Biodegradable 3D-Printed Conjunctival Inserts for the Treatment of Dry Eyes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
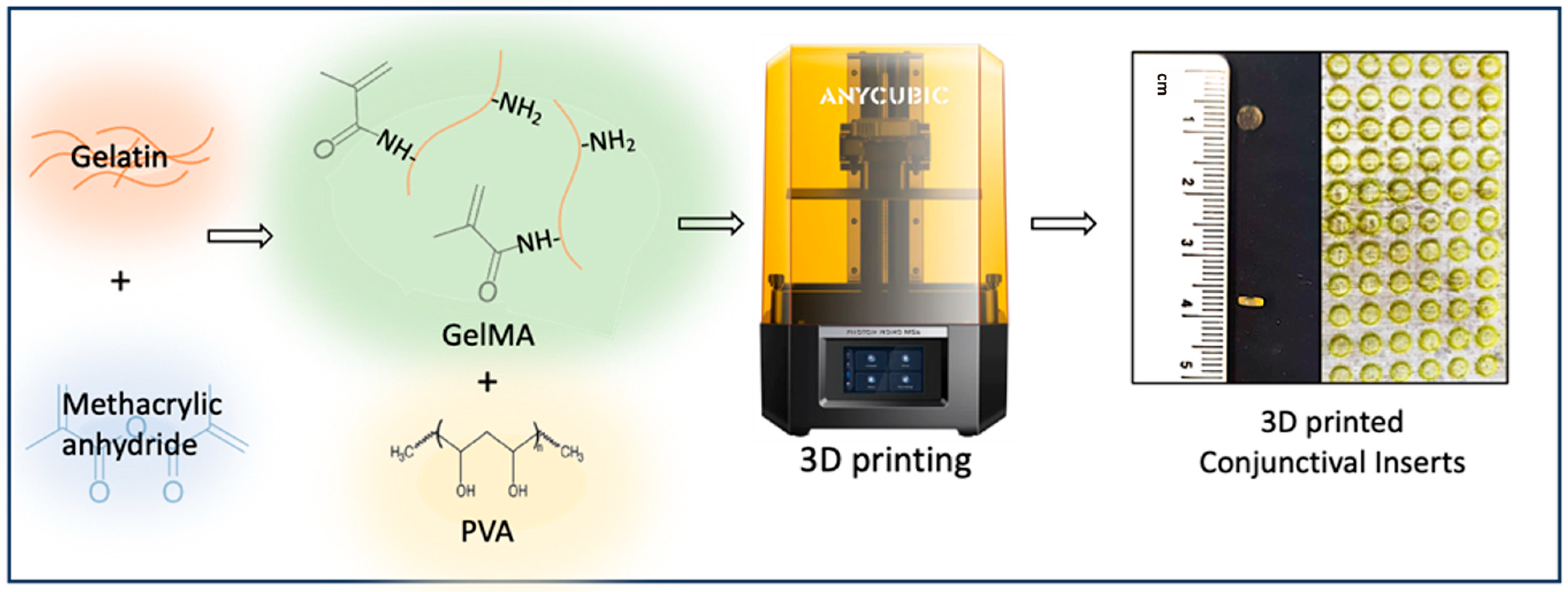
2.2. Synthesis of GelMA
2.3. Preparation GelMA and P-Gel-5% Ink
2.4. 3D Printing of P-Gel-5% Conjunctival Inserts
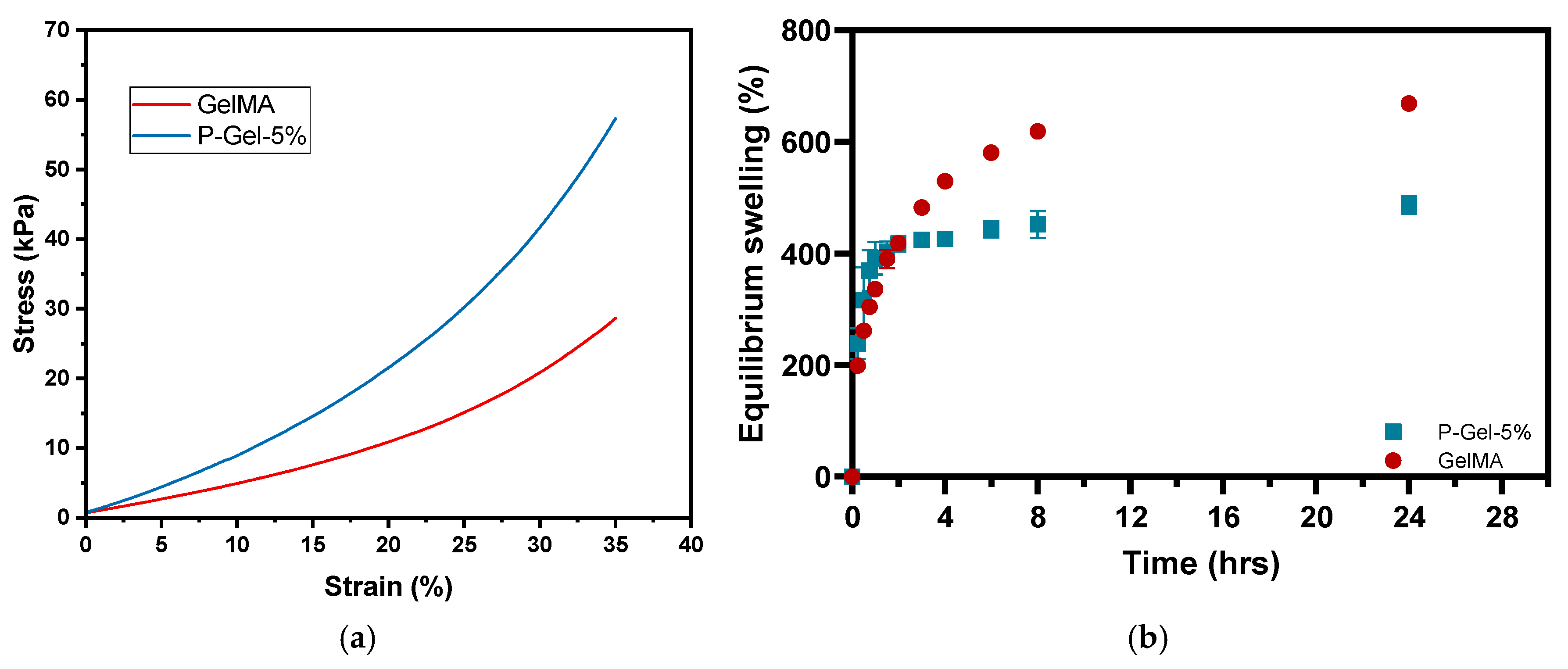
2.5. Mechanical Testing
2.6. Equilibrium Swelling
2.7. Water Contact Angle
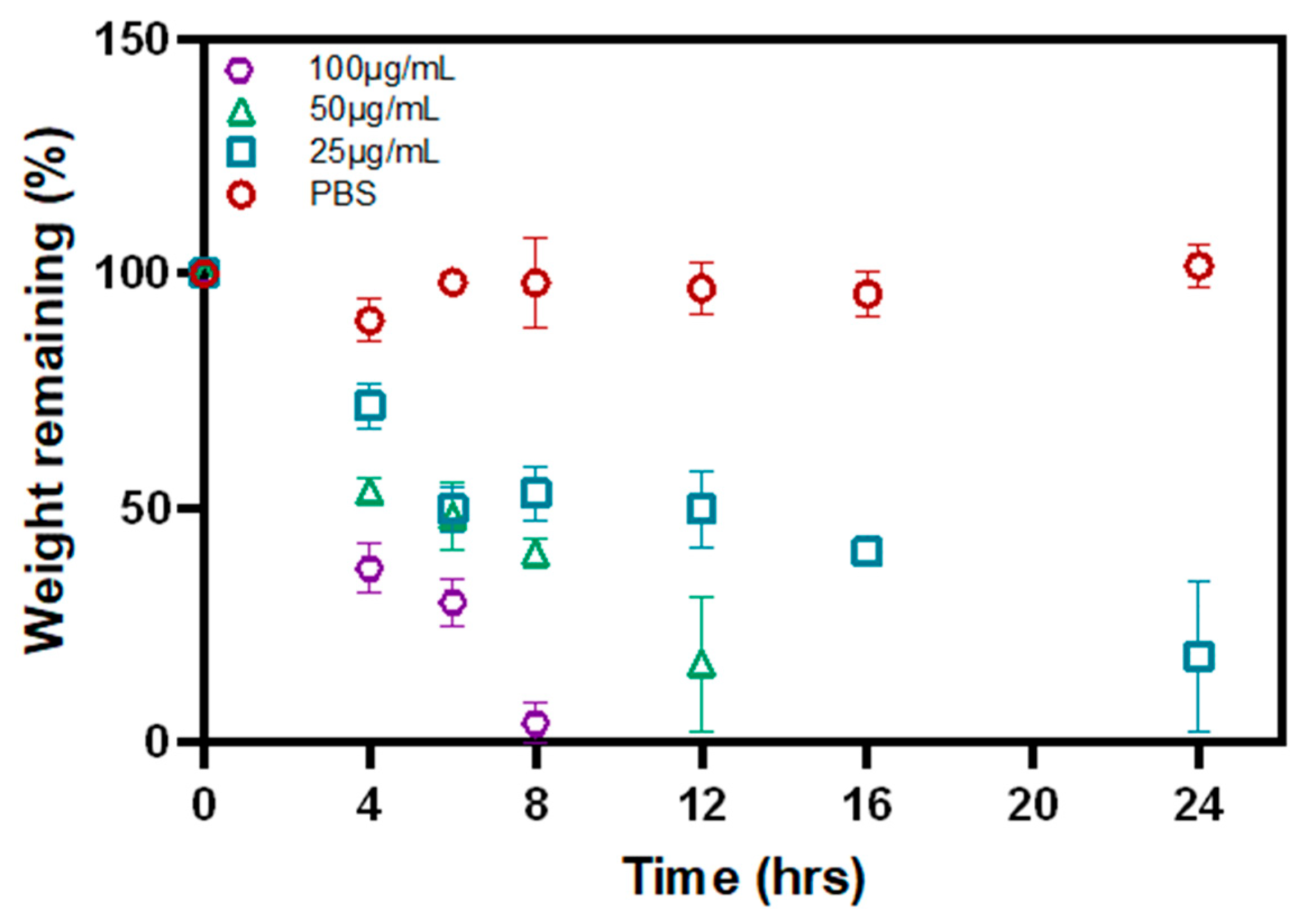
2.8. Biodegradation of P-Gel-5% Inserts by MMP9
2.9. Scanning Electron Microscopy
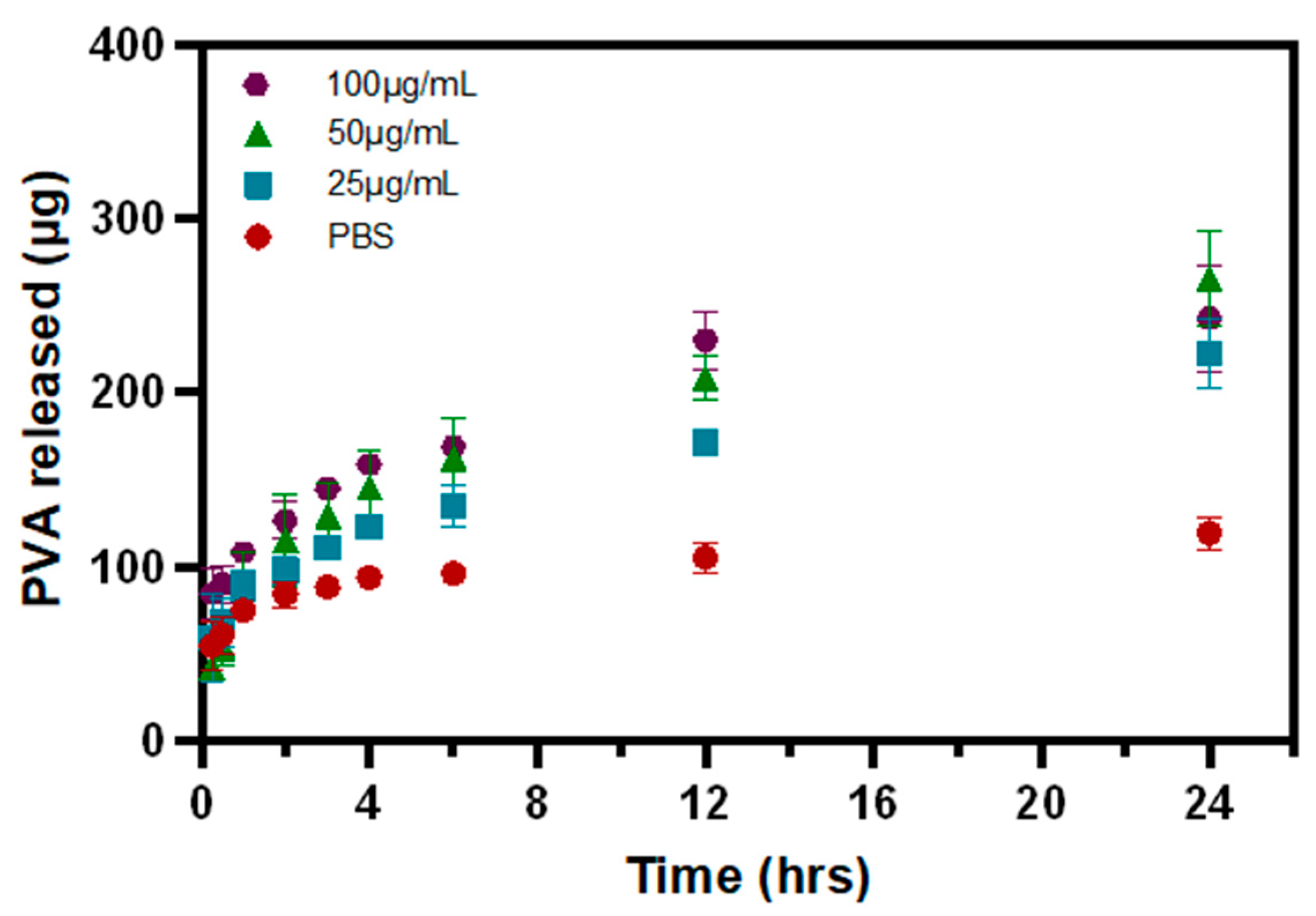
2.10. Release and Detection of PVA
2.11. Drug Release Kinetics via Mathematical Modeling
2.12. Statistical Analysis
3. Results and Discussion
3.1. The 3D Printing of Ocular Inserts
3.2. Mechanical Testing, Equilibrium Water Content (EWC), and Water Contact Angle
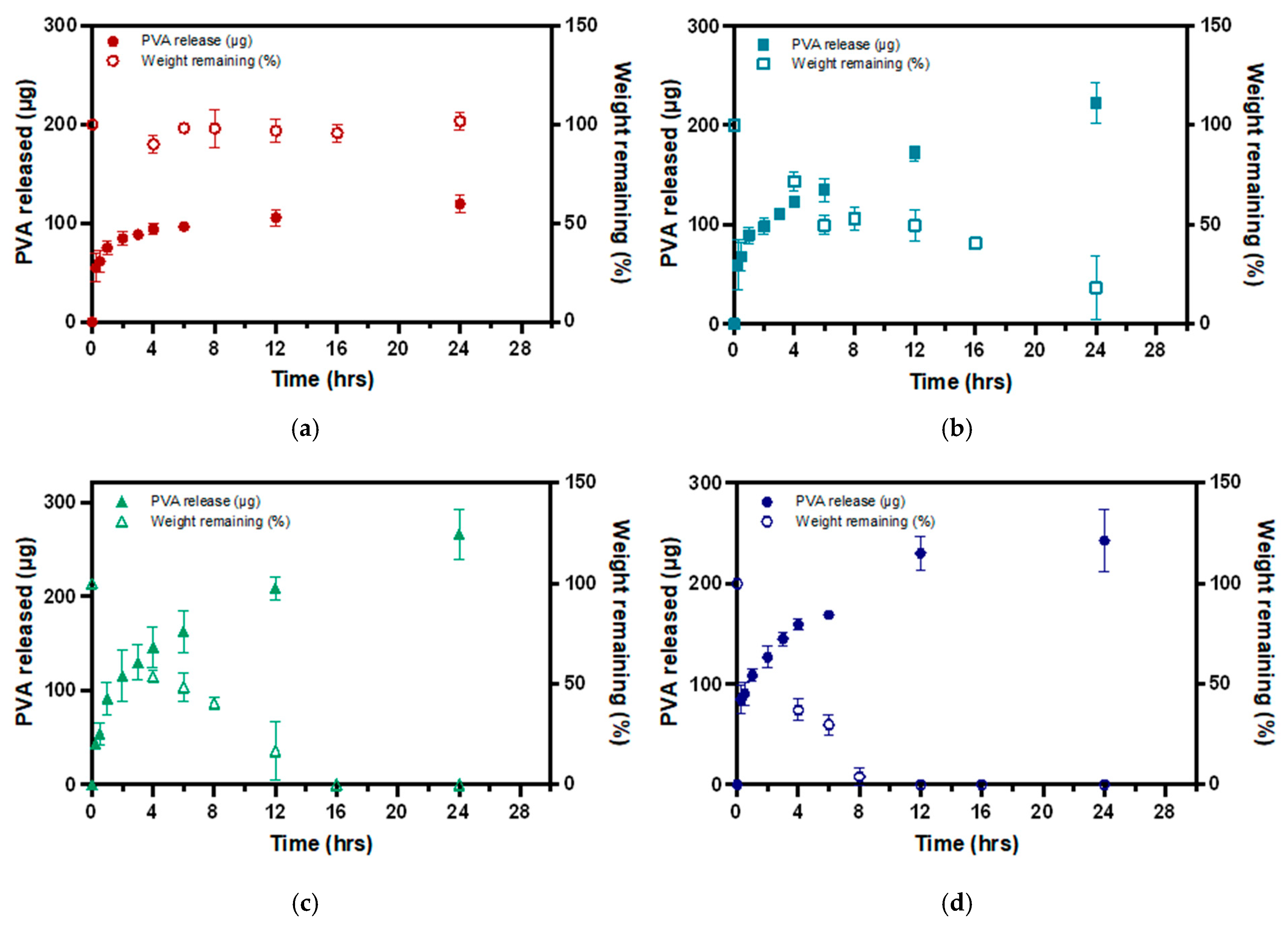
3.3. PVA Release from P-Gel-5% Inserts in the Presence of MMP9
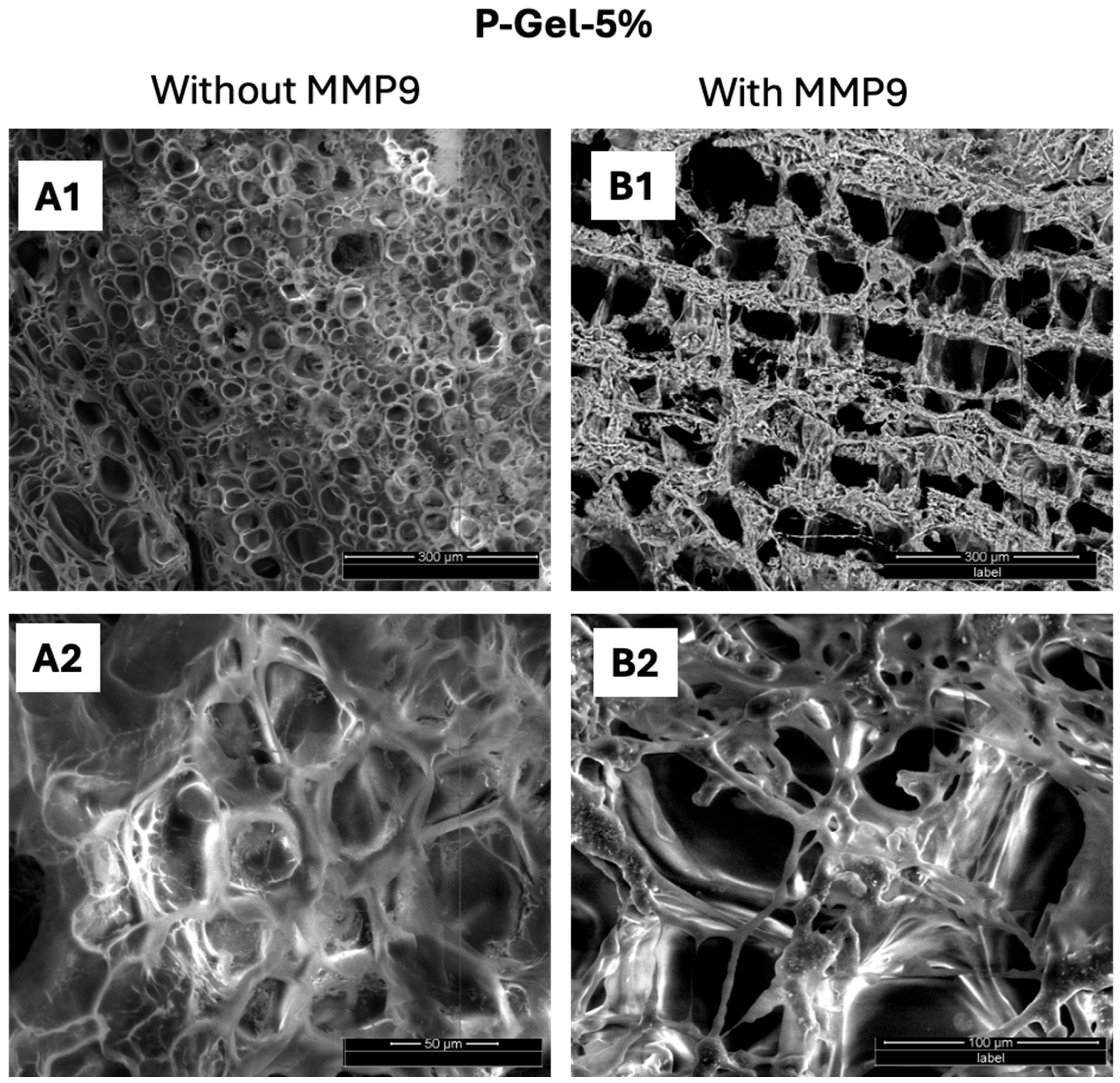
3.4. Biodegradation of P-Gel-5% Inserts in the Presence of MMP9
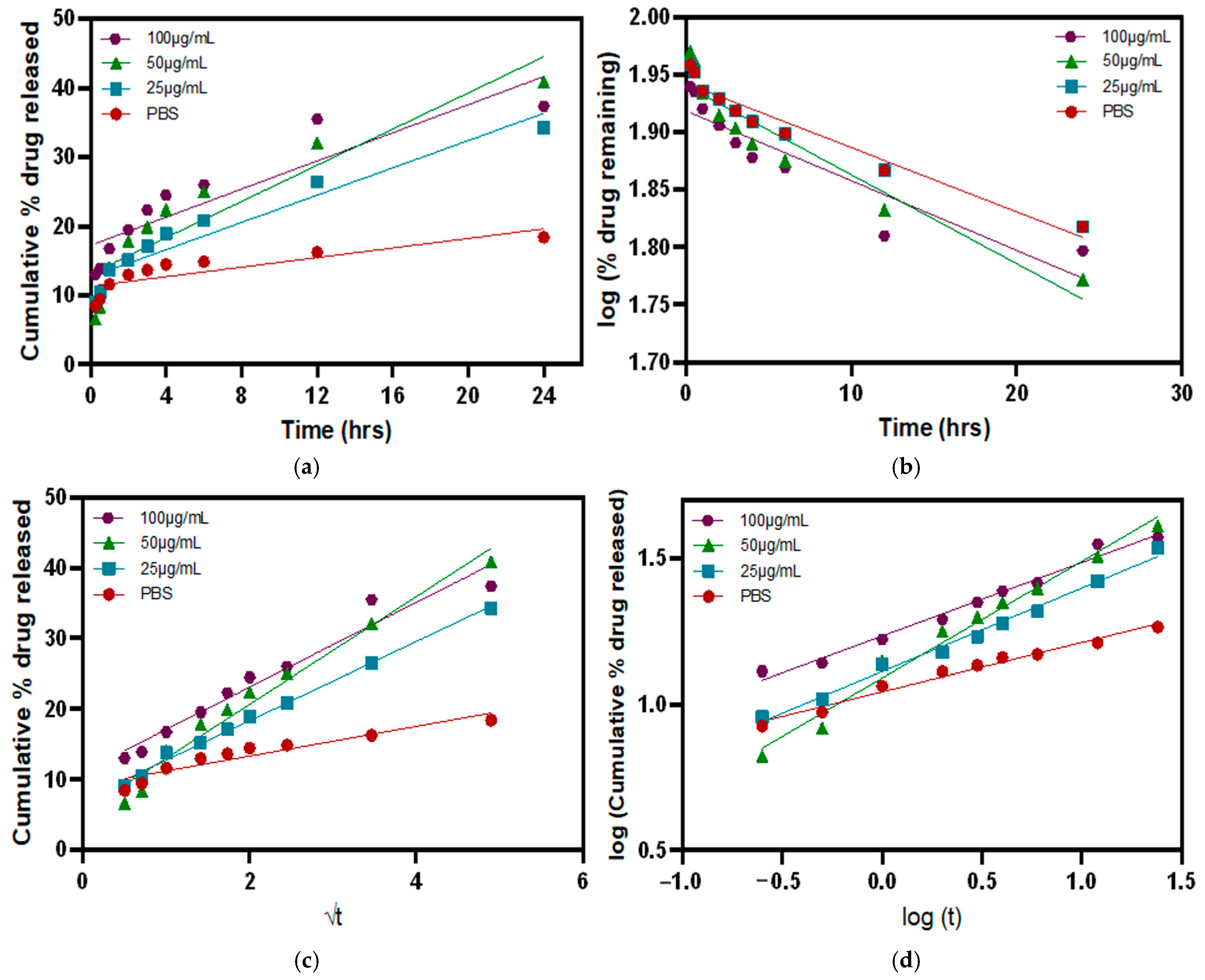
3.5. Release Kinetics in the Presence of MMP9 Enzymes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Craig, J.P.; Nichols, K.K.; Akpek, E.K.; Caffery, B.; Dua, H.S.; Joo, C.-K.; Liu, Z.; Nelson, J.D.; Nichols, J.J.; Tsubota, K.; et al. TFOS DEWS II definition and classification report. Ocul. Surf. 2017, 15, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Lemp, M.A.; Bron, A.J.; Baudouin, C.; Del Castillo, J.M.B.; Geffen, D.; Tauber, J.; Foulks, G.N.; Pepose, J.S.; Sullivan, B.D. Tear osmolarity in the diagnosis and management of dry eye disease. Am. J. Ophthalmol. 2011, 151, 792–798.e791. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, F.; Alves, M.; Bunya, V.Y.; Jalbert, I.; Lekhanont, K.; Malet, F.; Na, K.S.; Schaumberg, D.; Uchino, M.; Vehof, J.; et al. TFOS DEWS II Epidemiology Report. Ocul. Surf. 2017, 15, 334–365. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, B.D.; Crews, L.A.; Sönmez, B.; de la Paz, M.F.; Comert, E.; Charoenrook, V.; de Araujo, A.L.; Pepose, J.S.; Berg, M.S.; Kosheleff, V.P. Clinical utility of objective tests for dry eye disease: Variability over time and implications for clinical trials and disease management. Cornea 2012, 31, 1000–1008. [Google Scholar] [CrossRef]
- Rocha, G.; Gulliver, E.; Borovik, A.; Chan, C.C. Randomized, masked, in vitro comparison of three commercially available tear film osmometers. Clin. Ophthalmol. 2017, 11, 243–248. [Google Scholar] [CrossRef]
- Lee, Y.H.; Bang, S.-P.; Shim, K.-Y.; Son, M.-J.; Kim, H.; Jun, J.H. Association of tear matrix metalloproteinase 9 immunoassay with signs and symptoms of dry eye disease: A cross-sectional study using qualitative, semiquantitative, and quantitative strategies. PLoS ONE 2021, 16, e0258203. [Google Scholar] [CrossRef]
- Sambursky, R.; Davitt, W.F., III; Friedberg, M.; Tauber, S. Prospective, multicenter, clinical evaluation of point-of-care matrix metalloproteinase-9 test for confirming dry eye disease. Cornea 2014, 33, 812–818. [Google Scholar] [CrossRef]
- Di Zazzo, A.; Barabino, S.; Fasciani, R.; Aragona, P.; Giannaccare, G.; Villani, E.; Rolando, M. One Soul and Several Faces of Evaporative Dry Eye Disease. J. Clin. Med. 2024, 13, 1220. [Google Scholar] [CrossRef]
- de la Fuente, M.; Rodríguez-Agirretxe, I.; Vecino, E.; Astigarraga, E.; Acera, A.; Barreda-Gómez, G. Elevation of tear MMP-9 concentration as a biomarker of inflammation in ocular pathology by antibody microarray immunodetection assays. Int. J. Mol. Sci. 2022, 23, 5639. [Google Scholar] [CrossRef]
- Chotikavanich, S.; de Paiva, C.S.; Li, d.Q.; Chen, J.J.; Bian, F.; Farley, W.J.; Pflugfelder, S.C. Production and activity of matrix metalloproteinase-9 on the ocular surface increase in dysfunctional tear syndrome. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3203–3209. [Google Scholar] [CrossRef]
- Lanza, N.L.; McClellan, A.L.; Batawi, H.; Felix, E.R.; Sarantopoulos, K.D.; Levitt, R.C.; Galor, A. Dry eye profiles in patients with a positive elevated surface matrix metalloproteinase 9 point-of-care test versus negative patients. Ocul. Surf. 2016, 14, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Mu, C.; Shi, M.; Liu, P.; Chen, L.; Marriott, G. Daylight-mediated, passive, and sustained release of the glaucoma drug timolol from a contact lens. ACS Cent. Sci. 2018, 4, 1677–1687. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.Z.; Liu, X.X.; Wang, S.Y.; Liu, Y.; Pan, X.Y.; Wang, J.J.; Nan, K.H. Engineering Advanced Drug Delivery Systems for Dry Eye: A Review. Bioengineering 2022, 10, 53. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Sharma, P.K.; Garg, V.K.; Garg, G. Ocular inserts-Advancement in therapy of eye diseases. J. Adv. Pharm. Technol. Res. 2010, 1, 291–296. [Google Scholar] [CrossRef]
- Mariz, M.; Murta, J.; Gil, M.H.; Ferreira, P. An ocular insert with zero-order extended delivery: Release kinetics and mathematical models. Eur. J. Pharm. Biopharm. 2022, 181, 79–87. [Google Scholar] [CrossRef]
- Alzahrani, A.; Youssef, A.A.A.; Nyavanandi, D.; Tripathi, S.; Bandari, S.; Majumdar, S.; Repka, M.A. Design and optimization of ciprofloxacin hydrochloride biodegradable 3D printed ocular inserts: Full factorial design and in-vitro and ex-vivo evaluations: Part II. Int. J. Pharm. 2023, 631, 122533. [Google Scholar] [CrossRef]
- Duman, G.; Yıldır, İ.; Macit, M.; Genç, E.; Sümer, E.; Kale, S.; Deniz, İ. Development and evaluation of 3D-printed ocular insert containing liposomal moxifloxacin. J. Drug Deliv. Sci. Technol. 2024, 92, 105353. [Google Scholar] [CrossRef]
- Friederich, R. The pilocarpine Ocusert: A new drug delivery system. Ann. Ophthalmol. 1974, 6, 1279–1284. [Google Scholar]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef]
- Kimura, H.; Ogura, Y. Biodegradable polymers for ocular drug delivery. Ophthalmologica 2001, 215, 143–155. [Google Scholar] [CrossRef]
- Vert, M.; Doi, Y.; Hellwich, K.-H.; Hess, M.; Hodge, P.; Kubisa, P.; Rinaudo, M.; Schué, F. Terminology for biorelated polymers and applications (IUPAC Recommendations 2012). Pure Appl. Chem. 2012, 84, 377–410. [Google Scholar] [CrossRef]
- Lyu, S.; Untereker, D. Degradability of polymers for implantable biomedical devices. Int. J. Mol. Sci. 2009, 10, 4033–4065. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Qi, X.; Cai, T.; Fan, Z.; Wang, H.; Du, X. Gelatin hydrogel/contact lens composites as rutin delivery systems for promoting corneal wound healing. Drug Deliv. 2021, 28, 1951–1961. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, M.; Wang, J.; Zhang, W.; Lu, W.; Gao, Y.; Zhang, B.; Guo, Y. Development of a Photo-Crosslinking, Biodegradable GelMA/PEGDA Hydrogel for Guided Bone Regeneration Materials. Materials 2018, 11, 1345. [Google Scholar] [CrossRef] [PubMed]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef]
- Tse, J.W.; Rizwan, M.; Rasmussen, J.; Jones, L.; Yim, E.K.F. Gelatin Methacrylate as an Enzyme-Controlled Release Vehicle of Hyaluronic Acid for the Treatment of Recurrent Corneal Erosion. ACS Appl. Bio Mater. 2020, 3, 6214–6223. [Google Scholar] [CrossRef]
- Schwab, A.; Levato, R.; D’Este, M.; Piluso, S.; Eglin, D.; Malda, J. Printability and shape fidelity of bioinks in 3D bioprinting. Chem. Rev. 2020, 120, 11028–11055. [Google Scholar] [CrossRef]
- Leone, G.; Consumi, M.; Pepi, S.; Pardini, A.; Bonechi, C.; Tamasi, G.; Donati, A.; Rossi, C.; Magnani, A. Modified low molecular weight poly-vinyl alcohol as viscosity enhancer. Mater. Today Commun. 2019, 21, 100634. [Google Scholar] [CrossRef]
- Phan, C.-M.; Subbaraman, L.N.; Jones, L.W. Uptake and release of polyvinyl alcohol from hydrogel daily disposable contact lenses. Optom. Vis. Sci. 2019, 96, 180–186. [Google Scholar] [CrossRef]
- Pflugfelder, S.C.; Farley, W.; Luo, L.; Chen, L.Z.; de Paiva, C.S.; Olmos, L.C.; Li, D.Q.; Fini, M.E. Matrix metalloproteinase-9 knockout confers resistance to corneal epithelial barrier disruption in experimental dry eye. Am. J. Pathol. 2005, 166, 61–71. [Google Scholar] [CrossRef]
- Bhaskaran, R.; Palmier, M.O.; Lauer-Fields, J.L.; Fields, G.B.; Van Doren, S.R. MMP-12 catalytic domain recognizes triple helical peptide models of collagen V with exosites and high activity. J. Biol. Chem. 2008, 283, 21779–21788. [Google Scholar] [CrossRef] [PubMed]
- Lauer-Fields, J.L.; Sritharan, T.; Stack, M.S.; Nagase, H.; Fields, G.B. Selective hydrolysis of triple-helical substrates by matrix metalloproteinase-2 and -9. J. Biol. Chem. 2003, 278, 18140–18145. [Google Scholar] [CrossRef] [PubMed]
- Shokrollahi, P.; Garg, P.; Wulff, D.; Hui, A.; Phan, C.M.; Jones, L. Vat photopolymerization 3D printing optimization: Analysis of print conditions and print quality for complex geometries and ocular applications. Int. J. Pharm. 2025, 668, 124999. [Google Scholar] [CrossRef] [PubMed]
- Procházková, L.; Rodríguez-Muñoz, Y.; Procházka, J.; Wanner, J. Simple spectrophotometric method for determination of polyvinylalcohol in different types of wastewater. Int. J. Environ. Anal. Chem. 2014, 94, 399–410. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, Q.; Huo, Y.; Liu, C.; Li, B.; Li, Y. Construction of a mesoporous polydopamine@ GO/cellulose nanofibril composite hydrogel with an encapsulation structure for controllable drug release and toxicity shielding. ACS Appl. Mater. Interfaces 2020, 12, 57410–57420. [Google Scholar] [CrossRef]
- Garg, P.; Shokrollahi, P.; Darge, H.F.; Phan, C.-M.; Jones, L. Controlled PVA Release from Chemical-Physical Interpenetrating Networks to Treat Dry Eyes. ACS Omega ASAP 2024, 10, 1249–1260. [Google Scholar] [CrossRef]
- Yu, K.; Zhang, X.J.; Sun, Y.; Gao, Q.; Fu, J.Z.; Cai, X.J.; He, Y. Printability during projection-based 3D bioprinting. Bioact. Mater. 2022, 11, 254–267. [Google Scholar] [CrossRef]
- Bose, S.; Phan, C.M.; Rizwan, M.; Tse, J.W.; Yim, E.; Jones, L.; Pignatello, R.; Almeida, H.; Santonocito, D.; Puglia, C. Fabrication and Characterization of an Enzyme-Triggered, Therapeutic-Releasing Hydrogel Bandage Contact Lens Material. Pharmaceutics 2024, 16, 26. [Google Scholar] [CrossRef]
- Zhu, M.X.; Wang, Y.Y.; Ferracci, G.; Zheng, J.; Cho, N.J.; Lee, B.H. Gelatin methacryloyl and its hydrogels with an exceptional degree of controllability and batch-to-batch consistency. Sci. Rep. 2019, 9, 6863. [Google Scholar] [CrossRef]
- Heidarian, P.; Kouzani, A.Z.; Kaynak, A.; Paulino, M.; Nasri-Nasrabadi, B. Dynamic hydrogels and polymers as inks for three-dimensional printing. ACS Biomater. Sci. Eng. 2019, 5, 2688–2707. [Google Scholar] [CrossRef]
- Chen, Q.; Chen, H.; Zhu, L.; Zheng, J. Fundamentals of double network hydrogels. J. Mater. Chem. B 2015, 3, 3654–3676. [Google Scholar] [CrossRef] [PubMed]
- Mandrycky, C.; Wang, Z.; Kim, K.; Kim, D.-H. 3D bioprinting for engineering complex tissues. Biotechnol. Adv. 2016, 34, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Caprioli, M.; Roppolo, I.; Chiappone, A.; Larush, L.; Pirri, C.F.; Magdassi, S. 3D-printed self-healing hydrogels via Digital Light Processing. Nat. Commun. 2021, 12, 2462. [Google Scholar] [CrossRef] [PubMed]
- Ino, J.M.; Sju, E.; Ollivier, V.; Yim, E.K.; Letourneur, D.; Le Visage, C. Evaluation of hemocompatibility and endothelialization of hybrid poly (vinyl alcohol)(PVA)/gelatin polymer films. J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 101, 1549–1559. [Google Scholar] [CrossRef] [PubMed]
- Hassan, C.M.; Peppas, N.A. Structure and applications of poly (vinyl alcohol) hydrogels produced by conventional crosslinking or by freezing/thawing methods. In Biopolymers·PVA Hydrogels, Anionic Polymerisation Nanocomposites; Springer: Berlin/Heidelberg, Germany, 2000; pp. 37–65. Available online: https://link.springer.com/chapter/10.1007/3-540-46414-X_2 (accessed on 20 February 2025).
- Mandal, P.; Stokes, K.; Hernández, G.; Brandell, D.; Mindemark, J. Influence of binder crystallinity on the performance of si electrodes with poly (vinyl alcohol) binders. ACS Appl. Energy Mater. 2021, 4, 3008–3016. [Google Scholar] [CrossRef]
- Adelnia, H.; Ensandoost, R.; Moonshi, S.S.; Gavgani, J.N.; Vasafi, E.I.; Ta, H.T. Freeze/thawed polyvinyl alcohol hydrogels: Present, past and future. Eur. Polym. J. 2022, 164, 110974. [Google Scholar] [CrossRef]
- Liu, Y.; Vrana, N.; Cahill, P.; McGuinness, G. Physically crosslinked composite hydrogels of PVA with natural macromolecules: Structure, mechanical properties, and endothelial cell compatibility. J. Biomed. Mater. Res. Part B Appl. Biomater. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2009, 90, 492–502. [Google Scholar] [CrossRef]
- Thangprasert, A.; Tansakul, C.; Thuaksubun, N.; Meesane, J. Mimicked hybrid hydrogel based on gelatin/PVA for tissue engineering in subchondral bone interface for osteoarthritis surgery. Mater. Des. 2019, 183, 108113. [Google Scholar] [CrossRef]
- Bernal-Chávez, S.A.; Romero-Montero, A.; Hernández-Parra, H.; Peña-Corona, S.I.; Del Prado-Audelo, M.L.; Alcalá-Alcalá, S.; Cortés, H.; Kiyekbayeva, L.; Sharifi-Rad, J.; Leyva-Gómez, G. Enhancing chemical and physical stability of pharmaceuticals using freeze-thaw method: Challenges and opportunities for process optimization through quality by design approach. J. Biol. Eng. 2023, 17, 35. [Google Scholar] [CrossRef]
- Hu, Q.; Lu, R.; Liu, S.; Liu, Y.; Gu, Y.; Zhang, H. 3D printing GelMA/PVA interpenetrating polymer networks scaffolds mediated with CuO nanoparticles for angiogenesis. Macromol. Biosci. 2022, 22, 2200208. [Google Scholar] [CrossRef]
- Sivak, J.M.; Fini, M.E. MMPs in the eye: Emerging roles for matrix metalloproteinases in ocular physiology. Prog Retin Eye Res 2002, 21, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Toth, M.; Sohail, A.; Fridman, R. Assessment of gelatinases (MMP-2 and MMP-9) by gelatin zymography. Metastasis Res. Protoc. 2012, 57, 121–135. [Google Scholar]
- Loessner, D.; Meinert, C.; Kaemmerer, E.; Martine, L.C.; Yue, K.; Levett, P.A.; Klein, T.J.; Melchels, F.P.; Khademhosseini, A.; Hutmacher, D.W. Functionalization, preparation and use of cell-laden gelatin methacryloyl–based hydrogels as modular tissue culture platforms. Nat. Protoc. 2016, 11, 727–746. [Google Scholar] [CrossRef] [PubMed]
- Schuurman, W.; Levett, P.A.; Pot, M.W.; van Weeren, P.R.; Dhert, W.J.; Hutmacher, D.W.; Melchels, F.P.; Klein, T.J.; Malda, J. Gelatin-methacrylamide hydrogels as potential biomaterials for fabrication of tissue-engineered cartilage constructs. Macromol. Biosci. 2013, 13, 551–561. [Google Scholar] [CrossRef]
- Lalitha Sridhar, S.; Vernerey, F. Localized enzymatic degradation of polymers: Physics and scaling laws. Phys. Rev. Appl. 2018, 9, 031001. [Google Scholar] [CrossRef]
- Ho, B.; Phan, C.M.; Garg, P.; Shokrollahi, P.; Jones, L. A Rapid Screening Platform for Simultaneous Evaluation of Biodegradation and Therapeutic Release of an Ocular Hydrogel. Pharmaceutics 2023, 15, 2625. [Google Scholar] [CrossRef]
- Bruschi, M.L. Mathematical models of drug release. In Strategies to Modify the Drug Release from Pharmaceutical Systems; Bruschi, M.L., Ed.; Woodhead Publishing: Sawston, UK, 2015; pp. 63–86. [Google Scholar] [CrossRef]



| GelMA | PVA | LAP | Tartrazine | |
|---|---|---|---|---|
| GelMA ink | 10 | 0 | 0.6 | 0.3 |
| P-Gel-5% ink | 10 | 5 | 0.6 | 0.3 |
| Zero Order | First Order | Higuchi Model | Korsmeyer-Peppas Model | |
|---|---|---|---|---|
| Equation | Ct = Co + Kot | logCt = logCo − (K1/2.303)t | Mt/M∞ = Kht1/2 | log(Mt/M∞) = logKkp + n log t |
| Graph | cumulative drug release vs. time | log cumulative % drug remaining vs. time | cumulative % drug release vs. √t | log cumulative % drug release vs. log t |
| Kinetic Model | PBS | 25 µg/mL | 50 µg/mL | 100 µg/mL |
|---|---|---|---|---|
| Zero-order | 0.7143 | 0.9160 | 0.8490 | 0.8135 |
| First order | 0.9420 | 0.9420 | 0.8983 | 0.8399 |
| Higuchi | 0.8910 | 0.9947 | 0.9734 | 0.9528 |
| Korsmeyer–Peppas | 0.9785 | 0.9896 | 0.9812 | 0.9809 |
| Diffusion exponent (n-value) * | 0.1685 | 0.2852 | 0.3995 | 0.2518 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garg, P.; Shokrollahi, P.; Phan, C.-M.; Jones, L. Biodegradable 3D-Printed Conjunctival Inserts for the Treatment of Dry Eyes. Polymers 2025, 17, 623. https://doi.org/10.3390/polym17050623
Garg P, Shokrollahi P, Phan C-M, Jones L. Biodegradable 3D-Printed Conjunctival Inserts for the Treatment of Dry Eyes. Polymers. 2025; 17(5):623. https://doi.org/10.3390/polym17050623
Chicago/Turabian StyleGarg, Piyush, Parvin Shokrollahi, Chau-Minh Phan, and Lyndon Jones. 2025. "Biodegradable 3D-Printed Conjunctival Inserts for the Treatment of Dry Eyes" Polymers 17, no. 5: 623. https://doi.org/10.3390/polym17050623
APA StyleGarg, P., Shokrollahi, P., Phan, C.-M., & Jones, L. (2025). Biodegradable 3D-Printed Conjunctival Inserts for the Treatment of Dry Eyes. Polymers, 17(5), 623. https://doi.org/10.3390/polym17050623







