Chemically Polymerized Polypyrrole on Glucose-Porcine Skin Gelatin Nanofiber as Multifunctional Electrochemical Actuator-Sensor-Capacitor
Abstract
1. Introduction
2. Materials and Methods
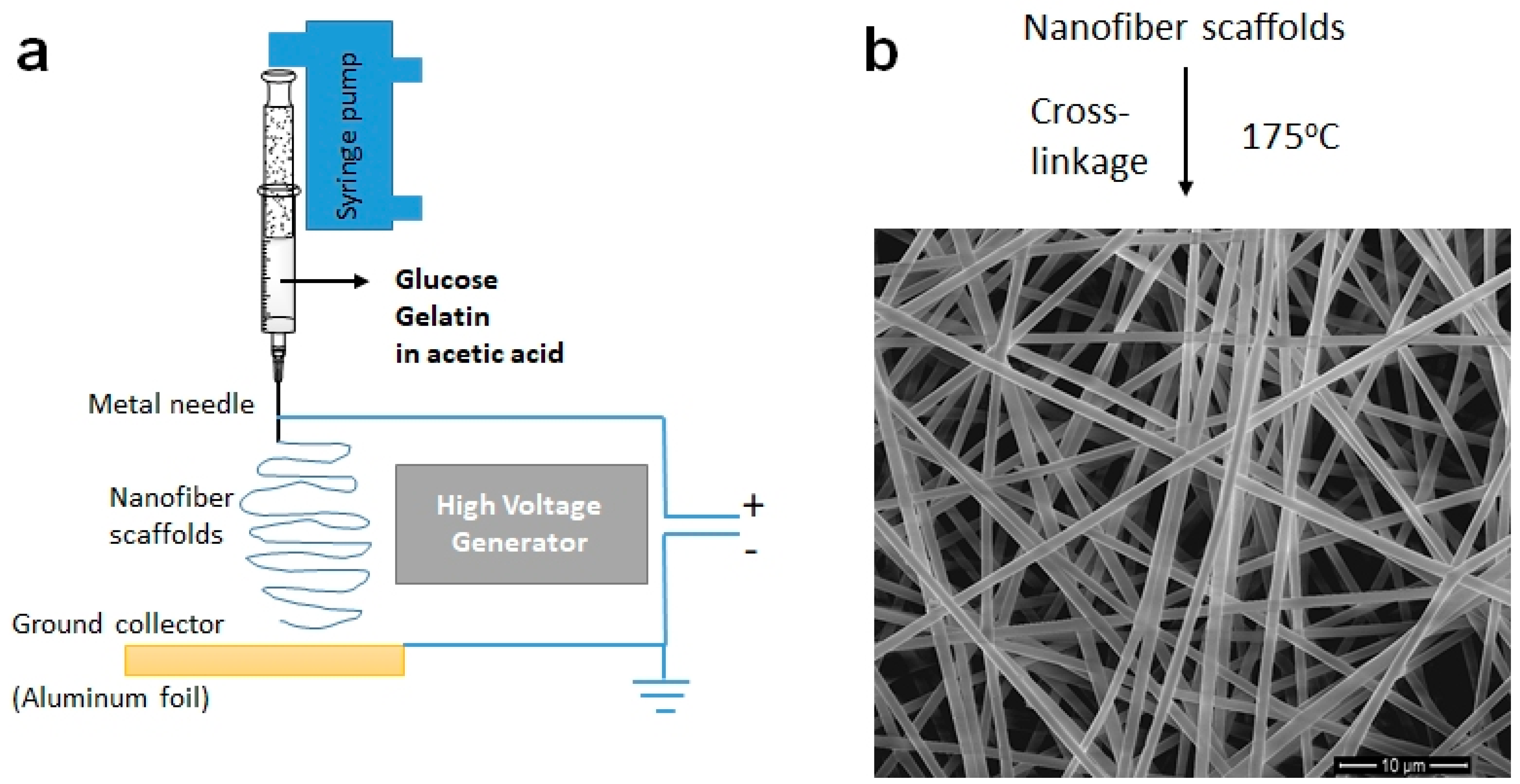
2.1. Getting NFs and Tissues
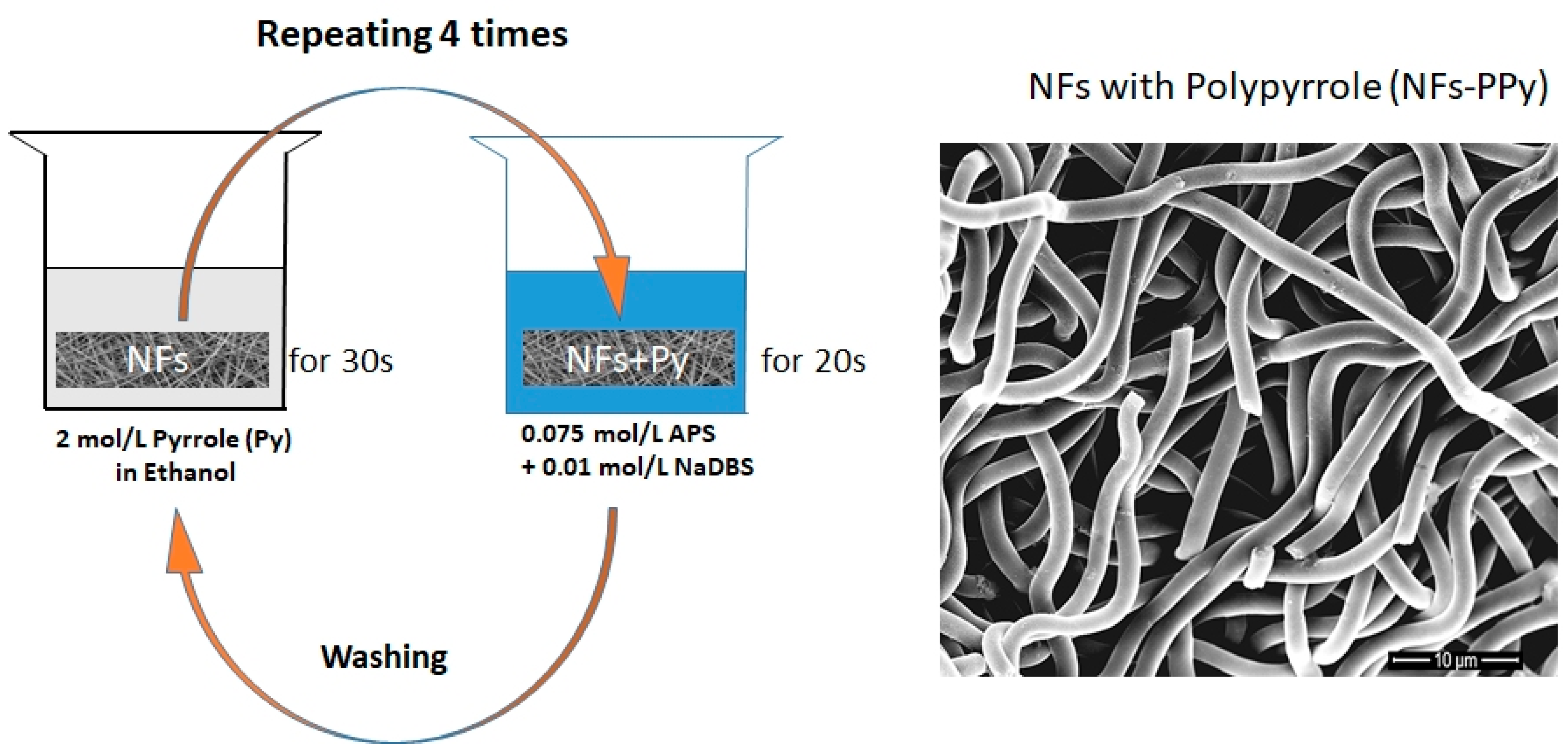
2.2. Coating the NFs with Chemically Oxidized PPy
2.3. ECMD Measurements of NFs-PPy
2.4. Microscopic, FTIR, EDX and Conductivity Characterizations
3. Results and Discussions
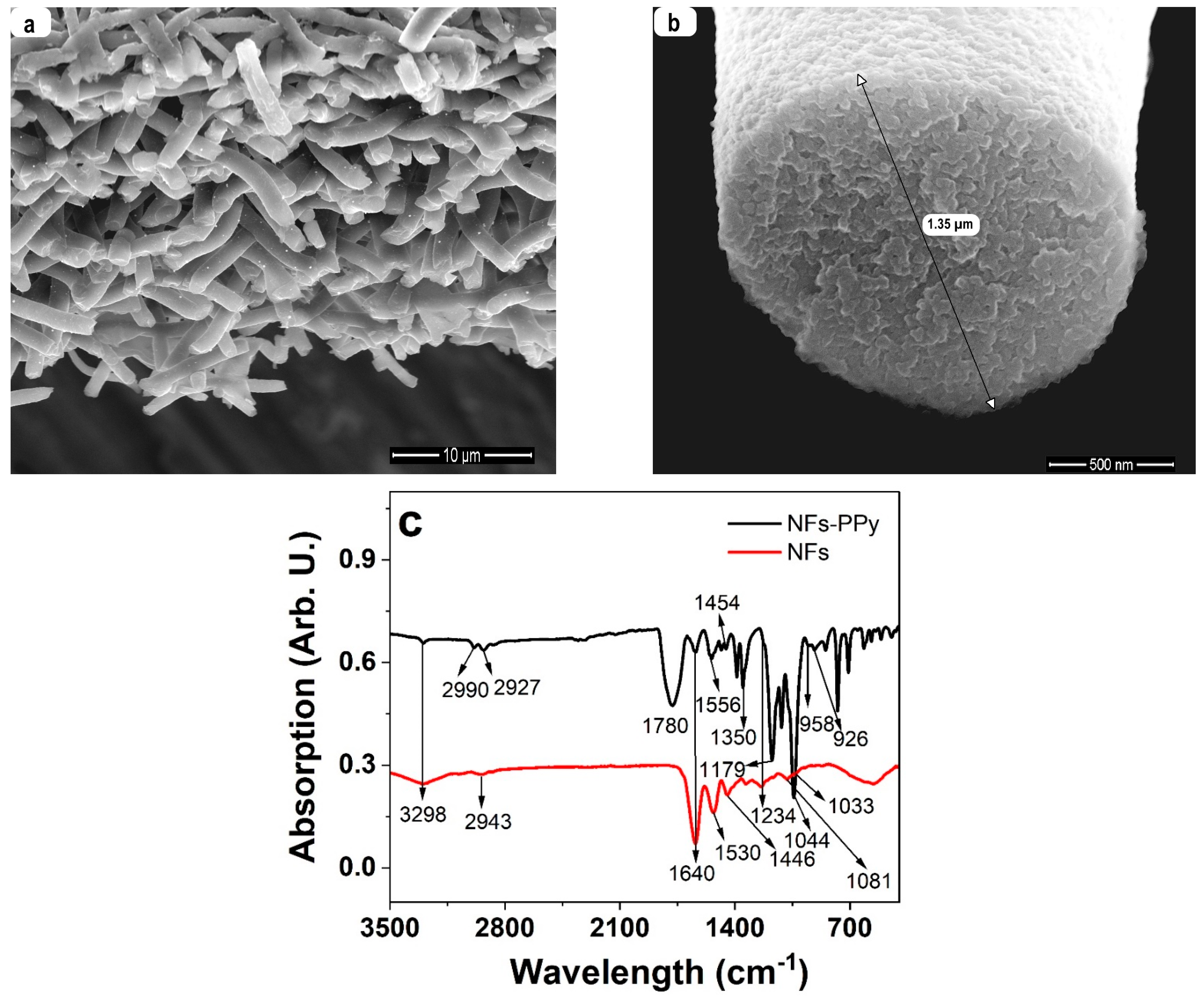
3.1. Characterization of NFs-PPy
3.2. Isometric and Isotonic ECMD Measurements
3.3. Chronopotentiometric Measurements of NFs-PPy
3.3.1. Energy Storage
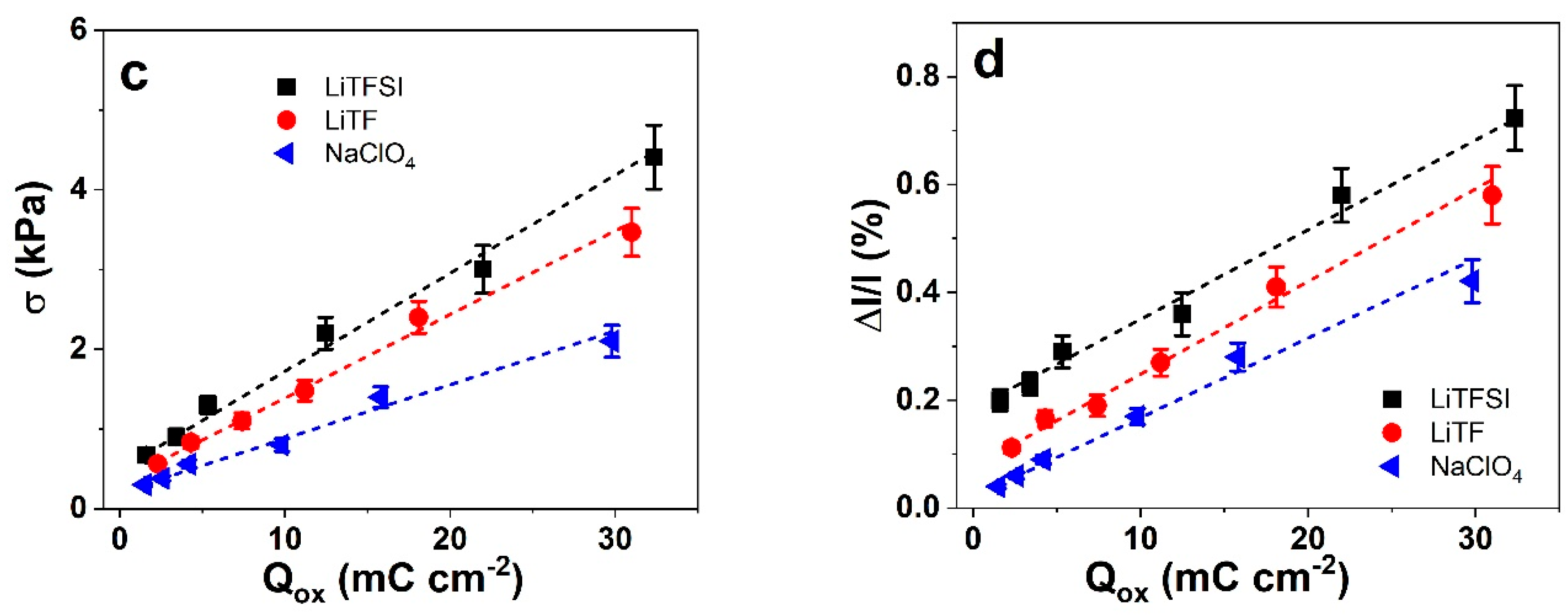
3.3.2. Electrical Sensor Calibrations in Different Electrolytes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Melling, D.; Martinez, J.G.; Jager, E.W.H. Conjugated Polymer Actuators and Devices: Progress and Opportunities. Adv. Mater. 2019, 31, 1808210. [Google Scholar] [CrossRef] [PubMed]
- Jager, E.W.H.; Inganas, O.; Lundstrom, I. Microrobots for Micrometer-Size Objects in Aqueous Media: Potential Tools for Single-Cell Manipulation. Science (80-) 2000, 288, 2335–2338. [Google Scholar] [CrossRef]
- García-Córdova, F.; Guerrero-González, A.; Zueco, J.; Cabrera-Lozoya, A. Simultaneous Sensing and Actuating Capabilities of a Triple-Layer Biomimetic Muscle for Soft Robotics. Sensors 2023, 23, 9132. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, A.; Han, Y.; Li, T. Sensors Based on Conductive Polymers and Their Composites: A Review. Polym. Int. 2020, 69, 7–17. [Google Scholar] [CrossRef]
- Shi, Y.; Peng, L.; Ding, Y.; Zhao, Y.; Yu, G. Nanostructured Conductive Polymers for Advanced Energy Storage. Chem. Soc. Rev. 2015, 44, 6684–6696. [Google Scholar] [CrossRef] [PubMed]
- Kiefer, R.; Harjo, M.; Otero, T.F.; Nguyen, N.T.; Le, Q.B. Linear Sensing Actuators of Polypyrrole Nanofiber Scaffolds. J. Appl. Polym. Sci. 2024, 141, e55300. [Google Scholar] [CrossRef]
- Hara, S.; Zama, T.; Takashima, W.; Kaneto, K. Artificial Muscles Based on Polypyrrole Actuators with Large Strain and Stress Induced Electrically. Polym. J. 2004, 36, 151–161. [Google Scholar] [CrossRef]
- Zama, T.; Tanaka, N.; Takashima, W.; Kaneto, K. Fast and Large Stretching Bis(trifluoromethylsulfonyl)imide (TFSI)-Doped Polypyrrole Actuators and Their Applications to Small Devices. Polym. J. 2006, 38, 669–677. [Google Scholar] [CrossRef]
- Hara, S.; Zama, T.; Takashima, W.; Kaneto, K. Free-Standing Polypyrrole Actuators with Response Rate of 10.8% S−1. Synth. Met. 2005, 149, 199–201. [Google Scholar] [CrossRef]
- Sadki, S.; Schottland, P.; Brodie, N.; Sabouraud, G. The Mechanisms of Pyrrole Electropolymerization. Chem. Soc. Rev. 2000, 29, 283–293. [Google Scholar] [CrossRef]
- Heinze, J.; Frontana-Uribe, B.A.; Ludwigs, S. Electrochemistry of Conducting PolymerssPersistent Models and New Concepts. Chem. Rev. 2010, 110, 4724–4771. [Google Scholar] [CrossRef]
- Yoon, C.O.; Sung, H.K.; Kim, J.H.; Barsoukov, E.; Kim, J.H.; Lee, H. The Effect of Low-Temperature Conditions on the Electrochemical Polymerization of Polypyrrole Films with High Density, High Electrical Conductivity and High Stability. Synth. Met. 1999, 99, 201–212. [Google Scholar] [CrossRef]
- Harjo, M.; Zondaka, Z.; Leemets, K.; Järvekülg, M.; Tamm, T.; Kiefer, R. Polypyrrole-Coated Fiber-Scaffolds: Concurrent Linear Actuation and Sensing. J. Appl. Polym. Sci. 2020, 137, 48533. [Google Scholar] [CrossRef]
- Stejskal, J.; Trchová, M. Conducting Polypyrrole Nanotubes: A Review; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; Volume 72, ISBN 0123456789. [Google Scholar]
- Hsu, C.F.; Zhang, L.; Peng, H.; Travas-Sejdic, J.; Kilmartin, P.A. Scavenging of DPPH Free Radicals by Polypyrrole Powders of Varying Levels of Overoxidation And/or Reduction. Synth. Met. 2008, 158, 946–952. [Google Scholar] [CrossRef]
- Trung, V.Q.; Hung, H.M.; Van Khoe, L.; Duc, L.M.; Bich Viet, N.T.; Linh, D.K.; Huong, V.T.; Dat, N.D.; Yen Oanh, D.T.; Luong, N.X.; et al. Synthesis and Characterization of Polypyrrole Film Doped with Both Molybdate and Salicylate and Its Application in the Corrosion Protection for Low Carbon Steel. ACS Omega 2022, 7, 19842–19852. [Google Scholar] [CrossRef]
- Bay, L.; Jacobsen, T.; Skaarup, S.; West, K. Mechanism of Actuation in Conducting Polymers: Osmotic Expansion. J. Phys. Chem. B 2001, 105, 8492–8497. [Google Scholar] [CrossRef]
- Otero, T.F.; Boyano, I. Comparative Study of Conducting Polymers by the ESCR Model. J. Phys. Chem. B 2003, 107, 6730–6738. [Google Scholar] [CrossRef]
- Otero, T.F.; Martinez, J.G. Physical and Chemical Awareness from Sensing Polymeric Artificial Muscles. Experiments and Modeling. Prog. Polym. Sci. 2015, 44, 62–78. [Google Scholar] [CrossRef]
- Misir, M.; Aydogdu, N.; Demir, E. Polymer Functionalized Materials for Design of Electrochemical Sensing Devices. In Real-Time Applications of Advanced Electrochemical Sensing Devices; Manjunatha, J.G., Ed.; IOP Publishing Ltd.: Bristol, UK, 2024; pp. 1–35. [Google Scholar]
- Siimon, K.; Reemann, P.; Pōder, A.; Pook, M.; Kangur, T.; Kingo, K.; Jaks, V.; Möeorg, U.; Järvekülg, M. Effect of Glucose Content on Thermally Cross-Linked Fibrous Gelatin Scaffolds for Tissue Engineering. Mater. Sci. Eng. C 2014, 42, 538–545. [Google Scholar] [CrossRef]
- Harjo, M.; Tamm, T.; Anbarjafari, G.; Kiefer, R. Hardware and Software Development for Isotonic Strain and Isometric Stress Measurements of Linear Ionic Actuators. Polymers 2019, 11, 1054. [Google Scholar] [CrossRef] [PubMed]
- Dehghanpour, H.; Yilmaz, K. The Relationship between Resistances Measured by Two-Probe, Wenner Probe and C1760-12 ASTM Methods in Electrically Conductive Concretes. SN Appl. Sci. 2020, 2, 1–10. [Google Scholar] [CrossRef]
- Temmer, R.; Must, I.; Kaasik, F.; Aabloo, A.; Tamm, T. Combined Chemical and Electrochemical Synthesis Methods for Metal-Free Polypyrrole Actuators. Sens. Actuators B Chem. 2012, 166–167, 411–418. [Google Scholar] [CrossRef]
- Harjo, M.; Kesküla, A.; Leemets, K.; Khorram, M.S.; Saar, R.; Järvekülg, M.; Tamm, T.; Kiefer, R. Polypyrrole Coatings on Gelatin Fiber Scaffolds: Material and Electrochemical Characterizations in Organic and Aqueous Electrolyte. Synth. Met. 2017, 232, 25–30. [Google Scholar] [CrossRef]
- Lee, J.Y.; Bashur, C.A.; Goldstein, A.S.; Schmidt, C.E. Polypyrrole-Coated Electrospun PLGA Nanofibers for Neural Tissue Applications. Biomaterials 2009, 30, 4325–4335. [Google Scholar] [CrossRef]
- Shafei, S.; Foroughi, J.; Chen, Z.; Wong, C.S.; Naebe, M. Short Oxygen Plasma Treatment Leading to Long-Term Hydrophilicity of Conductive PCL-PPy Nanofiber Scaffolds. Polymers 2017, 9, 614. [Google Scholar] [CrossRef] [PubMed]
- Zarei, M.; Samimi, A.; Khorram, M.; Abdi, M.M.; Golestaneh, S.I. Fabrication and Characterization of Conductive Polypyrrole/chitosan/collagen Electrospun Nanofiber Scaffold for Tissue Engineering Application. Int. J. Biol. Macromol. 2021, 168, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Wang, H.; Wang, R.; Chai, Y. Novel Cellulose-Gelatin Composite Films Made from Self-Dispersed Microgels: Structure and Properties. Int. J. Biol. Macromol. 2019, 123, 991–1001. [Google Scholar] [CrossRef] [PubMed]
- Azizi, M.; Azimzadeh, M.; Afzali, M.; Alafzadeh, M.; Mirhosseini, S.H. Characterization and Optimization of Using Calendula Officinalis Extract in The Fabrication of Polycaprolactone/Gelatin Electrospun Nanofibers for Wound Dressing Applications ARTICLE INFO ABSTRACT. J. Adv. Mater. Process. 2018, 6, 34–46. [Google Scholar]
- Ibrahim, M.; Alaam, M.; El-Haes, H.; Jalbout, A.F.; De Leon, A. Analysis of the Structure and Vibrational Spectra of Glucose and Fructose. Eclet. Quim. 2006, 31, 15–21. [Google Scholar] [CrossRef]
- Siimon, K.; Siimon, H.; Järvekülg, M. Mechanical Characterization of Electrospun Gelatin Scaffolds Cross-Linked by Glucose. J. Mater. Sci. Mater. Med. 2015, 26, 1–9. [Google Scholar] [CrossRef]
- Gibot, P.; Goetz, V. Polypyrrole Material for the Electrostatic Discharge Sensitivity Mitigation of Al/SnO2 Energetic Composites. J. Appl. Polym. Sci. 2021, 138, 50752. [Google Scholar] [CrossRef]
- Turczyn, R.; Krukiewicz, K.; Katunin, A.; Sroka, J.; Sul, P. Fabrication and Application of Electrically Conducting Composites for Electromagnetic Interference Shielding of Remotely Piloted Aircraft Systems. Compos. Struct. 2020, 232, 111498. [Google Scholar] [CrossRef]
- Kostić, R.; Raković, D.; Stepanyan, S.A.; Davidova, I.E.; Gribov, L.A. Vibrational Spectroscopy of Polypyrrole, Theoretical Study. J. Chem. Phys. 1995, 102, 3104–3109. [Google Scholar] [CrossRef]
- Yang, C.; Liu, P. Core-Shell Attapulgite@polypyrrole Composite with Well-Defined Corn Cob-like Morphology via Self-Assembling and in Situ Oxidative Polymerization. Synth. Met. 2009, 159, 2056–2062. [Google Scholar] [CrossRef]
- Fu, Y.; Manthiram, A. Core-Shell Structured Sulfur-Polypyrrole Composite Cathodes for Lithium-Sulfur Batteries. RSC Adv. 2012, 2, 5927–5929. [Google Scholar] [CrossRef]
- Morávková, Z.; Taboubi, O.; Minisy, I.M.; Bober, P. The Evolution of the Molecular Structure of Polypyrrole during Chemical Polymerization. Synth. Met. 2021, 271, 116608. [Google Scholar] [CrossRef]
- Kanaan, A.F.; Pinho, A.C.; Piedade, A.P. Electroactive Polymers Obtained by Conventional and Non-Conventional Technologies. Polymers 2021, 13, 2713. [Google Scholar] [CrossRef] [PubMed]
- Ue, M.; Murakami, A.; Nakamura, S. A Convenient Method to Estimate Ion Size for Electrolyte Materials Design. J. Electrochem. Soc. 2002, 149, A1385–A1388. [Google Scholar] [CrossRef]
- Marque, P.; Roncali, J.; Garnier, F. Electrolyte Effect on the Electrochemical Properties of poly(3-Methylthiophene) Thin Films. J. Electroanal. Chem. 1987, 218, 107–118. [Google Scholar] [CrossRef]
- Chaban, V. Solvation of the Fluorine Containing Anions and Their Lithium Salts in Propylene Carbonate and Dimethoxyethane. J. Mol. Model. 2015, 21, 172. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Balbuena, P.B. Theoretical Studies of Lithium Perchlorate in Ethylene Carbonate, Propylene Carbonate, and Their Mixtures. J. Electrochem. Soc. 1999, 146, 3613–3622. [Google Scholar] [CrossRef]
- Valero, L.; Otero, T.F.; Martinez, J.G.; Martínez, J.G. Exchanged Cations and Water during Reactions in Polypyrrole Macroions from Artificial Muscles. ChemPhysChem 2014, 15, 293–301. [Google Scholar] [CrossRef]
- Otero, T.F.; Martinez, J.G. Structural and Biomimetic Chemical Kinetics: Kinetic Magnitudes Include Structural Information. Adv. Funct. Mater. 2013, 23, 404–416. [Google Scholar] [CrossRef]
- West, B.J.; Otero, T.F.; Shapiro, B.; Smela, E. Chronoamperometric Study of Conformational Relaxation in PPy(DBS). J. Phys. Chem. B 2009, 113, 1277–1293. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Otero, T.F. Coulovoltammetric and Dynamovoltammetric Responses from Conducting Polymers and Bilayer Muscles as Tools to Identify Reaction-Driven Structural Changes. A Review. Electrochim. Acta 2016, 212, 440–457. [Google Scholar] [CrossRef]
- Martinez, J.G.; Otero, T.F.; Jager, E.W.H. Effect of the Electrolyte Concentration and Substrate on Conducting Polymer Actuators. Langmuir 2014, 30, 3894–3904. [Google Scholar] [CrossRef] [PubMed]
- Kiefer, R.; Martinez, J.G.; Kesküla, A.; Anbarjafari, G.; Aabloo, A.; Otero, T.F. Polymeric Actuators: Solvents Tune Reaction-Driven Cation to Reaction-Driven Anion Actuation. Sens. Actuators B Chem. 2016, 233, 328–336. [Google Scholar] [CrossRef]
- Kaempgen, M.; Chan, C.K.; Ma, J.; Cui, Y.; Gruner, G. Printable Thin Film Supercapacitors Using Single-Walled Carbon Nanotubes. Nano Lett. 2009, 9, 1872–1876. [Google Scholar] [CrossRef] [PubMed]
- Izadi-Najafabadi, A.; Tan, D.T.H.; Madden, J.D. Towards High Power Polypyrrole/carbon Capacitors. Synth. Met. 2005, 152, 129–132. [Google Scholar] [CrossRef]
- Kim, K.M.; Hur, J.W.; Jung, S.-I.; Kang, A.S. Electrochemical Characteristics of Activated carbon/Ppy Electrode Combined with P(VdF-Co-HFP)/PVP for EDLC. Electrochim. Acta 2004, 50, 863–872. [Google Scholar] [CrossRef]
- Le, Q.B.; Zondaka, Z.; Harjo, M.; Nguyen, N.T.; Kiefer, R. Role of Polyoxometalate Contents in Polypyrrole: Linear Actuation and Energy Storage. Materials 2022, 15, 3619. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Han, G.; Xiao, Y.; Chang, Y.; Yuan, W.; Li, Y.; Liu, C.; Zhang, Y. Polypyrrole Doped with Dodecyl Benzene Sulfonate Electrodeposited on Carbon Fibers for Flexible Capacitors with High-Performance. Electrochim. Acta 2015, 176, 594–603. [Google Scholar] [CrossRef]
- Sun, T.; Sun, Q.Q.; Yu, Y.; Zhang, X.B. Polypyrrole as an Ultrafast Organic Cathode for Dual-Ion Batteries. eScience 2021, 1, 186–193. [Google Scholar] [CrossRef]
- Lv, J.; Zhang, L.; Zhong, Y.; Sui, X.; Wang, B.; Chen, Z.; Feng, X.; Xu, H.; Mao, Z. High-Performance Polypyrrole Coated Knitted Cotton Fabric Electrodes for Wearable Energy Storage. Org. Electron. 2019, 74, 59–68. [Google Scholar] [CrossRef]
- Otero, T.F.; Cortés, M.T. Artificial Muscles with Tactile Sensitivity. Adv. Mater. 2003, 15, 279–282. [Google Scholar] [CrossRef]
- Otero, T.F.; Cortés, M.T. A Sensing Muscle. Sens. Actuators B Chem. 2003, 96, 152–156. [Google Scholar] [CrossRef]
- Shabeeba, A.; Ismail, Y.A. Chitosan/polypyrrole Hybrid Film as Multistep Electrochemical Sensor: Sensing Electrical, Thermal and Chemical Working Ambient. Mater. Res. Bull. 2022, 152, 111817. [Google Scholar] [CrossRef]
- Martinez, J.G.; Otero, T.F. Structural Electrochemistry. Chronopotentiometric Responses from Rising Compacted Polypyrrole Electrodes: Experiments and Model. RSC Adv. 2014, 4, 29139–29145. [Google Scholar] [CrossRef]
- Beer, P.D.; Gale, P.A. Anion Recognition and Sensing: The State of the Art and Future Perspectives. Angew. Chem. Int. Ed 2001, 40, 486–516. [Google Scholar] [CrossRef]
- Wu, X.; Gilchrist, A.M.; Gale, P.A. Prospects and Challenges in Anion Recognition and Transport. Chem 2020, 6, 1296–1309. [Google Scholar] [CrossRef]
- Dalisson, B.; Barralet, J. Bioinorganics and Wound Healing. Adv. Healthc. Mater. 2019, 8, 1–22. [Google Scholar] [CrossRef] [PubMed]






| NFs-PPy Electrolytes | Ue (J g−1) | Eox (V) | Stress σ (kPa) | Strain Δl/l (%) |
|---|---|---|---|---|
| LiTFSI | 4.42 ± 0.38 | 0.68 ± 0.06 | ||
| LiTF | 3.29 ± 0.31 | 0.49 ± 0.04 | ||
| NaClO4 | 2.27 ± 0.21 | 0.28 ± 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiefer, R.; Otero, T.F.; Harjo, M.; Le, Q.B. Chemically Polymerized Polypyrrole on Glucose-Porcine Skin Gelatin Nanofiber as Multifunctional Electrochemical Actuator-Sensor-Capacitor. Polymers 2025, 17, 631. https://doi.org/10.3390/polym17050631
Kiefer R, Otero TF, Harjo M, Le QB. Chemically Polymerized Polypyrrole on Glucose-Porcine Skin Gelatin Nanofiber as Multifunctional Electrochemical Actuator-Sensor-Capacitor. Polymers. 2025; 17(5):631. https://doi.org/10.3390/polym17050631
Chicago/Turabian StyleKiefer, Rudolf, Toribio F. Otero, Madis Harjo, and Quoc Bao Le. 2025. "Chemically Polymerized Polypyrrole on Glucose-Porcine Skin Gelatin Nanofiber as Multifunctional Electrochemical Actuator-Sensor-Capacitor" Polymers 17, no. 5: 631. https://doi.org/10.3390/polym17050631
APA StyleKiefer, R., Otero, T. F., Harjo, M., & Le, Q. B. (2025). Chemically Polymerized Polypyrrole on Glucose-Porcine Skin Gelatin Nanofiber as Multifunctional Electrochemical Actuator-Sensor-Capacitor. Polymers, 17(5), 631. https://doi.org/10.3390/polym17050631







