Low-Alpha-Cellulose-Based Membranes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
- (1)
- Bleached sulphate hardwood pulp grade “NS-Extra” with the following composition: 30% birch and 70% aspen (Cell 1);
- (2)
- Unbleached sulphate softwood pulp grade “Extra” with the following composition: 80% spruce and 20% pine (Cell 2);
- (3)
- Unbleached sulphate softwood pulp grade “Fiber” with the following composition: 80% spruce and 20% pine (Cell 3).
2.2. Formation of Membranes
- Cellulose sheets are crushed to a powder state with a particle size of up to 200 microns (Figure 1).
- Cellulose, propyl gallate and NMMO powders are mixed in the ratio required to obtain 16% solutions.
- The system is mechanically activated through intense shear deformation in a compressed state. This leads to the formation of H-complexes.
- The solution is transferred to a molten state and mixed at a temperature of up to 110–120 °C, using a laboratory twin-screw extruder from Haake Minilab II (ThermoFisher Scientific, Dreieich, Germany).
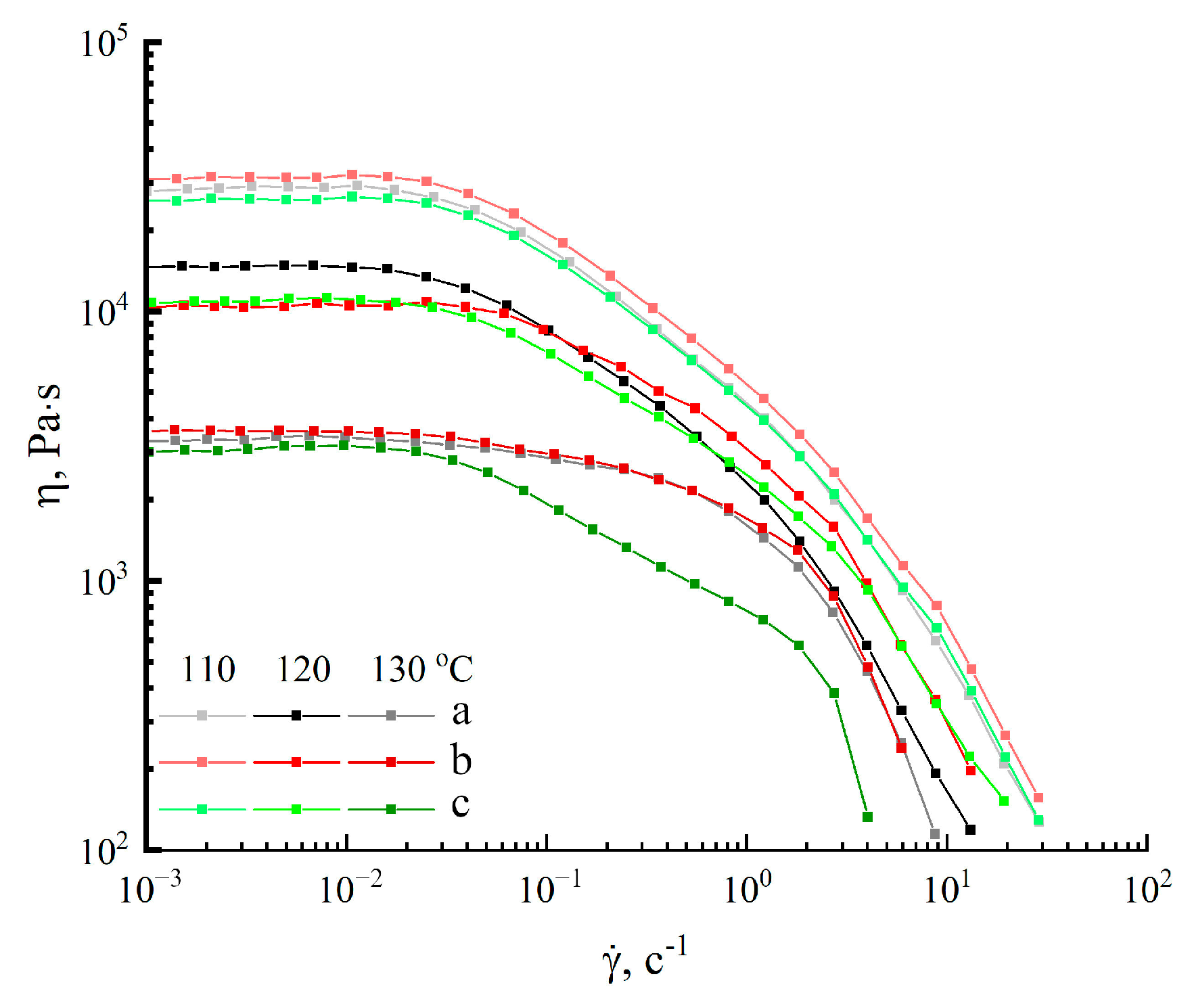
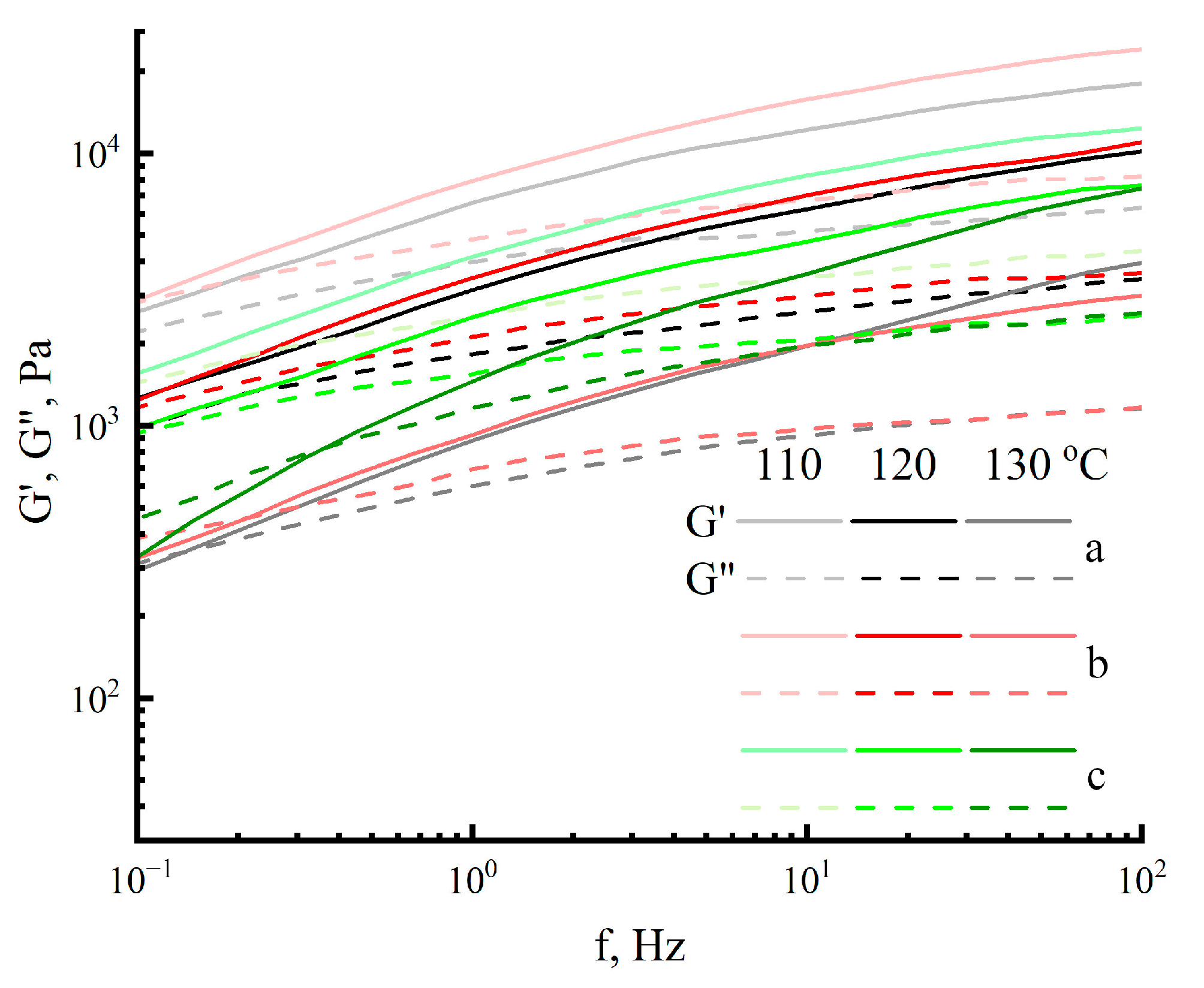
2.3. Morphology and Rheology of Solutions
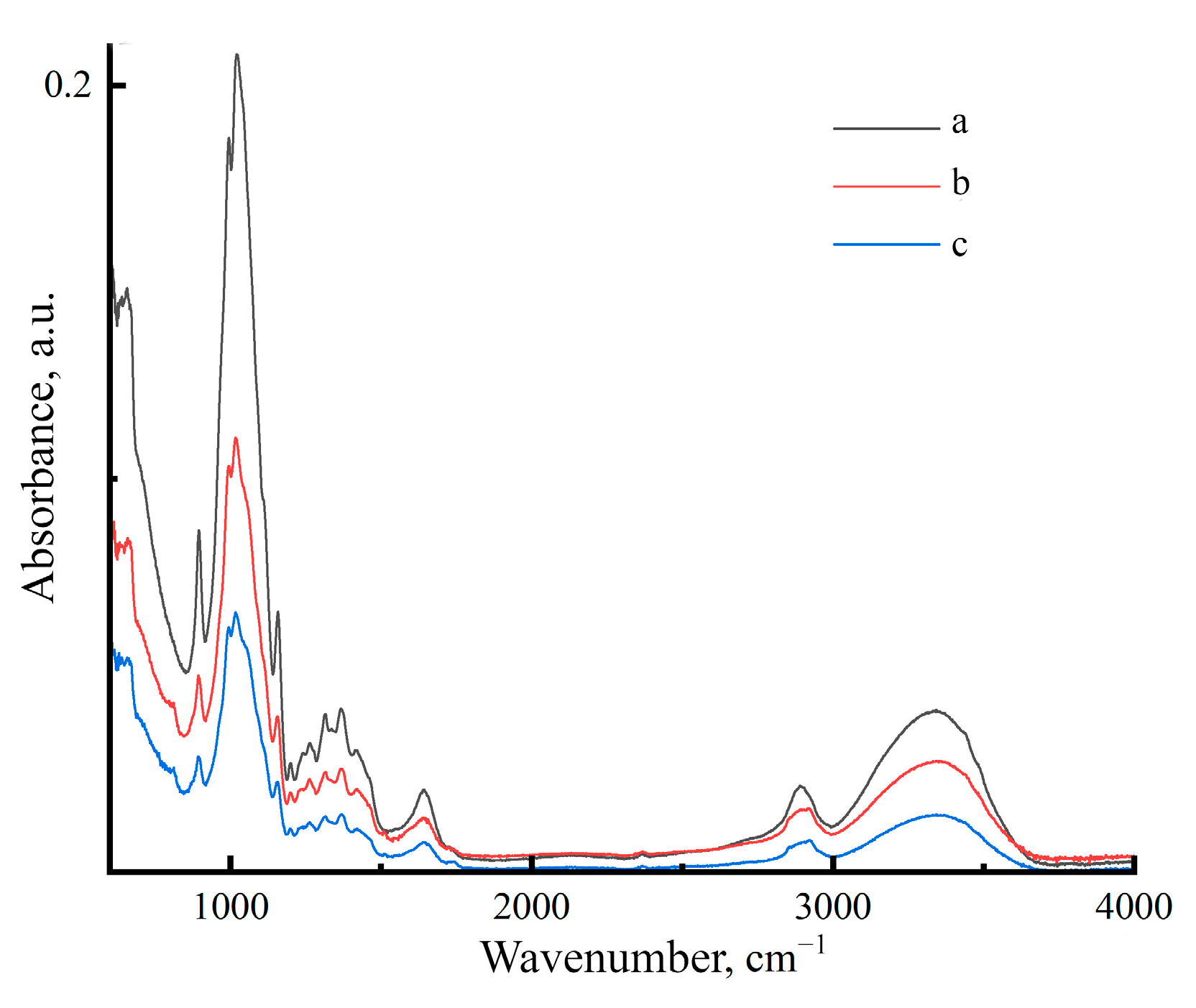
2.4. Structure and Chemical Composition of Membranes
2.5. Membranes Properties
2.6. Mechanical Properties
2.7. Contact Angle
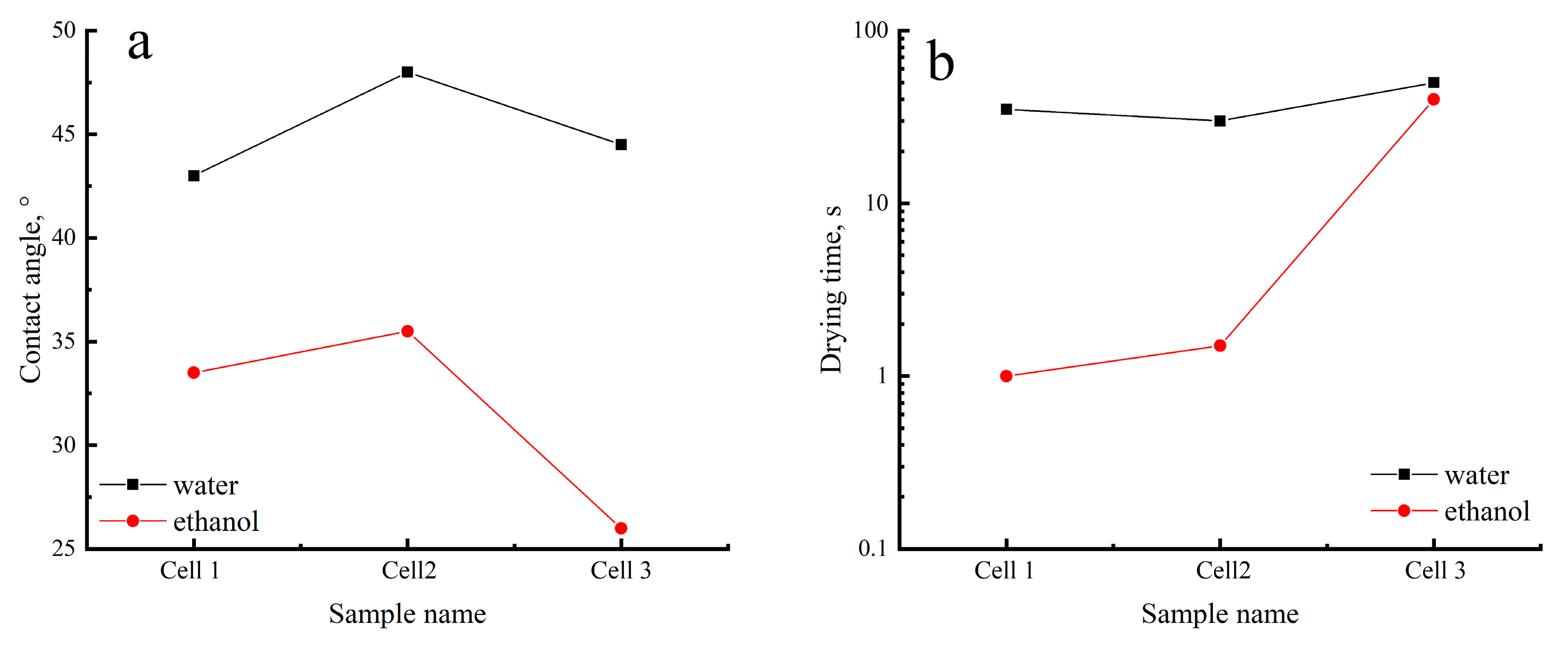
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gismatulina, Y.A.; Budaeva, V.V.; Veprev, S.G.; Sakovich, G.V.; Shumny, V.K. Cellulose from various parts of Soranovskii Miscanthus. Russ. J. Genet. Appl. Res. 2015, 5, 60–68. [Google Scholar] [CrossRef]
- Irimia, A.; Grigoraș, V.C.; Popescu, C.-M. Active Cellulose-Based Food Packaging and Its Use on Foodstuff. Polymers 2024, 16, 389. [Google Scholar] [CrossRef] [PubMed]
- Seddiqi, H.; Oliaei, E.; Honarkar, H.; Jin, J.; Geonzon, L.C.; Bacabac, R.G.; Klein-Nulend, J. Cellulose and its derivatives: Towards biomedical applications. Cellulose 2021, 28, 1893–1931. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, X.; Tian, J.; Zheng, C.; Khan, M.R.; Guo, J.; Zhu, W.; Jin, Y.; Xiao, H.; Song, J.; et al. High throughput disassembly of cellulose nanoribbons and colloidal stabilization of gel-like Pickering emulsions. Carbohydr. Polym. 2023, 315, 121000. [Google Scholar] [CrossRef]
- Vinogradov, M.I.; Makarov, I.S.; Golova, L.K.; Gromovykh, P.S.; Kulichikhin, V.G. Rheological Properties of Aqueous Dispersions of Bacterial Cellulose. Processes 2020, 8, 423. [Google Scholar] [CrossRef]
- Li, J.; Wang, Z.; Wang, P.; Tian, J.; Liu, T.; Guo, J.; Zhu, W.; Khan, M.R.; Xiao, H.; Song, J. Effects of hydrolysis conditions on the morphology of cellulose II nanocrystals (CNC-II) derived from mercerized microcrystalline cellulose. Int. J. Biol. Macromol. 2024, 258, 128936. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Langan, P.; Chanzy, H. Crystal Structure and Hydrogen-Bonding System in Cellulose Iβ from Synchrotron X-ray and Neutron Fiber Diffraction. J. Am. Chem. Soc. 2002, 124, 9074–9082. [Google Scholar] [CrossRef]
- Gardner, K.H.; Blackwell, J. Hydrogen Bonding in Native Cellulose. Biochim. Biophys. Acta 1974, 343, 232–237. [Google Scholar] [CrossRef]
- Poletto, M.; Ornaghi, H.L.J.; Zattera, A.J. Native Cellulose: Structure, Characterization and Thermal Properties. Materials 2014, 7, 6105–6119. [Google Scholar] [CrossRef]
- Makarov, I.S.; Shambilova, G.K.; Vinogradov, M.I.; Anokhina, T.S.; Bukanova, A.S.; Kairliyeva, F.B.; Bukanova, S.K.; Levin, I.S. Membranes Based on Cellulose and Copolymers of Acrylonitrile Prepared from Joint Solutions. Membranes 2023, 13, 667. [Google Scholar] [CrossRef]
- Pavlyuchenko, M.M.; Kaputsky, F.N.; Grinshpan, D.D. Effect of organic solvent nature on the interaction of cellulose with nitrogen tetroxide. J. Appl. Chem. 1975, 48, 1822–1825. [Google Scholar]
- Hammer, R.B.; Turbak, A.F. Production of Rayon from Solutions of Cellulose in N2O4-DMF in Solvent Spun Rayon, Modified Cellulose Fibers and Derivatives; American Chemical Society: Washington, DC, USA, 1977; Chapter 4. [Google Scholar]
- Yudianti, R.; Syampurwadi, A.; Onggo, H.; Karina, M.; Uyama, H.; Azuma, J. Properties of bacterial cellulose transparent film regenerated from dimethylacetamide–LiCl solution. Polym. Adv. Technol. 2016, 27, 1102. [Google Scholar] [CrossRef]
- Lu, X.; Shen, X. Solubility of bacteria cellulose in zinc chloride aqueous solutions. Carbohydr. Polym. 2011, 86, 239–244. [Google Scholar] [CrossRef]
- Grinshpan, D.D.; Gonchar, A.N.; Tsygankova, N.G.; Makarevich, S.E.; Savitskaya, T.A.; Sheimo, E.V. Rheological properties of concentrated solutions of cellulose and its mixtures with other polymers in orthophosphoric acid. J. Eng. Phys. Thermophys. 2011, 84, 594. [Google Scholar] [CrossRef]
- Boerstoel, H.; Maatman, H.; Westerink, J.B.; Koenders, B.M. Liquid crystalline solutions of cellulose in phosphoric acid. Polymer 2001, 42, 7371. [Google Scholar]
- Golova, L.K.; Romanov, V.V.; Lunina, O.B.; Platonov, V.A.; Papkov, S.P.; Khorozova, O.D.; Yakshin, V.V.; Belasheva, T.P.; Sokira, A.N. Method for Obtaining a Solution for Spinning. Fibers. Patent RF 1645308, 30 April 1991. [Google Scholar]
- Grzybek, P.; Dudek, G.; van der Bruggen, B. Cellulose-based films and membranes: A comprehensive review on preparation and applications. J. Chem. Eng. 2024, 495, 153500. [Google Scholar] [CrossRef]
- Cazón, P.; Vázquez, M.; Velazquez, G. Novel composite films based on cellulose reinforced with chitosan and polyvinyl alcohol: Effect on mechanical properties and water vapour permeability. Polym. Test. 2018, 69, 536–544. [Google Scholar] [CrossRef]
- Wu, Q.; Wu, B. Study of membrane morphology by image analysis of electron micrographs. J. Membr. Sci. 1995, 105, 113–120. [Google Scholar] [CrossRef]
- Zare, F.; Gonçalves, S.B.; Faria, M.; Gonçalves, M.C. Improving Structural Homogeneity, Hydraulic Permeability, and Mechanical Performance of Asymmetric Monophasic Cellulose Acetate/Silica Membranes: Spinodal Decomposition Mix. Membranes 2023, 13, 346. [Google Scholar] [CrossRef]
- Shen, L.; Cheng, R.; Yi, M.; Hung, W.-S.; Japip, S.; Tian, L.; Zhang, X.; Jiang, S.; Li, S.; Wang, Y. Polyamide-based membranes with structural homogeneity for ultrafast molecular sieving. Nat. Commun. 2022, 13, 500. [Google Scholar] [CrossRef]
- Liang, Y.; Zhu, Y.; Liu, C.; Lee, K.-R.; Hung, W.-S.; Wang, Z.; Li, Y.; Elimelech, M.; Jin, J.; Lin, S. Polyamide nanofiltration membrane with highly uniform sub-nanometre pores for sub-1 Å precision separation. Nat. Commun. 2020, 11, 2015. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Zhang, L.; Li, Y.; Xu, J. Fabrication and Properties of Cellulose Hydrated Membrane with Unique Structure. Macromol. Chem. Phys. 2007, 208, 594–602. [Google Scholar] [CrossRef]
- Yushkin, A.A.; Anokhina, T.S.; Volkov, A.V. Application of cellophane films as nanofiltration membranes. Pet. Chem. 2015, 55, 746–752. [Google Scholar] [CrossRef]
- Durmaz, E.N.; Çulfaz-Emecen, P.Z. Cellulose-based membranes via phase inversion using [EMIM]OAc-DMSO mixtures as solvent. Chem. Eng. Sci. 2018, 178, 93–103. [Google Scholar] [CrossRef]
- Kuzin, M.S.; Skvortsov, I.Y.; Gerasimenko, P.S.; Subbotin, A.V.; Malkin, A.Y. The role of the solvent nature in stretching polymer solutions (polyacrylonitrile spinning using different solvents). J. Mol. Liq. 2023, 392, 123516. [Google Scholar] [CrossRef]
- Makarov, I.S.; Golova, L.K.; Kuznetsova, L.K.; Antonov, S.V.; Kotsyuk, A.V.; Ignatenko, V.Y.; Kulichikhin, V.G. Influence of Precipitation and Conditioning Baths on the Structure, Morphology, and Properties of Cellulose Films. Fibre Chem. 2016, 48, 298–305. [Google Scholar] [CrossRef]
- Fink, H.-P.; Weigel, P.; Purz, H.J. Structure formation of regenerated cellulose materials from NMMO-Solutions. Prog. Polym. Sci. 2001, 26, 1473–1524. [Google Scholar] [CrossRef]
- Duolikun, T.; Ghazali, N.; Leo, B.F.; Lee, H.V.; Lai, C.W.; Johan, M.R.B. Asymmetric Cellulosic Membranes: Current and Future Aspects. Symmetry 2020, 12, 1160. [Google Scholar] [CrossRef]
- Makarov, I.S.; Golova, L.K.; Vinogradov, M.I.; Mironova, M.V.; Anokhina, T.S.; Arkharova, N.A. Morphology and transport properties of membranes obtained by coagulation of cellulose solutions in isobutanol. Carbohydr. Polym. 2021, 254, 117472. [Google Scholar] [CrossRef]
- Brasier, J. The Deceptively Versatile Non-Plastic. Mater. Des. 1986, 7, 65–67. [Google Scholar] [CrossRef]
- Huang, R.Y.M.; Jarvis, N.R. Separation of liquid mixtures by using polymer membranes. II. Permeation of aqueous alcohol solutions through cellophane and poly(vinyl alcohol). J. Appl. Polym. Sci. 1970, 14, 2341–2356. [Google Scholar] [CrossRef]
- Nagy, E.; Borlai, O.; Ujhidy, A. Membrane permeation of water—Alcohol binary mixtures. J. Membr. Sci. 1980, 7, 109–118. [Google Scholar] [CrossRef]
- Ghosh, I.; Sanyal, S.K.; Mukherjea, R.N. Pervaporation of methanol–ethylene glycol with cellophane membrane: Some mechanistic aspects. Ind. Eng. Chem. Res. 1988, 27, 1895. [Google Scholar] [CrossRef]
- Makarov, I.S.; Golova, L.K.; Bondarenko, G.N.; Anokhina, T.S.; Dmitrieva, E.S.; Levin, I.S.; Makhatova, V.E.; Galimova, N.Z.; Shambilova, G.K. Structure, Morphology, and Permeability of Cellulose Films. Membranes 2022, 12, 297. [Google Scholar] [CrossRef] [PubMed]
- Makarov, I.S.; Budaeva, V.V.; Gismatulina, Y.A.; Kashcheyeva, E.I.; Zolotukhin, V.N.; Gorbatova, P.A.; Sakovich, G.V.; Vinogradov, M.I.; Palchikova, E.E.; Levin, I.S.; et al. Preparation of Lyocell Fibers from Solutions of Miscanthus Cellulose. Polymers 2024, 16, 2915. [Google Scholar] [CrossRef]
- Protz, R.; Lehmann, A.; Ganster, J.; Fink, H.-P. Solubility and spinnability of cellulose-lignin blends in aqueous NMMO. Carbohydr. Polym. 2021, 251, 117027. [Google Scholar] [CrossRef]
- Byrne, N.; De Silva, R.; Ma, Y.; Sixta, H.; Hummel, M. Enhanced stabilization of cellulose-lignin hybrid filaments for carbon fiber production. Cellulose 2018, 25, 723–733. [Google Scholar] [CrossRef]
- Ma, Y.; Stubb, J.; Kontro, I.; Nieminen, K.; Hummel, M.; Sixta, H. Filament spinning of unbleached birch kraft pulps: Effect of pulping intensity on the processability and the fiber properties. Carbohydr. Polym. 2018, 179, 145–151. [Google Scholar] [CrossRef]
- GOST 6840-78; Cellulose. Method for Determination of Alpha Cellulose Content. Russian State Standard: Moscow, Russia, 1984. Available online: https://docs.cntd.ru/document/1200017933 (accessed on 7 November 2024).
- Golova, L.K. New Cellulose Fiber Lyocell. Russ. Chem. J. 2002, 46, 49–57. [Google Scholar]
- GOST R 5982-84; Sulfite Viscose Cellulose, Technical Specifications. Russian State Standard: Moscow, Russia, 1998. Available online: https://files.stroyinf.ru/Data2/1/4294823/4294823279.pdf (accessed on 7 November 2024). (In Russian)
- Salem, K.S.; Kasera, N.K.; Rahman, M.A.; Jameel, H.; Habibi, Y.; Eichhorn, S.J.; Lucia, L.A. Comparison and assessment of methods for cellulose crystallinity determination. Chem. Soc. Rev. 2023, 52, 6417. [Google Scholar] [CrossRef]
- Kostryukov, S.G.; Matyakubov, H.B.; Masterova, Y.Y.; Kozlov, A.S.; Pryanichnikova, M.K.; Pynenkov, A.A.; Khluchina, N.A. Determination of Lignin, Cellulose, and Hemicellulose in Plant Materials by FTIR Spectroscopy. J. Anal. Chem. 2023, 78, 718–727. [Google Scholar] [CrossRef]
- Makarov, I.; Palchikova, E.; Vinogradov, M.; Golubev, Y.; Legkov, S.; Gromovykh, P.; Makarov, G.; Arkharova, N.; Karimov, D.; Gainutdinov, R. Characterization of Structure and Morphology of Cellulose Lyocell Microfibers Extracted from PAN Matrix. Polysaccharides 2025, 6, 10. [Google Scholar] [CrossRef]
- Baskakov, S.A.; Baskakova, Y.V.; Kabachkov, E.N.; Kichigina, G.A.; Kushch, P.P.; Kiryukhin, D.P.; Krasnikova, S.S.; Badamshina, E.R.; Vasil’ev, S.G.; Soldatenkov, T.A. Cellulose from Annual Plants and Its Use for the Production of the Films Hydrophobized with Tetrafluoroethylene Telomers. Molecules 2022, 27, 6002. [Google Scholar] [CrossRef] [PubMed]
- Graessley, W.W. Polymeric Liquids and Networks: Dynamics and Rheology; Garland Science: New York, NY, USA, 2008. [Google Scholar]
- Makarov, I.S.; Golova, L.K.; Kuznetsova, L.K.; Mironova, M.V.; Vinogradov, M.I.; Bermeshev, M.V.; Levin, I.S.; Kulichikhin, V.G. Composite Fibers from Cellulose Solutions with Additives of Bis (Trimethylsilyl) Acetylene and Alkoxysilanes: Rheology, Structure and Properties. Fibre Chem. 2019, 51, 26–31. [Google Scholar] [CrossRef]
- Kaplan, D.L. Biopolymers from Renewable Resources; Springer Science & Business Media: New York, NY, USA, 2013. [Google Scholar]
- Nelson, M.L.; O’Connor, R.T. Relation of certain infrared bands to cellulose crystallinity and crystal lattice type. Part I. Spectra of lattice types I, II, III and amorphous cellulose. J. Appl. Polym. Sci. 1964, 8, 1311–1324. [Google Scholar] [CrossRef]
- Carrillo, F.; Colom, X.; Suñol, J.J.; Saurina, J. Structural FTIR analysis and thermal characterization of lyocell and viscose-type fibres. Eur. Polym. J. 2004, 40, 2229–2234. [Google Scholar] [CrossRef]
- Ornaghi, H.L.; Poletto, M.; Zattera, A.J.; Amico, S.C. Correlation of the thermal stability and the decomposition kinetics of six different vegetal fibers. Cellulose 2014, 21, 177–188. [Google Scholar] [CrossRef]
- Bashar, M.M.; Zhu, H.; Yamamoto, S.; Mitsuishi, M. Superhydrophobic surfaces with fluorinated cellulose nanofiber assemblies for oil–water separation. RSC Adv. 2017, 7, 37168. [Google Scholar] [CrossRef]
- Meng, X.; Dong, Y.; Zhao, Y.; Liang, L. Preparation and modification of cellulose sponge and application of oil/water separation. RSC Adv. 2020, 10, 41713. [Google Scholar] [CrossRef]
- Dankovich, T.A.; Gray, D.G. Contact Angle Measurements on Smooth Nanocrystalline Cellulose (I) Thin Films. JAST 2011, 25, 699–708. [Google Scholar] [CrossRef]
- Bruel, C.; Queffeulou, S.; Carreau, P.; Tavares, J.R.; Heuzey, M.-C. Orienting Cellulose Nanocrystal Functionalities Tunes the Wettability of Water-Cast Films. Langmuir 2020, 36, 12179–12189. [Google Scholar] [CrossRef]






| Cell 1 | Cell 2 | Cell 3 | |
|---|---|---|---|
| Viscosity (ISO 5251-2010), mL/g | 810 | 1090 | 995 |
| Lignin, % | 0 | 8.1 | 5.1 |
| Ash, % | 0.093 | 0.115 | 0.171 |
| Fiber length, mm | 1.05 | 3.05 | 2.95 |
| α-cellulose, % | 87 | 89 | 88 |
| Water, % | 5.73 | 6.57 | 6.52 |
| Element | Cell 1 | Cell 2 | Cell 3 |
|---|---|---|---|
| Al | 2.58 | 10.17 | 4.28 |
| Ca | 191.69 | 1109.38 | 1118.77 |
| Fe | 3.87 | 3.63 | 5.45 |
| K | ≤DL * | ≤DL * | 203.27 |
| Mg | 38.34 | 151.89 | 121.11 |
| Mn | 2.90 | 61.05 | 58.80 |
| Zn | ≤DL * | 4.00 | 10.90 |
| Sample | TCI | LOI | HBI |
|---|---|---|---|
| Cell 1 | 1.81 ± 0.02 | 0.34 ± 0.012 | 1.12 ± 0.01 |
| Cell 2 | 1.56 ± 0.05 | 0.41 ± 0.026 | 1.20 ± 0.02 |
| Cell 3 | 1.83 ± 0.03 | 0.36 ± 0.022 | 1.14 ± 0.01 |
| Membrane | Thickness, µm | Tensile Strength, MPa | Elongation at Break, % | Young Modulus, GPa |
|---|---|---|---|---|
| Cell 1 | 74–92 | 3.8–5.7 | 147–169 | 4.7–6.4 |
| Cell 2 | 86–92 | 1.3–3 | 105–143 | 2.8–5.1 |
| Cell 3 | 87–116 | 3.5–3.9 | 146–175 | 4.3–5.7 |
| Membrane | Thickness, µm | Tensile Strength, MPa | Elongation at Break, % | Young Modulus, GPa |
|---|---|---|---|---|
| Cell 1 | 30–50 | 67–83 | 4.2–13.5 | 1031–1937 |
| Cell 2 | 51–64 | 34–53 | 6.3–16.7 | 830–1142 |
| Cell 3 | 38–51 | 41–62 | 6.5–12.2 | 1047–1242 |
| Membrane | RRemazol, % | ROrange, % |
|---|---|---|
| Cell 1 | 5.6 | 2.9 |
| Cell 2 | 19.7 | 18.7 |
| Cell 3 | 39.7 | 20.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makarov, I.; Shambilova, G.; Bukanova, A.; Kairliyeva, F.; Bukanova, S.; Kadasheva, Z.; Gainutdinov, R.; Koksharov, A.; Komarov, I.; Song, J.; et al. Low-Alpha-Cellulose-Based Membranes. Polymers 2025, 17, 598. https://doi.org/10.3390/polym17050598
Makarov I, Shambilova G, Bukanova A, Kairliyeva F, Bukanova S, Kadasheva Z, Gainutdinov R, Koksharov A, Komarov I, Song J, et al. Low-Alpha-Cellulose-Based Membranes. Polymers. 2025; 17(5):598. https://doi.org/10.3390/polym17050598
Chicago/Turabian StyleMakarov, Igor, Gulbarshin Shambilova, Aigul Bukanova, Fazilat Kairliyeva, Saule Bukanova, Zhanar Kadasheva, Radmir Gainutdinov, Alexander Koksharov, Ivan Komarov, Junlong Song, and et al. 2025. "Low-Alpha-Cellulose-Based Membranes" Polymers 17, no. 5: 598. https://doi.org/10.3390/polym17050598
APA StyleMakarov, I., Shambilova, G., Bukanova, A., Kairliyeva, F., Bukanova, S., Kadasheva, Z., Gainutdinov, R., Koksharov, A., Komarov, I., Song, J., Legkov, S., & Nebesskaya, A. (2025). Low-Alpha-Cellulose-Based Membranes. Polymers, 17(5), 598. https://doi.org/10.3390/polym17050598






