Impact of Steam-Exploded Feather Incorporation on the Biodegradation Performance of Renewable Biocomposites
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Steam Explosion Treatment of Chicken Feathers
2.3. Preparation of Biocomposites
2.4. Characterization
2.4.1. Density
2.4.2. Thermogravimetry (TGA)
2.4.3. Field-Emission Scanning Electron Microscopy (FE-SEM)
2.4.4. Water Absorption of Biocomposites
2.4.5. Mechanical Testing
2.4.6. Biodegradation in Soil
2.4.7. Disintegration in Soil
2.4.8. Ecotoxicity Test in Soil
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Orzolek, M.D. A Guide to the Manufacture, Performance, and Potential of Plastics in Agriculture; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Assessment of Agricultural Plastics and Their Sustainability: A Call for Action; FAO: Rome, Italy, 2021; ISBN 978-92-5-135402-5.
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food Security: The Challenge of Feeding 9 Billion People. Science 2010, 327, 812–818. [Google Scholar] [CrossRef]
- Hofmann, T.; Ghoshal, S.; Tufenkji, N.; Adamowski, J.F.; Bayen, S.; Chen, Q.; Demokritou, P.; Flury, M.; Hüffer, T.; Ivleva, N.P.; et al. Plastics Can Be Used More Sustainably in Agriculture. Commun. Earth Environ. 2023, 4, 332. [Google Scholar] [CrossRef]
- Kumar, M.; Bolan, S.; Padhye, L.P.; Konarova, M.; Foong, S.Y.; Lam, S.S.; Wagland, S.; Cao, R.; Li, Y.; Batalha, N.; et al. Retrieving Back Plastic Wastes for Conversion to Value Added Petrochemicals: Opportunities, Challenges and Outlooks. Appl. Energy 2023, 345, 121307. [Google Scholar] [CrossRef]
- Ramos, L.; Berenstein, G.; Hughes, E.A.; Zalts, A.; Montserrat, J.M. Polyethylene Film Incorporation into the Horticultural Soil of Small Periurban Production Units in Argentina. Sci. Total Environ. 2015, 523, 74–81. [Google Scholar] [CrossRef]
- Chen, G.; Li, J.; Sun, Y.; Wang, Z.; Leeke, G.A.; Moretti, C.; Cheng, Z.; Wang, Y.; Li, N.; Mu, L.; et al. Replacing Traditional Plastics with Biodegradable Plastics: Impact on Carbon Emissions. Engineering 2024, 32, 152–162. [Google Scholar] [CrossRef]
- Reddy, N. Non-Food Industrial Applications of Poultry Feathers. Waste Manag. 2015, 45, 91–107. [Google Scholar] [CrossRef]
- Suzuki, Y.; Tsujimoto, Y.; Matsui, H.; Watanabe, K. Decomposition of Extremely Hard-to-Degrade Animal Proteins by Thermophilic Bacteria. J. Biosci. Bioeng. 2006, 102, 73–81. [Google Scholar] [CrossRef]
- Sinkiewicz, I.; Śliwińska, A.; Staroszczyk, H.; Kołodziejska, I. Alternative Methods of Preparation of Soluble Keratin from Chicken Feathers. Waste Biomass Valorization 2017, 8, 1043–1048. [Google Scholar] [CrossRef]
- Sharma, S.; Gupta, A. Sustainable Management of Keratin Waste Biomass: Applications and Future Perspectives. Braz. Arch. Biol. Technol. 2016, 59, e16150684. [Google Scholar] [CrossRef]
- Kurien, R.A.; Biju, A.; Raj, K.A.; Chacko, A.; Joseph, B.; Koshy, C.P. Chicken Feather Fiber Reinforced Composites for Sustainable Applications. Mater. Today Proc. 2022, 58, 862–866. [Google Scholar] [CrossRef]
- Li, Q. Perspectives on Converting Keratin-Containing Wastes Into Biofertilizers for Sustainable Agriculture. Front. Microbiol. 2022, 13, 918262. [Google Scholar] [CrossRef]
- Álvarez-del-Castillo, M.D.; Garrido-Soriano, N.; Casadesús, M.; Macanás, J.; Molins-Duran, G.; Carrillo-Navarrete, F. Environmental Impact of Chicken Feathers Based Polypropylene Composites Developed for Automotive and Stationary Applications and Comparison with Glass-Fibre Analogues. Waste Biomass Valorization 2022, 13, 4585–4598. [Google Scholar] [CrossRef]
- Mishra, A.; Jung, D.; Kim, N.K.; Bhattacharyya, D. Influence of Chicken Feather Fibre Processing Technique on Mechanical and Fire Performances of Flame-Retardant Polypropylene Composites. Compos. Part A Appl. Sci. Manuf. 2023, 165, 107338. [Google Scholar] [CrossRef]
- Ghani, S.A.; Tan, S.J.; Yeng, T.S. Properties of Chicken Feather Fiber-Filled Low-Density Polyethylene Composites: The Effect of Polyethylene Grafted Maleic Anhydride. Polym. Plast. Technol. Eng. 2013, 52, 495–500. [Google Scholar] [CrossRef]
- Baba, B.O.; Özmen, U. Preparation and Mechanical Characterization of Chicken Feather/PLA Composites. Polym. Compos. 2017, 38, 837–845. [Google Scholar] [CrossRef]
- Aranberri, I.; Montes, S.; Azcune, I.; Rekondo, A.; Grande, H.-J. Fully Biodegradable Biocomposites with High Chicken Feather Content. Polymers 2017, 9, 593. [Google Scholar] [CrossRef]
- Rodríguez, F.; Sanchez, A.; Parra, C. Role of Steam Explosion on Enzymatic Digestibility, Xylan Extraction, and Lignin Release of Lignocellulosic Biomass. ACS Sustain. Chem. Eng. 2017, 5, 5234–5240. [Google Scholar] [CrossRef]
- Vadillo, J.; Montes, S.; Grande, H.-J.; Verstichel, S.; Almqvist, J.; Wrześniewska-Tosik, K. Enhanced Biodegradability in Soil of Chicken Feather by Steam Explosion for Potential Application in Agricultural Biodegradable Plastics. Polymers 2023, 15, 3701. [Google Scholar] [CrossRef]
- ASTM D638-22; Standard Test Method for Tensile Properties of Plastics. ASTM International: West Conshohocken, PA, USA, 2022.
- ISO 9427:2003; Wood-Based Panels—Determination of Density. ISO: Geneva, Switzerland, 2003.
- ASTM D570-98; Standard Test Method for Water Absorption of Plastics. ASTM International: West Conshohocken, PA, USA, 1998.
- ISO 527-1:2019; Plastics—Determination of Tensile Properties—Part 1: General Principles. ISO: Geneva, Switzerland, 2019.
- ISO 17556:2019; Plastics—Determination of the Ultimate Aerobic Biodegradability of Plastic Materials in Soil by Measuring the Oxygen Demand in a Respirometer or the Amount of Carbon Dioxide Evolved. ISO: Geneva, Switzerland, 2019.
- Test No. 208: Terrestrial Plant Test: Seedling Emergence and Seedling Growth Test; OECD: Paris, France, 2006; ISBN 9789264070066.
- Bansal, G.; Singh, V.K. Water Absorption and Thickness Swelling Characterization of Chicken Feather Fiber and Extracted Fish Residue Powder Filled Epoxy Based Hybrid Biocomposite. Int. J. Waste Resour. 2016, 6, 1000237. [Google Scholar] [CrossRef]
- Pucciariello, R.; Villani, V.; Bonini, C.; D’Auria, M.; Vetere, T. Physical Properties of Straw Lignin-Based Polymer Blends. Polymers 2004, 45, 4159–4169. [Google Scholar] [CrossRef]
- Fu, S.-Y.; Feng, X.-Q.; Lauke, B.; Mai, Y.-W. Effects of Particle Size, Particle/Matrix Interface Adhesion and Particle Loading on Mechanical Properties of Particulate–Polymer Composites. Compos. B Eng. 2008, 39, 933–961. [Google Scholar] [CrossRef]
- Barone, J.R.; Gregoire, N.T. Characterisation of Fibre–Polymer Interactions and Transcrystallinity in Short Keratin Fibre–Polypropylene Composites. Plast. Rubber Compos. 2006, 35, 287–293. [Google Scholar] [CrossRef]
- Cañavate, J.; Aymerich, J.; Garrido, N.; Colom, X.; Macanás, J.; Molins, G.; Álvarez, M.; Carrillo, F. Properties and Optimal Manufacturing Conditions of Chicken Feathers/Poly(Lactic Acid) Biocomposites. J. Compos. Mater. 2016, 50, 1671–1683. [Google Scholar] [CrossRef]
- Ke, T.; Sun, S.X.; Seib, P. Blending of Poly(Lactic Acid) and Starches Containing Varying Amylose Content. J. Appl. Polym. Sci. 2003, 89, 3639–3646. [Google Scholar] [CrossRef]
- Hussain, M.; Khan, S.M.; Shafiq, M.; Abbas, N. A Review on PLA-Based Biodegradable Materials for Biomedical Applications. Giant 2024, 18, 100261. [Google Scholar] [CrossRef]
- Jin, A.; del Valle, L.J.; Puiggalí, J. Copolymers and Blends Based on 3-Hydroxybutyrate and 3-Hydroxyvalerate Units. Int. J. Mol. Sci. 2023, 24, 17250. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamamoto, H.; Yokote, Y.; Hattori, M. Thermal Behavior of Fowl Feather Keratin. Biosci. Biotechnol. Biochem. 2004, 68, 1875–1881. [Google Scholar] [CrossRef]
- Carrillo, F.; Rahhali, A.; Cañavate, J.; Colom, X. Biocomposites Using Waste Whole Chicken Feathers and Thermoplastic Matrices. J. Reinf. Plast. Compos. 2013, 32, 1419–1429. [Google Scholar]
- Cheng, S.; Lau, K.; Liu, T.; Zhao, Y.; Lam, P.-M.; Yin, Y. Mechanical and Thermal Properties of Chicken Feather Fiber/PLA Green Composites. Compos. B Eng. 2009, 40, 650–654. [Google Scholar] [CrossRef]
- Brebu, M. Environmental Degradation of Plastic Composites with Natural Fillers—A Review. Polymers 2020, 12, 166. [Google Scholar] [CrossRef]
- Rothon, R. Fillers from Organic Sources. In Fillers for Polymer Applications; Springer: Cham, Switzerland, 2017; pp. 315–325. [Google Scholar]
- Hubbe, M.A.; Lavoine, N.; Lucia, L.A.; Dou, C. Formulating Bioplastic Composites for Biodegradability, Recycling, and Performance: A Review. Bioresources 2020, 16, 2021–2083. [Google Scholar] [CrossRef]
- Bayerl, T.; Geith, M.; Somashekar, A.A.; Bhattacharyya, D. Influence of Fibre Architecture on the Biodegradability of FLAX/PLA Composites. Int. Biodeterior. Biodegrad. 2014, 96, 18–25. [Google Scholar] [CrossRef]
- Feijoo, P.; Marín, A.; Samaniego-Aguilar, K.; Sánchez-Safont, E.; Lagarón, J.M.; Gámez-Pérez, J.; Cabedo, L. Effect of the Presence of Lignin from Woodflour on the Compostability of PHA-Based Biocomposites: Disintegration, Biodegradation and Microbial Dynamics. Polymers 2023, 15, 2481. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, F.; Santana Pérez, O.; Maspoch, M. Kinetics of the Thermal Degradation of Poly(Lactic Acid) and Polyamide Bioblends. Polymers 2021, 13, 3996. [Google Scholar] [CrossRef]
- Boonmee, C.; Kositanont, C.; Leejarkpai, T. Degradation of Poly (Lactic Acid) under Simulated Landfill Conditions. Environ. Nat. Resour. J. 2016, 14, 1–9. [Google Scholar]
- Thakur, A.; Musioł, M.; Duale, K.; Kowalczuk, M. Exploring the Future of Polyhydroxyalkanoate Composites with Organic Fillers: A Review of Challenges and Opportunities. Polymers 2024, 16, 1768. [Google Scholar] [CrossRef]
- Nurdiawati, A.; Nakhshiniev, B.; Gonzales, H.B.; Yoshikawa, K. Nitrogen Mineralization Dynamics of Liquid Feather Hydrolysates Obtained by Hydrothermal Treatment. Appl. Soil. Ecol. 2019, 134, 98–104. [Google Scholar] [CrossRef]
- Da Silva Filho, J.C.F.; Batalha, J.A.B.; Lima, R.K.A.; Melo, R.S.; Matos, S.D.S.; Do Nascimento, N.M.; Dutra, A.F.; Da Silva Júnior, G.B. Excess Ammonium Causes Toxicity in Watermelon and Cucumber Plants Grown in Nutrient Solution. Científica 2021, 49, 144. [Google Scholar] [CrossRef]
- Sun, J.; Li, W.; Li, C.; Chang, W.; Zhang, S.; Zeng, Y.; Zeng, C.; Peng, M. Effect of Different Rates of Nitrogen Fertilization on Crop Yield, Soil Properties and Leaf Physiological Attributes in Banana Under Subtropical Regions of China. Front. Plant Sci. 2020, 11, 613760. [Google Scholar] [CrossRef]
- Anas, M.; Liao, F.; Verma, K.K.; Sarwar, M.A.; Mahmood, A.; Chen, Z.-L.; Li, Q.; Zeng, X.-P.; Liu, Y.; Li, Y.-R. Fate of Nitrogen in Agriculture and Environment: Agronomic, Eco-Physiological and Molecular Approaches to Improve Nitrogen Use Efficiency. Biol. Res. 2020, 53, 47. [Google Scholar] [CrossRef]
- Qin, C.; Yi, K.; Wu, P. Ammonium Affects Cell Viability to Inhibit Root Growth in Arabidopsis. J. Zhejiang Univ. Sci. B 2011, 12, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Zhang, H.; Zhang, F.; Wang, L.; Cui, S. Morphology of the Invasive Amphiphyte Alternanthera Philoxeroides Under Different Water Levels and Nitrogen Concentrations. Acta Biol. Cracoviensia S. Bot. 2015, 56, 136–147. [Google Scholar] [CrossRef]
- Rai, S.K.; Mukherjee, A.K. Optimization for Production of Liquid Nitrogen Fertilizer from the Degradation of Chicken Feather by Iron-Oxide (Fe3O4) Magnetic Nanoparticles Coupled β-Keratinase. Biocatal. Agric. Biotechnol. 2015, 4, 632–644. [Google Scholar] [CrossRef]
- Ambadi, A.; Krishnamurty, D.; Rao, S.; Desai, B.K.; Ravi, M.V.; Shubha, S. Influence of Varied Crop Residues and Green Biomass Composts to Rabi Sorghum Growing Soils on Uptake of Major Nutrients, Microbial Biomass and Soil Fertility Status. J. Appl. Nat. Sci. 2018, 10, 185–189. [Google Scholar] [CrossRef]
- Guo, T.; Bai, S.H.; Omidvar, N.; Wang, Y.; Chen, F.; Zhang, M. Insight into the Functional Mechanisms of Nitrogen-Cycling Inhibitors in Decreasing Yield-Scaled Ammonia Volatilization and Nitrous Oxide Emission: A Global Meta-Analysis. Chemosphere 2023, 338, 139611. [Google Scholar] [CrossRef]
- Tkaczyk, P.; Mocek-Płóciniak, A.; Skowrońska, M.; Bednarek, W.; Kuśmierz, S.; Zawierucha, E. The Mineral Fertilizer-Dependent Chemical Parameters of Soil Acidification under Field Conditions. Sustainability 2020, 12, 7165. [Google Scholar] [CrossRef]
- Grzyb, A.; Wolna-Maruwka, A.; Niewiadomska, A. The Significance of Microbial Transformation of Nitrogen Compounds in the Light of Integrated Crop Management. Agronomy 2021, 11, 1415. [Google Scholar] [CrossRef]
- Choi, J.-M.; Nelson, P.V. Developing a Slow-Release Nitrogen Fertilizer from Organic Sources: II. Using Poultry Feathers. J. Am. Soc. Hortic. Sci. 1996, 121, 634–638. [Google Scholar] [CrossRef]
- Duan, W.; Wang, S.; Zhang, H.; Xie, B.; Zhang, L. Plant Growth and Nitrate Absorption and Assimilation of Two Sweet Potato Cultivars with Different N Tolerances in Response to Nitrate Supply. Sci. Rep. 2024, 14, 21286. [Google Scholar] [CrossRef]
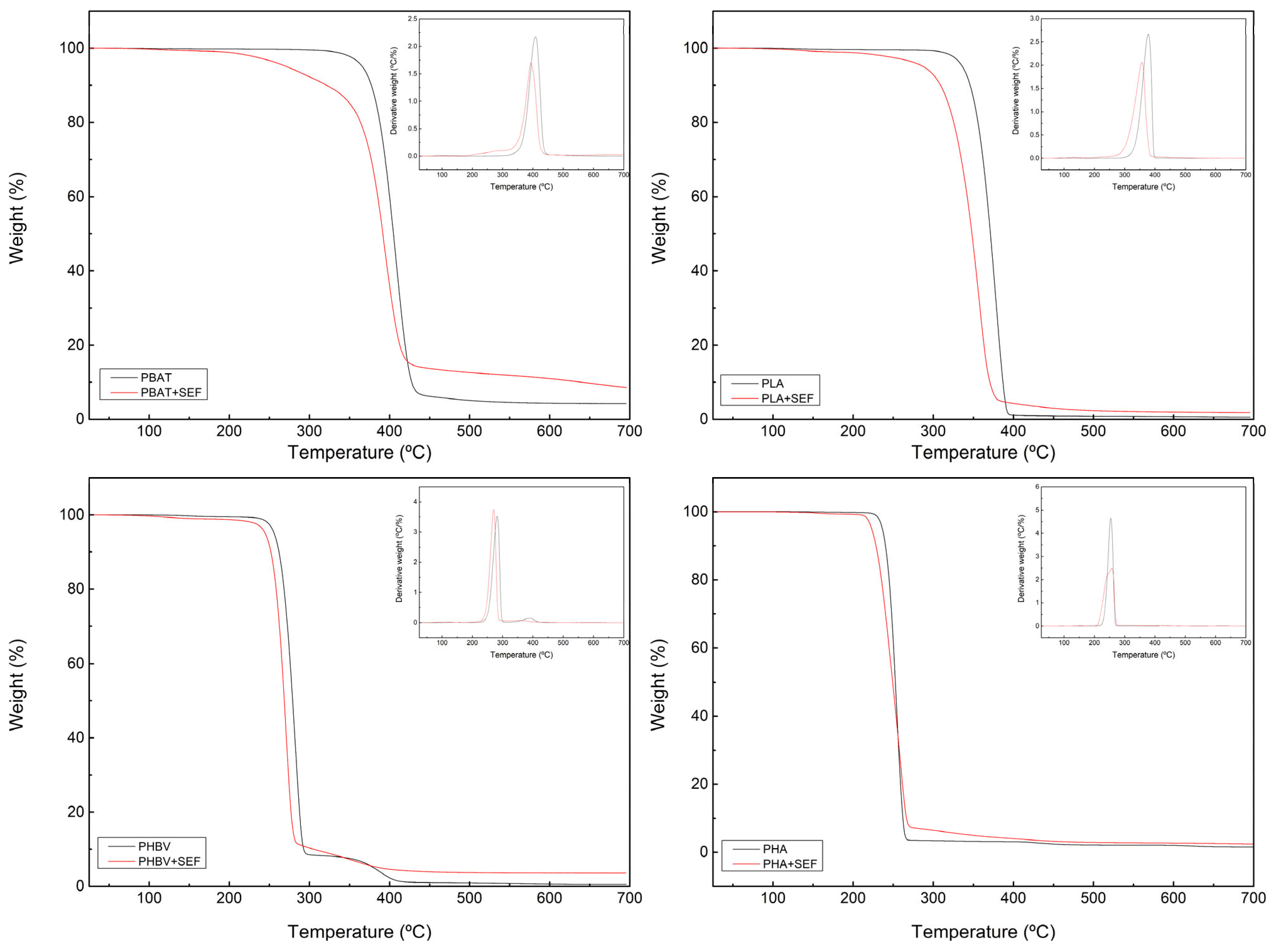
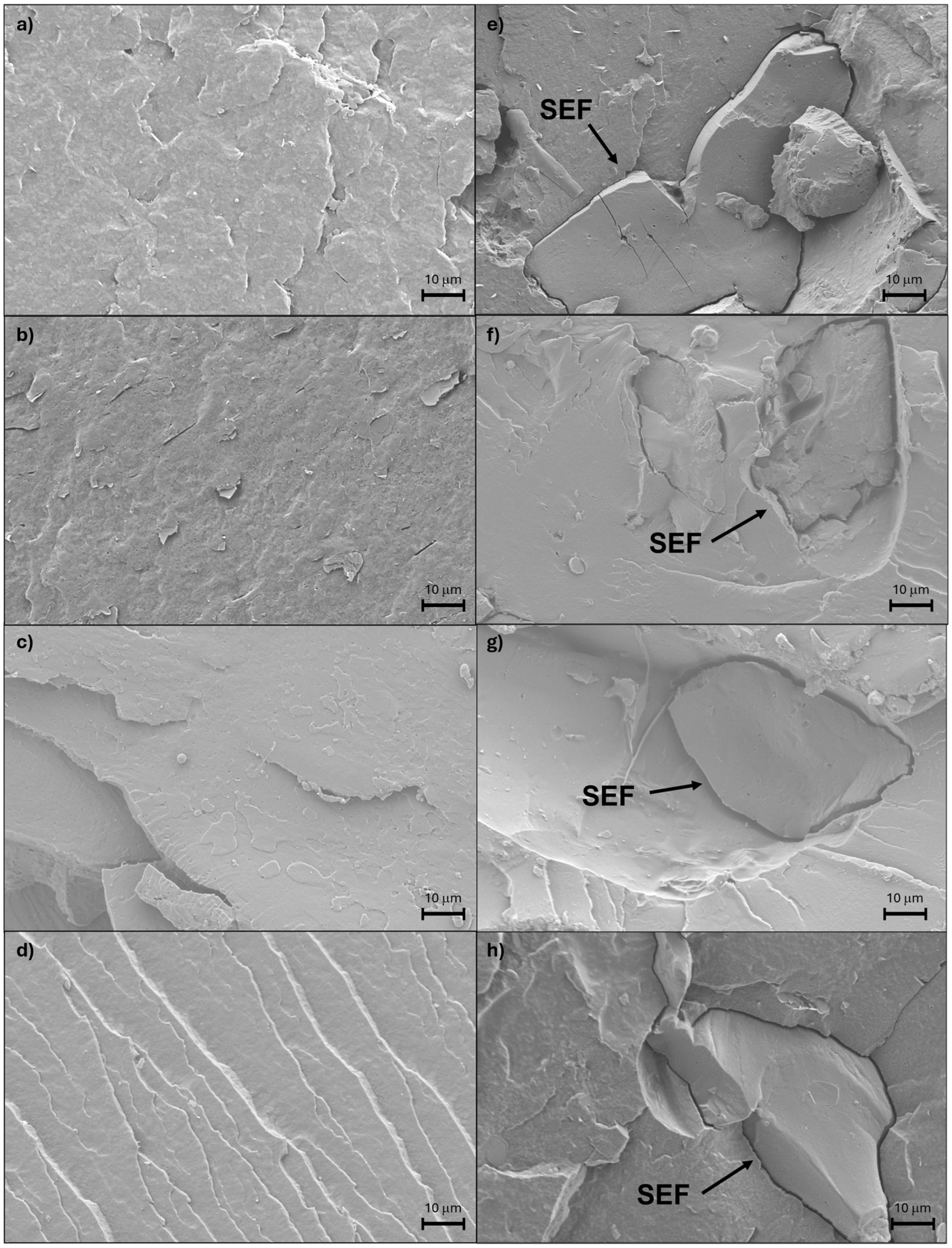



| System | SE Content (%) | Temperature (°C) | Speed (rpm) |
|---|---|---|---|
| PHB | - | 170 | 150 |
| PHB + SEF | 10 | 170 | 150 |
| PBAT | - | 165 | 150 |
| PBAT + SEF | 10 | 165 | 150 |
| PHBV | - | 165 | 150 |
| PHBV + SEF | 10 | 165 | 150 |
| PLA | - | 190 | 150 |
| PLA + SEF | 10 | 190 | 150 |
| Sample | Relative Density (g cm−3) | Swelling (%) | |
|---|---|---|---|
| t = 1 h | t = 24 h | ||
| PHB | 1.179 | 101 ± 1 | 103 ± 1 |
| PHB + SEF | 1.073 | 104 ± 1 | 107 ± 1 |
| PLA | 1.139 | 101 ± 1 | 102 ± 1 |
| PLA + SEF | 1.112 | 102 ± 1 | 104 ± 1 |
| PHBV | 1.207 | 102 ± 1 | 104 ± 1 |
| PHBV + SEF | 1.148 | 103 ± 1 | 106 ± 1 |
| PBAT | 1.202 | 101 ± 1 | 102 ± 1 |
| PBAT + SEF | 1.156 | 107 ± 2 | 109 ± 1 |
| Sample | Young Modulus (MPa) | Tensile Strength (MPa) | Strain at Break (%) |
|---|---|---|---|
| PHB | 2714 ± 156 | 29 ± 3 | 3 ± 1 |
| PHB + SEF | 2874 ± 75 | 26 ± 4 | 2 ± 1 |
| PLA | 2516 ± 178 | 65 ± 3 | 6 ± 1 |
| PLA + SEF | 2541 ± 226 | 40 ± 3 | 3 ± 1 |
| PHBV | 2231 ± 153 | 29 ± 2 | 4 ± 1 |
| PHBV + SEF | 3002 ± 101 | 25 ± 2 | 2 ± 1 |
| PBAT | 119 ± 7 | 18 ± 1 | 257 ± 7 |
| PBAT + SEF | 200 ± 21 | 8 ± 1 | 139 ± 12 |
| Sample | Disintegration Behaviour After 46 Weeks | Comparison (Week 0 vs. Week 40) |
|---|---|---|
| PHB | Pieces of varying size |  |
| PHB + SEF | 100% Disintegration in week 26 | - |
| PLA | All samples remain intact |  |
| PLA + SEF | All samples remain intact |  |
| PHBV | 100% Disintegration in week 24 | - |
| PHBV + SEF | 100% Disintegration in week 26 | - |
| PBAT | Small pieces of varying size |  |
| PBAT + SEF | 100% Disintegration in week 46 |  |
| Sample | Feather Content (%) | Nitrogen Content (g N/kg soil) | Cress | Barley | ||
|---|---|---|---|---|---|---|
| Germination Rate (%) | Fresh Weight Yield (g) | Germination Rate (%) | Fresh Weight Yield (g) | |||
| Blank soil with nutrient | - | - | 96 ± 2 | 7.7 ± 0.6 | 97 ± 1 | 13 ± 0 |
| Blank soil without nutrient | - | - | 93 ± 2 | 7.5 ± 0.6 | 98 ± 0 | 10 ± 0 |
| S1_SEF | 1 | 1.52 | 66 ± 7 | 1.5 ± 0.1 | 83 ± 3 | 3 ± 1 |
| S1_RF | 1 | 1.46 | 93 ± 5 | 3.0 ± 0.2 | 88 ± 1 | 5 ± 1 |
| S0.1_SEF | 0.1 | 0.15 | 96 ± 3 | 7.8 ± 0.7 | 97 ± 2 | 12 ± 0 |
| S0.1_RF | 0.1 | 0.15 | 97 ± 1 | 8.0 ± 0.5 | 96 ± 2 | 12 ± 1 |
| S0.05_SEF | 0.05 | 0.08 | 96 ± 3 | 7.6 ± 0.7 | 96 ± 1 | 11 ± 1 |
| S0.05_RF | 0.05 | 0.08 | 98 ± 2 | 8.4 ± 0.4 | 95 ± 1 | 11 ± 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vadillo, J.; Montes, S.; Grande, H.-J.; Beeckman, E.; Verstichel, S.; Almqvist, J. Impact of Steam-Exploded Feather Incorporation on the Biodegradation Performance of Renewable Biocomposites. Polymers 2025, 17, 910. https://doi.org/10.3390/polym17070910
Vadillo J, Montes S, Grande H-J, Beeckman E, Verstichel S, Almqvist J. Impact of Steam-Exploded Feather Incorporation on the Biodegradation Performance of Renewable Biocomposites. Polymers. 2025; 17(7):910. https://doi.org/10.3390/polym17070910
Chicago/Turabian StyleVadillo, Julen, Sarah Montes, Hans-Jürgen Grande, Eveline Beeckman, Steven Verstichel, and Jonna Almqvist. 2025. "Impact of Steam-Exploded Feather Incorporation on the Biodegradation Performance of Renewable Biocomposites" Polymers 17, no. 7: 910. https://doi.org/10.3390/polym17070910
APA StyleVadillo, J., Montes, S., Grande, H.-J., Beeckman, E., Verstichel, S., & Almqvist, J. (2025). Impact of Steam-Exploded Feather Incorporation on the Biodegradation Performance of Renewable Biocomposites. Polymers, 17(7), 910. https://doi.org/10.3390/polym17070910






