Advancing Textile Waste Recycling: Challenges and Opportunities Across Polymer and Non-Polymer Fiber Types
Abstract
1. Introduction
2. Raw Materials, Chemical Principles, and Manufacturing of Textiles
2.1. Fiber Production Routes
2.1.1. Natural Fibers
Natural Fibers of Natural Polymers
Natural Fibers of Non-Polymers
2.1.2. Man-Made Fibers
Man-Made Fibers of Natural Polymers
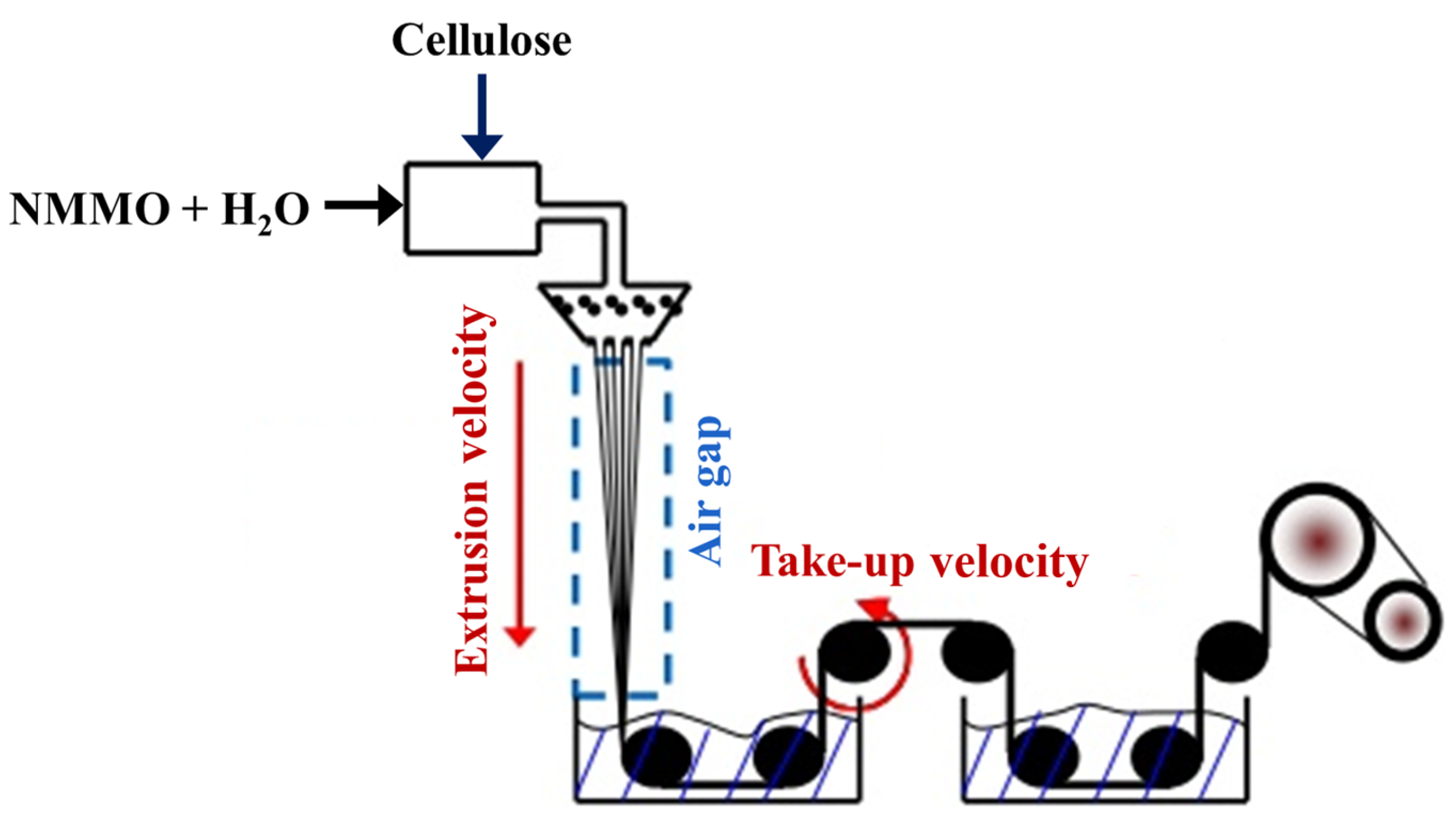
Man-Made Fibers of Synthetic Polymers
- Polyesters
| Polyester | Full Name | Structure | Tm (°C) 1 | Tg (°C) 2 | Assets | Ref. |
|---|---|---|---|---|---|---|
| PET | Polyethylene terephthalate |  | 250–265 | 70–80 | A common type of polyester | [134] |
| PTT | Polytrimethylene terephthalate |  | 220–230 | 30–40 | Superior softness and drape | [135] |
| PBT | Polybutylene terephthalate | 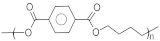 | 225–235 | 50–60 | Good mechanical properties and resistance to chemicals | [136] |
| PCDT | Poly(1,4-cyclohexylene-dimethylene terephthalate) | 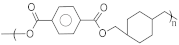 | 255–270 | 60–70 | High stretch and recovery properties | [137] |
| PEN | Polyethylene naphthalate | 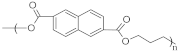 | 260–275 | 120–140 | High temperature resistance and excellent dimensional stability | [138] |
| PBN | Polybutylene naphthalate | 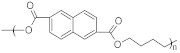 | 250–265 | 55–65 | High strength, stiffness, and thermal stability | [139] |
| PLA | Polylactic acid | 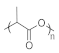 | 130–180 | 50–65 | A biodegradable polyester derived from renewable resources, such as cornstarch and sugarcane | [140] |
| PETG | Polyethylene Tere-phthalate Glycol | 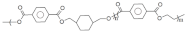 | 230–260 | 80–95 | Less shrinkage for printed parts | [141] |
- Polyamides
Man-Made Fibers of Non-Polymers
2.2. Blended Fiber Textiles
2.3. Fabrics
2.4. Textile Products
3. End-of-Life Options for Textile Waste
3.1. Collection and Sorting
3.1.1. Collection
3.1.2. Sorting

| IR Method | Algorithm | Fiber | Accuracy | Challenges | Advantages | Ref. |
|---|---|---|---|---|---|---|
| VNIR | Quadratic discriminant classifier, SVM, PCA | Cotton/viscose | 68% | A more extensive sample set is needed to guarantee a robust sorting system for all textile varieties. | Compared to the NIR range, the VNIR range offers higher spatial resolution, cheaper and compact cameras, and blue denim sorting. | [236] |
| PET | 70% | |||||
| Polyamide | 100% | |||||
| Wool | 100% | |||||
| Cotton and PET | Misclassified 50% as cotton and 40% as PET | |||||
| PET and cotton | Misclassified as cotton | |||||
| ATR-FTIR | PCA, CVA, k-NN | Polyamide, PET, viscose, cotton, linen, wool, silk | 100% | For real sorting machinery, implementing the specific software to establish a robust IR-spectra database for correctly classifying dirty-wet textile waste. | Automatically classified fiber samples with 100% accuracy and high speed, without any involvement of prior analytical treatment of the textile samples. | [24] |
| Post-sorting, a second sorting by color is required to reduce additional dyeing. | ||||||
| Strict maintenance protocol must be ensured for the contact between the sensor and the textile to register and compare the IR spectrum with the database for classification. | ||||||
| NIR | SVM, MLP, CNN | Pure PET slash, pure PET normal, pure wool, pure cotton normal, PET/polyamide, PET/wool, PET/cotton slash, PET/cotton, polyamide | 92–98% | To ensure higher prediction accuracy, a more comprehensive sample spectrum information is required to be included in the established standard spectral library. | The proposed method is simple and practical, presents a fast identification speed and a higher recognition rate, and can be applied to a wide range of applications. | [239] |
| NIR | SIMCA | Cotton | 93% | Low accuracy arises for cotton and polyester due to their relatively close spectral characteristics. | This is a nondestructive, simple, and fast method that can identify fibers with a total recognition rate higher than 95%. | [226] |
| PET | 92% | |||||
| PA6,6, acrylic, wool, silk | 100% | |||||
| NIR | PCA, SIMCA, LDA | Cotton, TencelTM, PP, PLA, PET, wool, cashmere | 100% | As there are many overlapping features in the chemical and physical analyses of wool and cashmere, there is lower accuracy for both textiles. | Using the mentioned algorithm for this method, seven textile fiber types were identified quickly and accurately. | [230] |
| ATR-FTIR | PCA | Wool, silk, cotton, linen, viscose, PET, PA6,6, acetate, TencelTM, acrylic, elastane, 15 two-component textiles | n.d. 1 | Mixed textiles are highly inhomogeneous. Thus, in such cases, homogeneity is tested using microscopic or IR-microspectroscopic analysis. | ATR-FTIR spectroscopy enables quick, easy, and nondestructive classification and semi-quantitative analysis of textiles. | [235] |
| NIR | PLS, ELM | Wool, PET, PAN, PA6,6 | Not mentioned | For the analysis, the number of spectral variables was reduced. However, the key to the successful utilization of an NIR-based analytical method is to construct a robust calibration model using samples with sufficient representativeness. | The ELM method is superior to the conventional PLS. | [238] |
| The developed procedure may have commercial and regulatory potential to avoid laborious, time-consuming, and expensive wet chemical analysis. | ||||||
| ATR-FTIR, r-FTIR, mATR-FTIR | Principal component-based discriminant analysis and random forest-based machine learning | Wool, silk, cotton, linen, jute, sisal, viscose, acetate, TencelTM, fiberglass, PET, PA6,6, acrylic, elastane, PE, PP | 99% (r-FTIR) | Obtaining a good-quality r-FTIR spectrum of elastane was problematic as the reflectance spectra of the used elastane thread was distorted. | r-FTIR is a suitable technique for the quick, easy, nondestructive, and non-invasive analysis of different types of textile samples. For analyzing very small threads (up to 10 individual fibers), a possible mATR-FTIR approach should be preferred. | [234] |
| 96% (mATR-FTIR) | For extraneous materials on sample fibers (additives or contaminants), the spectrum recorded is influenced by contaminants. | |||||
| FTIR | PCA, SIMCA | Viscose, PA6,6, acrylic, PET | 97% | The developed data-mining models can be made more extensive by adding more data of different fiber types, which will result in a sophisticated forensic tool for fiber discrimination. | The combination of spectroscopy and chemometrics has led to a highly desirable method for clustering and classifying 138 synthetic fiber samples into four groups. | [248] |
3.2. Reuse
3.3. Pretreatment Prior to Recycling
3.4. Mechanical Recycling
3.4.1. Fabric, Yarn, and Fiber Recycling
3.4.2. Polymer Recycling via Melting
3.4.3. Polymer Recycling via Dissolution
3.5. Chemical Recycling: Monomer/Oligomer Recycling
3.5.1. Degradation Mechanisms for Different Polymer Categories
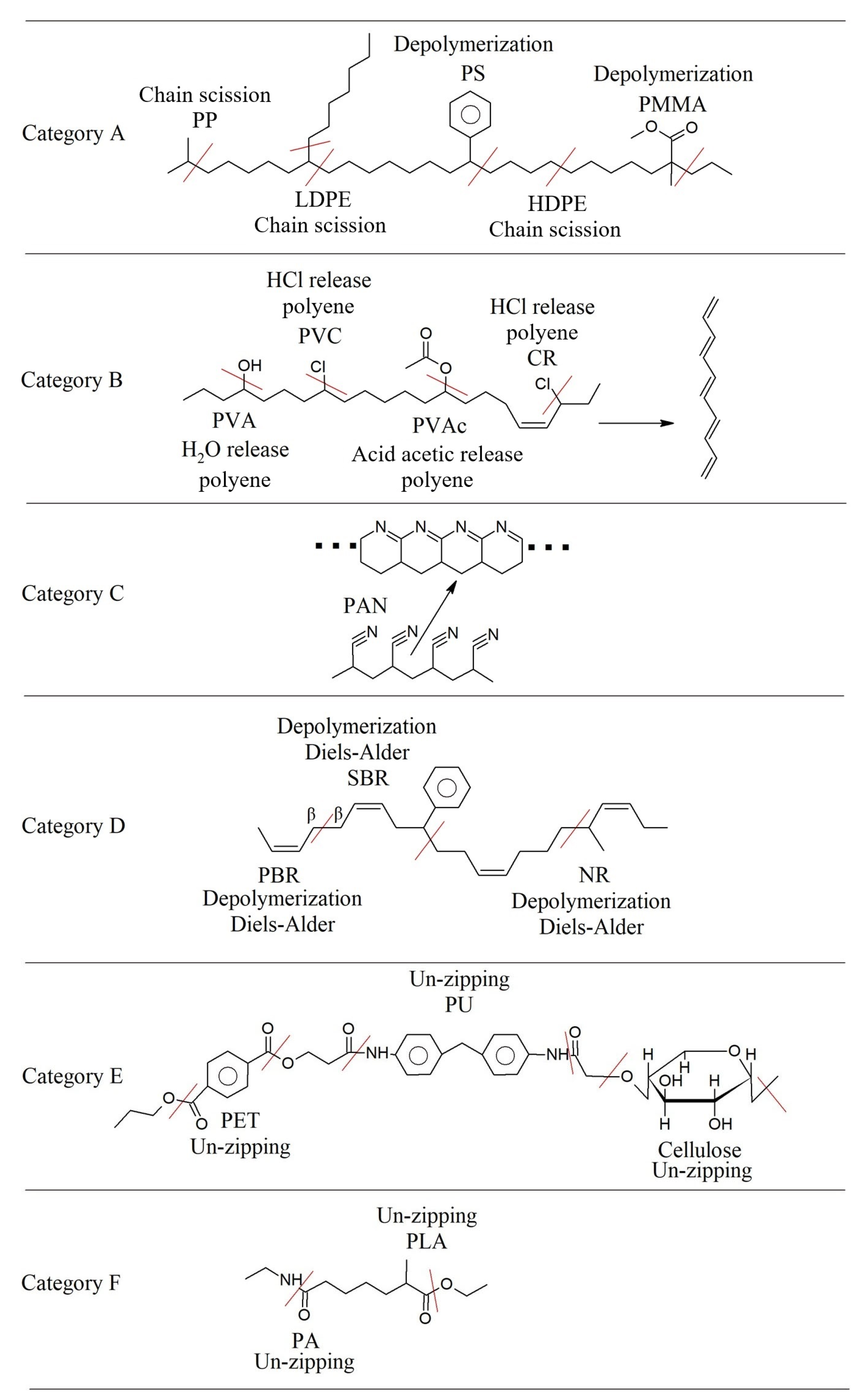
3.5.2. Biolysis
| Textile | Enzyme | Solvent for Pretreatment | Solvent:Polymer (vol/vol%) | T (°C) | t (h) | Buffer Solution | Glucose Yield (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Cotton-based waste (jeans) | Cellulase and Tricoderma reesei | H3PO4 | 50:1 | 50 | 96 | 0.33 mol·L−1 sodium citrate (pH: 4.8) | 86.1 | [419] |
| Cotton linters | Celluclast | NaOH/CH4N2O solution | 100:5 | 50 | 0.5–4 | Acetate (pH: 4.5) | 95.0 | [421] |
| Dyed cotton and cotton–polyester blend shirts | Cellulase AP3 | NMMO monohydrate, [BMIM]Cl, H3PO4, and NaOH/CH4N2O solution | 10:1–20:1 | 50 | 72 | 0.1 M·L−1 phosphate (pH: 5) with 0.02% sodium azide | 58.1 | [427] |
| Polyester–cotton blend shirts | Cellusoft L | Lutensol AT20 | NA 1 | 50 | 1 | 0.1 M·L−1 of acetate (pH: 5) | 100 | [428] |
| Undyed cotton T-shirt | Cellulase | [AMIM]Cl | 100:5 | 50 | NA | 2.5 M·L−1 citrate (pH: 5) | 86.0 | [429] |
| Cotton–polyester blend | Celluclast 1.5 L and β-glucosidase | NaOH/CH4N2O solution | NA | 50 | 96 | 0.2 M·L−1 sodium citrate (pH 5.1) | 98.7 | [430] |
| Cotton–PET blend | HiC and CTec2 | Mechanical treatment | NA | 55 | 24 | Sodium phosphate (pH: 7.4) | 83–87 | [431] |
| Polyester–cotton blend | Celluclast 1.5 L and β-glucosidase | NaOH/CH4N2O, NaOH thiourea, and NaOH/CH4N2O/thiourea | 95:5 | 45 | 72 | Sodium acetate and 0.5 g·L−1 sodium azide (pH: 4.8) | 91.0 | [294] |
| Cellulose–wool–polyester blend | Cellic CTec3 and Savinase 12T | mQ-H2O | NA | 50 | 48 | 0.67 M·L−1, Tris-HCl (pH: 9) | 50–90 | [426] |
| Bleached cotton fabric | Celluclast 1.5 L | Ultrasonic treatment | NA | 50 | 1.5 | 0.2 M·L−1, acetate (pH: 5) | 54.0 | [432] |
3.5.3. Pyrolysis
| Feed | Carrier Gas | Pyrolysis T (°C) | Catalysis T (°C) | Reactor | Catalyst | Cat/ Feed | Product Yield | Major Findings | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Polyamide | CO2 | 700 (10 °C·min−1) | 600 | Fixed bed | Ni/SiO2 | 1 | Syngas: | The production of syngas from CO2-assisted catalytic pyrolysis was seven times larger than the inert atmosphere of N2 arising from increased CO formation through the catalytic reaction of CO2 with pyrolytic products over the catalyst. CO2 also offered a great opportunity to suppress catalyst deactivation by suppressing coke deposition or removing the coke deposited on the catalyst surface. | |
| CO: 17.5 mol% | |||||||||
| H2: 11.9 mol% | |||||||||
| N2 | 700 (10 °C·min−1) | 600 | Fixed bed | Ni/SiO2 | 1 | Syngas: | |||
| CO: 5.7 mol% | |||||||||
| H2: 18.7 mol% | |||||||||
| PET | N2 | 600 (45 °C·min−1) | 600 (45 °C·min−1) | Tubular | Sulfated zirconia | 0.1 | Benzoic acid: 28.0 wt% | Increasing the catalyst/plastic ratio could increase the amount of other valuable products (e.g., light hydrocarbons [C1–C3]) recovered in the gas. | [455] |
| PET | N2 | 600 | 600 | Tubular | ZSM-5 | 6 | Solid residual: 10.5 wt% | ZSM-5 has little effect on the primary decomposition of PET, but it promotes the secondary volatile reactions. The use of NiCl2 as a catalyst will greatly improve the primary decomposition of PET to generate more liquid products. | [434] |
| Wax: 23 wt% | |||||||||
| N2 | 600 | 600 | Tubular | ZnCl2 | 1 | Wax: 68.9 wt% | |||
| HDPE | N2 | 500 | 500 | Spouted bed + fixed bed | HZSM-5 | 8 | Light olefins (C2~C4): 59 wt% | The low residence time in the catalytic reactor enhances the selectivity of light olefins and attenuates the secondary reactions of coke formation. | [457] |
| LDPE 1 | He | 550 | 600 | Micropyrolyzer | Phosphorus-modified and steam-treated HZSM-5 | 80 | Light olefins (C2~C4): 82.8 wt% | Phosphorus-modified and steam-treated HZSM-5 showed almost no deactivation due to the lower coking propensity during 130 runs, with stable conversion to C5+ aliphatics and high C2–C4 olefin selectivity (~75%) using post-consumer mixed polyolefins. | [361] |
| 23 wt% LLDPE 2, 7.5 wt% LDPE, 29.5 wt% HDPE, 40 wt% PP | He | 550 | 600 | Micropyrolyzer | Phosphorus-modified and steam-treated HZSM-5 | 80 | Light olefins (C2~C4): 78.8 wt% | ||
| Mixed polyolefins | He | 550 | 600 | Micropyrolyzer | Phosphorus steamed HZSM-5 | 80 | Light olefins (C2~C4): 73.2 wt% | ||
| Waste textiles | N2 | 800 (10 °C·min−1) | 800 (10 °C·min−1) | Fixed bed | Al2O3 | - | Oil: 37.5 wt% | Heavy metals in textile dyes were used as self-catalysts to accelerate the conversion process and decrease the pyrolysis time by 15% and to increase the bio-oil yield by ~20% compared to the textile samples treated without catalysts. | [458] |
| Gas: 44.7 wt% | |||||||||
| Char: 17.8 wt% | |||||||||
| Waste textiles (main component: PET) | CO2 | 720 (10 °C·min−1) | 650 | Tubular | Co/SiO2 | 1 | Gas: 80.9% | Catalytic pyrolysis over a Co-based catalyst with threefold- and eightfold-higher production of H2 and CO, respectively, compared to non-catalytic pyrolysis. This process also suppressed catalyst deactivation, converting more than 80 wt% of waste textile into syngas and CH4. | [456] |
| Char: 17.6% | |||||||||
| N2 | 720 (10 °C·min−1) | 650 | Tubular | Co/SiO2 | 1 | Gas: 73.9% | |||
| Char: 19.1% | |||||||||
| Waste mixed cloth (cotton fibers, acrylic fibers, and PET fibers) | He | 650 | 650 | Pyroprobe | HZSM-5 | 6 | Aromatics: 98.9% | The utilization of HZSM with Brønsted/Lewis acid sites on microporous and mesoporous structures significantly contributed to the production of monocyclic/dicyclic chemicals, mainly referring to monoaromatics and naphthalene-based derivatives. | [435] |
| He | 650 | - | Pyroprobe | - | - | Anhydrosugars: 45.7% | |||
| COVID-19 mask | N2 | 550 | 550 | Fixed bed | Hbeta | - | BTEX: 49.4% | The pore sizes of Hbeta and HY were larger than the kinetic diameters of the branched hydrocarbons, allowing the thermally derived branched hydrocarbons to diffuse inside the pores and thereby converting into the aromatic hydrocarbons over acid sites located mainly inside the pores. | [461] |
| N2 | 550 | 550 | Fixed bed | HY | - | BTEX: 35.2% | |||
| N2 | 550 | 550 | Fixed bed | HZSM-5 | - | BTEX: 21.1% | |||
| COVID-19 mask (PP, PE, polyamide) | N2 | 600 (10 °C·min−1) | 600 | Tubular | Ni/SiO2 | 1 | H2: 55.1 mol% | Pyrolysis over a Ni/SiO2 catalyst led to the substantial conversion of longer-chain (≥C2) HCs into H2 and CH4. | [462] |
| CH4: 18.2 mol% |
3.5.4. Gasification
3.5.5. Activated Carbon Production
| Feed | Reactor | Activation Process | Properties | Ref. | ||||
|---|---|---|---|---|---|---|---|---|
| T (°C) | Heating Rate (°C·min−1) | BET 1 Surface Area (m2·g−1) | Micropore Area (m2·g−1) | Micropore Volume (cm3·g−1) | Total Pore Volume (cm3·g−1) | |||
| Waste cotton | Tubular | 750–900 | 5 | 1044–2562 | - | 0.41–1.14 | 0.41–1.35 | [488] |
| Waste cotton | Tubular | 700 | 5 | 292 | 255 | 0.11 | 0.14 | [489] |
| Aramid woven fabric waste | Furnace | 800–1200 | 300 | 109–248 | - | - | - | [490] |
| Hemp and flax | Fixed bed | 350–900 | 2 | 200–900 | - | 0.2–0.35 | 0.10–0.72 | [491] |
| Acrylic waste | Fixed bed | 700, 800, 900 | 5 | 752 | - | 0.32 | - | [492] |
| Waste wool | Tubular | 1100 | 2 | 152 | - | - | 0.15 | [493] |
| PET waste | Muffle furnace | 900 | 10 | 230–1336 | 193–589 | 0.08–0.29 | - | [494] |
| PET waste | Muffle furnace | 900 | 10 | 171–951 | 98–480 | 0.04–0.20 | 0.15–1.68 | [495] |
| PET waste | Muffle furnace | 900 | 10 | 171–1364 | 98–527 | 0.04–0.23 | 0.15–2.91 | [496] |
| PET waste | Pipe furnace | 900 | 10 | 483–1307 | 24–323 | 0.01–0.12 | 1.53–3.56 | [485] |
| PET waste | Pipe furnace | 650 | 10 | 382–1415 | 837–1071 | 0.32–0.43 | 1.45–2.10 | [497] |
| Cotton wastes | Pipe furnace | 400–500 | 10 | 510–1855 | 274–440 | 0.13–0.20 | - | [498] |
3.5.6. Solvolysis
Glycolysis
Hydrolysis
Methanolysis
Aminolysis
Ammonolysis
Hydrogenolysis
Reductive Depolymerization
4. Technology Assessment of Textile Fiber Recycling
5. Life Cycle Assessment
6. Eco-Design
7. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Elsasser, V.H. Textiles: Concepts and Principles; Fairchild Publications: New York, NY, USA, 2005; Available online: https://books.google.be/books?id=HHWTNAAACAAJ (accessed on 19 February 2025).
- Horrocks, A.R.; Anand, S.C. Handbook of Technical Textiles: Technical Textile Applications; Elsevier Science: Amsterdam, The Netherlands, 2016; Available online: https://books.google.be/books?id=EjSiBQAAQBAJ (accessed on 19 February 2025).
- Hasan, M.M.; Salman, M.S.; Hasan, M.N.; Rehan, A.I.; Awual, M.E.; Rasee, A.I.; Waliullah, R.M.; Hossain, M.S.; Kubra, K.T.; Sheikh, M.C.; et al. Facial conjugate adsorbent for sustainable Pb(II) ion monitoring and removal from contaminated water. Colloids Surf. A Physicochem. Eng. Asp. 2023, 673, 131794. [Google Scholar] [CrossRef]
- Rehan, A.I.; Rasee, A.I.; Awual, M.E.; Waliullah, R.M.; Hossain, M.S.; Kubra, K.T.; Salman, M.S.; Hasan, M.M.; Hasan, M.N.; Sheikh, M.C.; et al. Improving toxic dye removal and remediation using novel nanocomposite fibrous adsorbent. Colloids Surf. A Physicochem. Eng. Asp. 2023, 673, 131859. [Google Scholar] [CrossRef]
- Kubra, K.T.; Hasan, M.M.; Hasan, M.N.; Salman, M.S.; Khaleque, M.A.; Sheikh, M.C.; Rehan, A.I.; Rasee, A.I.; Waliullah, R.M.; Awual, M.E.; et al. The heavy lanthanide of Thulium(III) separation and recovery using specific ligand-based facial composite adsorbent. Colloids Surf. A Physicochem. Eng. Asp. 2023, 667, 131415. [Google Scholar] [CrossRef]
- Loccufier, E.; Geltmeyer, J.; Daelemans, L.; D’hooge, D.R.; De Buysser, K.; De Clerck, K. Azeotrope Separation: Silica Nanofibrous Membranes for the Separation of Heterogeneous Azeotropes. Adv. Funct. Mater. 2018, 28, 1870313. [Google Scholar] [CrossRef]
- Swanckaert, B.; Geltmeyer, J.; Rabaey, K.; De Buysser, K.; Bonin, L.; De Clerck, K. A review on ion-exchange nanofiber membranes: Properties, structure and application in electrochemical (waste)water treatment. Sep. Purif. Technol. 2022, 287, 120529. [Google Scholar] [CrossRef]
- Swanckaert, B.; Loccufier, E.; Geltmeyer, J.; Rabaey, K.; De Buysser, K.; Bonin, L.; De Clerck, K. Sulfonated silica-based cation-exchange nanofiber membranes with superior self-cleaning abilities for electrochemical water treatment applications. Sep. Purif. Technol. 2023, 309, 123001. [Google Scholar] [CrossRef]
- Swanckaert, B.; Vande Velde, N.; Loccufier, E.; De Buysser, K.; Bonin, L.; De Clerck, K. High capacity, silica-based anion-exchange nanofiber membranes for the selective recovery of lactic acid. Sustain. Mater. Technol. 2023, 38, e00758. [Google Scholar] [CrossRef]
- Van Eygen, G.; Keuppens, S.; De Breuck, X.; Swankaert, B.; Boura, P.; Loccufier, E.; Kosek, J.; Ramasamy, D.; Nahra, F.; Buekenhoudt, A.; et al. Comparison of distinctive polymeric membrane structures as support materials for membrane extraction of chiral amines. Sep. Purif. Technol. 2025, 352, 128192. [Google Scholar] [CrossRef]
- Li, M.; Loccufier, E.; Geltmeyer, J.; D’hooge, D.R.; De Buysser, K.; De Clerck, K. Playing with Chlorine-Based Post-modification Strategies for Manufacturing Silica Nanofibrous Membranes Acting as Stable Hydrophobic Separation Barriers. Adv. Fiber Mater. 2024, 6, 145–157. [Google Scholar] [CrossRef]
- Siliņa, L.; Dāboliņa, I.; Lapkovska, E. Sustainable textile industry—Wishful thinking or the new norm: A review. J. Eng. Fibers Fabr. 2024, 19, 15589250231220359. [Google Scholar] [CrossRef]
- Estévez, S.; Mosca Angelucci, D.; Moreira, M.T.; Tomei, M.C. Techno-environmental and economic assessment of color removal strategies from textile wastewater. Sci. Total Environ. 2024, 913, 169721. [Google Scholar] [CrossRef] [PubMed]
- Brundtland Report. Report of the World Commission on Environment and Development: Our Common Future; World Commission on Environment and Development: Cape Town, South Africa, 1987; Available online: https://sustainabledevelopment.un.org/content/documents/5987our-common-future.pdf (accessed on 19 February 2025).
- Böttcher, T.P.; Empelmann, S.; Weking, J.; Hein, A.; Krcmar, H. Digital sustainable business models: Using digital technology to integrate ecological sustainability into the core of business models. Inf. Syst. J. 2024, 34, 736–761. [Google Scholar] [CrossRef]
- Elkington, J. Cannibals with Forks: The Triple Bottom Line of 21st Century Business; New Society Publishers: Gabriola Island, BC, Canada, 1998; Available online: https://books.google.be/books?id=dIJAbIM7XNcC (accessed on 19 February 2025).
- Statista. Distribution of Textile Fibers Production Worldwide in 2021, by Type 2021. Available online: https://www.statista.com/statistics/1250812/global-fiber-production-share-type/ (accessed on 1 January 2025).
- OEC. Textiles. 2021. Available online: https://oec.world/en/profile/hs/textiles?yearSelector1=2021 (accessed on 1 January 2025).
- Textile Market (by Raw-Material: Cotton, Chemical, Wool, Silk; by Product: Natural Fibers, Polyesters, Nylon; by Application: Household, Technical, Fashion & Clothing)—Global Industry Analysis, Size, Share, Growth, Trends, Revenue, Regional Outlook 20222030. Vision Research Reports. 2022. Available online: https://www.visionresearchreports.com/textile-market/39190 (accessed on 19 February 2025).
- Facts & Key Figures; Euratex: Brussels, Belgium, 2022; Available online: https://euratex.eu/wp-content/uploads/EURATEX_FactsKey_Figures_2022rev-1.pdf (accessed on 19 February 2025).
- Common Objective. Faces and Figures: Who Makes Our Clothes? Available online: https://www.commonobjective.co/article/faces-and-figures-who-makes-our-clothes (accessed on 1 January 2025).
- The World Bank. Labor Force, Total. 2023. Available online: https://data.worldbank.org/indicator/SL.TLF.TOTL.IN (accessed on 1 January 2025).
- Grace Annapoorani, S. Social Sustainability in Textile Industry. In Sustainability in the Textile Industry; Muthu, S.S., Ed.; Springer: Singapore, 2017; pp. 57–78. [Google Scholar] [CrossRef]
- Riba, J.-R.; Cantero, R.; Canals, T.; Puig, R. Circular economy of post-consumer textile waste: Classification through infrared spectroscopy. J. Clean. Prod. 2020, 272, 123011. [Google Scholar] [CrossRef]
- The Impact of Textile Production and Waste on the Environment (Infographic). European Parliament. 2022. Available online: https://www.eumonitor.eu/9353000/1/j9vvik7m1c3gyxp/vlf0bgvqy6vx?ctx=vjxzjv7ta8z1 (accessed on 19 February 2025).
- Abbate, S.; Centobelli, P.; Cerchione, R.; Nadeem, S.P.; Riccio, E. Sustainability trends and gaps in the textile, apparel and fashion industries. Environ. Dev. Sustain. 2024, 26, 2837–2864. [Google Scholar] [CrossRef]
- Textiles and the Environment: The Role of Design in Europe’s Circular Economy. European Environment Agency. 2022. Available online: https://www.eea.europa.eu/publications/textiles-and-the-environment-the/textiles-and-the-environment-the (accessed on 19 February 2025).
- A New Textiles Economy: Redesigning Fashion’s Future; Ellen MacArthur Foundation: Isle of Wight, UK, 2017; Available online: https://emf.thirdlight.com/file/24/uiwtaHvud8YIG_uiSTauTlJH74/A%20New%20Textiles%20Economy%3A%20Redesigning%20fashion%E2%80%99s%20future.pdf (accessed on 19 February 2025).
- Beans, C. Can nature inspire sustainable fashion? Proc. Natl. Acad. Sci. USA 2023, 120, e2306481120. [Google Scholar] [CrossRef]
- Napper, I.E.; Thompson, R.C. Release of synthetic microplastic plastic fibres from domestic washing machines: Effects of fabric type and washing conditions. Mar. Pollut. Bull. 2016, 112, 39–45. [Google Scholar] [CrossRef]
- Leal Filho, W.; Dinis, M.A.P.; Liakh, O.; Paço, A.; Dennis, K.; Shollo, F.; Sidsaph, H. Reducing the carbon footprint of the textile sector: An overview of impacts and solutions. Text. Res. J. 2024, 94, 00405175241236971. [Google Scholar] [CrossRef]
- Samant, L.; Pavan, M.; Goel, A.; Kaur, M. Impact of the Textile Industry on Global Climate Change. In Climate Action Through Eco-Friendly Textiles; Sadhna, K.R., Greeshma, S., Eds.; Springer Nature: Singapore, 2024; pp. 11–26. [Google Scholar] [CrossRef]
- Remy, N.; Speelman, E.; Swartz, S. Style That’s Sustainable: A New Fast-Fashion Formula. McKinsey Sustainability. 2016. Available online: https://www.mckinsey.com/capabilities/sustainability/our-insights/style-thats-sustainable-a-new-fast-fashion-formula (accessed on 19 February 2025).
- Soares, B.; Ramos, M.; Martinho, G. Factors to consider for the implementation of a municipal scheme for the separate collection of textile waste. Sustain. Futures 2024, 7, 100203. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Meidiana, C.; Goh, H.H.; Zhang, D.; Othman, M.H.D.; Aziz, F.; Anouzla, A.; Sarangi, P.K.; Pasaribu, B.; Ali, I. Unlocking synergies between waste management and climate change mitigation to accelerate decarbonization through circular-economy digitalization in Indonesia. Sustain. Prod. Consum. 2024, 46, 522–542. [Google Scholar] [CrossRef]
- Wang, S.; Salmon, S. Progress toward Circularity of Polyester and Cotton Textiles. Sustain. Chem. 2022, 3, 376–403. [Google Scholar] [CrossRef]
- Riba, J.-R.; Cantero, R.; Puig, R. Classification of Textile Samples Using Data Fusion Combining Near-and Mid-Infrared Spectral Information. Polymers 2022, 14, 3073. [Google Scholar] [CrossRef] [PubMed]
- Daelemans, L.; Van Paepegem, W.; D’hooge, D.R.; De Clerck, K. Excellent nanofiber adhesion for hybrid polymer materials with high toughness based on matrix interdiffusion during chemical conversion. Adv. Funct. Mater. 2019, 29, 1807434. [Google Scholar] [CrossRef]
- Tian, R.; Lv, Z.; Fan, Y.; Wang, T.; Sun, M.; Xu, Z. Qualitative classification of waste garments for textile recycling based on machine vision and attention mechanisms. Waste Manag. 2024, 183, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Phan, K.; Ügdüler, S.; Harinck, L.; Denolf, R.; Roosen, M.; O’Rourke, G.; De Vos, D.; Van Speybroeck, V.; De Clerck, K.; De Meester, S. Analysing the potential of the selective dissolution of elastane from mixed fiber textile waste. Resour. Conserv. Recycl. 2023, 191, 106903. [Google Scholar] [CrossRef]
- Delva, L.; Van Kets, K.; Kuzmanovic, M.; Demets, R.; Hubo, S.; Mys, N.; De Meester, S.; Ragaert, K. Mechanical Recycling of Polymers for Dummies. Capture-Plast. Resour. 2019. Available online: https://www.ugent.be/ea/match/cpmt/en/research/topics/circular-plastics/mechanicalrecyclingfordummiesv2.pdf (accessed on 19 February 2025).
- Bigambo, P.; Carr, C.M.; Sumner, M.; Rigout, M. Investigation into the removal of pigment, sulphur and vat colourants from cotton textiles and implications for waste cellulosic recycling. Color. Technol. 2021, 137, 604–614. [Google Scholar] [CrossRef]
- Kamble, Z.; Behera, B.K. Upcycling textile wastes: Challenges and innovations. Text. Prog. 2021, 53, 65–122. [Google Scholar] [CrossRef]
- Huang, X.; Tan, Y.; Huang, J.; Zhu, G.; Yin, R.; Tao, X.; Tian, X. Industrialization of open- and closed-loop waste textile recycling towards sustainability: A review. J. Clean. Prod. 2024, 436, 140676. [Google Scholar] [CrossRef]
- Yang, K.; Wang, M.; Wang, X.; Shan, J.; Zhang, J.; Tian, G.; Yang, D.; Ma, J. Polyester/Cotton-Blended Textile Waste Fiber Separation and Regeneration via a Green Chemistry Approach. ACS Sustain. Chem. Eng. 2024, 12, 4530–4538. [Google Scholar] [CrossRef]
- Amundarain, I.; López-Montenegro, S.; Fulgencio-Medrano, L.; Leivar, J.; Iruskieta, A.; Asueta, A.; Miguel-Fernández, R.; Arnaiz, S.; Pereda-Ayo, B. Improving the Sustainability of Catalytic Glycolysis of Complex PET Waste through Bio-Solvolysis. Polymers 2024, 16, 142. [Google Scholar] [CrossRef]
- Yadav, A.; Yadav, P.; Bojjagani, S.; Srivastava, J.K.; Raj, A. Investigation of the speciation and environmental risk of heavy metals in biochar produced from textile sludge waste by pyrolysis at different temperatures. Chemosphere 2024, 360, 142454. [Google Scholar] [CrossRef]
- Seifali Abbas-Abadi, M.; Nekoomanesh Haghighi, M.; McDonald, A.G.; Yeganeh, H. Estimation of pyrolysis product of LDPE degradation using different process parameters in a stirred reactor. Polyolefins J. 2015, 2, 39–47. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, Z.; Chen, T.; Jiang, S.; Evrendilek, F.; Huang, S.; Tang, X.; Ding, Z.; He, Y.; Xie, W.; et al. Energetic, bio-oil, biochar, and ash performances of co-pyrolysis-gasification of textile dyeing sludge and Chinese medicine residues in response to K2CO3, atmosphere type, blend ratio, and temperature. J. Environ. Sci. 2024, 136, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson, J.; Peterson, A.; Idström, A.; de la Motte, H.; Jedvert, K. Chemical Recycling of a Textile Blend from Polyester and Viscose, Part II: Mechanism and Reactivity during Alkaline Hydrolysis of Textile Polyester. Sustainability 2022, 14, 6911. [Google Scholar] [CrossRef]
- Provin, A.P.; Cubas, A.L.V.; Dutra, A.R.d.A.; Schulte, N.K. Textile industry and environment: Can the use of bacterial cellulose in the manufacture of biotextiles contribute to the sector? Clean Technol. Environ. Policy 2021, 23, 2813–2825. [Google Scholar] [CrossRef]
- Juanga-Labayen, J.P.; Labayen, I.V.; Yuan, Q. A Review on Textile Recycling Practices and Challenges. Textiles 2022, 2, 174–188. [Google Scholar] [CrossRef]
- Sandberg, E.; Pal, R. Exploring supply chain capabilities in textile-to-textile recycling—A European interview study. Clean. Logist. Supply Chain 2024, 11, 100152. [Google Scholar] [CrossRef]
- Pensupa, N. Recycling of end-of-life clothes. In Sustainable Technologies for Fashion and Textiles; Elsevier: Amsterdam, The Netherlands, 2020; pp. 251–309. [Google Scholar] [CrossRef]
- Sulakhe, V.N. Introduction to Semisynthetic and Synthetic Fiber Based Composites. In Natural and Synthetic Fiber Reinforced Composites: Synthesis, Properties and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2022; pp. 67–79. [Google Scholar] [CrossRef]
- Shabbir, M.; Naim, M. Introduction to Textiles and the Environment. In Textiles and Clothing; John Wiley & Sons: Hoboken, NJ, USA, 2019; pp. 1–9. [Google Scholar] [CrossRef]
- Rajak, D.K.; Wagh, P.H.; Linul, E. A review on synthetic fibers for polymer matrix composites: Performance, failure modes and applications. Materials 2022, 15, 4790. [Google Scholar] [CrossRef]
- McCauley, E.; Jestratijevic, I. Exploring the Business Case for Textile-to-Textile Recycling Using Post-Consumer Waste in the US: Challenges and Opportunities. Sustainability 2023, 15, 1473. [Google Scholar] [CrossRef]
- Loo, S.-L.; Yu, E.; Hu, X. Tackling critical challenges in textile circularity: A review on strategies for recycling cellulose and polyester from blended fabrics. J. Environ. Chem. Eng. 2023, 11, 110482. [Google Scholar] [CrossRef]
- Damayanti, D.; Wulandari, L.A.; Bagaskoro, A.; Rianjanu, A.; Wu, H.-S. Possibility Routes for Textile Recycling Technology. Polymers 2021, 13, 3834. [Google Scholar] [CrossRef]
- Shahid, M.A.; Hossain, M.T.; Habib, M.A.; Islam, S.; Sharna, K.; Hossain, I.; Mortuza Limon, M.G. Prospects and challenges of recycling and reusing post-consumer garments: A review. Clean. Eng. Technol. 2024, 19, 100744. [Google Scholar] [CrossRef]
- Tripathi, M.; Sharma, M.; Bala, S.; Thakur, V.K.; Singh, A.; Dashora, K.; Hart, P.; Gupta, V.K. Recent technologies for transforming textile waste into value-added products: A review. Curr. Res. Biotechnol. 2024, 7, 100225. [Google Scholar] [CrossRef]
- Iezzi, B.; Shtein, M.; Wang, T.; Rothschild, M. Fiber and Fabric-Integrated Tracing Technologies for Textile Sorting and Recycling. In Technology Innovation for the Circular Economy; John Wiley & Sons: Hoboken, NJ, USA, 2024; pp. 223–237. [Google Scholar] [CrossRef]
- Baldia, C.M.; Armitage, R.A. Archaeological Textiles as Secondary Plant and Animal Products. In Handbook of Archaeological Sciences; John Wiley & Sons: Hoboken, NJ, USA, 2023; pp. 797–811. [Google Scholar] [CrossRef]
- Miles, D.C.; Briston, J.H. Polymer Technology; Temple Press Books: London, UK, 1965; Available online: https://books.google.be/books/about/Polymer_Technology.html?id=dLeaAAAAIAAJ&redir_esc=y (accessed on 19 February 2025).
- Harmsen, P.; Scheffer, M.; Bos, H. Textiles for Circular Fashion: The Logic behind Recycling Options. Sustainability 2021, 13, 9714. [Google Scholar] [CrossRef]
- Textile Exchange. Materials Market Report. 2023. Available online: https://textileexchange.org/app/uploads/2023/11/Materials-Market-Report-2023.pdf (accessed on 19 February 2025).
- Wang, K.; Wang, Q.; Zou, L.; Su, Y.; Liu, K.; Li, W.; Zhang, K.; Wang, H.; Song, J. Study on thermal protection and temperature of PMMA plastic optical fiber for concentrated sunlight transmission in daylighting. Sol. Energy 2023, 253, 127–136. [Google Scholar] [CrossRef]
- Al-Furjan, M.S.H.; Shan, L.; Shen, X.; Zarei, M.S.; Hajmohammad, M.H.; Kolahchi, R. A review on fabrication techniques and tensile properties of glass, carbon, and Kevlar fiber reinforced rolymer composites. J. Mater. Res. Technol. 2022, 19, 2930–2959. [Google Scholar] [CrossRef]
- Sabbatini, B.; Cambriani, A.; Cespi, M.; Palmieri, G.F.; Perinelli, D.R.; Bonacucina, G. An Overview of Natural Polymers as Reinforcing Agents for 3D Printing. ChemEngineering 2021, 5, 78. [Google Scholar] [CrossRef]
- McIntyre, J.E.; Daniels, P.N.; Terms, T.I.T.; Committee, D. Textile Terms and Definitions; Taylor & Francis: Abingdon, UK, 1995; Available online: https://books.google.be/books?id=bt9-QgAACAAJ (accessed on 19 February 2025).
- Binczarski, M.J.; Malinowska, J.Z.; Berlowska, J.; Cieciura-Wloch, W.; Borowski, S.; Cieslak, M.; Puchowicz, D.; Witonska, I.A. Concept for the Use of Cotton Waste Hydrolysates in Fermentation Media for Biofuel Production. Energies 2022, 15, 2856. [Google Scholar] [CrossRef]
- TextileExchange. Available online: https://textileexchange.org/plant-fibers/ (accessed on 1 January 2025).
- Nagdeve, T.; Dhara, S.; Tandulkar, H.; Jangde, P.; Ukani, N.; Chakole, S. Design and Synthesis of Chassis of Automated Seed Sowing Robot for BT Cotton Seed. In Proceedings of the 2020 IEEE International Students’ Conference on Electrical, Electronics and Computer Science (SCEECS), Bhopal, India, 22–23 February 2020; pp. 1–4. [Google Scholar] [CrossRef]
- Frank, E.; Bauch, V.; Schultze-Gebhardt, F.; Herlinger, K.-H. Fibers, 1. Survey. In Ullmann’s Encyclopedia of Industrial Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar] [CrossRef]
- Feughelman, M. Natural protein fibers. J. Appl. Polym. Sci. 2002, 83, 489–507. [Google Scholar] [CrossRef]
- Li, Y.; Hu, Y.P.; Hu, C.J.; Yu, Y.H. Microstructures and Mechanical Properties of Natural Fibers. Adv. Mater. Res. 2008, 33–37, 553–558. [Google Scholar] [CrossRef]
- Djafari Petroudy, S.R. 3—Physical and mechanical properties of natural fibers. In Advanced High Strength Natural Fibre Composites in Construction; Fan, M., Fu, F., Eds.; Woodhead Publishing: Amsterdam, The Netherlands, 2017; pp. 59–83. [Google Scholar] [CrossRef]
- Lee, S.M. Handbook of Composite Reinforcements; John Wiley & Sons: Hoboken, NJ, USA, 1992; Available online: http://www.knovel.com/knovel2/Toc.jsp?BookID=2013 (accessed on 19 February 2025).
- Whitmer, M. Types of Asbestos. 2024. Available online: www.asbestos.com (accessed on 1 January 2025).
- Lee, T.; Mischler, S.E.; Wolfe, C. Classification of asbestos and their nonasbestiform analogues using FTIR and multivariate data analysis. J. Hazard. Mater. 2024, 469, 133874. [Google Scholar] [CrossRef]
- Gualtieri, A.F.; Tartaglia, A. Thermal decomposition of asbestos and recycling in traditional ceramics. J. Eur. Ceram. Soc. 2000, 20, 1409–1418. [Google Scholar] [CrossRef]
- Mustafayevich, J.S.; Xolmurodovich, O.S. Inorganic Heat Insulation Materials. Gospodarka i Innowacje. 2024. Available online: https://gospodarkainnowacje-pl.openconference.us/index.php/issue_view_32/article/download/2259/2092 (accessed on 19 February 2025).
- Algranti, E.; Ramos-Bonilla, J.P.; Terracini, B.; Santana, V.S.; Comba, P.; Pasetto, R.; Mazzeo, A.; Cavariani, F.; Trotta, A.; Marsili, D. Prevention of Asbestos Exposure in Latin America within a Global Public Health Perspective. Ann. Glob. Health 2019, 85, 49. [Google Scholar] [CrossRef] [PubMed]
- Naz, M.; Rafiq, A.; Ikram, M.; Haider, A.; Ahmad, S.O.A.; Haider, J.; Naz, S. Elimination of dyes by catalytic reduction in the absence of light: A review. J. Mater. Sci. 2021, 56, 15572–15608. [Google Scholar] [CrossRef]
- Liu, F.; Pan, L.; Liu, Y.; Zhai, G.; Sha, Z.; Zhang, X.; Zhang, Z.; Liu, Q.; Yu, S.; Zhu, L. Biobased fibers from natural to synthetic: Processing, manufacturing, and application. Matter 2024, 7, 1977–2010. [Google Scholar] [CrossRef]
- Gupta, V.B. Solution-spinning processes. In Manufactured Fibre Technology; Gupta, V.B., Kothari, V.K., Eds.; Springer: Dordrecht, The Netherlands, 1997; pp. 124–138. [Google Scholar] [CrossRef]
- Preston, J. Man-Made Fibre. Encyclopedia Britannica. 2022. Available online: https://www.britannica.com/technology/man-made-fiber (accessed on 19 February 2025).
- Gupta, V.B. Melt-spinning processes. In Manufactured Fibre Technology; Gupta, V.B., Kothari, V.K., Eds.; Springer: Dordrecht, The Netherlands, 1997; pp. 67–97. [Google Scholar] [CrossRef]
- Hufenus, R.; Yan, Y.; Dauner, M.; Kikutani, T. Melt-Spun Fibers for Textile Applications. Materials 2020, 13, 4298. [Google Scholar] [CrossRef]
- Qin, Y. 3—A brief description of textile fibers. In Medical Textile Materials; Qin, Y., Ed.; Woodhead Publishing: Amsterdam, The Netherlands, 2016; pp. 23–42. [Google Scholar] [CrossRef]
- Xia, L.; Xi, P.; Cheng, B. A comparative study of UHMWPE fibers prepared by flash-spinning and gel-spinning. Mater. Lett. 2015, 147, 79–81. [Google Scholar] [CrossRef]
- Hugill, R.; Ley, K.; Rademan, K. Coming Full Circle: Innovating Towards Sustainable Man-Made Cellulosic Fibres; FashionForGood: Amsterdam, The Netherlands, 2020; Available online: https://reports.fashionforgood.com/report/coming-full-circle-innovating-towards-sustainable-man-made-cellulosic-fibres/ (accessed on 19 February 2025).
- Gupta, N.; Kanth, N. Heat Transfer Model for Silk Finishing Calender. In Frontiers in Industrial and Applied Mathematics: Proceedings of the FIAM-2021, Punjab, India, 21–22 December 2021; Springer: Berlin/Heidelberg, Germany, 2023; pp. 309–320. [Google Scholar] [CrossRef]
- Gobalakrishnan, M.; Saravanan, D.; Das, S. Sustainable finishing process using natural ingredients. In Sustainability in the Textile and Apparel Industries: Production Process Sustainability; Springer: Berlin/Heidelberg, Germany, 2020; pp. 129–146. [Google Scholar] [CrossRef]
- Ramasamy, R.; Subramanian, R.B. Synthetic textile and microfiber pollution: A review on mitigation strategies. Environ. Sci. Pollut. Res. 2021, 28, 41596–41611. [Google Scholar] [CrossRef]
- Wang, S.; Lu, A.; Zhang, L. Recent advances in regenerated cellulose materials. Prog. Polym. Sci. 2016, 53, 169–206. [Google Scholar] [CrossRef]
- Shaikh, T.; Chaudhari, S.; Varma, A. Viscose rayon: A legendary development in the manmade textile. Int. J. Eng. Res. Appl. 2012, 2, 675–680. [Google Scholar]
- Balkissoon, S.; Andrew, J.; Sithole, B. Dissolving wood pulp production: A review. Biomass Convers. Biorefin. 2022, 13, 16607–16642. [Google Scholar] [CrossRef]
- Karthik, T.; Gopalakrishnan, D. Environmental analysis of textile value chain: An overview. In Roadmap to Sustainable Textiles and Clothing: Environmental and Social Aspects of Textiles and Clothing Supply Chain; Springer: Berlin/Heidelberg, Germany, 2014; pp. 153–188. [Google Scholar] [CrossRef]
- Mendes, I.S.; Prates, A.; Evtuguin, D.V. Production of rayon fibres from cellulosic pulps: State of the art and current developments. Carbohydr. Polym. 2021, 273, 118466. [Google Scholar] [CrossRef] [PubMed]
- Kuchtová, G.; Herink, P.; Herink, T.; Chýlková, J.; Mikulášek, P.; Dušek, L. From lab-scale to pilot-scale treatment of real wastewater from the production of rayon fiber. Process Saf. Environ. Prot. 2023, 171, 834–846. [Google Scholar] [CrossRef]
- Zainul Armir, N.A.; Zulkifli, A.; Gunaseelan, S.; Palanivelu, S.D.; Salleh, K.M.; Che Othman, M.H.; Zakaria, S. Regenerated cellulose products for agricultural and their potential: A review. Polymers 2021, 13, 3586. [Google Scholar] [CrossRef] [PubMed]
- El Seoud, O.A.; Kostag, M.; Jedvert, K.; Malek, N.I. Cellulose regeneration and chemical recycling: Closing the “cellulose gap” using environmentally benign solvents. Macromol. Mater. Eng. 2020, 305, 1900832. [Google Scholar] [CrossRef]
- Sayyed, A.J.; Deshmukh, N.A.; Pinjari, D.V. A critical review of manufacturing processes used in regenerated cellulosic fibres: Viscose, cellulose acetate, cuprammonium, LiCl/DMAc, ionic liquids, and NMMO based lyocell. Cellulose 2019, 26, 2913–2940. [Google Scholar] [CrossRef]
- Chawla, S.P.; Kanatt, S.R.; Sharma, A.K. Chitosan. In Polysaccharides: Bioactivity and Biotechnology; Ramawat, K.G., Mérillon, J.-M., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 219–246. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Kim, C.-H.; Park, S.J.; Yang, D.H.; Chun, H.J. Chitosan for Tissue Engineering. In Novel Biomaterials for Regenerative Medicine; Chun, H.J., Park, K., Kim, C.-H., Khang, G., Eds.; Springer: Singapore, 2018; pp. 475–485. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, C.; Duan, C.; Hu, H.; Li, H.; Li, J.; Liu, Y.; Ma, X.; Stavik, J.; Ni, Y. Regenerated cellulose by the lyocell process, a brief review of the process and properties. BioResources 2018, 13, 4577–4592. [Google Scholar] [CrossRef]
- Parisi, O.I.; Curcio, M.; Puoci, F. Polymer Chemistry and Synthetic Polymers. In Advanced Polymers in Medicine; Puoci, F., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 1–31. [Google Scholar] [CrossRef]
- Su, W.-F. Radical chain polymerization. In Principles of Polymer Design and Synthesis; Springer: Berlin/Heidelberg, Germany, 2013; pp. 137–183. [Google Scholar] [CrossRef]
- Braunecker, W.A.; Matyjaszewski, K. Controlled/living radical polymerization: Features, developments, and perspectives. Prog. Polym. Sci. 2007, 32, 93–146. [Google Scholar] [CrossRef]
- D’hooge, D.R.; Van Steenberge, P.H.M.; Reyniers, M.-F.; Marin, G.B. The strength of multi-scale modeling to unveil the complexity of radical polymerization. Prog. Polym. Sci. 2016, 58, 59–89. [Google Scholar] [CrossRef]
- Yilmaz, G.; Yagci, Y. Light-induced step-growth polymerization. Prog. Polym. Sci. 2020, 100, 101178. [Google Scholar] [CrossRef]
- Van Steenberge, P.H.M.; Vandenbergh, J.; Reyniers, M.-F.; Junkers, T.; D’hooge, D.R.; Marin, G.B. Kinetic Monte Carlo Generation of Complete Electron Spray Ionization Mass Spectra for Acrylate Macromonomer Synthesis. Macromolecules 2017, 50, 2625–2636. [Google Scholar] [CrossRef]
- Adegbola, T.; Agboola, O.; Fayomi, O. Review of polyacrylonitrile blends and application in manufacturing technology: Recycling and environmental impact. Results Eng. 2020, 7, 100144. [Google Scholar] [CrossRef]
- Smirnova, O.; Kharitonov, A.; Belentsov, Y. Influence of polyolefin fibers on the strength and deformability properties of road pavement concrete. J. Traffic Transp. Eng. Engl. Ed. 2019, 6, 407–417. [Google Scholar] [CrossRef]
- Gurera, D.; Bhushan, B. Fabrication of bioinspired superliquiphobic synthetic leather with self-cleaning and low adhesion. Colloids Surf. A Physicochem. Eng. Asp. 2018, 545, 130–137. [Google Scholar] [CrossRef]
- Agrawal, A.; Kaur, R.; Walia, R. PU foam derived from renewable sources: Perspective on properties enhancement: An overview. Eur. Polym. J. 2017, 95, 255–274. [Google Scholar] [CrossRef]
- Novikov, M.B.; Borodulina, T.A.; Kotomin, S.V.; Kulichikhin, V.G.; Feldstein, M.M. Relaxation properties of pressure-sensitive adhesives upon withdrawal of bonding pressure. J. Adhes. 2005, 81, 77–107. [Google Scholar] [CrossRef]
- Gadhave, R.V.; Vineeth, S. Synthesis of Microcrystalline Cellulose—Polyvinyl Alcohol Stabilized Polyvinyl Acetate Emulsion. Green Sustain. Chem. 2023, 13, 23–33. [Google Scholar] [CrossRef]
- Aydemir, D. The Lap Joint Shear Strength of Wood Materials Bonded by Cellulose Fiber-Reinforced Polyvinyl Acetate. Bioresources 2014, 9, 1179–1188. [Google Scholar] [CrossRef]
- Islam, M.S. Polyvinyl Alcohol and Polyvinyl Acetate. In Industrial Applications of Biopolymers and Their Environmental Impact; CRC Press: Boca Raton, FL, USA, 2020; pp. 135–152. [Google Scholar] [CrossRef]
- Bossion, A.; Heifferon, K.V.; Meabe, L.; Zivic, N.; Taton, D.; Hedrick, J.L.; Long, T.E.; Sardon, H. Opportunities for organocatalysis in polymer synthesis via step-growth methods. Prog. Polym. Sci. 2019, 90, 164–210. [Google Scholar] [CrossRef]
- Jaffe, M.; Easts, A.J.; Feng, X. Polyester fibers. In Thermal Analysis of Textiles and Fibers; Elsevier: Amsterdam, The Netherlands, 2020; pp. 133–149. [Google Scholar] [CrossRef]
- Fiorillo, C.; Edeleva, M.; Trossaert, L.; Van Steenberge, P.; Cardon, L.; D’hooge, D. Understanding the hydrolytic stability of the (co-)polyester polymer family. In Proceedings of the Annual Meeting of the Belgian Polymer Group 2022 (BPG 2022), Blankenberge, Belgium, 14–15 November 2022; Available online: http://hdl.handle.net/1854/LU-01GP3ASHRN3F72TAYVSYWTQWK6 (accessed on 19 February 2025).
- Trossaert, L.; De Vel, M.; Cardon, L.; Edeleva, M. Lifting the Sustainability of Modified Pet-Based Multilayer Packaging Material with Enhanced Mechanical Recycling Potential and Processing. Polymers 2022, 14, 196. [Google Scholar] [CrossRef]
- Rabiei, N.; Kish, M.H. Aminolysis of polyesters for cracking and structure clarifying: A review. Polym. Adv. Technol. 2022, 33, 3903–3919. [Google Scholar] [CrossRef]
- Heidrich, D.; Gehde, M. The 3-Phase Structure of Polyesters (PBT, PET) after Isothermal and Non-Isothermal Crystallization. Polymers 2022, 14, 793. [Google Scholar] [CrossRef]
- Vouyiouka, S.N.; Karakatsani, E.K.; Papaspyrides, C.D. Solid state polymerization. Prog. Polym. Sci. 2005, 30, 10–37. [Google Scholar] [CrossRef]
- Pang, K.; Kotek, R.; Tonelli, A. Review of conventional and novel polymerization processes for polyesters. Prog. Polym. Sci. 2006, 31, 1009–1037. [Google Scholar] [CrossRef]
- Thiele, U.K. Polyester Bottle Resins Production, Processing, Properties and Recycling; PETplanet Print: Heidelberg, Germany, 2007; Available online: https://www.gettextbooks.com/isbn/9783980749749/ (accessed on 19 February 2025).
- Duh, B. Reaction kinetics for solid-state polymerization of poly (ethylene terephthalate). J. Appl. Polym. Sci. 2001, 81, 1748–1761. [Google Scholar] [CrossRef]
- Ketema, A.; Worku, A. Review on intermolecular forces between dyes used for polyester dyeing and polyester fiber. J. Chem. 2020, 2020, 1–7. [Google Scholar] [CrossRef]
- Shogren, R.; Wood, D.; Orts, W.; Glenn, G. Plant-based materials and transitioning to a circular economy. Sustain. Prod. Consum. 2019, 19, 194–215. [Google Scholar] [CrossRef]
- Zhao, S.; Gao, Z.; Jiang, G.; Wang, J.; Miao, X.; Wan, A. Effect of the dyeing process on thermal and dyeing properties of poly(butylene terephthalate) fibers. Text. Res. J. 2021, 91, 580–588. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Dash, A.K. Sustainable polyester and caprolactam fibres. In Sustainable Fibres for Fashion and Textile Manufacturing; Elsevier: Amsterdam, The Netherlands, 2023; pp. 247–269. [Google Scholar] [CrossRef]
- Shukla, D.K.; Dey, A.; Singh, A.; Tripathi, S.N.; Bonda, S.; Saha, S.; Iyer, P.K.; Srivastava, V.K.; Jasra, R.V. Disentangled ultrahigh molecular weight polyethylene thin film as a transparent substrate for flexible flat panel display. J. Appl. Polym. Sci. 2022, 139, e52932. [Google Scholar] [CrossRef]
- Ding, Q.; Soccio, M.; Lotti, N.; Cavallo, D.; Androsch, R. Melt Crystallization of Poly(butylene 2,6-naphthalate). Chin. J. Polym. Sci. 2019, 38, 311–322. [Google Scholar] [CrossRef]
- de Albuquerque, T.L.; Júnior, J.E.M.; de Queiroz, L.P.; Ricardo, A.D.S.; Rocha, M.V.P. Polylactic acid production from biotechnological routes: A review. Int. J. Biol. Macromol. 2021, 186, 933–951. [Google Scholar] [CrossRef] [PubMed]
- Soleyman, E.; Aberoumand, M.; Rahmatabadi, D.; Soltanmohammadi, K.; Ghasemi, I.; Baniassadi, M.; Abrinia, K.; Baghani, M. Assessment of controllable shape transformation, potential applications, and tensile shape memory properties of 3D printed PETG. J. Mater. Res. Technol. 2022, 18, 4201–4215. [Google Scholar] [CrossRef]
- Vasanthan, N. Polyamide fiber formation: Structure, properties and characterization. In Handbook of Textile Fibre Structure; Elsevier: Amsterdam, The Netherlands, 2009; pp. 232–256. [Google Scholar] [CrossRef]
- Fan, W.; Wang, Y.; Liu, R.; Zou, J.; Yu, X.; Liu, Y.; Zhi, C.; Meng, J. Textile production by additive manufacturing and textile waste recycling: A review. Environ. Chem. Lett. 2024, 22, 1929–1987. [Google Scholar] [CrossRef]
- Vojdani, M.; Giti, R. Polyamide as a denture base material: A literature review. J. Dent. 2015, 16 (Suppl. S1), 1–9. Available online: https://pubmed.ncbi.nlm.nih.gov/26106628 (accessed on 19 February 2025).
- Shioya, M.; Kikutani, T. Chapter 7—Synthetic Textile Fibres: Non-polymer Fibres. In Textiles and Fashion; Sinclair, R., Ed.; Woodhead Publishing: Amsterdam, The Netherlands, 2015; pp. 139–155. [Google Scholar] [CrossRef]
- Polymer Science Learning Center. Is Inorganic Glass an Inorganic Polymer? Available online: https://pslc.ws/macrog/glass.htm (accessed on 1 January 2025).
- Chawla, K.K. Glass Fibers. In Encyclopedia of Materials: Science and Technology; Buschow, K.H.J., Cahn, R.W., Flemings, M.C., Ilschner, B., Kramer, E.J., Mahajan, S., Veyssière, P., Eds.; Elsevier: Oxford, UK, 2001; pp. 3541–3545. [Google Scholar] [CrossRef]
- Chaudhary, A.; Gupta, V.; Teotia, S.; Nimanpure, S.; Rajak, D.K. Electromagnetic Shielding Capabilities of Metal Matrix Composites. In Encyclopedia of Materials: Composites; Brabazon, D., Ed.; Elsevier: Oxford, UK, 2021; pp. 428–441. [Google Scholar] [CrossRef]
- Li, A.-j.; Xu, J.; Zhang, F.-z.; Song, Y.-h.; Wang, J.-h.; Ye, C.; Zhu, S.-p. A microstructure-based model for the thermal conductivity of carbon fibers. Mater. Sci. Eng. B 2024, 303, 117309. [Google Scholar] [CrossRef]
- Chennam, P.K.; Kachlík, M.; Říhová, M.; Čičmancová, V.; Maca, K.; Macak, J.M. Synthesis of centrifugally spun polyacrylonitrile-carbon fibers. J. Mater. Res. Technol. 2024, 28, 2199–2205. [Google Scholar] [CrossRef]
- Fazeli, M.; Islam, S.; Baniasadi, H.; Abidnejad, R.; Schlapp-Hackl, I.; Hummel, M.; Lipponen, J. Exploring the potential of regenerated Ioncell fiber composites: A sustainable alternative for high-strength applications. Green Chem. 2024, 26, 6822–6835. [Google Scholar] [CrossRef]
- Krithikaa, D.; Chandramohan, P.; Suresh, G.; Rathinasabapathi, G.; Madheswaran, D.K.; Faisal, A.M. A study on: Design and fabrication of E-glass fiber reinforced IPN composite chain plates for low duty chain drives. Mater. Today Proc. 2024. [Google Scholar] [CrossRef]
- Ilyas, R.; Zuhri, M.; Norrrahim, M.N.F.; Misenan, M.S.M.; Jenol, M.A.; Samsudin, S.A.; Nurazzi, N.; Asyraf, M.; Supian, A.; Bangar, S.P. Natural fiber-reinforced polycaprolactone green and hybrid biocomposites for various advanced applications. Polymers 2022, 14, 182. [Google Scholar] [CrossRef]
- Muthukumar, C.; Krishnasamy, S.; Thiagamani, S.M.K.; Nagarajan, R.; Siengchin, S. Thermal characterization of the natural fiber-based hybrid composites: An overview. In Natural Fiber-Reinforced Composites: Thermal Properties and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2022; pp. 1–15. [Google Scholar] [CrossRef]
- Kakati, A.; Banerjee, A.; Das, P.; Saha, B.; Goyary, D.; Karmakar, S.; Kishor, S.; Bhutia, Y.D.; Chattopadhyay, P. Development of insecticide-impregnated polyester/cotton blend fabric and assessment of their repellent characteristics against Cimex lectularius and dengue vectors Aedes albopictus and Aedes aegypti. Parasites Vectors 2023, 16, 122. [Google Scholar] [CrossRef]
- Li, Y.; Sun, L.; Wang, H.; Wang, S.; Jin, X.; Lu, Z.; Dong, C. A novel composite coating containing P/N/B and bio-based compounds for flame retardant modification of polyester/cotton blend fabrics. Colloids Surf. A Physicochem. Eng. Asp. 2023, 660, 130826. [Google Scholar] [CrossRef]
- Nesa, S.H.S.; Tarangini, K. A review on augmentation of natural fabric materials with novel bio/nanomaterials and their multifunctional perspectives. Hybrid Adv. 2023, 2, 100020. [Google Scholar] [CrossRef]
- Birkocak, D.T. Effects of Needle Size and Sewing Thread on Seam Quality of Traditional Fabrics. Text. Appar. 2022, 32, 277–287. [Google Scholar] [CrossRef]
- Wakida, T.; Tokuyama, T.; Doi, C.; Lee, M.; Jeong, D.S.; Ishida, S. Mechanical properties of polyester/cotton and polyester/rayon fabrics treated with ammonia-gas. Sen’i Gakkaishi 2004, 60, 34–37. [Google Scholar] [CrossRef][Green Version]
- İlhan, Ö. Effects of pre-and intermediate causticisation on pattern formation and fastness properties of three-and two-bath dyeings of woven polyester/cationic dyeable polyester/rayon fabrics. Text. Appar. 2013, 4, 369–373. Available online: https://dergipark.org.tr/en/download/article-file/220099 (accessed on 19 February 2025).
- Najar, S.S.; Amani, M.; Hasani, H. Analysis of blend irregularities and fiber migration index of wool/acrylic blended worsted yarns by using an image-analysis technique. J. Text. Inst. 2003, 94, 177–185. [Google Scholar] [CrossRef]
- Matayeva, A.; Madsen, A.S.; Biller, P. Evaluation of different fiber impurities on hydrothermal liquefaction of mixed textile waste. Resour. Conserv. Recycl. 2023, 190, 106833. [Google Scholar] [CrossRef]
- Koch, H.C.; Schmelzeisen, D.; Gries, T. 4D textiles made by additive manufacturing on pre-stressed textiles—An overview. Actuators 2021, 10, 31. [Google Scholar] [CrossRef]
- Panda, H. Modern Technology of Textile Dyes & Pigments; NIIR Project Consultancy Services: New Delhi, India, 2016; Available online: https://www.niir.org/books/book/modern-technology-textile-dyes-pigments-2nd-revised-edition/isbn-9789381039717/zb,,43,a,0,0,a/index.html (accessed on 19 February 2025).
- Özer, M.S.; Gaan, S. Recent developments in phosphorus based flame retardant coatings for textiles: Synthesis, applications and performance. Prog. Org. Coat. 2022, 171, 107027. [Google Scholar] [CrossRef]
- Gulati, R.; Sharma, S.; Sharma, R.K. Antimicrobial textile: Recent developments and functional perspective. Polym. Bull. 2022, 79, 5747–5771. [Google Scholar] [CrossRef]
- Sankaran, A.; Kamboj, A.; Samant, L.; Jose, S. Synthetic and natural UV protective agents for textile finishing. In Innovative and Emerging Technologies for Textile Dyeing and Finishing; John Wiley & Sons: Hoboken, NJ, USA, 2021; pp. 301–324. [Google Scholar] [CrossRef]
- Mahapatra, A.; Patil, S.; Arputharaj, A.; Gotmare, V.; Patil, P. Effect of textile softeners on BTCA treated cotton fabric. Indian J. Fibre Text. Res. 2020, 45, 96–101. [Google Scholar] [CrossRef]
- Haule, L.V.; Nambela, L. Advances in waterproof technologies in textiles. In Functional and Technical Textiles; Elsevier: Amsterdam, The Netherlands, 2023; pp. 275–291. [Google Scholar] [CrossRef]
- Almasry, S.; Rabea, H.; Hany, H.; Gerges, M.; Rafaat, Y.; Maamoun, D.; Mohamed, H.; Khattab, T.A. Eco-Friendly Multi-Finishing Properties of Polyester Fabrics. J. Text. Color. Polym. Sci. 2023, 20, 103–111. [Google Scholar] [CrossRef]
- Kahoush, M.; Kadi, N. Towards sustainable textile sector: Fractionation and separation of cotton/polyester fibers from blended textile waste. Sustain. Mater. Technol. 2022, 34, e00513. [Google Scholar] [CrossRef]
- Chen, Z.; Sun, H.; Kong, W.; Chen, L.; Zuo, W. Closed-loop utilization of polyester in the textile industry. Green Chem. 2023, 25, 4429–4437. [Google Scholar] [CrossRef]
- Stefan, D.S.; Bosomoiu, M.; Stefan, M. Methods for Natural and Synthetic Polymers Recovery from Textile Waste. Polymers 2022, 14, 3939. [Google Scholar] [CrossRef]
- Ruschel-Soares, R.; Contin, B.; Siqueira, M.U.; Fernandes, P.R.B.; Soares, N.R.; Baruque-Ramos, J. Environmental Impacts of Polyester-Cotton Blend Compared to Cotton Fiber in Brazil. Mater. Circ. Econ. 2022, 4, 13. [Google Scholar] [CrossRef]
- El Darai, T.; Ter-Halle, A.; Blanzat, M.; Despras, G.; Sartor, V.; Bordeau, G.; Lattes, A.; Franceschi, S.; Cassel, S.; Chouini-Lalanne, N.; et al. Chemical recycling of polyester textile wastes: Shifting towards sustainability. Green Chem. 2024, 26, 6857–6885. [Google Scholar] [CrossRef]
- Allen, E.; Henninger, C.E.; Garforth, A.; Asuquo, E. Microfiber Pollution: A Systematic Literature Review to Overcome the Complexities in Knit Design to Create Solutions for Knit Fabrics. Environ. Sci. Technol. 2024, 58, 4031–4045. [Google Scholar] [CrossRef]
- Franco Urquiza, E.A. Advances in Additive Manufacturing of Polymer-Fused Deposition Modeling on Textiles: From 3D Printing to Innovative 4D Printing—A Review. Polymers 2024, 16, 700. [Google Scholar] [CrossRef]
- Sawant, Y.; Admuthe, L. Characterization of needle-punched nonwoven fabric air filter using computer vision—A review. J. Text. Inst. 2024, 115, 151–158. [Google Scholar] [CrossRef]
- Barman, N.K.; Bhattacharya, S.S.; Alagirusamy, R. Textile structures in concrete reinforcement. Text. Prog. 2024, 56, 1–229. [Google Scholar] [CrossRef]
- Šajn Gorjanc, D.; Kostajnšek, K. Permeable Properties of Hygienic Nonwovens Bonded Using Mechanical, Chemical, and Thermal Techniques. Polymers 2024, 16, 1132. [Google Scholar] [CrossRef]
- Klemm, C.; Kaufman, S. The importance of circular attributes for consumer choice of fashion and textile products in Australia. Sustain. Prod. Consum. 2024, 45, 538–550. [Google Scholar] [CrossRef]
- Kilinc, M.; Korkmaz, G.; Kilinc, N.; Kut, D. Chapter 19—The use of wool fiber in technical textiles and recent developments. In The Wool Handbook; Jose, S., Thomas, S., Basu, G., Eds.; Woodhead Publishing: Amsterdam, The Netherlands, 2024; pp. 441–465. [Google Scholar] [CrossRef]
- Zhang, Q.; Cheng, H.; Zhang, S.; Li, Y.; Li, Z.; Ma, J.; Liu, X. Advancements and challenges in thermoregulating textiles: Smart clothing for enhanced personal thermal management. Chem. Eng. J. 2024, 488, 151040. [Google Scholar] [CrossRef]
- Maity, S.; Singha, K.; Pandit, P. 1—Introduction to functional and technical textiles. In Functional and Technical Textiles; Maity, S., Singha, K., Pandit, P., Eds.; Woodhead Publishing: Amsterdam, The Netherlands, 2023; pp. 1–30. [Google Scholar] [CrossRef]
- Panneerselvam, D.; Murugesan, P.; Moses, J.A. Silk fibroin and prospective applications in the food sector. Eur. Polym. J. 2024, 212, 113058. [Google Scholar] [CrossRef]
- Deutsches Institut für Normung. DIN EN ISO 5157, Textilien—Umweltaspekte—Begriffe (ISO 5157:2023): Textiles—Environmental Aspects—Vocabulary (ISO 5157:2023); Beuth Verlag GmbH: Berlin, Germany, 2023; Available online: https://books.google.be/books?id=9hOe0AEACAAJ (accessed on 19 February 2025).
- Dodampegama, S.; Hou, L.; Asadi, E.; Zhang, G.; Setunge, S. Revolutionizing construction and demolition waste sorting: Insights from artificial intelligence and robotic applications. Resour. Conserv. Recycl. 2024, 202, 107375. [Google Scholar] [CrossRef]
- ISO 472:2013; Plastics—Vocabulary. Kunststoffe–Fachwörterverzeichnis. ISO: Geneva, Switzerland, 2013. Available online: https://www.iso.org/obp/ui/#iso:std:iso:472:ed-4:v1:en (accessed on 19 February 2025).
- Sandin, G.; Peters, G.M. Environmental impact of textile reuse and recycling—A review. J. Clean. Prod. 2018, 184, 353–365. [Google Scholar] [CrossRef]
- Azad, A.K.; Haq, U.N.; Khairul Akter, M.M.; Uddin, M.A. Recycling Practices of Pre-Consumer Waste Generated from Textile Industry. In Sustainable Manufacturing Practices in the Textiles and Fashion Sector; Muthu, S.S., Ed.; Springer Nature: Cham, Switzerland, 2024; pp. 301–324. [Google Scholar] [CrossRef]
- ASTM-D5033; Standard Guide for Development of ASTM Standards Relating to Recycling and Use of Recycled Plastics. Document Center Inc.: Oakland, CA, USA, 2007. Available online: https://www.document-center.com/standards/show/ASTM-D5033 (accessed on 1 January 2025).
- Muthu, S.S.; Li, Y.; Hu, J.Y.; Ze, L. Carbon footprint reduction in the textile process chain: Recycling of textile materials. Fibers Polym. 2012, 13, 1065–1070. [Google Scholar] [CrossRef]
- Directive 2008/122/EC of the European parliament and of the council. In Fundamental Texts On European Private Law; Hart Publishing: Oxford, UK, 2020; pp. 3–30. [CrossRef]
- Stubbe, B.; Van Vrekhem, S.; Huysman, S.; Tilkin, R.G.; De Schrijver, I.; Vanneste, M. White Paper on Textile Fibre Recycling Technologies. Sustainability 2024, 16, 618. [Google Scholar] [CrossRef]
- Zamani, B.; Sandin, G.; Peters, G.M. Life cycle assessment of clothing libraries: Can collaborative consumption reduce the environmental impact of fast fashion? J. Clean. Prod. 2017, 162, 1368–1375. [Google Scholar] [CrossRef]
- Sood, K.; Gosselin, S.; Seifali Abbas-Abadi, M.; De Coensel, N.; Lizardo-Huerta, J.-C.; El Bakali, A.; Van Geem, K.M.; Gasnot, L.; Tran, L.-S. Experimental Detection of Oxygenated Aromatics in an Anisole-Blended Flame. Energy Fuels 2024, 38, 6355–6369. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Textiles: Material-Specific Data. Available online: https://www.epa.gov/facts-and-figures-about-materials-waste-and-recycling/textiles-material-specific-data (accessed on 1 January 2025).
- Baskan-Bayrak, H.; Karakas, H. Chapter 8—Morphology and chemical structure of a wool fiber. In The Wool Handbook; Jose, S., Thomas, S., Basu, G., Eds.; Woodhead Publishing: Amsterdam, The Netherlands, 2024; pp. 181–194. [Google Scholar] [CrossRef]
- Fazalur, R.; Fatima, A.; Adeel, S.; Qayyum, M.A.; Tanveer, H.A. Biosynthesis Application and Modification of Protein Fiber. In Biopolymers in the Textile Industry: Opportunities and Limitations; Ahmed, S., Shabbir, M., Eds.; Springer Nature: Singapore, 2024; pp. 273–313. [Google Scholar] [CrossRef]
- Kadam, V.; Saini, H.; Verma, K.; Dubey, I.; Verma, P. Chapter 26—Prospects of wool and woolen products. In The Wool Handbook; Jose, S., Thomas, S., Basu, G., Eds.; Woodhead Publishing: Amsterdam, The Netherlands, 2024; pp. 593–610. [Google Scholar] [CrossRef]
- Hole, G.; Hole, A.S. Improving recycling of textiles based on lessons from policies for other recyclable materials: A minireview. Sustain. Prod. Consum. 2020, 23, 42–51. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Ding, X. Textile supply chain waste management in China. J. Clean. Prod. 2021, 289, 125147. [Google Scholar] [CrossRef]
- Cura, K.; Rintala, N.; Kamppuri, T.; Saarimäki, E.; Heikkilä, P. Textile Recognition and Sorting for Recycling at an Automated Line Using Near Infrared Spectroscopy. Recycling 2021, 6, 11. [Google Scholar] [CrossRef]
- Karell, E.; Niinimäki, K. Addressing the dialogue between design, sorting and recycling in a circular economy. Des. J. 2019, 22, 997–1013. [Google Scholar] [CrossRef]
- Jordeva, S.; Tomovska, E.; Zhezhova, S.; Golomeova, S.; Dimitrijeva, V. Textile waste management practices. Contemporary trends and innovations in the textile industry CT&ITI 2020. Mech. Eng. 2020. Available online: https://eprints.ugd.edu.mk/id/eprint/26677 (accessed on 19 February 2025).
- Tang, K.H.D. State of the Art in Textile Waste Management: A Review. Textiles 2023, 3, 454–467. [Google Scholar] [CrossRef]
- Chavan, R. Environmental sustainability through textile recycling. J. Text. Sci. Eng. 2014, 2. Available online: https://api.semanticscholar.org/CorpusID:54720162 (accessed on 19 February 2025).
- Yalcin-Enis, I.; Kucukali-Ozturk, M.; Sezgin, H. Risks and Management of Textile Waste. In Nanoscience and Biotechnology for Environmental Applications; Gothandam, K.M., Ranjan, S., Dasgupta, N., Lichtfouse, E., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 29–53. [Google Scholar] [CrossRef]
- Duhoux, T.; Lingås, D. Volumes and destruction of returned and unsold textiles in Europe’s circular economy. ETC CE Rep. 2024. [Google Scholar] [CrossRef]
- Puglia, M.; Parker, L.; Clube, R.K.M.; Demirel, P.; Aurisicchio, M. The circular policy canvas: Mapping the European Union’s policies for a sustainable fashion textiles industry. Resour. Conserv. Recycl. 2024, 204, 107459. [Google Scholar] [CrossRef]
- Charnley, F.; Cherrington, R.; Mueller, F.; Jain, A.; Nelson, C.; Wendland, S.; Ventosa, S. Retaining product value in post-consumer textiles: How to scale a closed-loop system. Resour. Conserv. Recycl. 2024, 205, 107542. [Google Scholar] [CrossRef]
- Büyükaslan, E.; Jevšnık, S.; Kalaoglu, F. A Sustainable Approach to Collect Post-Consumer Textile Waste in Developing Countries. Marmara Fen Bilim. Derg. 2015, 27, 107–111. [Google Scholar] [CrossRef]
- Palm, D.; Elander, M.; Watson, D.; Kiørboe, N.; Salmenperä, H.; Dahlbo, H.; Moliis, K.; Lyng, K.A.; Valente, C.; Gíslason, S. Towards a Nordic Textile Strategy: Collection, Sorting, Reuse and Recycling of Textiles; Nordic Council of Ministers: Copenhagen, Denmark, 2014; Available online: https://books.google.be/books?id=H6TEAwAAQBAJ (accessed on 19 February 2025).
- Wang, Y. Fiber and Textile Waste Utilization. Waste Biomass Valorization 2010, 1, 135–143. [Google Scholar] [CrossRef]
- Manglani, H.; Hodge, G.L.; Oxenham, W. Application of the Internet of Things in the textile industry. Text. Prog. 2019, 51, 225–297. [Google Scholar] [CrossRef]
- Hack-Polay, D.; Rahman, M.; Billah, M.M.; Al-Sabbahy, H.Z. Big data analytics and sustainable textile manufacturing. Manag. Decis. 2020, 58, 1699–1714. [Google Scholar] [CrossRef]
- Wojnowska-Baryła, I.; Bernat, K.; Zaborowska, M.; Kulikowska, D. The Growing Problem of Textile Waste Generation—The Current State of Textile Waste Management. Energies 2024, 17, 1528. [Google Scholar] [CrossRef]
- Dursun, E.; Ulker, Y.; Gunalay, Y. Blockchain’s potential for waste management in textile industry. Manag. Environ. Qual. Int. J. 2023, 34, 1174–1197. [Google Scholar] [CrossRef]
- Martikkala, A.; Mayanti, B.; Helo, P.; Lobov, A.; Ituarte, I.F. Smart textile waste collection system—Dynamic route optimization with IoT. J. Environ. Manag. 2023, 335, 117548. [Google Scholar] [CrossRef]
- Lingås, D.; Manshoven, S.; Mortensen, L. EU exports of used textiles in Europe’s circular economy. ETC CE Rep. 2023. [Google Scholar] [CrossRef]
- Hicks, C.; Dietmar, R.; Eugster, M. The recycling and disposal of electrical and electronic waste in China—Legislative and market responses. Environ. Impact Assess. Rev. 2005, 25, 459–471. [Google Scholar] [CrossRef]
- Abagnato, S.; Rigamonti, L.; Grosso, M. Life cycle assessment applications to reuse, recycling and circular practices for textiles: A review. Waste Manag. 2024, 182, 74–90. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Hao, H.; Lu, H.; Chow, C.L.; Lau, D. Exploring the development and applications of sustainable natural fiber composites: A review from a nanoscale perspective. Compos. Part B Eng. 2024, 276, 111369. [Google Scholar] [CrossRef]
- Shu, D.; Li, W.; Han, B.; An, F.; Zhang, Y.; Cao, S.; Liu, R. Cleaner reactive dyeing with the recycled dyeing wastewater. J. Environ. Chem. Eng. 2024, 12, 113069. [Google Scholar] [CrossRef]
- Akter, M.; Anik, H.R.; Mahmud, S. Conversion of Textile Waste to Wealth and Their Industrial Utilization. In From Waste to Wealth; Arya, R.K., Verros, G.D., Verma, O.P., Hussain, C.M., Eds.; Springer Nature: Singapore, 2024; pp. 669–738. [Google Scholar] [CrossRef]
- Zhou, C.; Han, G.; Via, B.K.; Song, Y.; Gao, S.; Jiang, W. Rapid identification of fibers from different waste fabrics using the near-infrared spectroscopy technique. Text. Res. J. 2019, 89, 3610–3616. [Google Scholar] [CrossRef]
- Pettersson, A. Towards Recycling of Textile Fibers: Separation and Characterization of Textile Fibers and Blends; Chalmers University of Technology: Gothenburg, Sweden, 2015; Available online: https://api.semanticscholar.org/CorpusID:55263503 (accessed on 19 February 2025).
- Bianchi, S.; Bartoli, F.; Bruni, C.; Fernandez-Avila, C.; Rodriguez-Turienzo, L.; Mellado-Carretero, J.; Spinelli, D.; Coltelli, M.-B. Opportunities and Limitations in Recycling Fossil Polymers from Textiles. Macromol 2023, 3, 120–148. [Google Scholar] [CrossRef]
- Iezzi, B.; Coon, A.; Cantley, L.; Perkins, B.; Doran, E.; Wang, T.; Rothschild, M.; Shtein, M. Polymeric Photonic Crystal Fibers for Textile Tracing and Sorting. Adv. Mater. Technol. 2023, 8, 2201099. [Google Scholar] [CrossRef]
- Zhou, J.; Yu, L.; Ding, Q.; Wang, R. Textile Fiber Identification Using Near-Infrared Spectroscopy and Pattern Recognition. Autex Res. J. 2019, 19, 201–209. [Google Scholar] [CrossRef]
- Suciyati, S.W.; Manurung, P.; Sembiring, S.; Situmeang, R. Comparative study of Cladophora sp. cellulose by using FTIR and XRD. J. Phys. Conf. Ser. 2021, 1751, 012075. [Google Scholar] [CrossRef]
- Singh, R.K.; Ruj, B.; Sadhukhan, A.K.; Gupta, P. A TG-FTIR investigation on the co-pyrolysis of the waste HDPE, PP, PS and PET under high heating conditions. J. Energy Inst. 2020, 93, 1020–1035. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Zhang, S.; Gao, Q.; Lu, Q.; Peng, R.; Xu, P.; Shang, H.; Yuan, Y.; Zou, H. Micro-FTIR combined with curve fitting method to study cellulose crystallinity of developing cotton fibers. Anal. Bioanal. Chem. 2021, 413, 1313–1320. [Google Scholar] [CrossRef]
- Peets, P.; Kaupmees, K.; Vahur, S.; Leito, I. Reflectance FT-IR spectroscopy as a viable option for textile fiber identification. Herit. Sci. 2019, 7, 93. [Google Scholar] [CrossRef]
- Peets, P.; Leito, I.; Pelt, J.; Vahur, S. Identification and classification of textile fibres using ATR-FT-IR spectroscopy with chemometric methods. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 173, 175–181. [Google Scholar] [CrossRef]
- Blanch-Perez-del-Notario, C.; Saeys, W.; Lambrechts, A. Hyperspectral imaging for textile sorting in the visible–near infrared range. J. Spectr. Imaging 2019, 8, a17. [Google Scholar] [CrossRef]
- Alpert, C.; Turkowski, M.; Tasneem, T. Scalability Solutions for Automated Textile Sorting: A Case Study on How Dynamic Capabilities can Overcome Scalability Challenges; The Swedish School of Textiles—University of Borås: Borås, Sweden, 2021; Available online: https://www.diva-portal.org/smash/get/diva2:1593417/FULLTEXT01.pdf (accessed on 19 February 2025).
- Chen, H.; Tan, C.; Lin, Z. Quantitative determination of the fiber components in textiles by near-infrared spectroscopy and extreme learning machine. Anal. Lett. 2020, 53, 844–857. [Google Scholar] [CrossRef]
- Liu, Z.; Li, W.; Wei, Z. Qualitative classification of waste textiles based on near infrared spectroscopy and the convolutional network. Text. Res. J. 2019, 90, 1057–1066. [Google Scholar] [CrossRef]
- Mäkelä, M.; Geladi, P. Hyperspectral near infrared imaging quantifies the heterogeneity of carbon materials. Sci. Rep. 2018, 8, 10442. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, C. mRMR-based feature selection for classification of cotton foreign matter using hyperspectral imaging. Comput. Electron. Agric. 2015, 119, 191–200. [Google Scholar] [CrossRef]
- Du, W.; Zheng, J.; Li, W.; Liu, Z.; Wang, H.; Han, X. Efficient Recognition and Automatic Sorting Technology of Waste Textiles Based on Online Near infrared Spectroscopy and Convolutional Neural Network. Resour. Conserv. Recycl. 2022, 180, 106157. [Google Scholar] [CrossRef]
- Li, X.; Xu, X.; Liu, Z. Cryogenic grinding performance of scrap tire rubber by devulcanization treatment with ScCO2. Powder Technol. 2020, 374, 609–617. [Google Scholar] [CrossRef]
- Wang, Z.; Jiang, Y.; Pan, C. Mechanochemical devulcanization of waste tire rubber in high pressure water jet pulverization. Prog. Rubber Plast. Recycl. Technol. 2021, 37, 279–300. [Google Scholar] [CrossRef]
- Wang, Z.; Zeng, D. Preparation of devulcanized ground tire rubber with supercritical carbon dioxide jet pulverization. Mater. Lett. 2021, 282, 128878. [Google Scholar] [CrossRef]
- Abbas-Abadi, M.S.; Kusenberg, M.; Shirazi, H.M.; Goshayeshi, B.; Van Geem, K.M. Towards full recyclability of end-of-life tires: Challenges and opportunities. J. Clean. Prod. 2022, 374, 134036. [Google Scholar] [CrossRef]
- Grammelis, P.; Margaritis, N.; Dallas, P.; Rakopoulos, D.; Mavrias, G. A Review on Management of End of Life Tires (ELTs) and Alternative Uses of Textile Fibers. Energies 2021, 14, 571. [Google Scholar] [CrossRef]
- Aljannahi, A.; Alblooshi, R.A.; Alremeithi, R.H.; Karamitsos, I.; Ahli, N.A.; Askar, A.M.; Albastaki, I.M.; Ahli, M.M.; Modak, S. Forensic Analysis of Textile Synthetic Fibers Using a FT-IR Spectroscopy Approach. Molecules 2022, 27, 4281. [Google Scholar] [CrossRef]
- Rasheed, A. Classification of Technical Textiles. In Fibers for Technical Textiles; Ahmad, S., Rasheed, A., Nawab, Y., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 49–64. [Google Scholar] [CrossRef]
- Bartl, A. Chapter 16—End-of-Life Textiles. In Waste, 2nd ed.; Letcher, T.M., Vallero, D.A., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 323–336. [Google Scholar] [CrossRef]
- Rao, N.; Salvidge, C.; Doriza, A.; Downing, P. Citizen Insights: Estimating the Longevity of Home Textiles in the UK; WRAP: Oxford, UK, 2022; Available online: https://www.wrap.ngo/resources/report/citizen-insights-estimating-longevity-home-textiles-uk (accessed on 19 February 2025).
- Bukhari, M.A.; Carrasco-Gallego, R.; Ponce-Cueto, E. Developing a national programme for textiles and clothing recovery. Waste Manag. Res. 2018, 36, 321–331. [Google Scholar] [CrossRef]
- Paras, M.K.; Ekwall, D.; Pal, R.; Curteza, A.; Chen, Y.; Wang, L. An Exploratory Study of Swedish Charities to Develop a Model for the Reuse-Based Clothing Value Chain. Sustainability 2018, 10, 1176. [Google Scholar] [CrossRef]
- Palm, D.; Elander, M.; Watson, D.; Kiørboe, N.; Salmenperä, H.; Dahlbo, H.; Rubach, S.; Hanssen, O.J.; Gíslason, S.; Ingulfsvann, A.S. A Nordic Textile Strategy: Part II: A Proposal for Increased Collection, Sorting, Reuse and Recycling of Textiles; Nordic Council of Ministers: Copenhagen, Denmark, 2015; Available online: https://books.google.be/books?id=-vveBgAAQBAJ (accessed on 19 February 2025).
- Ivana, K. Waste Framework Directive: A More Sustainable Use of Natural Resources. EPRS: European Parliamentary Research Service. 2023. Available online: https://policycommons.net/artifacts/10880460/waste-framework-directive/11758402/ (accessed on 19 February 2025).
- Hardy, D.; Wickenden, R.; McLaren, A. Electronic textile reparability. J. Clean. Prod. 2020, 276, 124328. [Google Scholar] [CrossRef]
- All-Party Parliamentary Sustainable Resource Group. Remanufacturing: Towards a Resource Efficient Economy. 2014. Available online: https://www.policyconnect.org.uk/research/report-remanufacturing-towards-resource-efficient-economy (accessed on 19 February 2025).
- Lund, R.T. Remanufacturing: The Experience of the United States and Implications for Developing Countries; World Bank: Chicago, IL, USA, 1984; Available online: https://books.google.be/books?id=xPEeAQAAIAAJ (accessed on 19 February 2025).
- Sinha, P.; Muthu, S.S.; Dissanayake, G. The Remanufacturing Industry and Fashion. In Remanufactured Fashion; Sinha, P., Muthu, S.S., Dissanayake, G., Eds.; Springer: Singapore, 2016; pp. 1–9. [Google Scholar] [CrossRef]
- Stanescu, M.D. State of the art of post-consumer textile waste upcycling to reach the zero waste milestone. Environ. Sci. Pollut. Res. 2021, 28, 14253–14270. [Google Scholar] [CrossRef]
- Ghosh, B. Climate Change and the Female Sex: An Intangible Connection. In Gender, Environment and Sustainable Development; Routledge: Delhi, India, 2024; pp. 197–209. [Google Scholar] [CrossRef]
- Shirvanimoghaddam, K.; Motamed, B.; Ramakrishna, S.; Naebe, M. Death by waste: Fashion and textile circular economy case. Sci. Total Environ. 2020, 718, 137317. [Google Scholar] [CrossRef]
- Biermaier, C.; Petz, P.; Bechtold, T.; Pham, T. Investigation of the Functional Ageing of Conductive Coated Fabrics under Simulated Washing Conditions. Materials 2023, 16, 912. [Google Scholar] [CrossRef]
- Bresee, R.R. General effects of ageing on textiles. J. Am. Inst. Conserv. 1986, 25, 39–48. [Google Scholar] [CrossRef]
- Hawkins, W.L. Polymer Degradation and Stabilization; Springer: Berlin/Heidelberg, Germany, 1984; Available online: https://books.google.be/books?id=mOKdAQAACAAJ (accessed on 19 February 2025).
- Castellani, V.; Sala, S.; Mirabella, N. Beyond the throwaway society: A life cycle-based assessment of the environmental benefit of reuse. Integr. Environ. Assess. Manag. 2015, 11, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Commission, E.; Directorate-General for Internal Market, Industry, Entrepreneurship and SMEs; Duhoux, T.; Maes, E.; Hirschnitz-Garbers, M.; Peeters, K.; Asscherickx, L.; Christis, M.; Stubbe, B. Study on the Technical, Regulatory, Economic and Environmental Effectiveness of Textile Fibres Recycling—Final Report; Publications Office: Luxembourg, 2021; Available online: https://data.europa.eu/doi/10.2873/828412 (accessed on 19 February 2025).
- Terra Technical Monitoring of Optical Sorting, Recognition and Disassembly Technologies for Textiles at European Scale. Re-fashion. 2023. Available online: https://refashion.fr/pro/sites/default/files/rapport-etude/240428_Synth%C3%A8se_Veille-technos-tri-d%C3%A9lissage_VF-EN.pdf (accessed on 19 February 2025).
- Record, A.; Harscoet, E.; Chouvenc, S. Chemical and physico-chemical recycling of plastic waste. Tech. L’ingenieur 2022. [Google Scholar] [CrossRef]
- Egan, J.; Wang, S.; Shen, J.; Baars, O.; Moxley, G.; Salmon, S. Enzymatic textile fiber separation for sustainable waste processing. Resour. Environ. Sustain. 2023, 13, 100118. [Google Scholar] [CrossRef]
- Haule, L.V. Textile Recycling: A Review. 2016. Available online: https://api.semanticscholar.org/CorpusID:113949423 (accessed on 19 February 2025).
- Malik, R.K.; Goswami, K.K. 14—Processing and finishing in carpet. In Advances in Carpet Manufacture, 2nd ed.; Goswami, K.K., Ed.; Woodhead Publishing: Amsterdam, The Netherlands, 2018; pp. 387–418. [Google Scholar] [CrossRef]
- Maione, D. Recrafting Futures: Post-Material Transformations Toward Clothing Longevity; Carnegie Mellon University: Pittsburgh, PA, USA, 2023; Available online: https://www.proquest.com/openview/079668ee127d40c4533dd5989a8b48b0/1?pq-origsite=gscholar&cbl=18750&diss=y (accessed on 19 February 2025).
- Ecole Nationale Supérieure des Arts et Industries Textiles. Étude des Perturbateurs et Facilitateurs au Recyclage des Textiles et Linges de Maison. Refashion. 2014. Available online: https://refashion.fr/eco-design/sites/default/files/fichiers/%C3%89tude%20des%20perturbateurs%20et%20facilitateurs%20au%20recyclage%20des%20textiles%20et%20linges%20de%20maison.pdf (accessed on 19 February 2025).
- Valvan. Textile Sorting Solutions for the Recycling Industry. Available online: https://www.valvan.com/en/solutions/textile-sorting-recycling (accessed on 1 January 2025).
- CETIA. Innovation Platform Dedicated to the Recyclability of Textile and Leather Articles. Available online: https://cetia.tech/home-en/ (accessed on 1 January 2025).
- Schuch, A. The chemical recycle of cotton. Rev. Produção E Desenvolv. 2016, 2, 64–76. [Google Scholar] [CrossRef]
- Sharma, S.; Kaur, A. Various methods for removal of dyes from industrial effluents—A review. Indian J. Sci. Technol. 2018, 11, 1–21. [Google Scholar] [CrossRef]
- Verma, A.K.; Dash, R.R.; Bhunia, P. A review on chemical coagulation/flocculation technologies for removal of colour from textile wastewaters. J. Environ. Manag. 2012, 93, 154–168. [Google Scholar] [CrossRef]
- Hossain, M.S.; Shenashen, M.A.; Awual, M.E.; Rehan, A.I.; Rasee, A.I.; Waliullah, R.M.; Kubra, K.T.; Salman, M.S.; Sheikh, M.C.; Hasan, M.N.; et al. Benign separation, adsorption, and recovery of rare-earth Yb(III) ions with specific ligand-based composite adsorbent. Process Saf. Environ. Prot. 2024, 185, 367–374. [Google Scholar] [CrossRef]
- Awual, M.E.; Salman, M.S.; Hasan, M.M.; Hasan, M.N.; Kubra, K.T.; Sheikh, M.C.; Rasee, A.I.; Rehan, A.I.; Waliullah, R.M.; Hossain, M.S.; et al. Ligand imprinted composite adsorbent for effective Ni(II) ion monitoring and removal from contaminated water. J. Ind. Eng. Chem. 2024, 131, 585–592. [Google Scholar] [CrossRef]
- Awual, M.R. Innovative composite material for efficient and highly selective Pb(II) ion capturing from wastewater. J. Mol. Liq. 2019, 284, 502–510. [Google Scholar] [CrossRef]
- Rasee, A.I.; Awual, E.; Rehan, A.I.; Hossain, M.S.; Waliullah, R.M.; Kubra, K.T.; Sheikh, M.C.; Salman, M.S.; Hasan, M.N.; Hasan, M.M.; et al. Efficient separation, adsorption, and recovery of Samarium(III) ions using novel ligand-based composite adsorbent. Surf. Interfaces 2023, 41, 103276. [Google Scholar] [CrossRef]
- Yousef, S.; Tatariants, M.; Tichonovas, M.; Kliucininkas, L.; Lukošiūtė, S.-I.; Yan, L. Sustainable green technology for recovery of cotton fibers and polyester from textile waste. J. Clean. Prod. 2020, 254, 120078. [Google Scholar] [CrossRef]
- Slama, N.E.H.; Masmoudi, G.; Fizer, M.; Mariychuk, R.; Dhaouadi, H. Comprehensive study of Fenton reaction efficiency on textile wastewater treatment from dye solution to real effluent with emphasis on Fukui function analysis. J. Mol. Liq. 2024, 402, 124773. [Google Scholar] [CrossRef]
- Liu, X.; Wang, J. Decolorization and degradation of crystal violet dye by electron beam radiation: Performance, degradation pathways, and synergetic effect with peroxymonosulfate. Environ. Pollut. 2024, 350, 124037. [Google Scholar] [CrossRef]
- Powar, A. LCA and Eco-Design in the Field of Chemicals Removal from Textile Waste for Textile Recycling; Université de Lille: Lill, France; Universitatea Tehnică” Gheorghe Asachi”: Iaşi, Romania, 2021; Available online: https://theses.hal.science/tel-04047038 (accessed on 19 February 2025).
- Määttänen, M.; Gunnarsson, M.; Wedin, H.; Stibing, S.; Olsson, C.; Köhnke, T.; Asikainen, S.; Vehviläinen, M.; Harlin, A. Pre-treatments of pre-consumer cotton-based textile waste for production of textile fibres in the cold NaOH(aq) and cellulose carbamate processes. Cellulose 2021, 28, 3869–3886. [Google Scholar] [CrossRef]
- Baloyi, R.B.; Gbadeyan, O.J.; Sithole, B.; Chunilall, V. Recent advances in recycling technologies for waste textile fabrics: A review. Text. Res. J. 2024, 94, 508–529. [Google Scholar] [CrossRef]
- Le, K. Textile Recycling Technologies, Colouring and Finishing Methods. UBC Sustainability Scholars Report. 2018. Available online: https://sustain.ubc.ca/about/resources/textile-recycling-technologies-colouring-and-finishing-methods (accessed on 19 February 2025).
- Schmidt, C.; Berghahn, E.; Ilha, V.; Granada, C.E. Biodegradation potential of Citrobacter cultures for the removal of amaranth and congo red azo dyes. Int. J. Environ. Sci. Technol. 2019, 16, 6863–6872. [Google Scholar] [CrossRef]
- Haslinger, S.; Wang, Y.; Rissanen, M.; Lossa, M.B.; Tanttu, M.; Ilen, E.; Määttänen, M.; Harlin, A.; Hummel, M.; Sixta, H. Recycling of vat and reactive dyed textile waste to new colored man-made cellulose fibers. Green Chem. 2019, 21, 5598–5610. [Google Scholar] [CrossRef]
- Björquist, S. Separation for Regeneration-Chemical Recycling of Cotton and Polyester Textiles; The Swedish School of Textiles—University of Borås: Borås, Sweden, 2017; Available online: https://hb.diva-portal.org/smash/record.jsf?pid=diva2%3A1121304 (accessed on 19 February 2025).
- Gholamzad, E.; Karimi, K.; Masoomi, M. Effective conversion of waste polyester–cotton textile to ethanol and recovery of polyester by alkaline pretreatment. Chem. Eng. J. 2014, 253, 40–45. [Google Scholar] [CrossRef]
- Haule, L.V.; Carr, C.M.; Rigout, M. Investigation into the removal of an easy-care crosslinking agent from cotton and the subsequent regeneration of lyocell-type fibres. Cellulose 2014, 21, 2147–2156. [Google Scholar] [CrossRef]
- Rescoll. INDAR Debonding Process: Structural Debondable Adhesive Used for Ground Testing of GAIA Segments. Available online: https://rescoll.fr/indar-debonding-process-structural-debondable-adhesive-used-for-ground-testing-of-gaia-segments__trashed/ (accessed on 1 January 2025).
- Olive, M.; Bergara, T.; Di-Tomaso, J.; Plouraboue, T. Latest Achievements in the Field of Assembling Metals and Composites. Available online: https://rescoll.fr/wp-content/uploads/2015/12/MMP2015-28-paper.x66374.pdf (accessed on 19 February 2025).
- Collins, D.M. Separating Polymer from Composite Structures. Available online: https://patents.google.com/patent/WO2018035565A1/en (accessed on 1 January 2025).
- Ball, D.L.; Hance, M.H. Process for Recycling Denim Waste. Available online: https://patents.google.com/patent/US5369861A/en (accessed on 1 January 2025).
- Sheikh, M.C.; Hasan, M.M.; Hasan, M.N.; Salman, M.S.; Kubra, K.T.; Awual, M.E.; Waliullah, R.M.; Rasee, A.I.; Rehan, A.I.; Hossain, M.S.; et al. Toxic cadmium(II) monitoring and removal from aqueous solution using ligand-based facial composite adsorbent. J. Mol. Liq. 2023, 389, 122854. [Google Scholar] [CrossRef]
- Yurtaslan, Ö.; Altun Kurtoğlu, Ş.; Yılmaz, D. Closed-loop Mechanical Recycling Opportunities in Industrial Cotton Wastes. J. Nat. Fibers 2022, 19, 11802–11817. [Google Scholar] [CrossRef]
- Panda, S.K.B.C.; Sen, K.; Mukhopadhyay, S. Sustainable pretreatments in textile wet processing. J. Clean. Prod. 2021, 329, 129725. [Google Scholar] [CrossRef]
- Piribauer, B.; Bartl, A. Textile recycling processes, state of the art and current developments: A mini review. Waste Manag. Res. 2019, 37, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S.; Raja, A.; Arputharaj, A. Challenges in sustainable wet processing of textiles. In Textiles and Clothing Sustainability: Sustainable Textile Chemical Processes; Spring: Berlin/Heidelberg, Germany, 2017; pp. 43–79. [Google Scholar] [CrossRef]
- Allen, T.W. Garnett Machine. Available online: https://patents.google.com/patent/US1630158A/en (accessed on 1 January 2025).
- Lambert, M. ’Cast-off Wearing Apparell’: The consumption and distribution of second-hand clothing in northern England during the long eighteenth century. Text. Hist. 2004, 35, 1–26. [Google Scholar] [CrossRef]
- Pensupa, N.; Leu, S.-Y.; Hu, Y.; Du, C.; Liu, H.; Jing, H.; Wang, H.; Lin, C.S.K. Recent Trends in Sustainable Textile Waste Recycling Methods: Current Situation and Future Prospects. Top. Curr. Chem. 2017, 375, 76. [Google Scholar] [CrossRef]
- Wang, Y. Recycling in Textiles; Elsevier Science: Amsterdam, The Netherlands, 2006; Available online: https://books.google.be/books?id=4uVRAwAAQBAJ (accessed on 19 February 2025).
- Gulich, B. 9—Development of products made of reclaimed fibres. In Recycling in Textiles; Woodhead Publishing: Amsterdam, The Netherlands, 2006. [Google Scholar] [CrossRef]
- Mowafi, S.; Mashaly, H.; El-Sayed, H. Towards water-saving textile wet processing. Part 1: Scouring and dyeing. Egypt. J. Chem. 2020, 63, 3343–3353. [Google Scholar] [CrossRef]
- Lindström, K.; Sjöblom, T.; Persson, A.; Kadi, N. Improving Mechanical Textile Recycling by Lubricant Pre-Treatment to Mitigate Length Loss of Fibers. Sustainability 2020, 12, 8706. [Google Scholar] [CrossRef]
- Payne, A. 6—Open- and closed-loop recycling of textile and apparel products. In Handbook of Life Cycle Assessment (LCA) of Textiles and Clothing; Muthu, S.S., Ed.; Woodhead Publishing: Amsterdam, The Netherlands, 2015; pp. 103–123. [Google Scholar] [CrossRef]
- Madhav, S.; Ahamad, A.; Singh, P.; Mishra, P.K. A review of textile industry: Wet processing, environmental impacts, and effluent treatment methods. Environ. Qual. Manag. 2018, 27, 31–41. [Google Scholar] [CrossRef]
- Khan, W.S.; Asmatulu, E.; Uddin, M.N.; Asmatulu, R. Recycling and Reusing of Engineering Materials: Recycling for Sustainable Developments; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar] [CrossRef]
- Langley, K.D.; Kim, Y.K. Manufacturing nonwovens and other products using recycled fibers containing spandex. In Recycling in Textiles; Woodhead Publishing: Amsterdam, The Netherlands, 2006; pp. 137–164. [Google Scholar] [CrossRef]
- Textile Exchange. Textile Exchange Guide to Recycled Inputs. 2021. Available online: https://textileexchange.org/app/uploads/2021/09/GRS-202-V1.0-Textile-Exchange-Guide-to-Recycled-Inputs.pdf (accessed on 19 February 2025).
- Dissanayake, D.G.K.; Weerasinghe, D.U. Fabric Waste Recycling: A Systematic Review of Methods, Applications, and Challenges. Mater. Circ. Econ. 2021, 3, 24. [Google Scholar] [CrossRef]
- Tshifularo, C.A.; Patnaik, A. 13—Recycling of plastics into textile raw materials and products. In Sustainable Technologies for Fashion and Textiles; Nayak, R., Ed.; Woodhead Publishing: Amsterdam, The Netherlands, 2020; pp. 311–326. [Google Scholar] [CrossRef]
- Cao, H.; Cobb, K.; Yatvitskiy, M.; Wolfe, M.; Shen, H. Textile and Product Development from End-of-Use Cotton Apparel: A Study to Reclaim Value from Waste. Sustainability 2022, 14, 8553. [Google Scholar] [CrossRef]
- Esteve-Turrillas, F.A.; de la Guardia, M. Environmental impact of Recover cotton in textile industry. Resour. Conserv. Recycl. 2017, 116, 107–115. [Google Scholar] [CrossRef]
- Aronsson, J.; Persson, A. Tearing of post-consumer cotton T-shirts and jeans of varying degree of wear. J. Eng. Fibers Fabr. 2020, 15, 1558925020901322. [Google Scholar] [CrossRef]
- De Smit, K.; Wieme, T.; Marien, Y.W.; Van Steenberge, P.H.M.; D’Hooge, D.R.; Edeleva, M. Multi-scale reactive extrusion modelling approaches to design polymer synthesis, modification and mechanical recycling. React. Chem. Eng. 2022, 7, 245–263. [Google Scholar] [CrossRef]
- Ragaert, K.; Delva, L.; Van Geem, K. Mechanical and chemical recycling of solid plastic waste. Waste Manag. 2017, 69, 24–58. [Google Scholar] [CrossRef]
- Ogutgen, M.K.; Vatansevdi, M.K.; Ozturk, B.; Caliskan Akduman, M. Plastic Recycling in a R&d Office: Recycling of Plastic Waste as Granules and Fibre. In Proceedings of the 11th Global Conference on Global Warming (GCGW 2023), İstanbul, Turkey, 14–16 June 2023. [Google Scholar] [CrossRef]
- Kunchi Mon, S.Z.B. Polyamide 6 Fibre Recycling by Twin-Screw Melt Extrusion of Mixed Thermoplastic Polymers; University of Leeds: Leeds, UK, 2019; Available online: https://etheses.whiterose.ac.uk/26494/ (accessed on 19 February 2025).
- Ozmen, S.C.; Ozkoc, G.; Serhatli, I.E. Effect of reactive extrusion process parameters on thermal, mechanical, and physical properties of recycled polyamide-6: Comparison of two novel chain extenders. J. Macromol. Sci. Part B 2021, 60, 350–367. [Google Scholar] [CrossRef]
- Luiken, A.; Brinks, G.; BMA-Techne; Almelo. Reflow Amsterdam Circular Textiles Pilot-A Primer on Textile Recycling. Available online: https://reflowproject.eu/wp-content/uploads/2021/05/REFLOW_BOOKLET_TEXTILE_WHEEL-compressed.pdf (accessed on 19 February 2025).
- Altun, S.; Ulcay, Y. Improvement of Waste Recycling in PET Fiber Production. J. Polym. Environ. 2004, 12, 231–237. [Google Scholar] [CrossRef]
- Qin, Y.; Qu, M.; Kaschta, J.; Allen, V.; Schubert, D.W. Studies on recycled polyester. In Recycled Polyester: Manufacturing, Properties, Test Methods, and Identification; Springer: Singapore, 2020; pp. 29–67. [Google Scholar] [CrossRef]
- Ceretti, D.V.; Edeleva, M.; Cardon, L.; D’hooge, D.R. Molecular pathways for polymer degradation during conventional processing, additive manufacturing, and mechanical recycling. Molecules 2023, 28, 2344. [Google Scholar] [CrossRef]
- Jang, J.Y.; Sadeghi, K.; Seo, J. Chain-extending modification for value-added recycled PET: A review. Polym. Rev. 2022, 62, 860–889. [Google Scholar] [CrossRef]
- Guillén-Mallette, J.; Ríos-Soberanis, C.R.; Enríquez-Reyes, J. Discoloration of green PET bottles recycled with chemical agents by reactive extrusion. J. Elastomers Plast. 2021, 53, 1090–1104. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, S.; Guang, S.; Wang, Y.; Zhang, X.; Chen, W. Continuous post-polycondensation of high-viscosity poly (ethylene terephthalate) in the molten state. J. Appl. Polym. Sci. 2019, 136, 47484. [Google Scholar] [CrossRef]
- Benvenuta-Tapia, J.J.; Vivaldo-Lima, E.; Guerrero-Santos, R. Effect of copolymers synthesized by nitroxide-mediated polymerization as chain extenders of postconsumer poly(ethylene terephthalate) waste. Polym. Eng. Sci. 2019, 59, 2255–2264. [Google Scholar] [CrossRef]
- De Smit, K.; Marien, Y.W.; Van Steenberge, P.H.M.; D’Hooge, D.R.; Edeleva, M. Playing with process conditions to increase the industrial sustainability of poly(lactic acid)-based materials. React. Chem. Eng. 2023, 8, 1598–1612. [Google Scholar] [CrossRef]
- Edeleva, M.; De Smit, K.; Debrie, S.; Verberckmoes, A.; Marien, Y.W.; D’Hooge, D.R. Molecular scale-driven upgrading of extrusion technology for sustainable polymer processing and recycling. Curr. Opin. Green Sustain. Chem. 2023, 43, 100848. [Google Scholar] [CrossRef]
- Wu, W.-J.; Sun, X.-L.; Chen, Q.; Qian, Q. Recycled Poly(Ethylene Terephthalate) from Waste Textiles with Improved Thermal and Rheological Properties by Chain Extension. Polymers 2022, 14, 510. [Google Scholar] [CrossRef]
- Yang, Z.; Xin, C.; Mughal, W.; Li, X.; He, Y. High-melt-elasticity poly(ethylene terephthalate) produced by reactive extrusion with a multi-functional epoxide for foaming. J. Appl. Polym. Sci. 2018, 135, 45805. [Google Scholar] [CrossRef]
- Ozmen, S.C.; Ozkoc, G.; Serhatli, E. Thermal, mechanical and physical properties of chain extended recycled polyamide 6 via reactive extrusion: Effect of chain extender types. Polym. Degrad. Stab. 2019, 162, 76–84. [Google Scholar] [CrossRef]
- Tuna, B.; Benkreira, H. Reactive extrusion of polyamide 6 using a novel chain extender. Polym. Eng. Sci. 2019, 59, E25–E31. [Google Scholar] [CrossRef]
- Li, J.; Li, B.; Huang, S.; Luo, S.; He, S.; Gao, C.; Liu, S. Epoxy chain extender grafted pyrophyllite/poly(ethylene terephthalate) composites with enhanced crystallinity and mechanical properties. Polym. Compos. 2022, 43, 6404–6415. [Google Scholar] [CrossRef]
- Santos, R.M.; Costa, A.R.; Almeida, Y.M.; Carvalho, L.H.; Delgado, J.M.; Lima, E.S.; Magalhães, H.L.; Gomez, R.S.; Leite, B.E.; Rolim, F.D. Thermal and Rheological Characterization of Recycled PET/Virgin HDPE Blend Compatibilized with PE-g-MA and an Epoxy Chain Extender. Polymers 2022, 14, 1144. [Google Scholar] [CrossRef]
- Scremin, D.M.; Miyazaki, D.Y.; Lunelli, C.E.; Silva, S.A.; Zawadzki, S.F. PET recycling by alcoholysis using a new heterogeneous catalyst: Study and its use in polyurethane adhesives preparation. Macromol. Symp. 2019, 383, 1800027. [Google Scholar] [CrossRef]
- Berg, D.; Pich, A.; Möller, M. Post-Consumer Poly (Ethylene Terephthalate)-Properties, Problems During Reprocessing, and Modification by Reactive Extrusion; Universitätsbibliothek der RWTH: Aachen, Germany, 2018; Available online: https://publications.rwth-aachen.de/record/753165/files/753165.pdf (accessed on 19 February 2025).
- Lee, S.J.; Hahm, W.G.; Kikutani, T.; Kim, B.C. Effects of clay and POSS nanoparticles on the quiescent and shear-induced crystallization behavior of high molecular weight polyethylene terephthalate. Polym. Eng. Sci. 2009, 49, 317–323. [Google Scholar] [CrossRef]
- Makkam, S.; Harnnarongchai, W. Rheological and mechanical properties of recycled PET modified by reactive extrusion. Energy Procedia 2014, 56, 547–553. [Google Scholar] [CrossRef]
- Johnson, S.; Echeverria, D.; Venditti, R.; Jameel, H.; Yao, Y. Supply Chain of Waste Cotton Recycling and Reuse: A Review. AATCC J. Res. 2020, 7, 19–31. [Google Scholar] [CrossRef]
- Liu, W.; Liu, S.; Liu, T.; Liu, T.; Zhang, J.; Liu, H. Eco-friendly post-consumer cotton waste recycling for regenerated cellulose fibers. Carbohydr. Polym. 2019, 206, 141–148. [Google Scholar] [CrossRef]
- Ma, Y.; Zeng, B.; Wang, X.; Byrne, N. Circular Textiles: Closed Loop Fiber to Fiber Wet Spun Process for Recycling Cotton from Denim. ACS Sustain. Chem. Eng. 2019, 7, 11937–11943. [Google Scholar] [CrossRef]
- Regel. Available online: https://regel.world/ (accessed on 1 January 2025).
- OBBOTEC-SPEX. SPEX: Recycle Plastic by Dissolution. Available online: https://obbotec-spex.com/en/spex-technologie/ (accessed on 19 February 2025).
- PureCycle. Available online: https://www.purecycle.com/ (accessed on 1 January 2025).
- TeijinAramid. Available online: https://www.teijinaramid.com/en/sustainability (accessed on 1 January 2025).
- Antonini, P.G.; Palluau, M.; Safran, C.H.B. Recyclage Chimique des Plastiques Application aux Plastiques Issus des DEEE. 2020. Available online: https://www.arbe-regionsud.org/Block/download/?id=175234&filename=ecosystem-recyclage-physique-des-deee-2020.pdf (accessed on 19 February 2025).
- Biswas, M.C.; Dwyer, R.; Jimenez, J.; Su, H.-C.; Ford, E. Strengthening Regenerated Cellulose Fibers Sourced from Recycled Cotton T-Shirt Using Glucaric Acid for Antiplasticization. Polysaccharides 2021, 2, 138–153. [Google Scholar] [CrossRef]
- Liu, R.-G.; Shen, Y.-Y.; Shao, H.-L.; Wu, C.-X.; Hu, X.-C. An Analysis of Lyocell Fiber Formation as a Melt–spinning Process. Cellulose 2001, 8, 13–21. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Tang, J.; Lan, A.; Yang, Y.; Gibril, M.; Yu, M. Efficient preparation of high concentration cellulose solution with complex DMSO/ILs solvent. J. Polym. Res. 2016, 23, 32. [Google Scholar] [CrossRef]
- A Study on Technologies for Recycling and Re-Use of Textile Scraps. Tex-Med Alliances. Available online: https://www.enicbcmed.eu/sites/default/files/2023-01/A%20Study%20on%20technologies%20for%20recycling%20and%20re-use%20of%20textile%20scraps%20-%20TMA%20WP6.pdf (accessed on 19 February 2025).
- Triebert, D.; Hanel, H.; Bundt, M.; Wohnig, K. Solvent-based recycling. In Circular Economy of Polymers: Topics in Recycling Technologies; ACS Publications: Washington, DC, USA, 2021; pp. 33–59. [Google Scholar] [CrossRef]
- Cecon, V.S.; Da Silva, P.F.; Curtzwiler, G.W.; Vorst, K.L. The challenges in recycling post-consumer polyolefins for food contact applications: A review. Resour. Conserv. Recycl. 2021, 167, 105422. [Google Scholar] [CrossRef]
- Eschenbacher, A.; Varghese, R.J.; Delikonstantis, E.; Mynko, O.; Goodarzi, F.; Enemark-Rasmussen, K.; Oenema, J.; Abbas-Abadi, M.S.; Stefanidis, G.D.; Van Geem, K.M. Highly selective conversion of mixed polyolefins to valuable base chemicals using phosphorus-modified and steam-treated mesoporous HZSM-5 zeolite with minimal carbon footprint. Appl. Catal. B Environ. 2022, 309, 121251. [Google Scholar] [CrossRef]
- Mu, B.; Yang, Y. Complete separation of colorants from polymeric materials for cost-effective recycling of waste textiles. Chem. Eng. J. 2022, 427, 131570. [Google Scholar] [CrossRef]
- Abbas-Abadi, M.S.; Haghighi, M.N.; Yeganeh, H.; McDonald, A.G. Evaluation of pyrolysis process parameters on polypropylene degradation products. J. Anal. Appl. Pyrolysis 2014, 109, 272–277. [Google Scholar] [CrossRef]
- Wang, L.; Huang, S.; Wang, Y. Recycling of Waste Cotton Textile Containing Elastane Fibers through Dissolution and Regeneration. Membranes 2022, 12, 355. [Google Scholar] [CrossRef]
- Sherwood, J. Closed-loop recycling of polymers using solvents: Remaking plastics for a circular economy. Johns. Matthey Technol. Rev. 2020, 64, 4–15. [Google Scholar] [CrossRef]
- Serad, S.L. Polyester Dissolution for Polyester/Cotton Blend Recycle. Available online: https://patents.google.com/patent/US5342854A/en (accessed on 1 January 2025).
- Brinks, G.J.; Bouwhuis, G.H.; Agrawal, P.B.; Gooijer, H. Processing of Cotton-Polyester Waste Textile. Available online: https://patents.google.com/patent/WO2014081291A1/en (accessed on 1 January 2025).
- WornAgain. Available online: https://wornagain.co.uk/ (accessed on 1 January 2025).
- TextileChange. Available online: https://textilechange.com/ (accessed on 1 January 2025).
- Sarian, A.K.; Handermann, A.C.; Jones, S.; Davis, E.A.; Adhya, A. Recovery of Polyamides from Composite Articles. Available online: https://patents.google.com/patent/US5849804A/en (accessed on 1 January 2025).
- Haeggblom, J.; Budde, I. Circular Design as a Key Driver for Sustainability in Fashion and Textiles. In Sustainable Textile and Fashion Value Chains; Springer: Cham, Switzerland, 2021; pp. 35–45. [Google Scholar] [CrossRef]
- ECOPET. Available online: https://www.ecopet.info/en/ (accessed on 1 January 2025).
- Tournier, V.; Topham, C.M.; Gilles, A.; David, B.; Folgoas, C.; Moya-Leclair, E.; Kamionka, E.; Desrousseaux, M.L.; Texier, H.; Gavalda, S.; et al. An engineered PET depolymerase to break down and recycle plastic bottles. Nature 2020, 580, 216–219. [Google Scholar] [CrossRef]
- Yousef, S.; Tatariants, M.; Tichonovas, M.; Sarwar, Z.; Jonuškienė, I.; Kliucininkas, L. A new strategy for using textile waste as a sustainable source of recovered cotton. Resour. Conserv. Recycl. 2019, 145, 359–369. [Google Scholar] [CrossRef]
- Haslinger, S.; Hummel, M.; Anghelescu-Hakala, A.; Määttänen, M.; Sixta, H. Upcycling of cotton polyester blended textile waste to new man-made cellulose fibers. Waste Manag. 2019, 97, 88–96. [Google Scholar] [CrossRef]
- De Silva, R.; Wang, X.; Byrne, N. Recycling textiles: The use of ionic liquids in the separation of cotton polyester blends. RSC Adv. 2014, 4, 29094–29098. [Google Scholar] [CrossRef]
- Jeihanipour, A.; Karimi, K.; Niklasson, C.; Taherzadeh, M.J. A novel process for ethanol or biogas production from cellulose in blended-fibers waste textiles. Waste Manag. 2010, 30, 2504–2509. [Google Scholar] [CrossRef]
- Xia, G.; Han, W.; Xu, Z.; Zhang, J.; Kong, F.; Zhang, J.; Zhang, X.; Jia, F. Complete recycling and valorization of waste textiles for value-added transparent films via an ionic liquid. J. Environ. Chem. Eng. 2021, 9, 106182. [Google Scholar] [CrossRef]
- Abbas-Abadi, M.S.; Ureel, Y.; Eschenbacher, A.; Vermeire, F.H.; Varghese, R.J.; Oenema, J.; Stefanidis, G.D.; Van Geem, K.M. Challenges and opportunities of light olefin production via thermal and catalytic pyrolysis of end-of-life polyolefins: Towards full recyclability. Prog. Energy Combust. Sci. 2023, 96, 101046. [Google Scholar] [CrossRef]
- Abbas-Abadi, M.S.; Zayoud, A.; Kusenberg, M.; Roosen, M.; Vermeire, F.; Yazdani, P.; Van Waeyenberg, J.; Eschenbacher, A.; Hernandez, F.J.A.; Kuzmanović, M.; et al. Thermochemical recycling of end-of-life and virgin HDPE: A pilot-scale study. J. Anal. Appl. Pyrolysis 2022, 166, 105614. [Google Scholar] [CrossRef]
- De Keer, L.; Kilic, K.I.; Van Steenberge, P.H.M.; Daelemans, L.; Kodura, D.; Frisch, H.; De Clerck, K.; Reyniers, M.-F.; Barner-Kowollik, C.; Dauskardt, R.H.; et al. Computational prediction of the molecular configuration of three-dimensional network polymers. Nat. Mater. 2021, 20, 1422–1430. [Google Scholar] [CrossRef]
- Zayoud, A.; Thi, H.D.; Kusenberg, M.; Eschenbacher, A.; Kresovic, U.; Alderweireldt, N.; Djokic, M.; Van Geem, K.M. Pyrolysis of end-of-life polystyrene in a pilot-scale reactor: Maximizing styrene production. Waste Manag. 2022, 139, 85–95. [Google Scholar] [CrossRef]
- Godiya, C.B.; Gabrielli, S.; Materazzi, S.; Pianesi, M.S.; Stefanini, N.; Marcantoni, E. Depolymerization of waste poly (methyl methacrylate) scraps and purification of depolymerized products. J. Environ. Manag. 2019, 231, 1012–1020. [Google Scholar] [CrossRef]
- Moens, E.K.C.; De Smit, K.; Marien, Y.W.; Trigilio, A.D.; Van Steenberge, P.H.M.; Van Geem, K.M.; Dubois, J.-L.; D’hooge, D.R. Progress in Reaction Mechanisms and Reactor Technologies for Thermochemical Recycling of Poly(methyl methacrylate). Polymers 2020, 12, 1667. [Google Scholar] [CrossRef]
- Dogu, O.; Eschenbacher, A.; John Varghese, R.; Dobbelaere, M.; D’Hooge, D.R.; Van Steenberge, P.H.M.; Van Geem, K.M. Bayesian tuned kinetic Monte Carlo modeling of polystyrene pyrolysis: Unraveling the pathways to its monomer, dimers, and trimers formation. Chem. Eng. J. 2023, 455, 140708. [Google Scholar] [CrossRef]
- De Smit, K.; Marien, Y.W.; Van Geem, K.M.; Van Steenberge, P.H.M.; D’Hooge, D.R. Connecting polymer synthesis and chemical recycling on a chain-by-chain basis: A unified matrix-based kinetic Monte Carlo strategy. React. Chem. Eng. 2020, 5, 1909–1928. [Google Scholar] [CrossRef]
- Goshayeshi, B.; Kumar, R.; Wang, Y.; Varghese, R.J.; Roy, S.; Baruah, B.; Lemonidou, A.A.; Van Geem, K.M. Enhancing Polystyrene Recycling: Temperature-Responsive of Pyrolysis in a Pilot-Scale Vortex Reactor. J. Anal. Appl. Pyrolysis 2025, 187, 107016. [Google Scholar] [CrossRef]
- Kusenberg, M.; Zayoud, A.; Roosen, M.; Thi, H.D.; Abbas-Abadi, M.S.; Eschenbacher, A.; Kresovic, U.; De Meester, S.; Van Geem, K.M. A comprehensive experimental investigation of plastic waste pyrolysis oil quality and its dependence on the plastic waste composition. Fuel Process. Technol. 2022, 227, 107090. [Google Scholar] [CrossRef]
- Abbas-Abadi, M.S.; Haghighi, M.N.; Yeganeh, H. Evaluation of pyrolysis product of virgin high density polyethylene degradation using different process parameters in a stirred reactor. Fuel Process. Technol. 2013, 109, 90–95. [Google Scholar] [CrossRef]
- Abbas-Abadi, M.S.; Haghighi, M.N.; Yeganeh, H. The effect of temperature, catalyst, different carrier gases and stirrer on the produced transportation hydrocarbons of LLDPE degradation in a stirred reactor. J. Anal. Appl. Pyrolysis 2012, 95, 198–204. [Google Scholar] [CrossRef]
- Eschenbacher, A.; Goodarzi, F.; Varghese, R.J.; Enemark-Rasmussen, K.; Kegnæs, S.; Abbas-Abadi, M.S.; Van Geem, K.M. Boron-Modified Mesoporous ZSM-5 for the Conversion of Pyrolysis Vapors from LDPE and Mixed Polyolefins: Maximizing the C2–C4 Olefin Yield with Minimal Carbon Footprint. ACS Sustain. Chem. Eng. 2021, 9, 14618–14630. [Google Scholar] [CrossRef]
- Abbas-Abadi, M.S. The effect of process and structural parameters on the stability, thermo-mechanical and thermal degradation of polymers with hydrocarbon skeleton containing PE, PP, PS, PVC, NR, PBR and SBR. J. Therm. Anal. Calorim. 2021, 143, 2867–2882. [Google Scholar] [CrossRef]
- Monsores, K.G.d.C.; Silva, A.O.d.; Oliveira, S.d.S.A.; Rodrigues, J.G.P.; Weber, R.P. Influence of ultraviolet radiation on polymethylmethacrylate (PMMA). J. Mater. Res. Technol. 2019, 8, 3713–3718. [Google Scholar] [CrossRef]
- Abbas-Abadi, M.S.; Haghighi, M.N.; Yeganeh, H. Effect of the melt flow index and melt flow rate on the thermal degradation kinetics of commercial polyolefins. J. Appl. Polym. Sci. 2012, 126, 1739–1745. [Google Scholar] [CrossRef]
- Abudabbus, M.M.; Jevremović, I.; Nešović, K.; Perić-Grujić, A.; Rhee, K.Y.; Mišković-Stanković, V. In situ electrochemical synthesis of silver-doped poly (vinyl alcohol)/graphene composite hydrogels and their physico-chemical and thermal properties. Compos. Part B Eng. 2018, 140, 99–107. [Google Scholar] [CrossRef]
- Abbas-Abadi, M.S.; Van Geem, K.M.; Alvarez, J.; Lopez, G. The pyrolysis study of polybutadiene rubber under different structural and process parameters: Comparison with polyvinyl chloride degradation. J. Therm. Anal. Calorim. 2022, 147, 1237–1249. [Google Scholar] [CrossRef]
- Hamou, K.B.; Kaddami, H.; Dufresne, A.; Boufi, S.; Magnin, A.; Erchiqui, F. Impact of TEMPO-oxidization strength on the properties of cellulose nanofibril reinforced polyvinyl acetate nanocomposites. Carbohydr. Polym. 2018, 181, 1061–1070. [Google Scholar] [CrossRef]
- Ahire, J.J.; Neveling, D.P.; Dicks, L.M.T. Polyacrylonitrile (PAN) nanofibres spun with copper nanoparticles: An anti-Escherichia coli membrane for water treatment. Appl. Microbiol. Biotechnol. 2018, 102, 7171–7181. [Google Scholar] [CrossRef] [PubMed]
- Tamri, Z.; Yazdi, A.V.; Haghighi, M.N.; Abbas-Abadi, M.S.; Heidarinasab, A. The effect of temperature, heating rate, initial cross-linking and zeolitic catalysts as key process and structural parameters on the degradation of natural rubber (NR) to produce the valuable hydrocarbons. J. Anal. Appl. Pyrolysis 2018, 134, 35–42. [Google Scholar] [CrossRef]
- Seifali Abbas-Abadi, M.; Nekoomanesh Haghighi, M. The Consideration of Different Effective Zeolite Based Catalysts and Heating Rate on the Pyrolysis of Styrene Butadiene Rubber (SBR) in a Stirred Reactor. Energy Fuels 2017, 31, 12358–12363. [Google Scholar] [CrossRef]
- Salmasi, S.S.Z.; Abbas-Abadi, M.S.; Haghighi, M.N.; Abedini, H. The effect of different zeolite based catalysts on the pyrolysis of poly butadiene rubber. Fuel 2015, 160, 544–548. [Google Scholar] [CrossRef]
- Lubna, M.M.; Salem, K.S.; Sarker, M.; Khan, M.A. Modification of Thermo-Mechanical Properties of Recycled PET by Vinyl Acetate (VAc) Monomer Grafting Using Gamma Irradiation. J. Polym. Environ. 2018, 26, 83–90. [Google Scholar] [CrossRef]
- Pramoda, K.P.; Liu, T.; Liu, Z.; He, C.; Sue, H.-J. Thermal degradation behavior of polyamide 6/clay nanocomposites. Polym. Degrad. Stab. 2003, 81, 47–56. [Google Scholar] [CrossRef]
- Trovati, G.; Sanches, E.A.; Neto, S.C.; Mascarenhas, Y.P.; Chierice, G.O. Characterization of polyurethane resins by FTIR, TGA, and XRD. J. Appl. Polym. Sci. 2010, 115, 263–268. [Google Scholar] [CrossRef]
- Zhou, H.; Long, Y.; Meng, A.; Chen, S.; Li, Q.; Zhang, Y. A novel method for kinetics analysis of pyrolysis of hemicellulose, cellulose, and lignin in TGA and macro-TGA. Rsc Adv. 2015, 5, 26509–26516. [Google Scholar] [CrossRef]
- Mofokeng, J.P.; Luyt, A.S. Morphology and thermal degradation studies of melt-mixed poly(lactic acid) (PLA)/poly(ε-caprolactone) (PCL) biodegradable polymer blend nanocomposites with TiO2 as filler. Polym. Test. 2015, 45, 93–100. [Google Scholar] [CrossRef]
- Denardin, E.L.; Samios, D.; Janissek, P.R.; de Souza, G.P. Thermal degradation of aged chloroprene rubber studied by thermogravimetric analysis. Rubber Chem. Technol. 2001, 74, 622–629. [Google Scholar] [CrossRef]
- Ye, Q.; Ma, X.; Li, B.; Jin, Z.; Xu, Y.; Fang, C.; Zhou, X.; Ge, Y.; Ye, F. Development and Investigation of Lanthanum Sulfadiazine with Calcium Stearate and Epoxidised Soyabean Oil as Complex Thermal Stabilizers for Stabilizing Poly (vinyl chloride). Polymers 2019, 11, 531. [Google Scholar] [CrossRef] [PubMed]
- Yusof, N.; Ismail, A. Post spinning and pyrolysis processes of polyacrylonitrile (PAN)-based carbon fiber and activated carbon fiber: A review. J. Anal. Appl. Pyrolysis 2012, 93, 1–13. [Google Scholar] [CrossRef]
- Seifali Abbas-Abadi, M.; Fathi, M.; Ghadiri, M. Effect of Different Process Parameters on the Pyrolysis of Iranian Oak Using a Fixed Bed Reactor and TGA Instrument. Energy Fuels 2019, 33, 11226–11234. [Google Scholar] [CrossRef]
- Kumagai, S.; Yamasaki, R.; Kameda, T.; Saito, Y.; Watanabe, A.; Watanabe, C.; Teramae, N.; Yoshioka, T. Tandem μ-reactor-GC/MS for online monitoring of aromatic hydrocarbon production via CaO-catalysed PET pyrolysis. React. Chem. Eng. 2017, 2, 776–784. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Cho, J.; Tompsett, G.A.; Westmoreland, P.R.; Huber, G.W. Kinetics and mechanism of cellulose pyrolysis. J. Phys. Chem. C 2009, 113, 20097–20107. [Google Scholar] [CrossRef]
- Jomaa, G.; Goblet, P.; Coquelet, C.; Morlot, V. Kinetic modeling of polyurethane pyrolysis using non-isothermal thermogravimetric analysis. Thermochim. Acta 2015, 612, 10–18. [Google Scholar] [CrossRef]
- Barnard, E.; Arias, J.J.R.; Thielemans, W. Chemolytic depolymerisation of PET: A review. Green Chem. 2021, 23, 3765–3789. [Google Scholar] [CrossRef]
- Quicker, P.; Seitz, M.; Vogel, J. Chemical recycling: A critical assessment of potential process approaches. Waste Manag. Res. 2022, 40, 1494–1504. [Google Scholar] [CrossRef] [PubMed]
- Filip, D.; Macocinschi, D.; Vlad, S. Thermogravimetric study for polyurethane materials for biomedical applications. Compos. Part B Eng. 2011, 42, 1474–1479. [Google Scholar] [CrossRef]
- Ge, Y.; Zhang, Q.; Zhang, Y.; Liu, F.; Han, J.; Wu, C. High-performance natural rubber latex composites developed by a green approach using ionic liquid-modified multiwalled carbon nanotubes. J. Appl. Polym. Sci. 2018, 135, 46588. [Google Scholar] [CrossRef]
- Hanif, M.U.; Zwawi, M.; Algarni, M.; Bahadar, A.; Iqbal, H.; Capareda, S.C.; Hanif, M.A.; Waqas, A.; Hossain, N.; Siddiqui, M.T.H. The effects of using pretreated cotton gin trash on the production of biogas from anaerobic co-digestion with cow manure and sludge. Energies 2022, 15, 490. [Google Scholar] [CrossRef]
- Shen, F.; Xiao, W.; Lin, L.; Yang, G.; Zhang, Y.; Deng, S. Enzymatic saccharification coupling with polyester recovery from cotton-based waste textiles by phosphoric acid pretreatment. Bioresour. Technol. 2013, 130, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Venkatramanan, V.; Aravinth, S.; Prabhu, C.S.; Nithya, M.; Bama, K.S. Bioethanol production from cotton waste using cellulase extracted from Fusarium species. Int. J. ChemTech Res. 2014, 6, 4061–4069. Available online: https://api.semanticscholar.org/CorpusID:9502360 (accessed on 19 February 2025).
- Wang, Y.; Zhao, Y.; Deng, Y. Effect of enzymatic treatment on cotton fiber dissolution in NaOH/urea solution at cold temperature. Carbohydr. Polym. 2008, 72, 178–184. [Google Scholar] [CrossRef]
- Vecchiato, S.; Skopek, L.; Jankova, S.; Pellis, A.; Ipsmiller, W.; Aldrian, A.; Mueller, B.; Herrero Acero, E.; Guebitz, G.M. Enzymatic Recycling of High-Value Phosphor Flame-Retardant Pigment and Glucose from Rayon Fibers. ACS Sustain. Chem. Eng. 2018, 6, 2386–2394. [Google Scholar] [CrossRef]
- Wojnowska-Baryła, I.; Bernat, K.; Zaborowska, M. Strategies of recovery and organic recycling used in textile waste management. Int. J. Environ. Res. Public Health 2022, 19, 5859. [Google Scholar] [CrossRef]
- Navone, L.; Speight, R. Understanding the dynamics of keratin weakening and hydrolysis by proteases. PLoS ONE 2018, 13, e0202608. [Google Scholar] [CrossRef]
- Navone, L.; Moffitt, K.; Hansen, K.-A.; Blinco, J.; Payne, A.; Speight, R. Closing the textile loop: Enzymatic fibre separation and recycling of wool/polyester fabric blends. Waste Manag. 2020, 102, 149–160. [Google Scholar] [CrossRef]
- Quartinello, F.; Vecchiato, S.; Weinberger, S.; Kremenser, K.; Skopek, L.; Pellis, A.; Guebitz, G.M. Highly selective enzymatic recovery of building blocks from wool-cotton-polyester textile waste blends. Polymers 2018, 10, 1107. [Google Scholar] [CrossRef]
- Kuo, C.; Lin, P.; Lee, C. Enzymatic saccharification of dissolution pretreated waste cellulosic fabrics for bacterial cellulose production by Gluconacetobacter xylinus. J. Chem. Technol. Biotechnol. 2010, 85, 1346–1352. [Google Scholar] [CrossRef]
- Vasconcelos, A.; Cavaco-Paulo, A. Enzymatic removal of cellulose from cotton/polyester fabric blends. Cellulose 2006, 13, 611–618. [Google Scholar] [CrossRef]
- Hong, F.; Guo, X.; Zhang, S.; Han, S.-F.; Yang, G.; Jönsson, L.J. Bacterial cellulose production from cotton-based waste textiles: Enzymatic saccharification enhanced by ionic liquid pretreatment. Bioresour. Technol. 2012, 104, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Karbalaei, S.; Golieskardi, A.; Watt, D.U.; Boiret, M.; Hanachi, P.; Walker, T.R.; Karami, A. Analysis and inorganic composition of microplastics in commercial Malaysian fish meals. Mar. Pollut. Bull. 2019, 150, 110687. [Google Scholar] [CrossRef] [PubMed]
- Kaabel, S.; Arciszewski, J.; Borchers, T.H.; Therien, J.P.D.; Friščić, T.; Auclair, K. Solid-State Enzymatic Hydrolysis of Mixed PET/Cotton Textiles**. ChemSusChem 2022, 16, e202201613. [Google Scholar] [CrossRef]
- Szabo, O.E.; Csiszar, E. Some factors affecting efficiency of the ultrasound-aided enzymatic hydrolysis of cotton cellulose. Carbohydr. Polym. 2017, 156, 357–363. [Google Scholar] [CrossRef]
- Czernik, S.; Elam, C.C.; Evans, R.J.; Meglen, R.R.; Moens, L.; Tatsumoto, K. Catalytic pyrolysis of nylon-6 to recover caprolactam. J. Anal. Appl. Pyrolysis 1998, 46, 51–64. [Google Scholar] [CrossRef]
- Jia, H.; Ben, H.; Luo, Y.; Wang, R. Catalytic fast pyrolysis of poly (ethylene terephthalate)(PET) with zeolite and nickel chloride. Polymers 2020, 12, 705. [Google Scholar] [CrossRef]
- Zhang, J.; Gu, J.; Yuan, H.; Chen, Y. Catalytic fast pyrolysis of waste mixed cloth for the production of value-added chemicals. Waste Manag. 2021, 127, 141–146. [Google Scholar] [CrossRef]
- Lee, S.; Jung, S.; Lin, K.-Y.A.; Tsang, Y.F.; Kwon, E.E. Use of CO2 and nylon as the raw materials for flammable gas production through a catalytic thermo-chemical process. Green Chem. 2021, 23, 8922–8931. [Google Scholar] [CrossRef]
- Kaminsky, W. Chemical recycling of plastics by fluidized bed pyrolysis. Fuel Commun. 2021, 8, 100023. [Google Scholar] [CrossRef]
- Akin, O.; Varghese, R.J.; Eschenbacher, A.; Oenema, J.; Abbas-Abadi, M.S.; Stefanidis, G.D.; Van Geem, K.M. Chemical recycling of plastic waste to monomers: Effect of catalyst contact time, acidity and pore size on olefin recovery in ex-situ catalytic pyrolysis of polyolefin waste. J. Anal. Appl. Pyrolysis 2023, 172, 106036. [Google Scholar] [CrossRef]
- Goshayeshi, B.; Alexandros Theofanidis, S.; Abbas-Abadi, M.S.; Mahmoudi, E.; Akin, O.; John Varghese, R.; Lemonidou, A.; Van Geem, K.M. Selective catalytic conversion of model olefin and diolefin compounds of waste plastic pyrolysis oil: Insights for light olefin production and coke minimization. Chem. Eng. J. 2024, 500, 156987. [Google Scholar] [CrossRef]
- Dogu, O.; Pelucchi, M.; Van de Vijver, R.; Van Steenberge, P.H.M.; D’Hooge, D.R.; Cuoci, A.; Mehl, M.; Frassoldati, A.; Faravelli, T.; Van Geem, K.M. The chemistry of chemical recycling of solid plastic waste via pyrolysis and gasification: State-of-the-art, challenges, and future directions. Prog. Energy Combust. Sci. 2021, 84, 100901. [Google Scholar] [CrossRef]
- Abbas-Abadi, M.S.; Kusenberg, M.; Zayoud, A.; Roosen, M.; Vermeire, F.; Madanikashani, S.; Kuzmanović, M.; Parvizi, B.; Kresovic, U.; De Meester, S.; et al. Thermal pyrolysis of waste versus virgin polyolefin feedstocks: The role of pressure, temperature and waste composition. Waste Manag. 2023, 165, 108–118. [Google Scholar] [CrossRef]
- Green, A.; Sadrameli, S. Analytical representations of experimental polyethylene pyrolysis yields. J. Anal. Appl. Pyrolysis 2004, 72, 329–335. [Google Scholar] [CrossRef]
- De Smit, K.; Edeleva, M.; Trigilio, A.D.; Marien, Y.W.; Van Steenberge, P.H.M.; D’Hooge, D.R. Kinetic Monte Carlo residence time distributions and kinetics in view of extrusion-based polymer modification and recycling. React. Chem. Eng. 2023, 8, 563–576. [Google Scholar] [CrossRef]
- Milne, B.J.; Behie, L.A.; Berruti, F. Recycling of waste plastics by ultrapyrolysis using an internally circulating fluidized bed reactor. J. Anal. Appl. Pyrolysis 1999, 51, 157–166. [Google Scholar] [CrossRef]
- Kusenberg, M.; Eschenbacher, A.; Djokic, M.R.; Zayoud, A.; Ragaert, K.; De Meester, S.; Van Geem, K.M. Opportunities and challenges for the application of post-consumer plastic waste pyrolysis oils as steam cracker feedstocks: To decontaminate or not to decontaminate? Waste Manag. 2022, 138, 83–115. [Google Scholar] [CrossRef]
- Frączak, D. Chemical Recycling of Polyolefins (PE, PP): Modern Technologies and Products. In Waste Material Recycling in the Circular Economy-Challenges and Developments; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Ouyang, Y.; Manzano, M.N.; Beirnaert, K.; Heynderickx, G.J.; Van Geem, K.M. Micromixing in a gas–liquid vortex reactor. AIChE J. 2021, 67, e17264. [Google Scholar] [CrossRef]
- Lang, X.J.; Ouyang, Y.; Vandewalle, L.A.; Goshayeshi, B.; Chen, S.Y.; Madanikashani, S.; Perreault, P.; Van Geem, K.M. Gas-solid hydrodynamics in a stator-rotor vortex chamber reactor. Chem. Eng. J. 2022, 446, 137323. [Google Scholar] [CrossRef]
- Orozco, S.; Alvarez, J.; Lopez, G.; Artetxe, M.; Bilbao, J.; Olazar, M. Pyrolysis of plastic wastes in a fountain confined conical spouted bed reactor: Determination of stable operating conditions. Energy Convers. Manag. 2021, 229, 113768. [Google Scholar] [CrossRef]
- Pan, X.; Lian, W.; Yang, J.; Wang, J.; Zhang, Z.; Hao, X.; Abudula, A.; Guan, G. Downer reactor simulation and its application on coal pyrolysis: A review. Carbon Resour. Convers. 2022, 5, 35–51. [Google Scholar] [CrossRef]
- Luo, H.; Wang, X.; Liu, X.; Wu, X.; Shi, X.; Xiong, Q. A review on CFD simulation of biomass pyrolysis in fluidized bed reactors with emphasis on particle-scale models. J. Anal. Appl. Pyrolysis 2022, 162, 105433. [Google Scholar] [CrossRef]
- Moltó, J.; Font, R.; Conesa, J.A. Study of the organic compounds produced in the pyrolysis and combustion of used polyester fabrics. Energy Fuels 2006, 20, 1951–1958. [Google Scholar] [CrossRef]
- Marco, I.d.; Caballero, B.; Torres, A.; Laresgoiti, M.F.; Chomon, M.J.; Cabrero, M.A. Recycling polymeric wastes by means of pyrolysis. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 2002, 77, 817–824. [Google Scholar] [CrossRef]
- Kumagai, S.; Yamasaki, R.; Kameda, T.; Saito, Y.; Watanabe, A.; Watanabe, C.; Teramae, N.; Yoshioka, T. Catalytic Pyrolysis of Poly (ethylene terephthalate) in the Presence of Metal Oxides for Aromatic Hydrocarbon Recovery Using Tandem μ-Reactor-GC/MS. Energy Fuels 2020, 34, 2492–2500. [Google Scholar] [CrossRef]
- Diaz-Silvarrey, L.S.; McMahon, A.; Phan, A.N. Benzoic acid recovery via waste poly(ethylene terephthalate) (PET) catalytic pyrolysis using sulphated zirconia catalyst. J. Anal. Appl. Pyrolysis 2018, 134, 621–631. [Google Scholar] [CrossRef]
- Kwon, D.; Yi, S.; Jung, S.; Kwon, E.E. Valorization of synthetic textile waste using CO2 as a raw material in the catalytic pyrolysis process. Environ. Pollut. 2021, 268, 115916. [Google Scholar] [CrossRef]
- Artetxe, M.; Lopez, G.; Amutio, M.; Elordi, G.; Bilbao, J.; Olazar, M. Cracking of high density polyethylene pyrolysis waxes on HZSM-5 catalysts of different acidity. Ind. Eng. Chem. Res. 2013, 52, 10637–10645. [Google Scholar] [CrossRef]
- Yousef, S.; Eimontas, J.; Striūgas, N.; Tatariants, M.; Abdelnaby, M.A.; Tuckute, S.; Kliucininkas, L. A sustainable bioenergy conversion strategy for textile waste with self-catalysts using mini-pyrolysis plant. Energy Convers. Manag. 2019, 196, 688–704. [Google Scholar] [CrossRef]
- Phan, A.N.; Ryu, C.; Sharifi, V.N.; Swithenbank, J. Characterisation of slow pyrolysis products from segregated wastes for energy production. J. Anal. Appl. Pyrolysis 2008, 81, 65–71. [Google Scholar] [CrossRef]
- Kim, S.; Lee, N.; Lee, S.W.; Kim, Y.T.; Lee, J. Upcycling of waste teabags via catalytic pyrolysis in carbon dioxide over HZSM-11. Chem. Eng. J. 2021, 412, 128626. [Google Scholar] [CrossRef]
- Lee, S.B.; Lee, J.; Tsang, Y.F.; Kim, Y.-M.; Jae, J.; Jung, S.-C.; Park, Y.-K. Production of value-added aromatics from wasted COVID-19 mask via catalytic pyrolysis. Environ. Pollut. 2021, 283, 117060. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Lee, S.; Dou, X.; Kwon, E.E. Valorization of disposable COVID-19 mask through the thermo-chemical process. Chem. Eng. J. 2021, 405, 126658. [Google Scholar] [CrossRef]
- Wang, M.; Mao, M.; Zhang, M.; Wen, G.; Yang, Q.; Su, B.; Ren, Q. Highly efficient treatment of textile dyeing sludge by CO2 thermal plasma gasification. Waste Manag. 2019, 90, 29–36. [Google Scholar] [CrossRef]
- Athanasopoulos, P.; Zabaniotou, A. Post-consumer textile thermochemical recycling to fuels and biocarbon: A critical review. Sci. Total Environ. 2022, 834, 155387. [Google Scholar] [CrossRef]
- Li, S.; Vela, I.C.; Järvinen, M.; Seemann, M. Polyethylene terephthalate (PET) recycling via steam gasification–The effect of operating conditions on gas and tar composition. Waste Manag. 2021, 130, 117–126. [Google Scholar] [CrossRef]
- Gholami, Z.; Gholami, F.; Tišler, Z.; Tomas, M.; Vakili, M. A review on production of light olefins via fluid catalytic cracking. Energies 2021, 14, 1089. [Google Scholar] [CrossRef]
- Dhaka, A.K.; Kaushal, R.; Pal, Y. Assessing the power generation potential and quality of producer gas from blended of the cotton stalk and pistachio shell in an open core downdraft gasifier. Int. J. Ambient Energy 2022, 43, 8351–8360. [Google Scholar] [CrossRef]
- Abdpour, S.; Santos, R.M. Recent advances in heterogeneous catalysis for supercritical water oxidation/gasification processes: Insight into catalyst development. Process Saf. Environ. Prot. 2021, 149, 169–184. [Google Scholar] [CrossRef]
- Wu, Y.; Wen, C.; Chen, X.; Jiang, G.; Liu, G.; Liu, D. Catalytic pyrolysis and gasification of waste textile under carbon dioxide atmosphere with composite Zn-Fe catalyst. Fuel Process. Technol. 2017, 166, 115–123. [Google Scholar] [CrossRef]
- Jeong, Y.-S.; Kim, J.-W.; Ra, H.W.; Seo, M.W.; Mun, T.-Y.; Kim, J.-S. Characteristics of Air Gasification of 10 Different Types of Plastic in a Two-Stage Gasification Process. ACS Sustain. Chem. Eng. 2022, 10, 4705–4716. [Google Scholar] [CrossRef]
- Bai, B.; Liu, Y.; Zhang, H.; Zhou, F.; Han, X.; Wang, Q.; Jin, H. Experimental investigation on gasification characteristics of polyethylene terephthalate (PET) microplastics in supercritical water. Fuel 2020, 262, 116630. [Google Scholar] [CrossRef]
- Li, W.; Wanninayake, N.; Gao, X.; Li, M.; Pu, Y.; Kim, D.-Y.; Ragauskas, A.J.; Shi, J. Mechanistic insight into lignin slow pyrolysis by linking pyrolysis chemistry and carbon material properties. ACS Sustain. Chem. Eng. 2020, 8, 15843–15854. [Google Scholar] [CrossRef]
- Abbas-Abadi, M.S.; Van Geem, K.M.; Fathi, M.; Bazgir, H.; Ghadiri, M. The pyrolysis of oak with polyethylene, polypropylene and polystyrene using fixed bed and stirred reactors and TGA instrument. Energy 2021, 232, 121085. [Google Scholar] [CrossRef]
- Ghadiri, M.; Ghasemzadeh, N.; Behrooz Sarand, A.; Seifali Abbas Abadi, M. Wastewater treatment by new high-performance activated carbon from Semecarpus Anacardium and Quercus Infectoria nutshells: Applications- kinetic and equilibrium studies. Iran. J. Chem. Chem. Eng. 2024, 43, 2737–2749. [Google Scholar] [CrossRef]
- Bazgir, H.; Rostami, M.R.; Tavakkol, S.; Issaabadi, Z.; Shirazi, H.M.; Goshayeshi, B.; Van Geem, K.M.; Haghighi, M.N.; Abbas-Abadi, M.S. The chemical process of producing activated carbon using walnut shells and plastic wastes. J. Therm. Anal. Calorim. 2023, 148, 10125–10138. [Google Scholar] [CrossRef]
- Xu, Z.; Sun, Z.; Zhou, Y.; Chen, W.; Zhang, T.; Huang, Y.; Zhang, D. Insights into the pyrolysis behavior and adsorption properties of activated carbon from waste cotton textiles by FeCl3-activation. Colloids Surf. A Physicochem. Eng. Asp. 2019, 582, 123934. [Google Scholar] [CrossRef]
- Akkouche, F.; Boudrahem, F.; Yahiaoui, I.; Vial, C.; Audonnet, F.; Aissani-Benissad, F. Cotton textile waste valorization for removal of tetracycline and paracetamol alone and in mixtures from aqueous solutions: Effects of H3PO4 as an oxidizing agent. Water Environ. Res. 2021, 93, 464–478. [Google Scholar] [CrossRef]
- Gumus, H.; Buyukkidan, B. A Simple and Green Preparation Route of Waste Textile Based Photocatalytic Biochars for Pollution Removal. Chem. Afr. 2023, 6, 629–642. [Google Scholar] [CrossRef]
- Parmakoğlu, E.Ü.; Çay, A.; Yanık, J. Valorization of Solid Wastes from Textile Industry as an Adsorbent Through Activated Carbon Production. AATCC J. Res. 2023, 10, 133–143. [Google Scholar] [CrossRef]
- Zou, Z.; Liu, X.; Ding, J.; Chen, T.; Wang, X. Activated carbon powder derived from cashmere guard hair. J. Ind. Text. 2020, 50, 599–615. [Google Scholar] [CrossRef]
- Deng, H.; Mao, Z.; Xu, H.; Zhang, L.; Zhong, Y.; Sui, X. Synthesis of fibrous LaFeO3 perovskite oxide for adsorption of Rhodamine B. Ecotoxicol. Environ. Saf. 2019, 168, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Beyan, S.M.; Prabhu, S.V.; Sissay, T.T.; Getahun, A.A. Sugarcane bagasse based activated carbon preparation and its adsorption efficacy on removal of BOD and COD from textile effluents: RSM based modeling, optimization and kinetic aspects. Bioresour. Technol. Rep. 2021, 14, 100664. [Google Scholar] [CrossRef]
- Keawploy, N.; Venkatkarthick, R.; Wangyao, P.; Zhang, X.; Liu, R.; Qin, J. Eco-friendly conductive cotton-based textile electrodes using silver-and carbon-coated fabrics for advanced flexible supercapacitors. Energy Fuels 2020, 34, 8977–8986. [Google Scholar] [CrossRef]
- Nieto-Delgado, C.; Partida-Gutierrez, D.; Rangel-Mendez, J.R. Preparation of activated carbon cloths from renewable natural fabrics and their performance during the adsorption of model organic and inorganic pollutants in water. J. Clean. Prod. 2019, 213, 650–658. [Google Scholar] [CrossRef]
- Xu, Z.; Yuan, Z.; Zhang, D.; Chen, W.; Huang, Y.; Zhang, T.; Tian, D.; Deng, H.; Zhou, Y.; Sun, Z. Highly mesoporous activated carbon synthesized by pyrolysis of waste polyester textiles and MgCl2: Physiochemical characteristics and pore-forming mechanism. J. Clean. Prod. 2018, 192, 453–461. [Google Scholar] [CrossRef]
- Silva, T.L.; Cazetta, A.L.; Souza, P.S.C.; Zhang, T.; Asefa, T.; Almeida, V.C. Mesoporous activated carbon fibers synthesized from denim fabric waste: Efficient adsorbents for removal of textile dye from aqueous solutions. J. Clean. Prod. 2018, 171, 482–490. [Google Scholar] [CrossRef]
- Rabbi, A.; Dadashian, F. Simultaneous improvement in tensile strength and adsorption capacity of activated carbon fibers during stabilization and activation of acrylic fibers. Diam. Relat. Mater. 2019, 95, 174–184. [Google Scholar] [CrossRef]
- Kim, J.; Kwon, W.; Bai, B.C.; Jeong, E. Recycling of cotton clothing into activated carbon fibers. Carbon Lett. 2022, 32, 1315–1327. [Google Scholar] [CrossRef]
- Wanassi, B.; Hariz, I.B.; Ghimbeu, C.M.; Vaulot, C.; Hassen, M.B.; Jeguirim, M. Carbonaceous adsorbents derived from textile cotton waste for the removal of Alizarin S dye from aqueous effluent: Kinetic and equilibrium studies. Environ. Sci. Pollut. Res. 2017, 24, 10041–10055. [Google Scholar] [CrossRef] [PubMed]
- Karthik, D.; Baheti, V.; Militky, J.; Naeem, M.S.; Tunakova, V.; Ali, A. Activated Carbon Derived from Carbonization of Kevlar Waste Materials: A Novel Single Stage Method. Materials 2021, 14, 6433. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.T.; Reed, A.R. Pre-formed activated carbon matting derived from the pyrolysis of biomass natural fibre textile waste. J. Anal. Appl. Pyrolysis 2003, 70, 563–577. [Google Scholar] [CrossRef]
- Nahil, M.A.; Williams, P.T. Surface chemistry and porosity of nitrogen-containing activated carbons produced from acrylic textile waste. Chem. Eng. J. 2012, 184, 228–237. [Google Scholar] [CrossRef]
- Zhu, X.; Li, Q.; Qiu, S.; Liu, X.; Xiao, L.; Ai, X.; Yang, H.; Cao, Y. Hard Carbon Fibers Pyrolyzed from Wool as High-Performance Anode for Sodium-Ion Batteries. JOM 2016, 68, 2579–2584. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, D.; Yuan, Z.; Chen, W.; Zhang, T.; Tian, D.; Deng, H. Physicochemical and adsorptive characteristics of activated carbons from waste polyester textiles utilizing MgO template method. Environ. Sci. Pollut. Res. 2017, 24, 22602–22612. [Google Scholar] [CrossRef]
- Yuan, Z.; Xu, Z.; Zhang, D.; Chen, W.; Huang, Y.; Zhang, T.; Tian, D.; Deng, H.; Zhou, Y.; Sun, Z. Mesoporous activated carbons synthesized by pyrolysis of waste polyester textiles mixed with Mg-containing compounds and their Cr(VI) adsorption. Colloids Surfaces A Physicochem. Eng. Asp. 2018, 549, 86–93. [Google Scholar] [CrossRef]
- Yuan, Z.; Xu, Z.; Zhang, D.; Chen, W.; Zhang, T.; Huang, Y.; Gu, L.; Deng, H.; Tian, D. Box-Behnken design approach towards optimization of activated carbon synthesized by co-pyrolysis of waste polyester textiles and MgCl2. Appl. Surf. Sci. 2018, 427, 340–348. [Google Scholar] [CrossRef]
- Xu, Z.; Tian, D.; Sun, Z.; Zhang, D.; Zhou, Y.; Chen, W.; Deng, H. Highly porous activated carbon synthesized by pyrolysis of polyester fabric wastes with different iron salts: Pore development and adsorption behavior. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 565, 180–187. [Google Scholar] [CrossRef]
- Xia, M.; Shao, X.; Sun, Z.; Xu, Z. Conversion of cotton textile wastes into porous carbons by chemical activation with ZnCl2, H3PO4, and FeCl3. Environ. Sci. Pollut. Res. 2020, 27, 25186–25196. [Google Scholar] [CrossRef]
- Kumar, S. Modeling PET Solvolysis; APS March Meeting Abstracts; APS: Minneapolis, MN, USA, 2022; Available online: https://ui.adsabs.harvard.edu/abs/2022APS..MARB16006K/abstract (accessed on 19 February 2025).
- Kárpáti, L.; Fogarassy, F.; Kovácsik, D.; Vargha, V. One-pot depolymerization and polycondensation of PET based random oligo-and polyesters. J. Polym. Environ. 2019, 27, 2167–2181. [Google Scholar] [CrossRef]
- Pham, D.D.; Cho, J. Low-energy catalytic methanolysis of poly (ethyleneterephthalate). Green Chem. 2021, 23, 511–525. [Google Scholar] [CrossRef]
- Shirazimoghaddam, S.; Amin, I.; Faria Albanese, J.A.; Shiju, N.R. Chemical Recycling of Used PET by Glycolysis Using Niobia-Based Catalysts. ACS Eng. Au 2023, 3, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Štrukil, V. Highly Efficient Solid-State Hydrolysis of Waste Polyethylene Terephthalate by Mechanochemical Milling and Vapor-Assisted Aging. ChemSusChem 2021, 14, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.; Wang, L.; Zheng, R.; Guo, L.; Chen, Y.; Qian, X. Research and progress of chemical depolymerization of waste PET and high-value application of its depolymerization products. RSC Adv. 2022, 12, 31564–31576. [Google Scholar] [CrossRef]
- Gupta, P.; Bhandari, S. Chemical depolymerization of PET bottles via ammonolysis and aminolysis. In Recycling of Polyethylene Terephthalate Bottles; Elsevier: Amsterdam, The Netherlands, 2019; pp. 109–134. [Google Scholar] [CrossRef]
- Pegoretti, A. Towards sustainable structural composites: A review on the recycling of continuous-fiber-reinforced thermoplastics. Adv. Ind. Eng. Polym. Res. 2021, 4, 105–115. [Google Scholar] [CrossRef]
- Vollmer, I.; Jenks, M.J.; Roelands, M.C.; White, R.J.; van Harmelen, T.; de Wild, P.; van Der Laan, G.P.; Meirer, F.; Keurentjes, J.T.; Weckhuysen, B.M. Beyond mechanical recycling: Giving new life to plastic waste. Angew. Chem. Int. Ed. 2020, 59, 15402–15423. [Google Scholar] [CrossRef]
- Liu, B.; Fu, W.; Lu, X.; Zhou, Q.; Zhang, S. Lewis Acid–Base Synergistic Catalysis for Polyethylene Terephthalate Degradation by 1,3-Dimethylurea/Zn(OAc)2 Deep Eutectic Solvent. ACS Sustain. Chem. Eng. 2018, 7, 3292–3300. [Google Scholar] [CrossRef]
- Guo, Z.; Adolfsson, E.; Tam, P.L. Nanostructured micro particles as a low-cost and sustainable catalyst in the recycling of PET fiber waste by the glycolysis method. Waste Manag. 2021, 126, 559–566. [Google Scholar] [CrossRef]
- Jehanno, C.; Flores, I.; Dove, A.P.; Müller, A.J.; Ruipérez, F.; Sardon, H. Organocatalysed depolymerisation of PET in a fully sustainable cycle using thermally stable protic ionic salt. Green Chem. 2018, 20, 1205–1212. [Google Scholar] [CrossRef]
- Fang, P.; Liu, B.; Xu, J.; Zhou, Q.; Zhang, S.; Ma, J.; Lu, X. High-efficiency glycolysis of poly(ethylene terephthalate) by sandwich-structure polyoxometalate catalyst with two active sites. Polym. Degrad. Stab. 2018, 156, 22–31. [Google Scholar] [CrossRef]
- Scé, F.; Cano, I.; Martin, C.; Beobide, G.; Castillo, Ó.; de Pedro, I. Comparing conventional and microwave-assisted heating in PET degradation mediated by imidazolium-based halometallate complexes. New J. Chem. 2019, 43, 3476–3485. [Google Scholar] [CrossRef]
- Liu, Y.; Yao, X.; Yao, H.; Zhou, Q.; Xin, J.; Lu, X.; Zhang, S. Degradation of poly(ethylene terephthalate) catalyzed by metal-free choline-based ionic liquids. Green Chem. 2020, 22, 3122–3131. [Google Scholar] [CrossRef]
- Bin Jin, S.; Jeong, J.-M.; Son, S.G.; Park, S.H.; Lee, K.G.; Choi, B.G. Synthesis of two-dimensional holey MnO2/graphene oxide nanosheets with high catalytic performance for the glycolysis of poly(ethylene terephthalate). Mater. Today Commun. 2021, 26, 101857. [Google Scholar] [CrossRef]
- Wang, R.; Wang, T.; Yu, G.; Chen, X. A new class of catalysts for the glycolysis of PET: Deep eutectic solvent@ZIF-8 composite. Polym. Degrad. Stab. 2020, 183, 109463. [Google Scholar] [CrossRef]
- Lalhmangaihzuala, S.; Laldinpuii, Z.; Lalmuanpuia, C.; Vanlaldinpuia, K. Glycolysis of Poly(Ethylene Terephthalate) Using Biomass-Waste Derived Recyclable Heterogeneous Catalyst. Polymers 2020, 13, 37. [Google Scholar] [CrossRef]
- Cano, I.; Martin, C.; Fernandes, J.A.; Lodge, R.W.; Dupont, J.; Casado-Carmona, F.A.; Lucena, R.; Cardenas, S.; Sans, V.; de Pedro, I. Paramagnetic ionic liquid-coated SiO2@Fe3O4 nanoparticles—The next generation of magnetically recoverable nanocatalysts applied in the glycolysis of PET. Appl. Catal. B Environ. 2020, 260, 118110. [Google Scholar] [CrossRef]
- Fuentes, C.A.; Gallegos, M.V.; García, J.R.; Sambeth, J.; Peluso, M.A. Catalytic Glycolysis of Poly (ethylene terephthalate) Using Zinc and Cobalt Oxides Recycled from Spent Batteries. Waste Biomass Valorization 2019, 11, 4991–5001. [Google Scholar] [CrossRef]
- Laldinpuii, Z.T.; Khiangte, V.; Lalhmangaihzuala, S.; Lalmuanpuia, C.; Pachuau, Z.; Lalhriatpuia, C.; Vanlaldinpuia, K. Methanolysis of PET Waste Using Heterogeneous Catalyst of Bio-waste Origin. J. Polym. Environ. 2022, 30, 1600–1614. [Google Scholar] [CrossRef]
- Du, J.-T.; Sun, Q.; Zeng, X.-F.; Wang, D.; Wang, J.-X.; Chen, J.-F. ZnO nanodispersion as pseudohomogeneous catalyst for alcoholysis of polyethylene terephthalate. Chem. Eng. Sci. 2020, 220, 115642. [Google Scholar] [CrossRef]
- Jiang, Z.; Yan, D.; Xin, J.; Li, F.; Guo, M.; Zhou, Q.; Xu, J.; Hu, Y.; Lu, X. Poly(ionic liquid)s as efficient and recyclable catalysts for methanolysis of PET. Polym. Degrad. Stab. 2022, 199, 109905. [Google Scholar] [CrossRef]
- Stanica-Ezeanu, D.; Matei, D. Natural depolymerization of waste poly(ethylene terephthalate) by neutral hydrolysis in marine water. Sci. Rep. 2021, 11, 4431. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.J.; Yu, H.J.; Jegal, J.; Kim, H.S.; Gil Cha, H. Depolymerization of PET into terephthalic acid in neutral media catalyzed by the ZSM-5 acidic catalyst. Chem. Eng. J. 2020, 398, 125655. [Google Scholar] [CrossRef]
- Bäckström, E.; Odelius, K.; Hakkarainen, M. Ultrafast microwave assisted recycling of PET to a family of functional precursors and materials. Eur. Polym. J. 2021, 151, 110441. [Google Scholar] [CrossRef]
- Karpati, L.; Fejer, M.; Kalocsai, D.; Molnar, J.; Vargha, V. Synthesis and characterization of isophorondiamine based epoxy hardeners from aminolysis of PET. Express Polym. Lett. 2019, 13, 618–631. [Google Scholar] [CrossRef]
- Nica, S.; Duldner, M.; Hanganu, A.; Iancu, S.; Cursaru, B.; Sarbu, A.; Filip, P.; Bartha, E. Functionalized 1,5,7-triazabicyclo [4.4.0] dec-5-ene (TBD) as Novel Organocatalyst for Efficient Depolymerization of Polyethylene Terephthalate (PET) Wastes. Rev. Chim. 2018, 69, 2613–2616. [Google Scholar] [CrossRef]
- Fukushima, K.; Lecuyer, J.M.; Wei, D.S.; Horn, H.W.; Jones, G.O.; Al-Megren, H.A.; Alabdulrahman, A.M.; Alsewailem, F.D.; McNeil, M.A.; Rice, J.E.; et al. Advanced chemical recycling of poly(ethylene terephthalate) through organocatalytic aminolysis. Polym. Chem. 2012, 4, 1610–1616. [Google Scholar] [CrossRef]
- Krall, E.M.; Klein, T.W.; Andersen, R.J.; Nett, A.J.; Glasgow, R.W.; Reader, D.S.; Dauphinais, B.C.; Mc Ilrath, S.P.; Fischer, A.A.; Carney, M.J.; et al. Controlled hydrogenative depolymerization of polyesters and polycarbonates catalyzed by ruthenium (II) PNN pincer complexes. Chem. Commun. 2014, 50, 4884–4887. [Google Scholar] [CrossRef]
- Feghali, E.; Cantat, T. Room temperature organocatalyzed reductive depolymerization of waste polyethers, polyesters, and polycarbonates. ChemSusChem 2015, 8, 980–984. [Google Scholar] [CrossRef]
- Wu, P.; Lu, G.; Cai, C. Cobalt–molybdenum synergistic catalysis for the hydrogenolysis of terephthalate-based polyesters. Green Chem. 2021, 23, 8666–8672. [Google Scholar] [CrossRef]
- Kratish, Y.; Marks, T.J. Efficient polyester hydrogenolytic deconstruction via tandem catalysis. Angew. Chem. Int. Ed. 2022, 61, e202112576. [Google Scholar] [CrossRef] [PubMed]
- Kamimura, A.; Shiramatsu, Y.; Kawamoto, T. Depolymerization of polyamide 6 in hydrophilic ionic liquids. Green Energy Environ. 2019, 4, 166–170. [Google Scholar] [CrossRef]
- Patil, D.B.; Madhamshettiwar, S.V. Kinetics and Thermodynamic Studies of Depolymerization of Nylon Waste by Hydrolysis Reaction. J. Appl. Chem. 2014, 2014, 286709. [Google Scholar] [CrossRef]
- Wang, L.; Nelson, G.A.; Toland, J.; Holbrey, J.D. Glycolysis of PET Using 1,3-Dimethylimidazolium-2-Carboxylate as an Organocatalyst. ACS Sustain. Chem. Eng. 2020, 8, 13362–13368. [Google Scholar] [CrossRef]
- Shafaghat, H.; Lee, H.W.; Tsang, Y.F.; Oh, D.; Jae, J.; Jung, S.-C.; Ko, C.H.; Lam, S.S.; Park, Y.-K. In-situ and ex-situ catalytic pyrolysis/co-pyrolysis of empty fruit bunches using mesostructured aluminosilicate catalysts. Chem. Eng. J. 2019, 366, 330–338. [Google Scholar] [CrossRef]
- Choudhury, K.; Tsianou, M.; Alexandridis, P. Recycling of Blended Fabrics for a Circular Economy of Textiles: Separation of Cotton, Polyester, and Elastane Fibers. Sustainability 2024, 16, 6206. [Google Scholar] [CrossRef]
- Wu, Y.; Che, Y.; Wei, X.; Hu, Q.; Xu, J.; Guo, B.; Niu, Z. Nondestructive Recovery of Cotton from Waste Polycotton Textiles by Catalytic Hydrolysis. ACS Sustain. Chem. Eng. 2024, 12, 10446–10454. [Google Scholar] [CrossRef]
- Andini, E.; Bhalode, P.; Gantert, E.; Sadula, S.; Vlachos, D.G. Chemical recycling of mixed textile waste. Sci. Adv. 2024, 10, eado6827. [Google Scholar] [CrossRef]
- Shojaei, B.; Abtahi, M.; Najafi, M. Chemical recycling of PET: A stepping-stone toward sustainability. Polym. Adv. Technol. 2020, 31, 2912–2938. [Google Scholar] [CrossRef]
- Wu, H.-S. Strategic possibility routes of recycled PET. Polymers 2021, 13, 1475. [Google Scholar] [CrossRef]
- Thiyagarajan, S.; Maaskant-Reilink, E.; Ewing, T.A.; Julsing, M.K.; Van Haveren, J. Back-to-monomer recycling of polycondensation polymers: Opportunities for chemicals and enzymes. RSC Adv. 2022, 12, 947–970. [Google Scholar] [CrossRef] [PubMed]
- Ügdüler, S.; Van Geem, K.M.; Denolf, R.; Roosen, M.; Mys, N.; Ragaert, K.; De Meester, S. Towards closed-loop recycling of multilayer and coloured PET plastic waste by alkaline hydrolysis. Green Chem. 2020, 22, 5376–5394. [Google Scholar] [CrossRef]
- Zhang, Y.H.P.; Lynd, L.R. Toward an aggregated understanding of enzymatic hydrolysis of cellulose: Noncomplexed cellulase systems. Biotechnol. Bioeng. 2004, 88, 797–824. [Google Scholar] [CrossRef] [PubMed]
- Palme, A.; Peterson, A.; de la Motte, H.; Theliander, H.; Brelid, H. Development of an efficient route for combined recycling of PET and cotton from mixed fabrics. Text. Cloth. Sustain. 2017, 3, 4. [Google Scholar] [CrossRef]
- Yang, Y.; Lu, Y.; Xiang, H.; Xu, Y.; Li, Y. Study on methanolytic depolymerization of PET with supercritical methanol for chemical recycling. Polym. Degrad. Stab. 2002, 75, 185–191. [Google Scholar] [CrossRef]
- Fockink, D.H.; Maceno, M.A.C.; Ramos, L.P. Production of cellulosic ethanol from cotton processing residues after pretreatment with dilute sodium hydroxide and enzymatic hydrolysis. Bioresour. Technol. 2015, 187, 91–96. [Google Scholar] [CrossRef]
- Sanchis-Sebastiá, M.; Ruuth, E.; Stigsson, L.; Galbe, M.; Wallberg, O. Novel sustainable alternatives for the fashion industry: A method of chemically recycling waste textiles via acid hydrolysis. Waste Manag. 2021, 121, 248–254. [Google Scholar] [CrossRef]
- Trache, D.; Hussin, M.H.; Haafiz, M.M.; Thakur, V.K. Recent progress in cellulose nanocrystals: Sources and production. Nanoscale 2017, 9, 1763–1786. [Google Scholar] [CrossRef]
- Kamimura, A.; Yamamoto, S. An Efficient Method To Depolymerize Polyamide Plastics: A New Use of Ionic Liquids. Org. Lett. 2007, 9, 2533–2535. [Google Scholar] [CrossRef]
- Han, M. 5—Depolymerization of PET Bottle via Methanolysis and Hydrolysis. In Recycling of Polyethylene Terephthalate Bottles; Thomas, S., Rane, A., Kanny, K., V.K, A., Thomas, M.G., Eds.; William Andrew Publishing: Norwich, NY, USA, 2019; pp. 85–108. [Google Scholar] [CrossRef]
- Sinha, V.; Patel, M.R.; Patel, J.V. Pet Waste Management by Chemical Recycling: A Review. J. Polym. Environ. 2010, 18, 8–25. [Google Scholar] [CrossRef]
- Uekert, T.; Singh, A.; DesVeaux, J.S.; Ghosh, T.; Bhatt, A.; Yadav, G.; Afzal, S.; Walzberg, J.; Knauer, K.M.; Nicholson, S.R. Technical, Economic, and Environmental Comparison of Closed-Loop Recycling Technologies for Common Plastics. ACS Sustain. Chem. Eng. 2023, 11, 965–978. [Google Scholar] [CrossRef]
- Liu, Q.; Li, R.; Fang, T. Investigating and modeling PET methanolysis under supercritical conditions by response surface methodology approach. Chem. Eng. J. 2015, 270, 535–541. [Google Scholar] [CrossRef]
- Tang, S.; Li, F.; Liu, J.; Guo, B.; Tian, Z.; Lv, J. MgO/NaY as modified mesoporous catalyst for methanolysis of polyethylene terephthalate wastes. J. Environ. Chem. Eng. 2022, 10, 107927. [Google Scholar] [CrossRef]
- Chan, K.; Zinchenko, A. Conversion of waste bottles’ PET to a hydrogel adsorbent via PET aminolysis. J. Environ. Chem. Eng. 2021, 9, 106129. [Google Scholar] [CrossRef]
- Shukla, S.R.; Harad, A.M. Aminolysis of polyethylene terephthalate waste. Polym. Degrad. Stab. 2006, 91, 1850–1854. [Google Scholar] [CrossRef]
- Musale, R.M.; Shukla, S.R. Deep eutectic solvent as effective catalyst for aminolysis of polyethylene terephthalate (PET) waste. Int. J. Plast. Technol. 2016, 20, 106–120. [Google Scholar] [CrossRef]
- Metelski, P.D. Chemical Recycle of PET. Industrial Arene Chemistry: Markets, Technologies, Sustainable Processes and Cases Studies of Aromatic Commodities; John Wiley & Sons: Hoboken, NJ, USA, 2023; Volume 4, pp. 2101–2115. [Google Scholar] [CrossRef]
- Radadiya, R.; Shahabuddin, S.; Gaur, R. Waste to Best: Chemical Recycling of Polyethylene Terephthalate (PET) for Generation of Useful Molecules. In Tailored Functional Materials: Select Proceedings of MMETFP 2021; Springer: Berlin/Heidelberg, Germany, 2022; pp. 245–258. [Google Scholar] [CrossRef]
- Loccufier, E.; Debecker, D.P.; D’hooge, D.R.; De Buysser, K.; De Clerck, K. Fibrous Material Structure Developments for Sustainable Heterogeneous Catalysis—An Overview. ChemCatChem 2024, 16, e202301563. [Google Scholar] [CrossRef]
- Kratish, Y.; Li, J.; Liu, S.; Gao, Y.; Marks, T.J. Polyethylene Terephthalate Deconstruction Catalyzed by a Carbon-Supported Single-Site Molybdenum-Dioxo Complex. Angew. Chem. 2020, 132, 20029–20033. [Google Scholar] [CrossRef]
- Ye, M.; Li, Y.; Yang, Z.; Yao, C.; Sun, W.; Zhang, X.; Chen, W.; Qian, G.; Duan, X.; Cao, Y. Ruthenium/TiO2-Catalyzed Hydrogenolysis of Polyethylene Terephthalate: Reaction Pathways Dominated by Coordination Environment. Angew. Chem. 2023, 62, e202301024. [Google Scholar] [CrossRef]
- Kumar, A.; von Wolff, N.; Rauch, M.; Zou, Y.-Q.; Shmul, G.; Ben-David, Y.; Leitus, G.; Avram, L.; Milstein, D. Hydrogenative Depolymerization of Nylons. J. Am. Chem. Soc. 2020, 142, 14267–14275. [Google Scholar] [CrossRef]
- Fernandes, A.C. Reductive depolymerization as an efficient methodology for the conversion of plastic waste into value-added compounds. Green Chem. 2021, 23, 7330–7360. [Google Scholar] [CrossRef]
- Monsigny, L.; Berthet, J.-C.; Cantat, T. Depolymerization of Waste Plastics to Monomers and Chemicals Using a Hydrosilylation Strategy Facilitated by Brookhart’s Iridium(III) Catalyst. ACS Sustain. Chem. Eng. 2018, 6, 10481–10488. [Google Scholar] [CrossRef]
- Balaraman, E.; Gnanaprakasam, B.; Shimon, L.J.W.; Milstein, D. Direct Hydrogenation of Amides to Alcohols and Amines under Mild Conditions. J. Am. Chem. Soc. 2010, 132, 16756–16758. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.J.; Chang, G.S.; Kim, S.H.; Hahm, W.G.; Park, S.Y. Effects of recycling processes on physical, mechanical and degradation properties of PET yarns. Fibers Polym. 2013, 14, 2083–2087. [Google Scholar] [CrossRef]
- Gizem, C.; Gamze, D.T.; Fulya, Y. Limitations of Textile Recycling: The Reason behind the Development of Alternative Sustainable Fibers. In Next-Generation Textiles; Hassan, I., Ed.; IntechOpen: Rijeka, Italy, 2022. [Google Scholar] [CrossRef]
- Subramanian, K.; Chopra, S.S.; Cakin, E.; Li, X.T.; Lin, C.S.K. Environmental life cycle assessment of textile bio-recycling—valorizing cotton-polyester textile waste to pet fiber and glucose syrup. Resour. Conserv. Recy. 2020, 161, 104989. [Google Scholar] [CrossRef]
- ISO 14040; Environmental Management—Life Cycle Assessment—Principles and Framework. ISO: Geneva, Switzerland, 2006. Available online: https://www.iso.org/obp/ui/en/#iso:std:iso:14040:ed-2:v1:en (accessed on 1 January 2025).
- Shen, L.; Worrell, E.; Patel, M.K. Open-loop recycling: A LCA case study of PET bottle-to-fibre recycling. Resour. Conserv. Recy. 2010, 55, 34–52. [Google Scholar] [CrossRef]
- Fazio, S.; Castellani, V.; Sala, S.; Schau, E.M.; Secchi, M.; Zampori, L.; Diaconu, E. Supporting Information to the Characterisation Factors of Recommended EF Life Cycle Impact Assessment Methods; EU publications: Luxembourg, 2018; p. 42. Available online: https://data.europa.eu/doi/10.2760/002447 (accessed on 19 February 2025).
- Paunonen, S.; Kamppuri, T.; Katajainen, L.; Hohenthal, C.; Heikkila, P.; Harlin, A. Environmental impact of cellulose carbamate fibers from chemically recycled cotton. J. Clean. Prod. 2019, 222, 871–881. [Google Scholar] [CrossRef]
- Wernet, G.; Bauer, C.; Steubing, B.; Reinhard, J.; Moreno-Ruiz, E.; Weidema, B. The ecoinvent database version 3 (part I): Overview and methodology. Int. J. Life Cycle Assess. 2016, 21, 1218–1230. [Google Scholar] [CrossRef]
- Otto, K.N.; Wood, K.L. Product Design: Techniques in Reverse Engineering and New Product Development; Tsinghua University Press: Beijing, China, 2003; Available online: https://www.google.be/books/edition/Product_Design/0X54fSKq7bkC?hl=en&gbpv=0 (accessed on 19 February 2025).
- Varshney, P.; Swami, C. Sustainable Apparel Design for Longevity: Review and Analysis, Proceedings of online International Conference on Fashion Apparel & Textile (INCFAT ‘22) (2022) 27. Available online: https://www.amity.edu/asft/pdf/INCFAT22-proceedings.pdf#page=42 (accessed on 19 February 2025).
- Valuing Our Clothes: The True Cost of How We Design, Use and Dispose of Clothing in the UK; WRAP: Oxford, UK, 2012; Available online: https://www.wrap.ngo/resources/report/valuing-our-clothes-true-cost-how-we-design-use-and-dispose-clothing-uk-2012 (accessed on 19 February 2025).
- Niinimäki, K.; Peters, G.; Dahlbo, H.; Perry, P.; Rissanen, T.; Gwilt, A. The environmental price of fast fashion. Nat. Rev. Earth Environ. 2020, 1, 189–200. [Google Scholar] [CrossRef]
- Resortecs. Available online: https://resortecs.com/technology/ (accessed on 1 January 2025).
- Wear2. The Circular Process. Available online: https://wear2.com/en/various/the-circular-process/ (accessed on 1 January 2025).
- Laitala, K.; Klepp, I.G. Care and Production of Clothing in Norwegian Homes: Environmental Implications of Mending and Making Practices. Sustainability 2018, 10, 2899. [Google Scholar] [CrossRef]
- Arrigo, E.; Flavio, G. Take-Back Programs for Fashion Brands’ Garments in Sustainable Manufacturing Systems. In Sustainable Manufacturing Practices in the Textiles and Fashion Sector; Muthu, S.S., Ed.; Springer Nature: Cham, Switzerland, 2024; pp. 95–102. [Google Scholar] [CrossRef]
- Circular Business Models for Fashion and Textiles. Wrap. Available online: https://www.wrap.ngo/taking-action/textiles/actions/circular-business-models-fashion-textiles (accessed on 19 February 2025).
- Leonas, K.K. The Use of Recycled Fibers in Fashion and Home Products. In Textiles and Clothing Sustainability: Recycled and Upcycled Textiles and Fashion; Muthu, S.S., Ed.; Springer: Singapore, 2017; pp. 55–77. [Google Scholar] [CrossRef]
- Singh, S.; Jana, P. Decoding the Science Behind the Chemical Recycling of Textiles. In Functional Textiles and Clothing 2023; Springer: Singapore, 2024; pp. 295–320. [Google Scholar] [CrossRef]



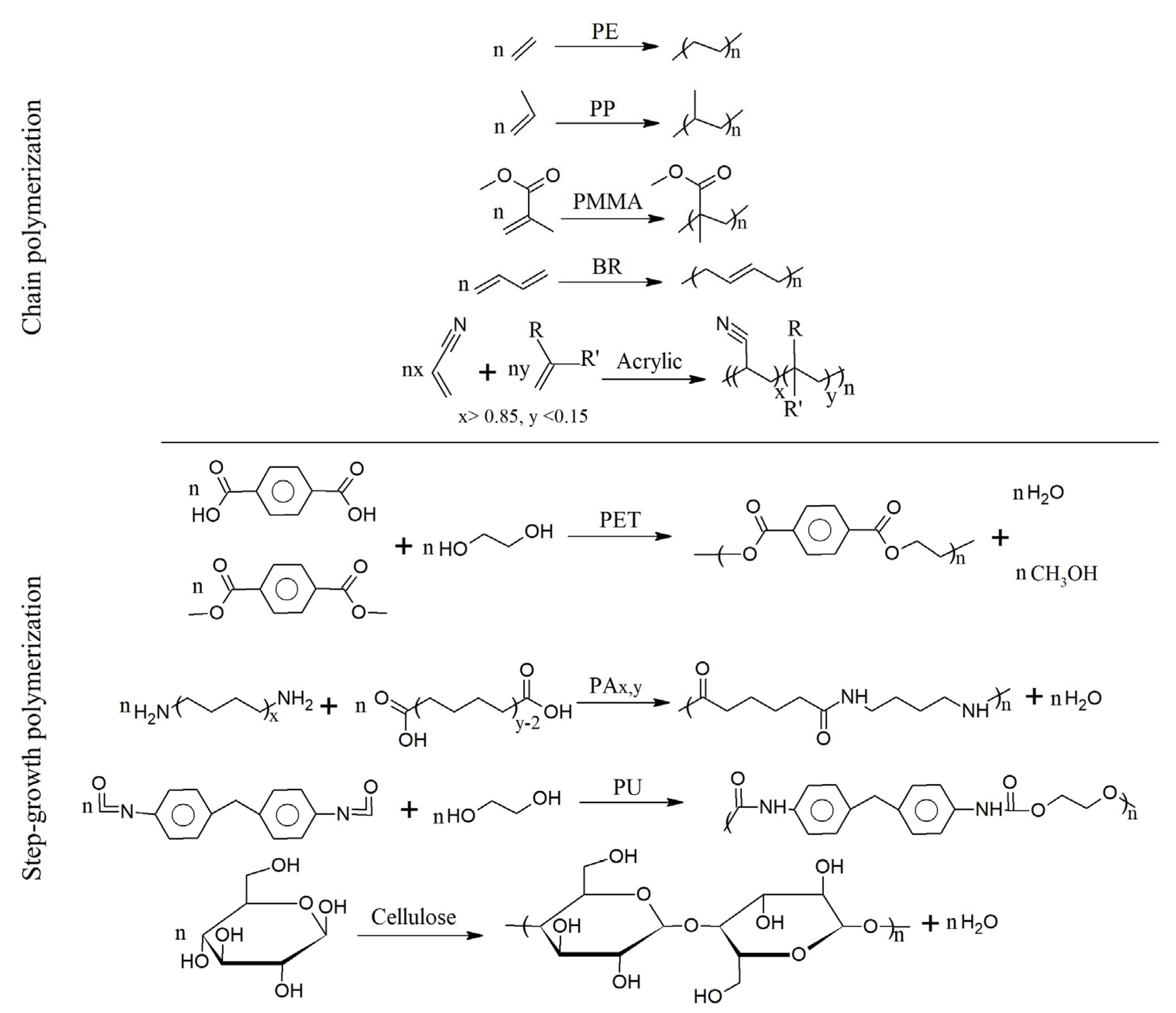
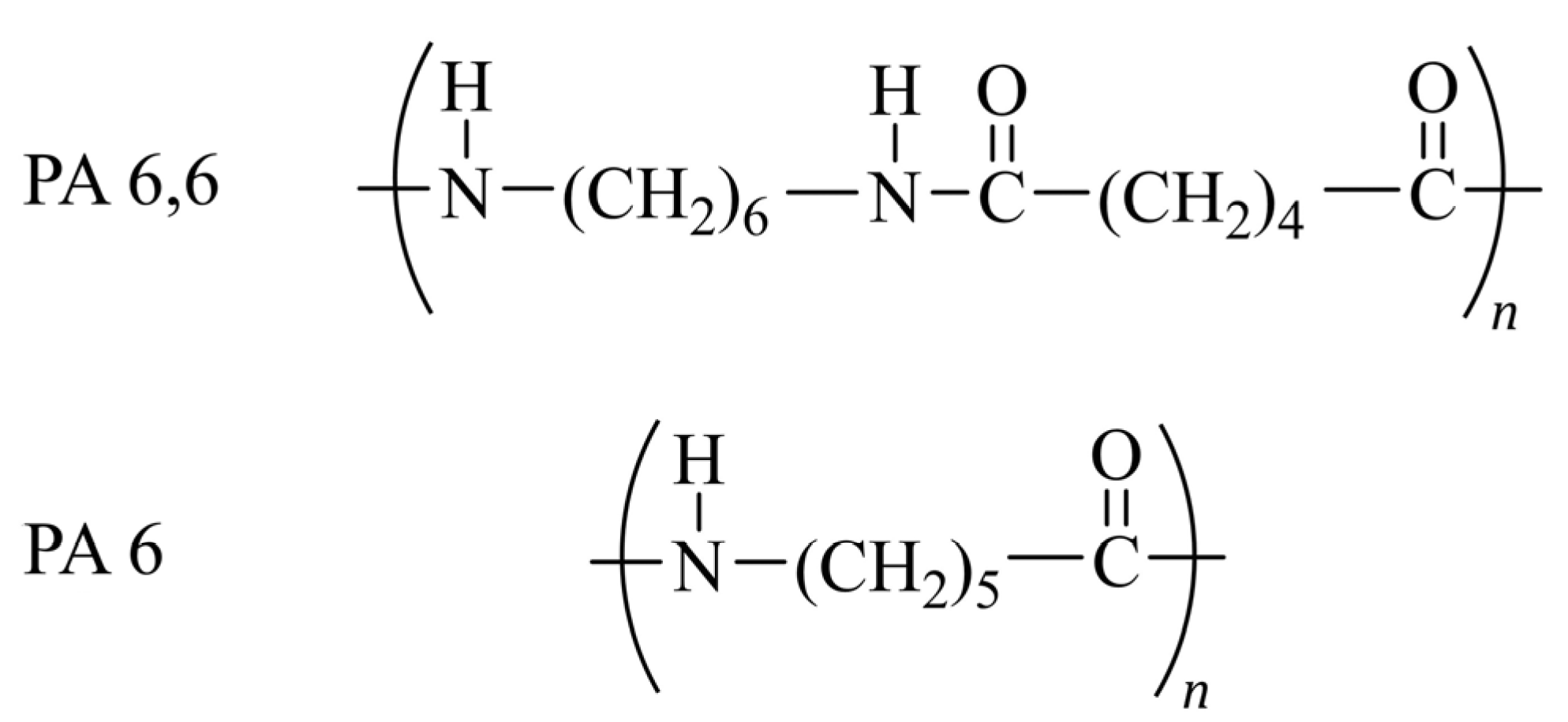
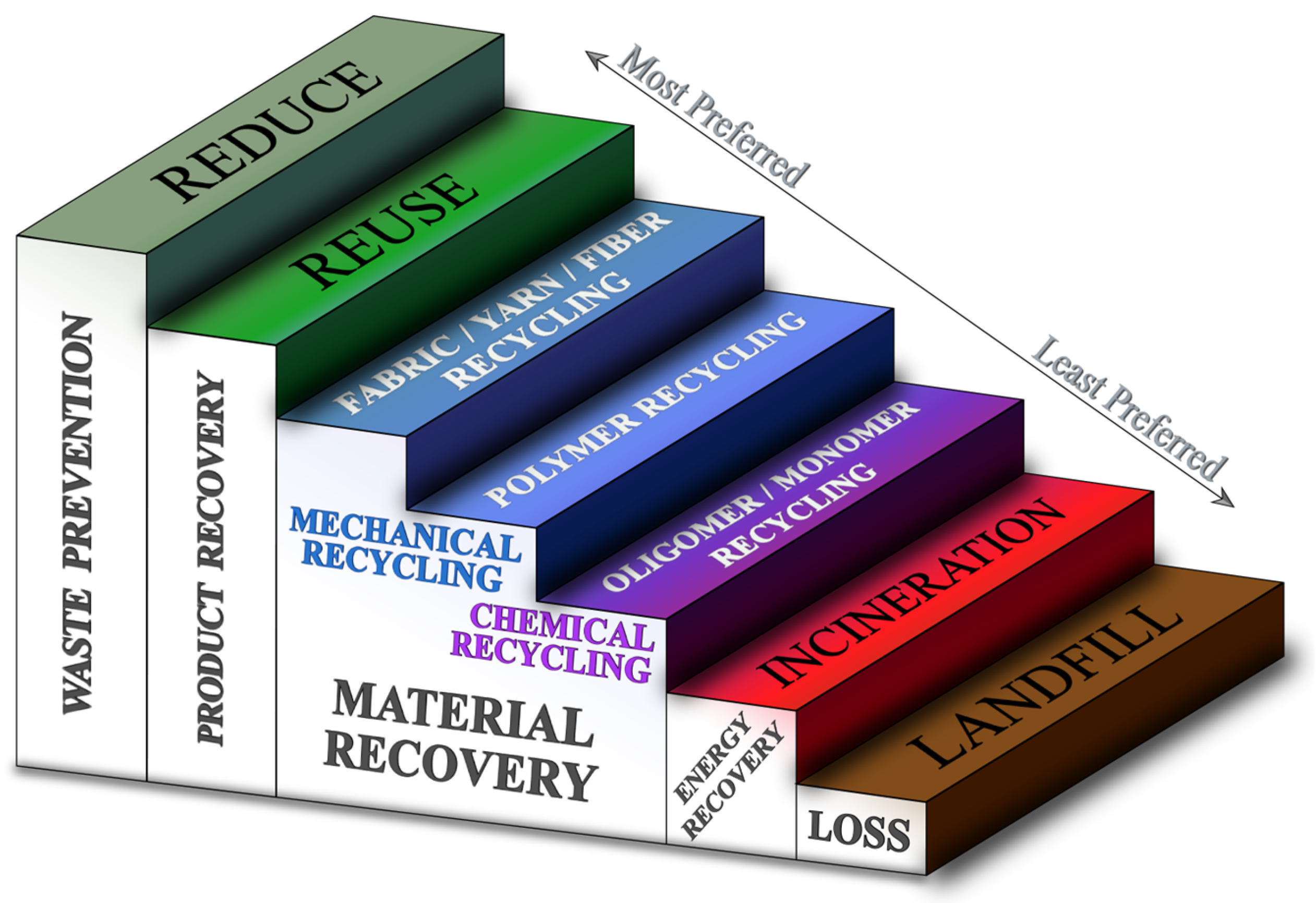


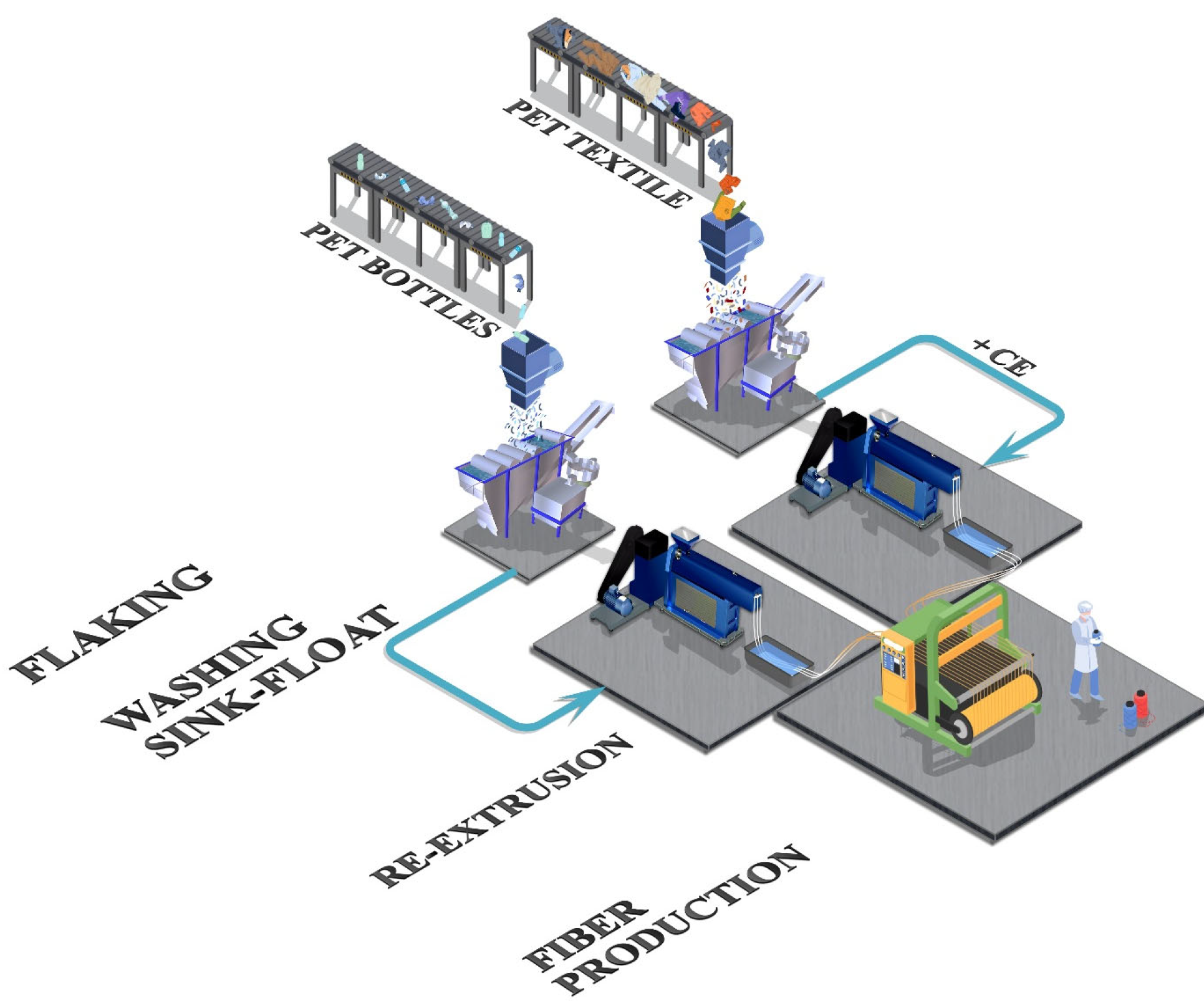
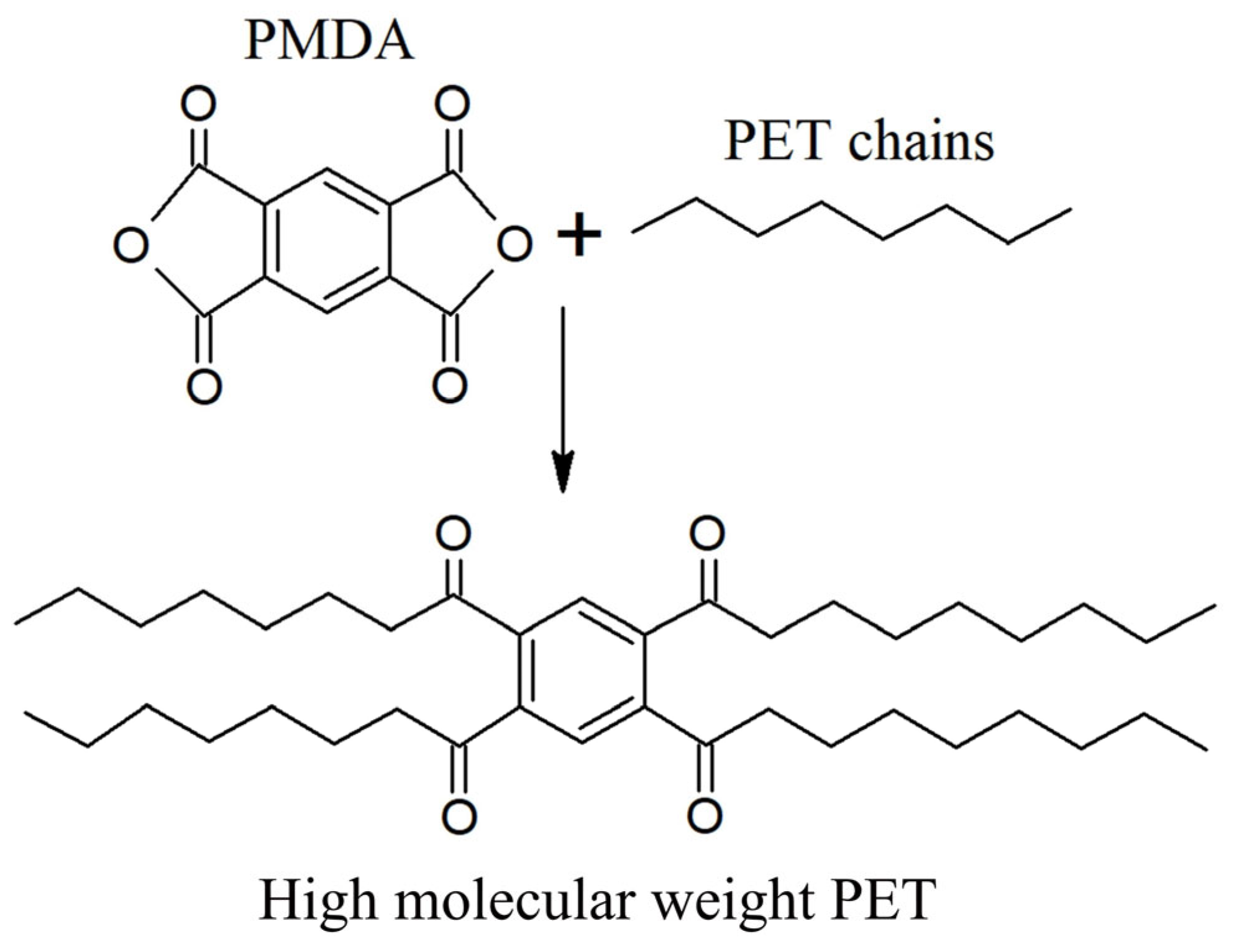
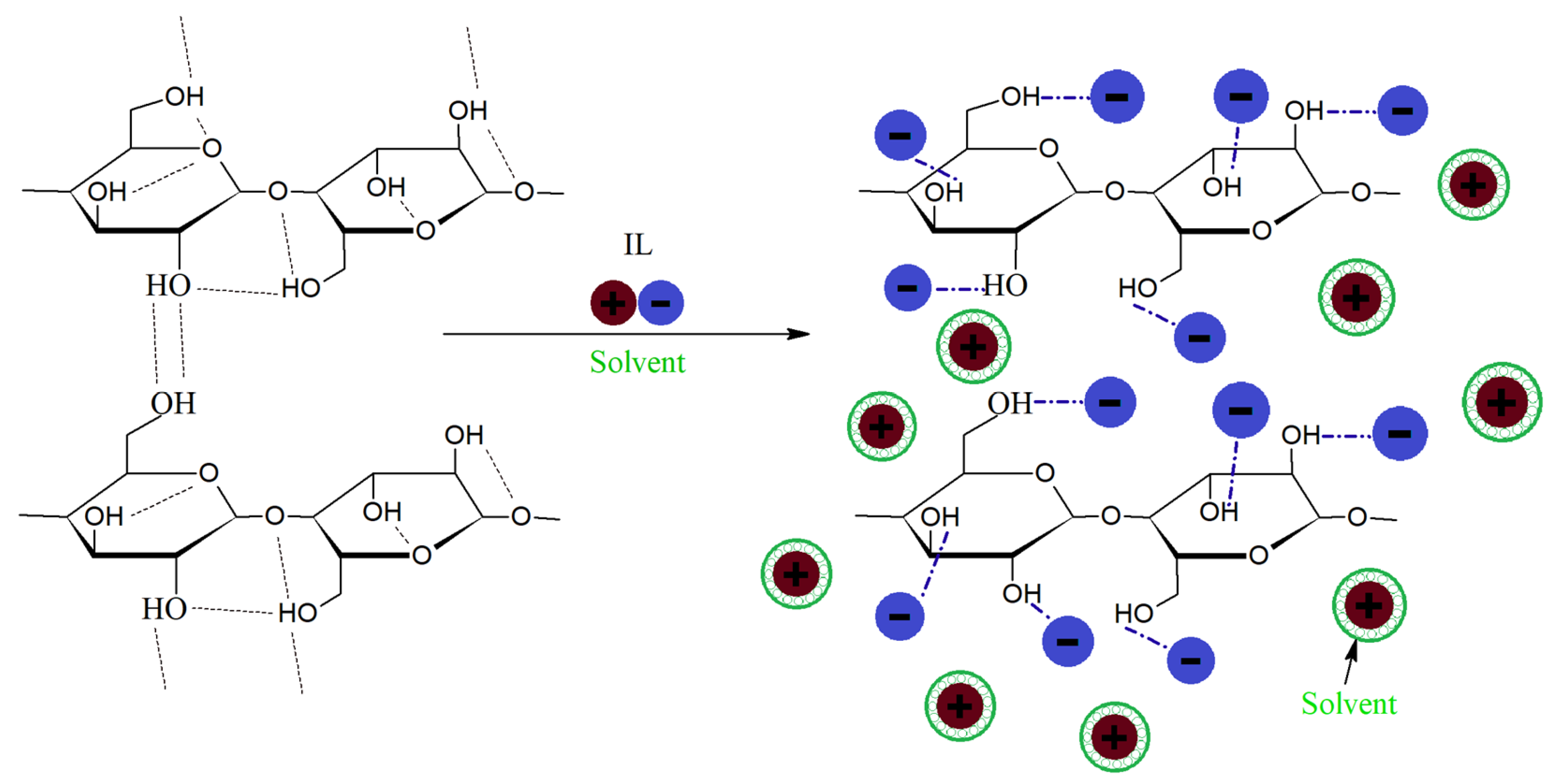

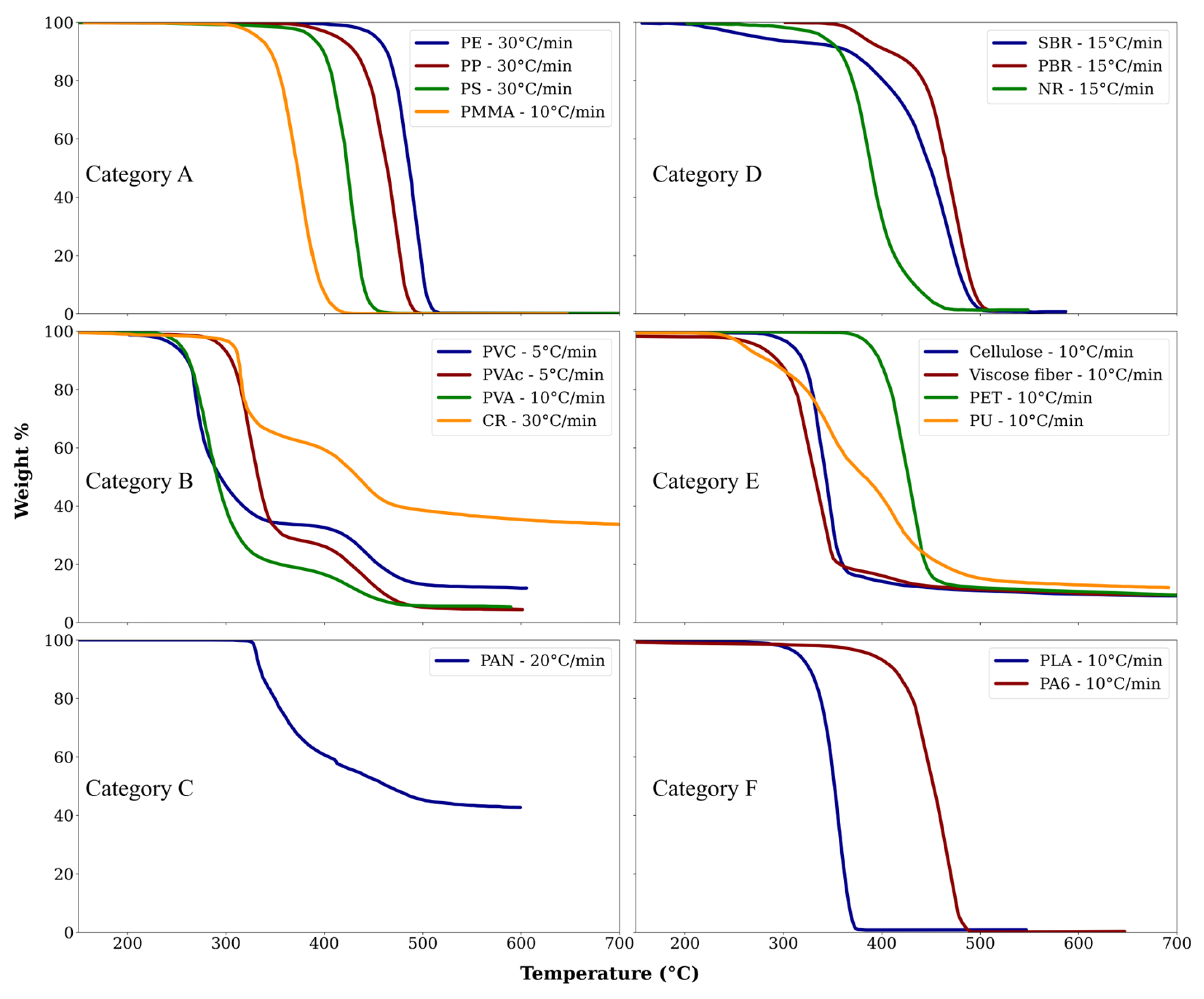
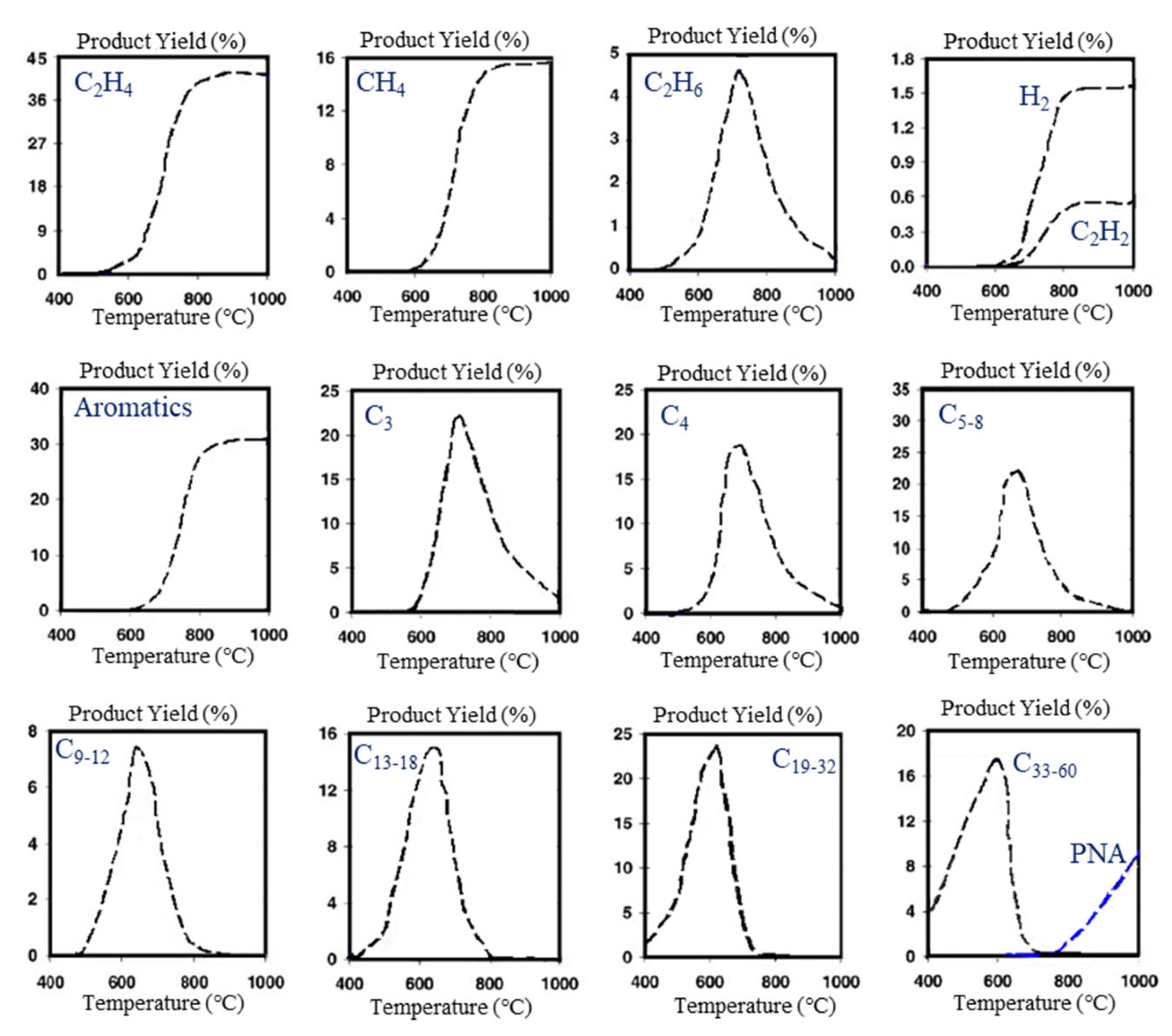

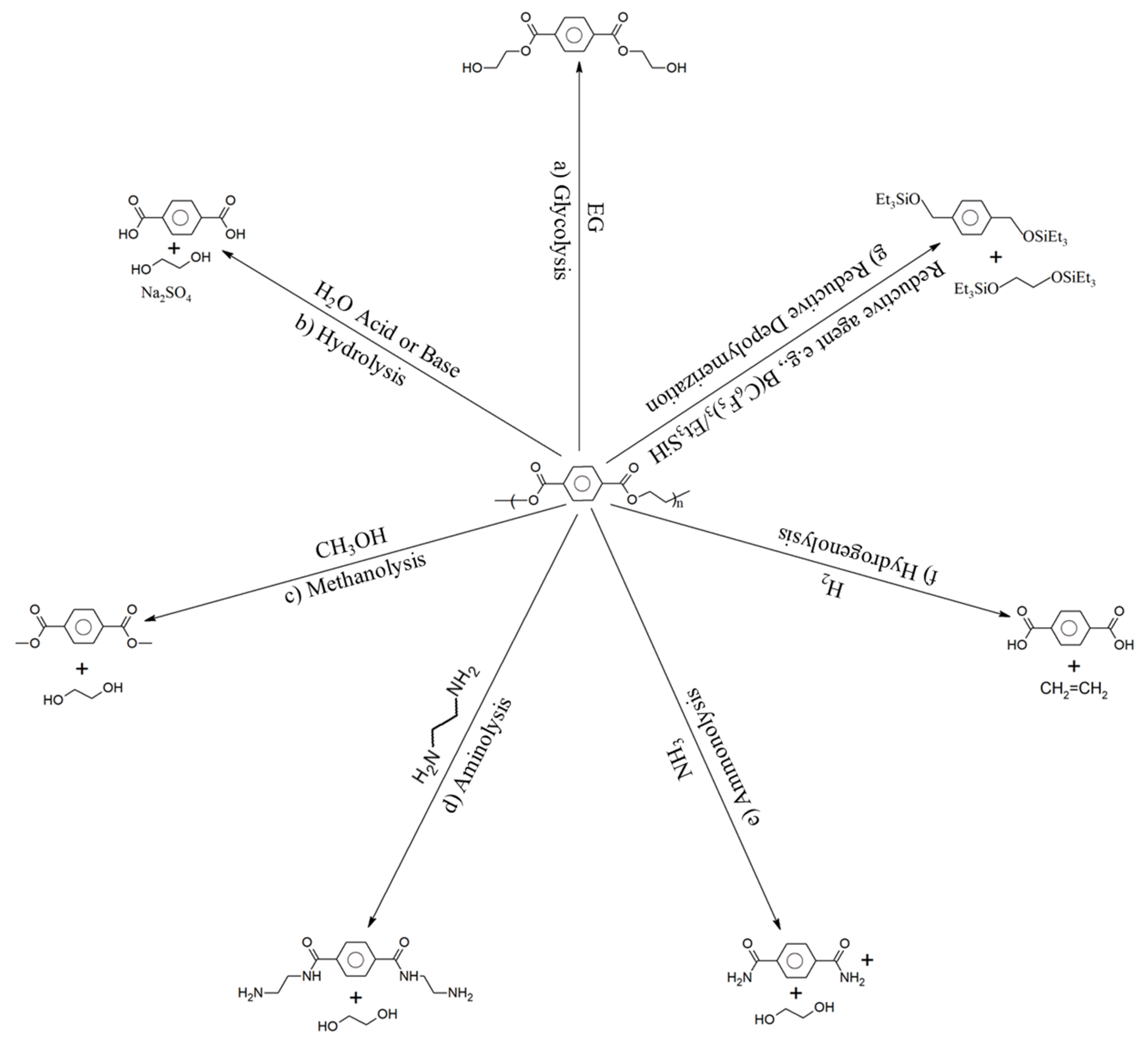
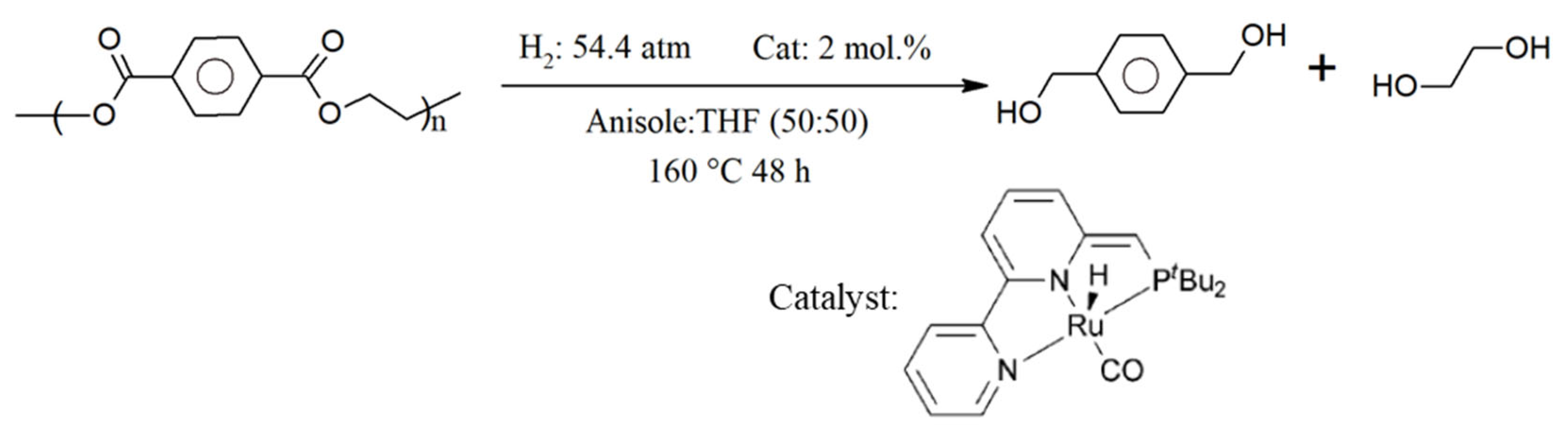


| Fiber | Solvent | Feed/Solvent | Process Conditions | Complementary Processes and Pretreatments | Products | Advantages | Ref. |
|---|---|---|---|---|---|---|---|
| Blends of polyester and cotton | AMIMCl 1 | 2–10 wt% | 80 °C, 6 h | - | Cotton, polyester | The IL was recovered. Cotton can be regenerated using water as the coagulated solvent. Selective dissolution of the cotton component using ionic liquid. Fully recovered and almost-pure polyester and cotton. | [376] |
| An orange 50:50 polyester–cotton blend and a blue 40:60 polyester–viscose blend | NMMO 2 | 18 wt% | 120 °C, 2 h | Two-day enzymatic hydrolysis (yield: 85%) and one-day fermentation (yield: 89%) of the man-made cotton and viscose | Polyester, cotton, viscose, ethanol, biogas | The polyesters were purified as fibers after the NMMO treatments. Up to 95% of the cellulose fibers were regenerated and collected. It is possible to recover the solvent efficiently. This process might be economically feasible. Possible NMMO degradation and cellulose oxidation, and requires antioxidants for stabilization. | [377] |
| Waste polyester-cotton jeans | AmimCl 3 | 4 wt% | 80 °C, 3 h | - | PET, cotton | Cotton components can be directly dissolved and regenerated. The recovered PET fibers can be recycled and reused in textiles. The resulting cellulose solution dope can be used for dry-jet wet spinning to obtain lyocell-type fibers. | [378] |
| Dyed PET, cotton, PA6,6 fibers | DMSO, ethylene carbonate, glycerol, tetramethylurea | 20 wt% | 80–100 °C, 0.5 h | - | Decolorized fibers | One hundred percent of the disperse dyes, acid dyes, and direct dyes were separated from PET, PA6,6, and cotton, respectively. Dye removal did not change the structures or dyeability of the dyes. The molecular weights of the polymers after dye removal were almost the same. | [362] |
| Colored waste garment | [Bmim]OAc/DMSO | Max. 18 wt% | 80 °C, 0.5 h | NaOH hydrolysis, wet spinning | Decolorized fibers | The solubility of a solvent system is also strongly influenced by the molecular weight of the cellulose. The use of a binary solvent containing a low IL amount reduces the drawbacks (e.g., high dope viscosity). The binary solvent reduces the cost of the solvent by 77%. The solvent can be recovered and reused via distillation. | [349] |
| Blue waste jeans (80/20 cotton/polyester) | DMCHA 4 | 1/40–1/120 g·mL−1 | 50 °C, full dissolution | Dye leaching (HNO3) | Decolorized cotton fiber and polyester with high purity | A green dissolution process was employed to dissolve polyester. Polyester was extracted from the solution by changing the hydrophilicity of the solvent. The recycling rate of the technology was >96%. The economic returns are up to $1629/ton of waste, and the carbon footprint is reduced by 1440 kg of CO2-eq/t of waste. | [284] |
| Polymer | Method | Agent | Catalyst | Catalyst: Polymer | T (°C) | t (min) | Reactor | P (atm) | Yield % | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| PET | Glycolysis | EG | Di-n-butylamine | 1:4 (mol%) | 160 | 90 | Three-necked flask | 1 | 76.8 BHET | [508] |
| Fe3O4-boosted 1 MWCNT | 1:19 (wt%) | 190 | 120 | Stainless steel cylinder | 1 | 100 BHET | [509] | |||
| 2 TBD:3 MSA | 1:99 (wt%) | 180 | 120 | Schlenck flask | 1 | 91.0 BHET | [510] | |||
| 4 POM:5 WZn3 | 1:2 (wt%) | 190 | 40 | Three-necked flask | 84.5 BHET | [511] | ||||
| (Dimim)[FeCl4] | 1:4 (wt%) | 170 | 120 | Three-necked flask | 1 | 99.0 BHET | [512] | |||
| 6 [Ch][For] | 1:19 (wt%) | 180 | 180 | Three-necked flask | 1 | 84.5 BHET | [513] | |||
| 7 [Ch][OAC] | 1:19 (wt%) | 180 | 240 | 85.2 BHET | ||||||
| MnO2/holey GO nanosheets | 1:10,000 (wt%) | 200 | 10 | Three-necked flask | 100 BHET | [514] | ||||
| Acetaminde/ZnCl2 | 1:250 (wt%) | 195 | 25 | Three-necked flask | 1 | 83.2 BHET | [515] | |||
| DES@Zif-8 | ||||||||||
| Orange peel ash | 1:10 (wt%) | 190 | 90 | Two-necked flask | 1 | 79.0 BHET | [516] | |||
| Fe3O4@SiO2@ | 3:20 (wt%) | 180 | 1440 | Round bottom flask | 100 BHET | [517] | ||||
| (mim)[FeCl4] | ||||||||||
| RZnO | 1:100 (wt%) | 196 | 120 | Three-necked flask | 50.0 BHET | [518] | ||||
| Co/RZnO | 1:100 (wt%) | 196 | 120 | 80.0 BHET | ||||||
| Methanolysis | MeOH | BLA | 1:5 (wt%) | 200 | 120 | Autoclave | 1 | 78.0 DMT | [519] | |
| Modified ZnO | 7:1000 (wt%) | 170 | 15 | Autoclave | 95.0 DMT | [520] | ||||
| PIL-Zn2+ | 1:50 (wt%) | 170 | 60 | Bottle reactor | 90.3 DMT | [521] | ||||
| MeOH/CH2Cl2 | K2CO3 | 1:5 (mol%) | 25 | 1440 | Round bottom flask | 1 | 93.1 DMT | [501] | ||
| CH3OK | 1:5 (mol%) | 25 | 1440 | 81.9 DMT | ||||||
| Hydrolysis | H2O | Marine water (metallic ions) | 7:20 (wt%) | 205 | 120 | Stirred reactor | 32.5 | 96.0 TPA | [522] | |
| NaHCO3 + KHCO3 | 8:20 (wt%) | 195 | 120 | Stirred reactor | 34.5 | 95.7 TPA | ||||
| - | - | 230 | 30 | Microwave | ~29 | 77.0 TPA | [523] | |||
| - | - | 200 | 30 | Microwave | 12.0 TPA | |||||
| H+@ZSM-5 | 1:2 (wt%) | 230 | 30 | Microwave | 98.5 TPA | |||||
| H+@ZSM-5 | 1:2 (wt%) | 200 | 30 | Microwave | 87.0 TPA | |||||
| Aminolysis | Hexyl-amine | - | - | 180 | 30 | Microwave | 1 | 64.0 8 DHTA | [524] | |
| Ethanol-amine | - | - | 200 | 10 | Microwave | 91.0 9 BHTA | ||||
| Furfuryl-amine | - | - | 200 | 60 | Microwave | 82.0 10 BFTA | ||||
| Allyl-amine | - | - | 280 | 15 | Microwave | 61.0 11 DAA | ||||
| Isophorenediamine | Zn(OAc)2 | 1:10 (wt%) | 200 | 240 | Four-necked flask | 90.0 TPA | [525] | |||
| Ethanol-amine | 12 mHpb | 5:32 (wt%) | 190 | 3 | Three-necked flask | 1 | 100 13 BHETA | [526] | ||
| Ethanol-amine | TBD | 7:192 (wt%) | 120 | 120 | Schlenk flask | 1 | 93.0 TPA | [527] | ||
| Reductive depolymerization | H2 | Ruthenium(II) PNN pincer | 2:100 (mol%) | 160 | 2880 | Schlenk tube | 54.4 | >99 TPA, EG | [528] | |
| Et3SiH | B(C6F5)3 | 2:100 (mol%) | 25 | 180 | Stirred reactor | 91.0 BDM-SI | [529] | |||
| Hydrogenolysis | H2 | CoMo@NC | 3:10 (wt%) | 260 | 1200 | Sealed tube | 1 | 91.0 TPA | [530] | |
| H2 | Hf-(OTf)4: Pd/C | Pd/Hf/PET: 1:6:400 | 180 | 1440 | Schlenk flask | 1 | 95–98 TPA | [531] | ||
| PA6 | 14 IL | - | - | 280 | 60 | Microwave | 1 | 0 caprolactam | [532] | |
| IL | - | - | 300 | 60 | Microwave | 36 caprolactam | ||||
| IL | 15 DMAP | 1:10 (wt%) | 300 | 60 | Microwave | 55 caprolactam | ||||
| IL | DMAP | 1:10 (wt%) | 300 | 30 | Microwave | 24 caprolactam | ||||
| IL | DMAP | 1:10 (wt%) | 310 | 60 | Microwave | 54 caprolactam | ||||
| Acid hydrolysis | HCl | - | - | 80 | 120 | Round bottom flask | 1 | 72.2 16 DBHMD | [533] |
| Fiber Type | |||
|---|---|---|---|
| Closed-loop end-of-life option | Natural fibers | Synthetic fibers | Blends elastane + cotton/polyamide/PET |
| Reuse | If textiles or fibers are reusable, they are a more suitable option than the other alternatives [189]. | ||
| Fiber recycling | Good option, but shortens fiber length [60,270,310] | Good option, but needs high purity and shortens fiber length [289,567,568] | Elastane makes this incompatible [312,315]. |
| Polymer recycling (re-extrusion) via melting | - | Good option [322,325,326] | - |
| Polymer recycling (re-extrusion) via dissolution | Good option [287,365] | Good option for removing additives and contaminants in polymers, but high energy consumption; under research [194,359,362] | Good option for separating cotton [40,364] |
| Monomer recycling via solvolysis | - | Good option for pure PET and polyamide [499,500,515,532,533] | Selectivity elastane vs. PET needed (complicated but possible) [59,536,538] |
| Monomer recycling via pyrolysis | - | Good option for polyolefins [434,437,438,441] | - |
| Monomer recycling via biological processes | Good option [419,421,426,428] | - | - |
| Monomer recycling via gasification | When options are limited due to low purity and high degree of contamination, gasification can be a suitable alternative [467,469,470,471]. | ||
| Combination of dissolution + solvolysis | - | - | Good option [289,536] |
| Combination of biological processes + solvolysis | - | - | Good option [66,536] |
| S/L Ratio | Estimated GHG Emissions [kg CO2 eq/kg of Recycled PET] |
|---|---|
| 0.02 | 5.6 |
| 0.03 | 3.9 |
| 0.04 | 3.0 |
| 0.05 | 2.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seifali Abbas-Abadi, M.; Tomme, B.; Goshayeshi, B.; Mynko, O.; Wang, Y.; Roy, S.; Kumar, R.; Baruah, B.; De Clerck, K.; De Meester, S.; et al. Advancing Textile Waste Recycling: Challenges and Opportunities Across Polymer and Non-Polymer Fiber Types. Polymers 2025, 17, 628. https://doi.org/10.3390/polym17050628
Seifali Abbas-Abadi M, Tomme B, Goshayeshi B, Mynko O, Wang Y, Roy S, Kumar R, Baruah B, De Clerck K, De Meester S, et al. Advancing Textile Waste Recycling: Challenges and Opportunities Across Polymer and Non-Polymer Fiber Types. Polymers. 2025; 17(5):628. https://doi.org/10.3390/polym17050628
Chicago/Turabian StyleSeifali Abbas-Abadi, Mehrdad, Brecht Tomme, Bahman Goshayeshi, Oleksii Mynko, Yihan Wang, Sangram Roy, Rohit Kumar, Bhargav Baruah, Karen De Clerck, Steven De Meester, and et al. 2025. "Advancing Textile Waste Recycling: Challenges and Opportunities Across Polymer and Non-Polymer Fiber Types" Polymers 17, no. 5: 628. https://doi.org/10.3390/polym17050628
APA StyleSeifali Abbas-Abadi, M., Tomme, B., Goshayeshi, B., Mynko, O., Wang, Y., Roy, S., Kumar, R., Baruah, B., De Clerck, K., De Meester, S., D’hooge, D. R., & Van Geem, K. M. (2025). Advancing Textile Waste Recycling: Challenges and Opportunities Across Polymer and Non-Polymer Fiber Types. Polymers, 17(5), 628. https://doi.org/10.3390/polym17050628












