Bellini, P.; Generali, L.; Consolo, U.; Breschi, L.; Mazzoni, A. The Role of Matrix Metalloproteinases in Periodontal Disease. Int. J. Environ. Res. Public Health 2020, 17, 4923. [Google Scholar] [CrossRef]
- Maravic, T.; Mazzoni, A.; Comba, A.; Scotti, N.; Checchi, V.; Breschi, L. How Stable Is Dentin as a Substrate for Bonding? Curr. Oral Health Rep. 2017, 4, 248–257. [Google Scholar] [CrossRef]
- Turco, G.; Cadenaro, M.; Maravić, T.; Frassetto, A.; Marsich, E.; Mazzoni, A.; Di Lenarda, R.; Tay, F.R.; Pashley, D.H.; Breschi, L. Release of ICTP and CTX Telopeptides from Demineralized Dentin Matrices: Effect of Time, Mass and Surface Area. Dent. Mater. 2018, 34, 452–459. [Google Scholar] [CrossRef]
- Comba, A.; Maravic, T.; Valente, L.; Girlando, M.; Cunha, S.R.; Checchi, V.; Salgarello, S.; Tay, F.R.; Scotti, N.; Breschi, L.; et al. Effect of Benzalkonium Chloride on Dentin Bond Strength and Endogenous Enzymatic Activity. J. Dent. 2019, 85, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Pashley, D.H.; Tay, F.R.; Breschi, L.; Tjäderhane, L.; Carvalho, R.M.; Carrilho, M.; Tezvergil-Mutluay, A. State of the Art Etch-and-Rinse Adhesives. Dent. Mater. 2011, 27, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Breschi, L.; Maravic, T.; Cunha, S.R.; Comba, A.; Cadenaro, M.; Tjäderhane, L.; Pashley, D.H.; Tay, F.R.; Mazzoni, A. Dentin Bonding Systems: From Dentin Collagen Structure to Bond Preservation and Clinical Applications. Dent. Mater. 2018, 34, 78–96. [Google Scholar] [CrossRef] [PubMed]
- Arcari, G.M.; Araújo, E.; Baratieri, L.N.; Lopes, G.C. Microtensile Bond Strength of a Nanofilled Composite Resin to Human Dentin after Nonvital Tooth Bleaching. J. Adhes. Dent. 2007, 9, 333–340. [Google Scholar]
- Attin, T.; Hannig, C.; Wiegand, A.; Attin, R. Effect of Bleaching on Restorative Materials and Restorations—A Systematic Review. Dent. Mater. 2004, 20, 852–861. [Google Scholar] [CrossRef]
- Boccuzzi, M.; Nota, A.; Cosola, S.; De Simone, G.; Iozzo, R.; Pittari, L.; Hwang, M.H.; Bosco, F.; Polizzi, E.; Tecco, S. Effect of Bleaching Treatments on the Adhesion of Orthodontic Brackets: A Systematic Review. BMC Oral Health 2023, 23, 758. [Google Scholar] [CrossRef]
- Ari, H.; Erdemir, A.; Belli, S. Evaluation of the Effect of Endodontic Irrigation Solutions on the Microhardness and the Roughness of Root Canal Dentin. J. Endod. 2004, 30, 792–795. [Google Scholar] [CrossRef]
- Kielbassa, A.M.; Attin, T.; Hellwig, E. Diffusion Behavior of Eugenol from Zinc Oxide-Eugenol Mixtures through Human and Bovine Dentin in Vitro. Oper. Dent. 1997, 22, 15–20. [Google Scholar]
- Carvalho, C.N.; de Oliveira Bauer, J.R.; Loguercio, A.D.; Reis, A. Effect of ZOE Temporary Restoration on Resin-Dentin Bond Strength Using Different Adhesive Strategies. J. Esthet. Restor. Dent. 2007, 19, 144–152. [Google Scholar] [CrossRef]
- Serafino, C.; Gallina, G.; Cumbo, E.; Ferrari, M. Surface Debris of Canal Walls after Post Space Preparation in Endodontically Treated Teeth: A Scanning Electron Microscopic Study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2004, 97, 381–387. [Google Scholar] [CrossRef]
- Serafino, C.; Gallina, G.; Cumbo, E.; Monticelli, F.; Goracci, C.; Ferrari, M. Ultrasound Effects after Post Space Preparation: An SEM Study. J. Endod. 2006, 32, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Coniglio, I.; Magni, E.; Goracci, C.; Radovic, I.; Carvalho, C.A.; Grandini, S.; Ferrari, M. Post Space Cleaning Using a New Nickel Titanium Endodontic Drill Combined with Different Cleaning Regimens. J. Endod. 2008, 34, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, E.M.; Carvalho, C.N.; Loguercio, A.D.; Lima, D.M.; Bauer, J. Effect of Temporary Cements on the Microtensile Bond Strength of Self-Etching and Self-Adhesive Resin Cement. Acta Odontol. Scand. 2014, 72, 762–769. [Google Scholar] [CrossRef]
- Feiz, A.; Mosleh, H.; Nazeri, R. Evaluating the Effect of Antioxidant Agents on Shear Bond Strength of Tooth-Colored Restorative Materials after Bleaching: A Systematic Review. J. Mech. Behav. Biomed. Mater. 2017, 71, 156–164. [Google Scholar] [CrossRef]
- Cadenaro, M.; Maravic, T.; Comba, A.; Mazzoni, A.; Fanfoni, L.; Hilton, T.; Ferracane, J.; Breschi, L. The Role of Polymerization in Adhesive Dentistry. Dent. Mater. 2018, 35, e1–e22. [Google Scholar] [CrossRef]
- Comba, A.; Maravić, T.; Villalta, V.; Tozzola, S.; Mazzitelli, C.; Checchi, V.; Mancuso, E.; Scotti, N.; Tay, F.R.; Breschi, L.; et al. Effect of an Ethanol Cross-Linker on Universal Adhesive. Dent. Mater. 2020, 36, 1645–1654. [Google Scholar] [CrossRef]
- Mazzoni, A.; Angeloni, V.; Comba, A.; Maravic, T.; Cadenaro, M.; Tezvergil-Mutluay, A.; Pashley, D.H.; Tay, F.R.; Breschi, L. Cross-Linking Effect on Dentin Bond Strength and MMPs Activity. Dent. Mater. 2018, 34, 288–295. [Google Scholar] [CrossRef]
- Mazzoni, A.; Angeloni, V.; Sartori, N.; Duarte, S.; Maravic, T.; Tjäderhane, L.; Pashley, D.H.; Tay, F.R.; Breschi, L. Substantivity of Carbodiimide Inhibition on Dentinal Enzyme Activity over Time. J. Dent. Res. 2017, 96, 902–908. [Google Scholar] [CrossRef]
- Maravic, T.; Mancuso, E.; Comba, A.; Checchi, V.; Generali, L.; Mazzitelli, C.; Josic, U.; Hass, V.; Reis, A.; Loguercio, A.D.; et al. Dentin Cross-Linking Effect of Carbodiimide after 5 Years. J. Dent. Res. 2021, in press. [Google Scholar] [CrossRef]
- Josic, U.; Mazzitelli, C.; Maravic, T.; Comba, A.; Cadenaro, M.; Radovic, I.; Sebold, M.; Turco, G.; Breschi, L.; Mazzoni, A. The Effect of Carbodiimide on Push-out Bond Strength of Fiber Posts and Endogenous Enzymatic Activity. BMC Oral Health 2023, 23, 399. [Google Scholar] [CrossRef]
- Heckler, B.; Yao, X.; Wang, Y. Proanthocyanidins Alter Adhesive/Dentin Bonding Strengths When Included in a Bonding System. Am. J. Dent. 2012, 25, 276–280. [Google Scholar]
- Seseogullari-Dirihan, R.; Apollonio, F.; Mazzoni, A.; Tjaderhane, L.; Pashley, D.; Breschi, L.; Tezvergil-Mutluay, A. Use of Crosslinkers to Inactivate Dentin MMPs. Dent. Mater. 2016, 32, 423–432. [Google Scholar] [CrossRef]
- Broyles, A.C.; Pavan, S.; Bedran-Russo, A.K. Effect of Dentin Surface Modification on the Microtensile Bond Strength of Self-Adhesive Resin Cements. J. Prosthodont. 2013, 22, 59–62. [Google Scholar] [CrossRef]
- Breschi, L.; Maravic, T.; Comba, A.; Cunha, S.R.; Loguercio, A.D.; Reis, A.; Hass, V.; Cadenaro, M.; Mancuso, E.; Mayer-Santos, E.; et al. Chlorhexidine Preserves the Hybrid Layer in Vitro after 10-Years Aging. Dent. Mater. 2020, 36, 672–680. [Google Scholar] [CrossRef]
- Cadenaro, M.; Pashley, D.H.; Marchesi, G.; Carrilho, M.; Antoniolli, F.; Mazzoni, A.; Tay, F.R.; Di Lenarda, R.; Breschi, L. Influence of Chlorhexidine on the Degree of Conversion and E-Modulus of Experimental Adhesive Blends. Dent. Mater. 2009, 25, 1269–1274. [Google Scholar] [CrossRef]
- Magne, P. Immediate Dentin Sealing: A Fundamental Procedure for Indirect Bonded Restorations. J. Esthet. Restor. Dent. 2005, 17, 144–154. [Google Scholar] [CrossRef]
- Alghauli, M.A.; Alqutaibi, A.Y.; Borzangy, S. Clinical Benefits of Immediate Dentin Sealing: A Systematic Review and Meta-Analysis. J. Prosthet. Dent. 2024. ahead of print. [Google Scholar] [CrossRef]
- Breschi, L.; Maravic, T.; Mazzitelli, C.; Josic, U.; Mancuso, E.; Cadenaro, M.; Pfeifer, C.S.; Mazzoni, A. The Evolution of Adhesive Dentistry: From Etch-and-Rinse to Universal Bonding Systems. Dent. Mater. 2024, 41, 141–158. [Google Scholar] [CrossRef]
- Varadan, P.; Balaji, L.; Manaswini, D.Y.; Rajan, R.M. Reinforced Immediate Dentin Sealing vs Conventional Immediate Dentin Sealing on Adhesive Behavior of Indirect Restorations: A Systematic Review. J. Contemp. Dent. Pract. 2022, 23, 1066–1075. [Google Scholar] [CrossRef]
- Elbishari, H.; Elsubeihi, E.S.; Alkhoujah, T.; Elsubeihi, H.E. Substantial In-Vitro and Emerging Clinical Evidence Supporting Immediate Dentin Sealing. Jpn. Dent. Sci. Rev. 2021, 57, 101–110. [Google Scholar] [CrossRef]
- Mueller, B.; Pilecco, R.O.; Valandro, L.F.; Ruschel, V.C.; Pereira, G.K.R.; Bernardon, J.K. Effect of Immediate Dentin Sealing on Load-Bearing Capacity under Accelerated Fatigue of Thin Occlusal Veneers Made of CAD-CAM Glass-Ceramic and Resin Composite Material. Dent. Mater. 2023, 39, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Josic, U.; Sebold, M.; Lins, R.; Savovic, J.; Mazzitelli, C.; Maravic, T.; Mazzoni, A.; Breschi, L. Does Immediate Dentin Sealing Influence Postoperative Sensitivity in Teeth Restored with Indirect Restorations? A Systematic Review and Meta-analysis. J. Esthet. Restor. Dent. 2022, 34, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.C.V.; Coelho, P.G.; Janal, M.N.; Silva, N.R.F.A.; Monteiro, A.J.; Fernandes, C.A.O. The Influence of Temporary Cements on Dental Adhesive Systems for Luting Cementation. J. Dent. 2011, 39, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Takimoto, M.; Ishii, R.; Iino, M.; Shimizu, Y.; Tsujimoto, A.; Takamizawa, T.; Ando, S.; Miyazaki, M. Influence of Temporary Cement Contamination on the Surface Free Energy and Dentine Bond Strength of Self-Adhesive Cements. J. Dent. 2012, 40, 131–138. [Google Scholar] [CrossRef]
- AlZain, S.; Kattadiyil, M.T.; AlHelal, A.; Alqahtani, A. Effect of Intraoral Mechanical Cleaning Techniques on Bond Strength of Cast Crowns to Metal Cores. J. Prosthodont. 2017, 29, 69–73. [Google Scholar] [CrossRef]
- Song, M.-Y.; An, H.; Park, E.-J. The Effect of Temporary Cement Cleaning Methods on the Retention of Crowns. J. Prosthodont. 2019, 28, e210–e215. [Google Scholar] [CrossRef]
- Hammad, I.A.; Al Amri, M. The Effect of Two Fit-Indicating Materials and Various Subsequent Cleaning Methods on the Retention of Simulated Crowns. J. Prosthet. Dent. 2008, 99, 46–53. [Google Scholar] [CrossRef]
- Fonseca, R.B.; Martins, L.R.M.; Quagliatto, P.S.; Soares, C.J. Influence of Provisional Cements on Ultimate Bond Strength of Indirect Composite Restorations to Dentin. J. Adhes. Dent. 2005, 7, 225–230. [Google Scholar]
- Iwama, H.; Ishii, R.; Takamizawa, T.; Aoki, R.; Watanabe, S.; Hayashi, K.; Kamimoto, A.; Miyazaki, M. Influence of Surface Pretreatment on the Bond Strength of a Resin Luting Cement to Saliva-Contaminated Enamel and Dentin. Oper. Dent. 2024, 49, 586–596. [Google Scholar] [CrossRef]
- Tian, F.; Jett, K.; Flaugher, R.; Arora, S.; Bergeron, B.; Shen, Y.; Tay, F. Effects of Dentine Surface Cleaning on Bonding of a Self-Etch Adhesive to Root Canal Sealer-Contaminated Dentine. J. Dent. 2021, 112, 103766. [Google Scholar] [CrossRef]
- Toledano, M.; Osorio, E.; Espigares, J.; González-Fernández, J.F.; Osorio, R. Effects of an MDP-Based Surface Cleaner on Dentin Structure, Morphology and Nanomechanical Properties. J. Dent. 2023, 138, 104734. [Google Scholar] [CrossRef] [PubMed]
- Fichera, G.; Mazzitelli, C.; Picciariello, V.; Maravic, T.; Josic, U.; Mazzoni, A.; Breschi, L. Structurally Compromised Teeth. Part I: Clinical Considerations and Novel Classification Proposal. J. Esthet. Restor. Dent. 2024, 36, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Fichera, G.; Mazzitelli, C.; Picciariello, V.; Maravic, T.; Josic, U.; Mazzoni, A.; Breschi, L. Structurally Compromised Teeth. Part II: A Novel Approach to Peripheral Build up Procedures. J. Esthet. Restor. Dent. 2024, 36, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.; Mannocci, F.; Vichi, A.; Goracci, G. Bond Strengths of a Porcelain Material to Different Abutment Substrates. Oper. Dent. 2000, 25, 299–305. [Google Scholar]
- Mendonça, L.M.D.; Pegoraro, L.F.; Lanza, M.D.S.; Pegoraro, T.A.; Carvalho, R.M.D. Effects of Coronal Substrates and Water Storage on the Microhardness of a Resin Cement Used for Luting Ceramic Crowns. J. Appl. Oral Sci. 2014, 22, 287–293. [Google Scholar] [CrossRef][Green Version]
- Yanagida, H.; Tanoue, N.; Hodate, K.; Muraguchi, K.; Uenodan, A.; Minesaki, Y.; Minami, H. Evaluation of the Effects of Three Pretreatment Conditioners and a Surface Preparation System on the Bonding Durability of Composite Resin Adhesive to a Gold Alloy. Dent. Mater. J. 2021, 40, 1388–1393. [Google Scholar] [CrossRef]
- Özcan, M.; Barbosa, S.H.; Melo, R.M.; Galhano, G.Á.P.; Bottino, M.A. Effect of Surface Conditioning Methods on the Microtensile Bond Strength of Resin Composite to Composite after Aging Conditions. Dent. Mater. 2007, 23, 1276–1282. [Google Scholar] [CrossRef]
- Padipatvuthikul, P.; Mair, L.H. Bonding of Composite to Water Aged Composite with Surface Treatments. Dent. Mater. 2007, 23, 519–525. [Google Scholar] [CrossRef]
- Nakabayashi, N.; Pashley, D.H. Hybridization of Dental Hard Tissues; Quintessence Pub. Co.: London, UK, 1998; ISBN 4874175759. [Google Scholar]
- Sano, H.; Takatsu, T.; Ciucchi, B.; Horner, J.A.; Matthews, W.G.; Pashley, D.H. Nanoleakage: Leakage within the Hybrid Layer. Oper. Dent. 1995, 20, 18–25. [Google Scholar]
- Hanks, C.T.; Strawn, S.E.; Watahai, J.C.; Craig, R.G. Cytotoxic Effects of Resin Components on Cultured Mammalian Fibroblasts. J. Dent. Res. 1991, 70, 1450–1455. [Google Scholar] [CrossRef]
- Schneider, T.R.; Hakami-Tafreshi, R.; Tomasino-Perez, A.; Tayebi, L.; Lobner, D. Effects of Dental Composite Resin Monomers on Dental Pulp Cells. Dent. Mater. J. 2019, 38, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Bouillaguet, S.; Duroux, B.; Ciucchi, B.; Sano, H. Ability of Adhesive Systems to Seal Dentin Surfaces: An in Vitro Study. J. Adhes. Dent. 2000, 2, 201–208. [Google Scholar]
- Longo, D.L.; Paula-Silva, F.W.G.; Faccioli, L.H.; Gatón-Hernández, P.M.; de Queiroz, A.M.; da Silva, L.A.B. Cytotoxicity and Cytokine Expression Induced by Silorane and Methacrylate-Based Composite Resins. J. Appl. Oral Sci. 2016, 24. [Google Scholar] [CrossRef]
- da Fonseca Roberti Garcia, L.; Pontes, E.C.V.; Basso, F.G.; Hebling, J.; de Souza Costa, C.A.; Soares, D.G. Transdentinal Cytotoxicity of Resin-Based Luting Cements to Pulp Cells. Clin. Oral Investig. 2016, 20, 1559–1566. [Google Scholar] [CrossRef]
- Bianchi, L.; Ribeiro, A.P.D.; de Oliveira Carrilho, M.R.; Pashley, D.H.; de Souza Costa, C.A.; Hebling, J. Transdentinal Cytotoxicity of Experimental Adhesive Systems of Different Hydrophilicity Applied to Ethanol-Saturated Dentin. Dent. Mater. 2013, 29, 980–990. [Google Scholar] [CrossRef]
- Özcan, M.; Mese, A. Adhesion of Conventional and Simplified Resin-Based Luting Cements to Superficial and Deep Dentin. Clin. Oral Investig. 2012, 16, 1081–1088. [Google Scholar] [CrossRef]
- Josic, U.; Teti, G.; Ionescu, A.; Maravic, T.; Mazzitelli, C.; Cokic, S.; Van Meerbeek, B.; Falconi, M.; Brambilla, E.; Mazzoni, A.; et al. Cytotoxicity and Microbiological Behavior of Universal Resin Composite Cements. Dent. Mater. 2024, 40, 1515–1523. [Google Scholar] [CrossRef]
- Breschi, L. Buonocore Memorial Lecture 2023: Changing Operative Mindsets with Universal Adhesives and Cements. Oper. Dent. 2025, 50, 12–32. [Google Scholar] [CrossRef]
- Fugolin, A.P.; Lewis, S.; Logan, M.G.; Ferracane, J.L.; Pfeifer, C.S. Methacrylamide-Methacrylate Hybrid Monomers for Dental Applications. Dent. Mater. 2020, 36, 1028–1037. [Google Scholar] [CrossRef]
- Borges, L.; Logan, M.; Weber, S.; Lewis, S.; Fang, C.; Correr-Sobrinho, L.; Pfeifer, C. Multi-Acrylamides Improve Bond Stability through Collagen Reinforcement under Physiological Conditions. Dent. Mater. 2024, 40, 993–1001. [Google Scholar] [CrossRef]
- Marzouk, T.; Sathyanarayana, S.; Kim, A.S.; Seminario, A.L.; McKinney, C.M. A Systematic Review of Exposure to Bisphenol A from Dental Treatment. JDR Clin. Trans. Res. 2019, 4, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Breschi, L.; Josic, U.; Maravic, T.; Mancuso, E.; Del Bianco, F.; Baldissara, P.; Mazzoni, A.; Mazzitelli, C. Selective Adhesive Luting: A Novel Technique for Improving Adhesion Achieved by Universal Resin Cements. J. Esthet. Restor. Dent. 2023, 35, 1030–1038. [Google Scholar] [CrossRef] [PubMed]
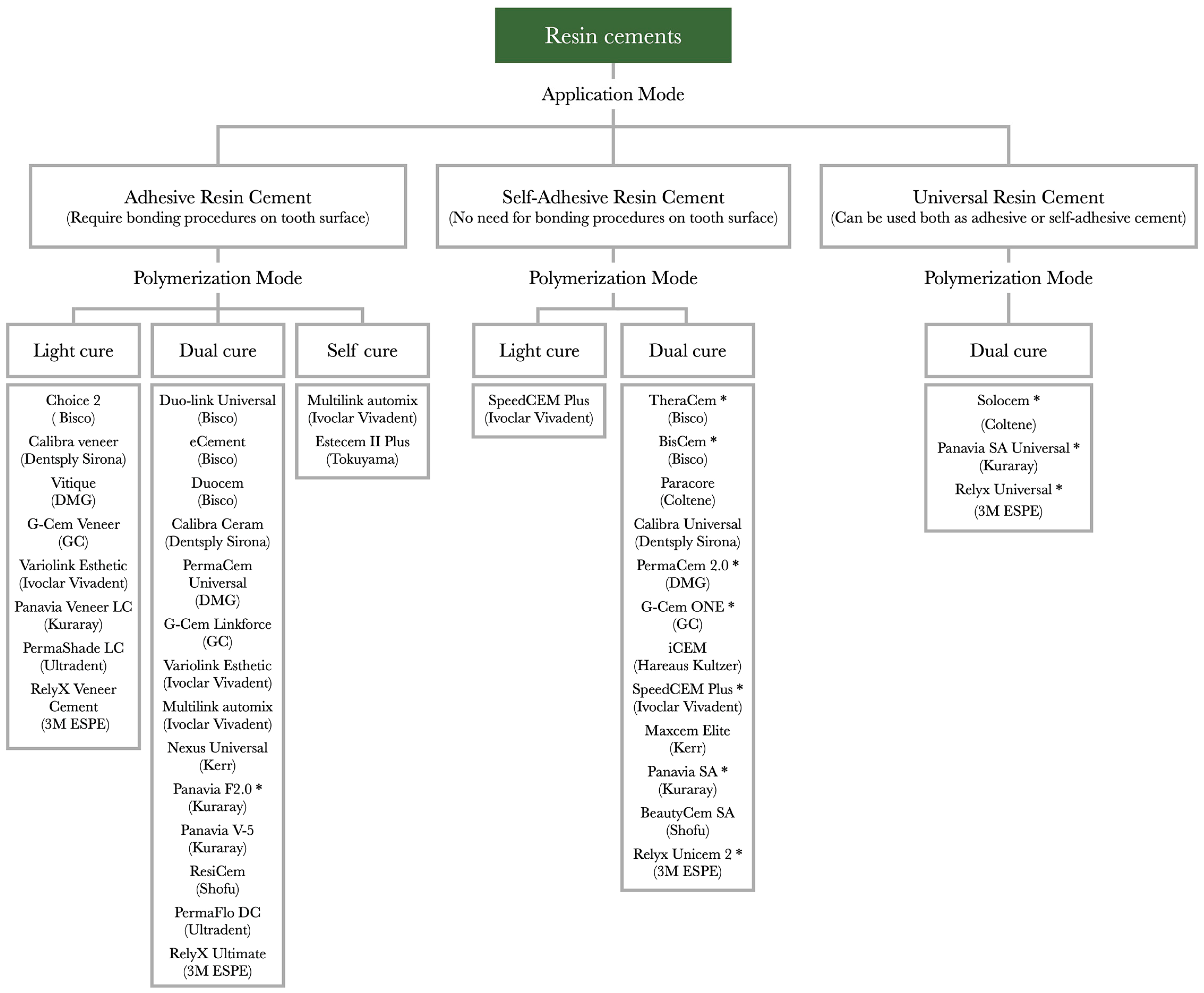



| Substrate | Preparation | Methacrylate-Based Cements (Self-Adhesive Cements, Adhesive Cements That Require a Separate Bonding Agent Application, Universal Cements—Can Be Used with or Without an Adhesive System) | Conventional Cements (Zinc Phosphate, Zinc-Oxide–Eugenol Cement, Zinc Polycarboxylate Cement, GIC, Resin-Modified GIC) |
|---|---|---|---|
| Enamel |
|
|
|
| Superficial dentin |
|
|
|
| Deep dentin |
|
|
|
| Root dentin |
|
|
|
| Enamel and dentin |
|
|
|
| Build ups |
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maravic, T.; Mazzitelli, C.; Mayer-Santos, E.; Mancuso, E.; Gracis, S.; Breschi, L.; Fuzzi, M. Current Trends for Cementation in Prosthodontics: Part 1—The Substrate. Polymers 2025, 17, 566. https://doi.org/10.3390/polym17050566
Maravic T, Mazzitelli C, Mayer-Santos E, Mancuso E, Gracis S, Breschi L, Fuzzi M. Current Trends for Cementation in Prosthodontics: Part 1—The Substrate. Polymers. 2025; 17(5):566. https://doi.org/10.3390/polym17050566
Chicago/Turabian StyleMaravic, Tatjana, Claudia Mazzitelli, Eric Mayer-Santos, Edoardo Mancuso, Stefano Gracis, Lorenzo Breschi, and Massimo Fuzzi. 2025. "Current Trends for Cementation in Prosthodontics: Part 1—The Substrate" Polymers 17, no. 5: 566. https://doi.org/10.3390/polym17050566
APA StyleMaravic, T., Mazzitelli, C., Mayer-Santos, E., Mancuso, E., Gracis, S., Breschi, L., & Fuzzi, M. (2025). Current Trends for Cementation in Prosthodontics: Part 1—The Substrate. Polymers, 17(5), 566. https://doi.org/10.3390/polym17050566











