Alginate Beads with Encapsulated Date Palm Pollen Extract: Development, Characterization and Their Potential Role in Hepato-Protection and Fertility-Stimulating Hormones Improvement in Bisphenol A-Treated Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of DPP Extract
2.2.2. Preparation of Alginate/DPP Extract Solution
2.2.3. Measuring of Particle Size Distribution and Zeta Potential of Alginate/DPP Extract Solution
2.2.4. Preparation of Alginate/DPP Extract Beads
2.2.5. Encapsulation Efficiency (EE)
2.2.6. Scanning Electron Microscopy (SEM)
2.2.7. Extraction Procedure of the Alginate/DPP Beads
2.2.8. High Performance Liquid Chromatography (HPLC) of the Beads Extract
2.2.9. Determination of the Total Phenolic Content (TPC) of the Beads Extract
2.2.10. Determination of the Total Flavonoid Content (TFC) of the Beads Extract
2.2.11. In Vitro Determination of Antioxidant Activity of the Beads Extract
DPPH Radical Scavenging Activity Assay
Thiobarbituric Acid Reactive Substances (TBARS) Assay
2.2.12. Assessment of the Controlled Release of Phenolic Compounds from the Beads
2.2.13. In Vivo Assay
Animals and Experimental Design
Biochemical Analysis
Oxidative Stress Markers
Histopathological Examination
Statistical Analysis
3. Results and Discussion
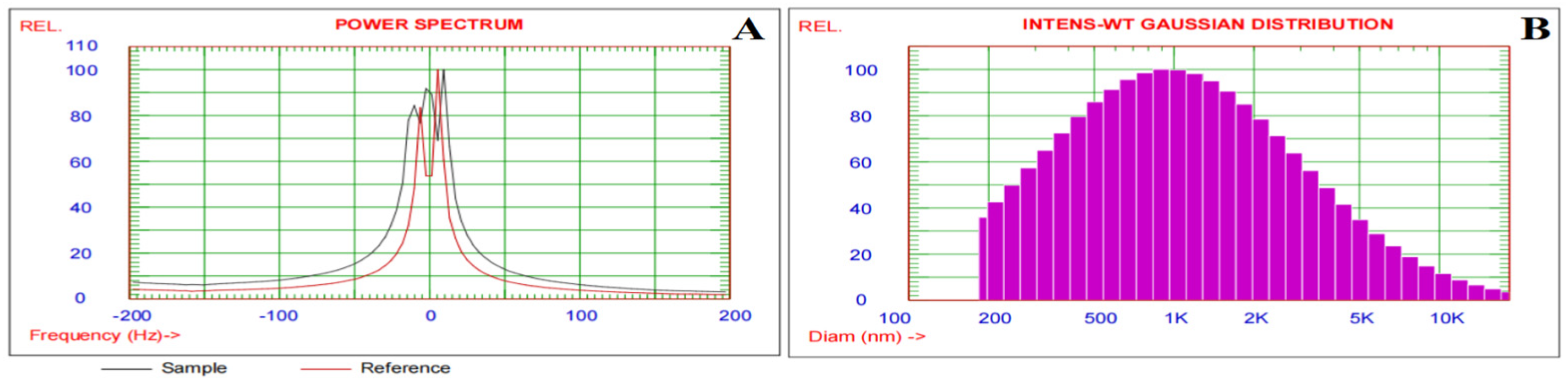
3.1. Zeta Potential and Particle Size Distribution of the Alginate/DPP Extract Solution
3.2. EE % of the Beads
3.3. SEM of the Beads
3.4. HPLC of Beads Extract
3.5. TPC and TFC of Beads Extract
3.6. In Vitro Antioxidant Activity of the Beads Extract
3.7. Release of Phenolic Compounds from the Beads
3.8. In Vivo Findings
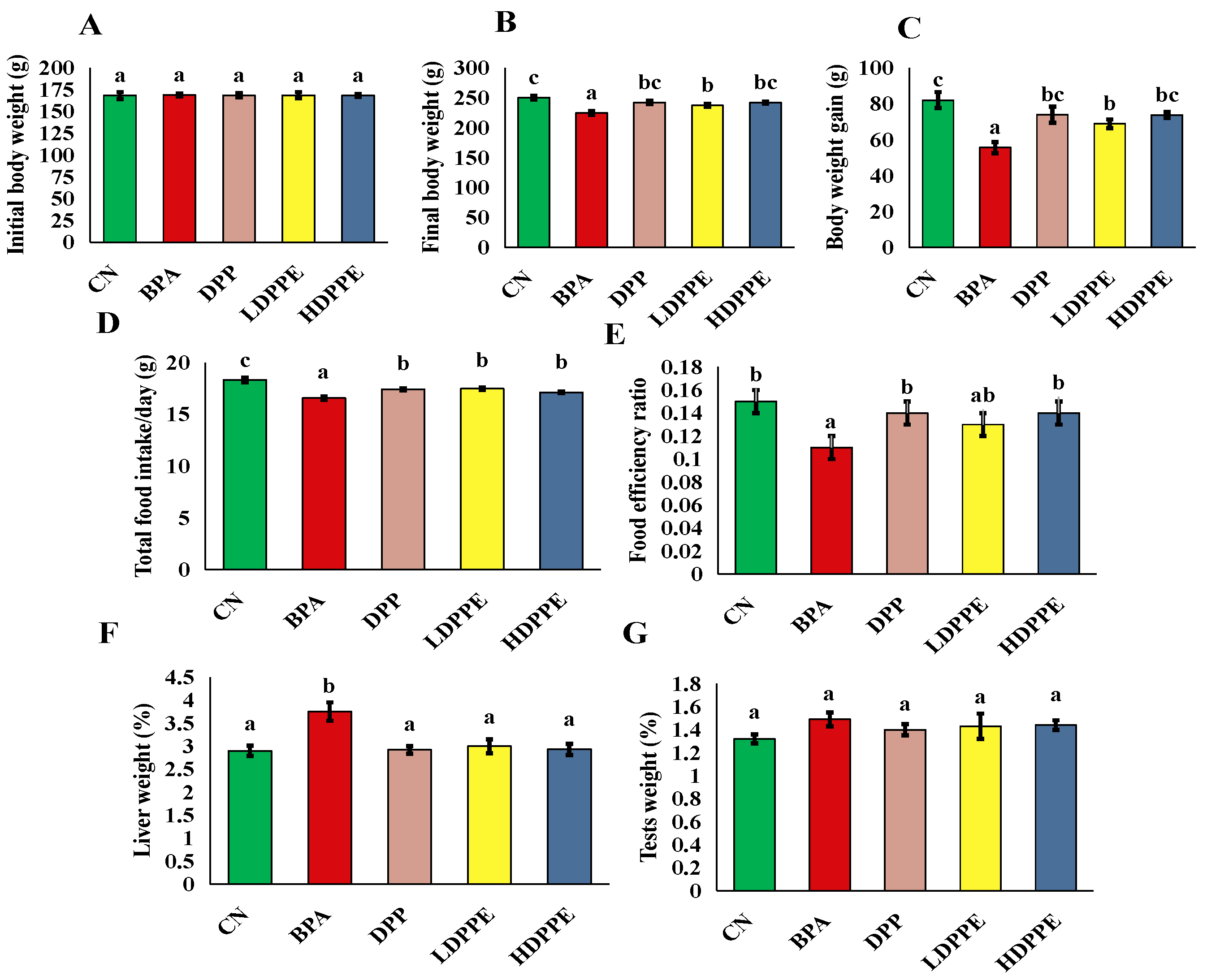
3.8.1. Effect of Alginate/DPP Extract Beads on the Growth Performance
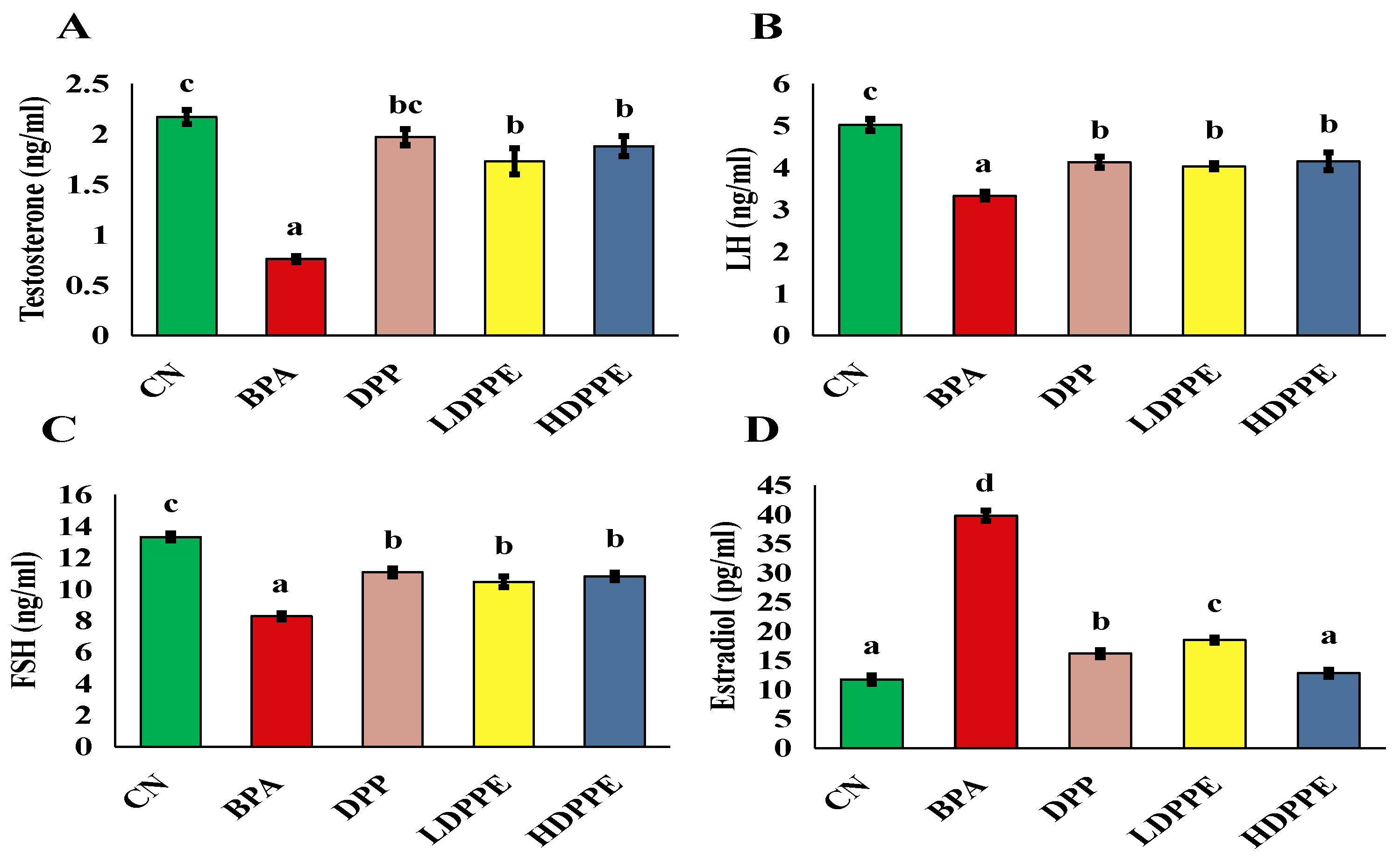
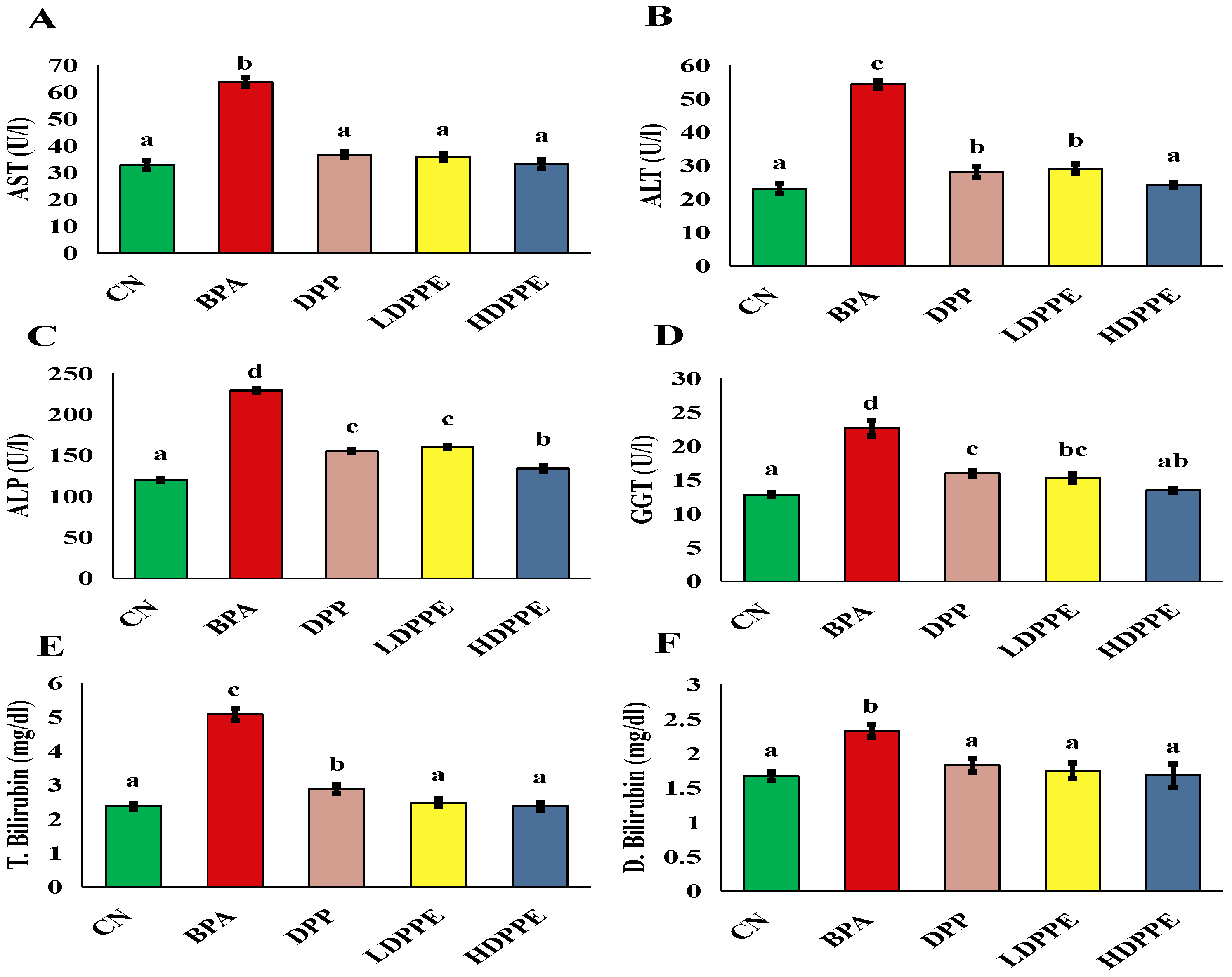
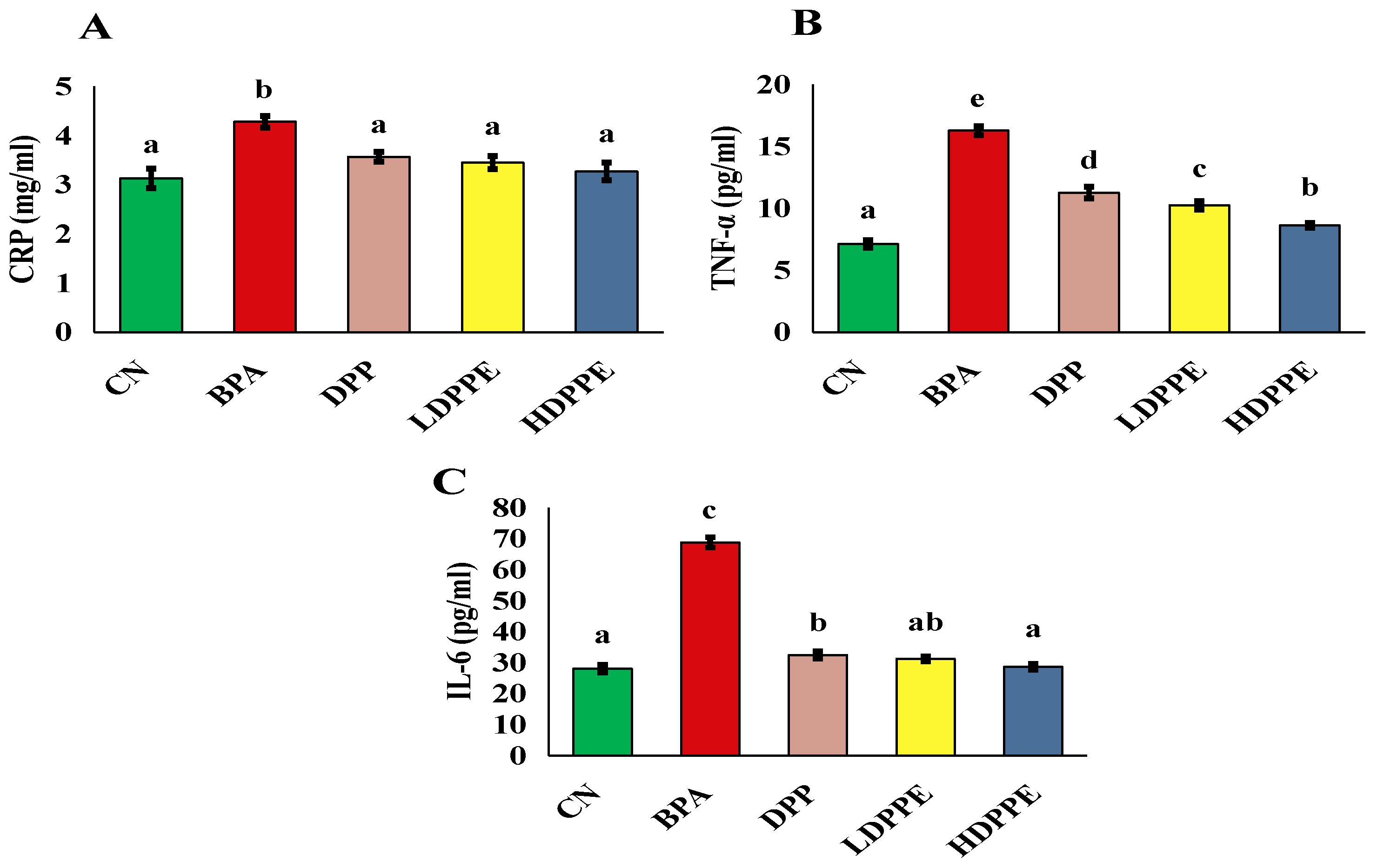
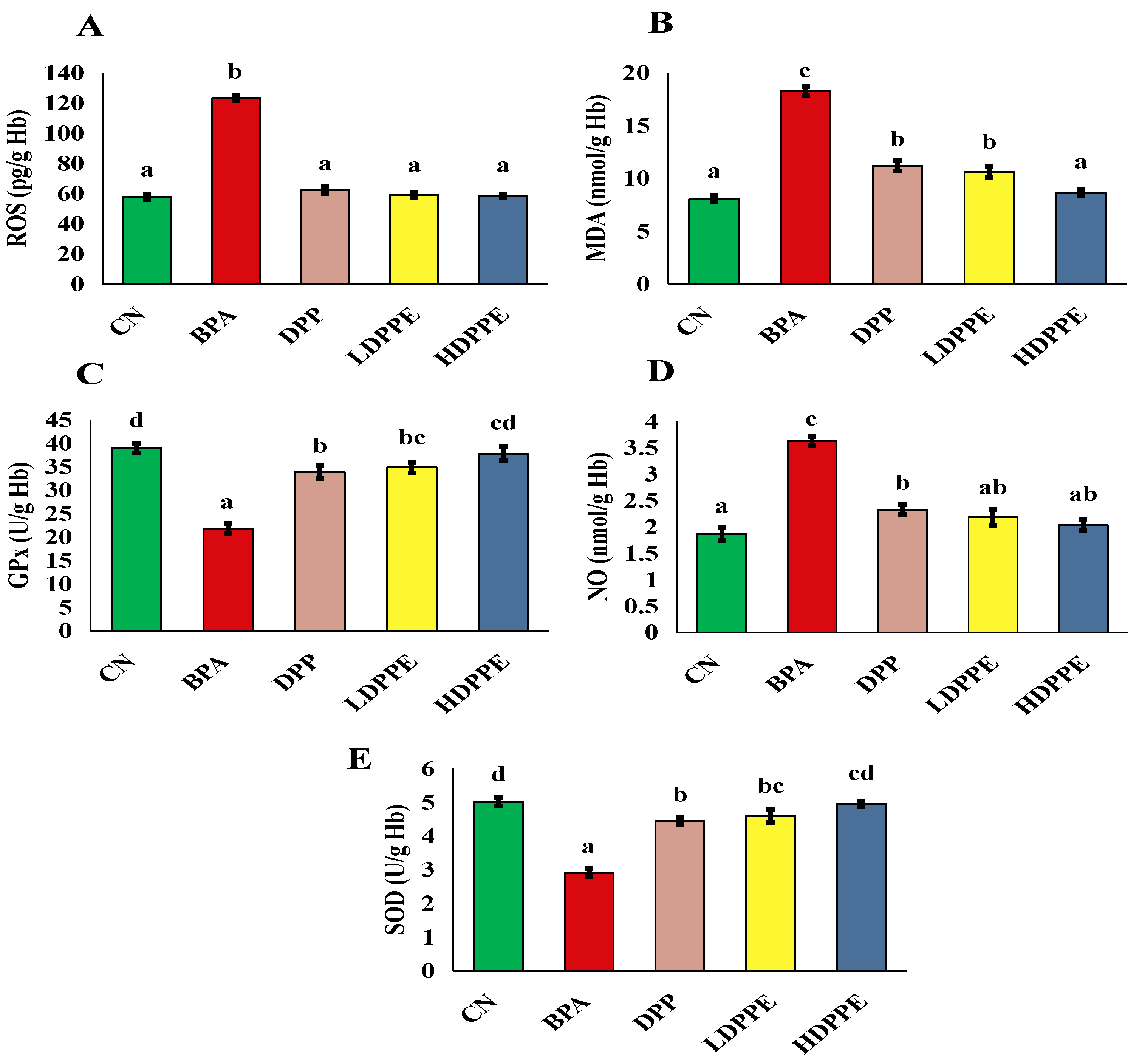
3.8.2. Effect of Alginate/DPP Extract Beads on the Biochemical Parameters
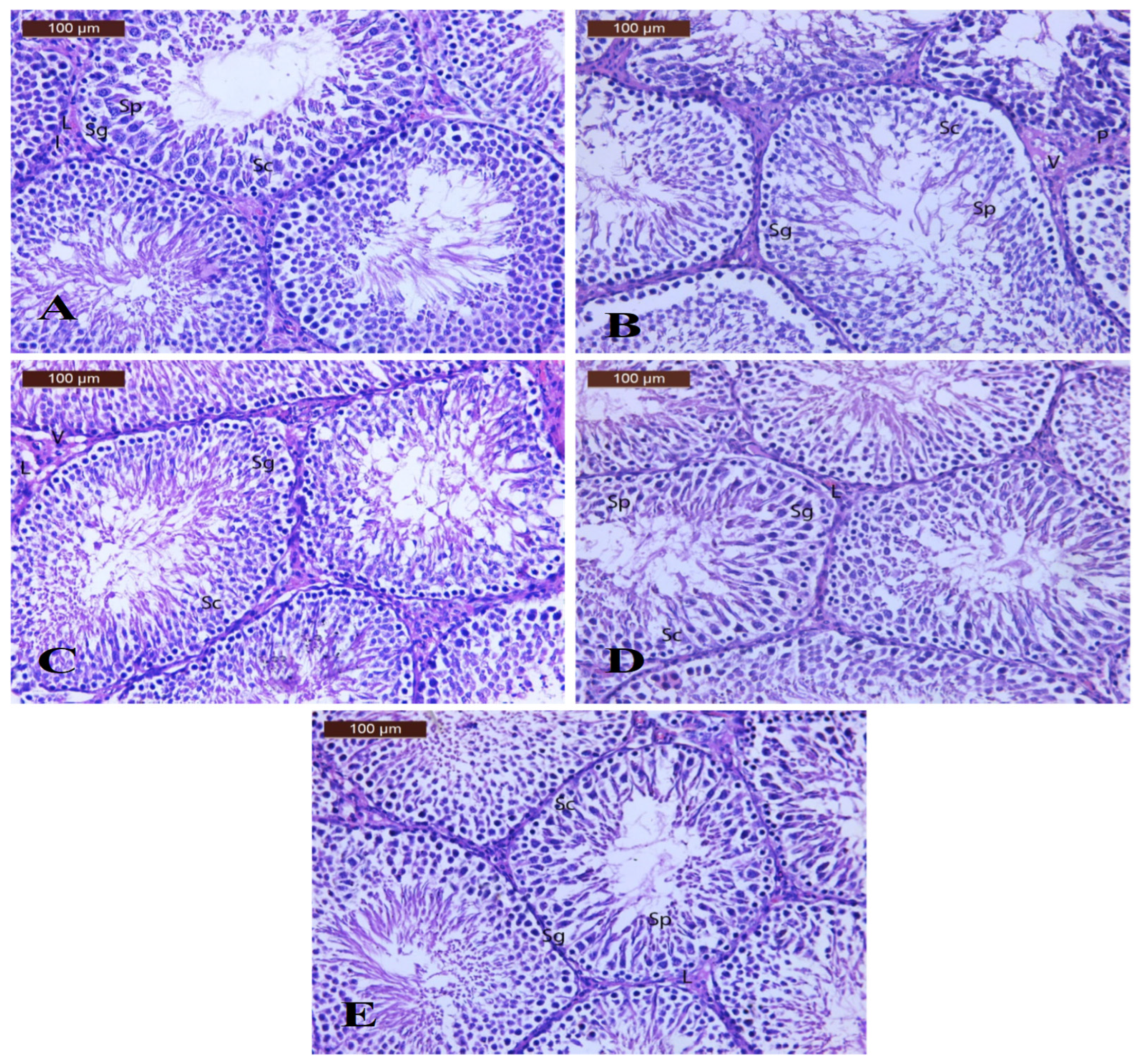
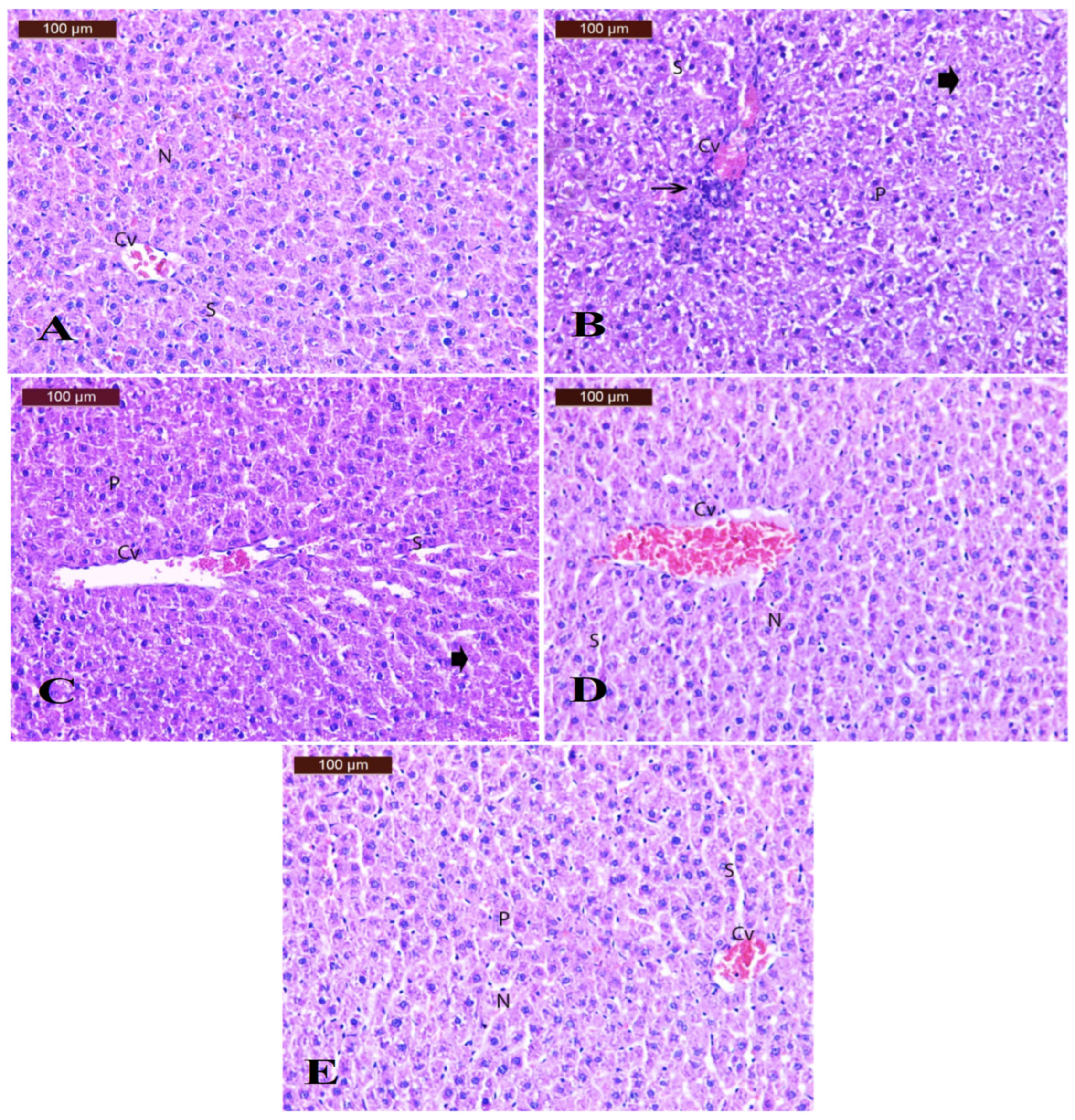
3.8.3. Effect of Alginate/DPP Extract Beads on the Histopathological Changes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Valenzuela, K.S.V.; Araujo, K.V.A.; Casillas, P.E.G.; González, C.C. Protective Encapsulation of a Bioactive Compound in Starch–Polyethylene Glycol-Modified Microparticles: Degradation Analysis with Enzymes. Polymers 2024, 16, 2075. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Wang, Q.; Xu, W.; Wang, X.; Zheng, Q.; Liang, X.; Dong, X.; Li, F.; Peng, L. A bifunctional endolytic alginate lyase with two different lyase catalytic domains from Vibrio sp. H204. Front. Microbiol. 2024, 15, 1509599. [Google Scholar] [CrossRef] [PubMed]
- Stachowiak, N.; Kowalonek, J.; Kozlowska, J.; Burkowska-But, A. Stability Studies, Biodegradation Tests, and Mechanical Properties of Sodium Alginate and Gellan Gum Beads Containing Surfactant. Polymers 2023, 15, 2568. [Google Scholar] [CrossRef] [PubMed]
- Salomón-Torres, R.; Krueger, R.; García-Vázquez, J.P.; Villa-Angulo, R.; Villa-Angulo, C.; Ortiz-Uribe, N.; Sol-Uribe, J.A.; Samaniego-Sandoval, L. Date Palm Pollen: Features, Production, Extraction and Pollination Methods. Agronomy 2021, 11, 504. [Google Scholar] [CrossRef]
- Al-Asmari, A.K.; Al-Said, M.S.; Abbasmanthiri, R.; Al-Buraidi, A.; Ibrahim, K.E.; Rafatullah, S. Impact of date palm pollen (Phoenix dactylifera) treatment on paracetamol-induced hepatorenal toxicity in rats. Clin. Phytosci. 2020, 6, 16. [Google Scholar] [CrossRef]
- Salhi, S.; Rahim, A.; Chentouf, M.; Harrak, H.; Bister, J.L.; Hamidallah, N.; El Amiri, B. Reproductive Enhancement through Phytochemical Characteristics and Biological Activities of Date Palm Pollen: A Comprehensive Review on Potential Mechanism Pathways. Metabolites 2024, 14, 166. [Google Scholar] [CrossRef]
- Mirzaei, M.; Asbagh, F.A.; Safavi, M.; Yekaninejad, M.S.; Rahimi, R.; Pourmand, G.; Karimi, M.; Farshbaf-Khalili, A.; Sarrafi, S. Phoenix dactylifera L. pollen versus pentoxifylline on improvement of sperm parameters in idiopathic male infertility: A randomized clinical trial. J. Ethnopharmacol. 2024, 330, 118168. [Google Scholar] [CrossRef]
- Liu, R.; Liu, B.; Tian, L.; Jiang, X.; Li, X.; Cai, D.; Sun, J.; Bai, W.; Jin, Y. Exposure to Bisphenol A Caused Hepatoxicity and Intestinal Flora Disorder in Rats. Int. J. Mol. Sci. 2022, 23, 8042. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, Y.; Li, Z.; Sun, L.; Zhang, M.; Yu, L.; Wu, S. BPA disrupts 17-estradiol-mediated hepatic protection against ischemia/reperfusion injury in rat liver by upregulating the Ang II/AT1R signaling pathway. Mol. Med. Rep. 2020, 22, 416–422. [Google Scholar] [CrossRef]
- Bordbar, H.; Yahyavi, S.S.; Noorafshan, A.; Aliabadi, E.; Naseh, M. Resveratrol ameliorates bisphenol A-induced testicular toxicity in adult male rats: A stereological and functional study. Basic Clin. Androl. 2023, 33, 1. [Google Scholar] [CrossRef]
- El-Neweshy, M.S.; El-Maddawy, Z.K.; El-Sayed, Y.S. Therapeutic effects of date palm (Phoenix dactylifera L.) pollen extract on cadmium-induced testicular toxicity. Andrologia 2013, 45, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, R.S.; Fouda, K.; Salama, A.; Akl, E.M. Peanut meal-derived bioactive compounds: Extraction, co-extrusion encapsulation and neuroprotection against aluminum-induced Alzheimer’s disease via in silico and in vivo studies. Med. Plus 2024, 4, 100588. [Google Scholar] [CrossRef]
- Siles-Sánchez, M.N.; Jaime, L.; Villalva, M.; Santoyo, S. Encapsulation of Marjoram Phenolic Compounds Using Chitosan to Improve Its Colon Delivery. Foods 2022, 11, 3657. [Google Scholar] [CrossRef]
- Mare, R.; Pujia, R.; Maurotti, S.; Greco, S.; Cardamone, A.; Coppoletta, A.R.; Bonacci, S.; Procopio, A.; Pujia, A. Assessment of Mediterranean Citrus Peel Flavonoids and Their Antioxidant Capacity Using an Innovative UV-Vis Spectrophotometric Approach. Plants 2023, 12, 4046. [Google Scholar] [CrossRef]
- Corrêa, R.C.G.; de Souza, A.H.P.; Calhelha, R.C.; Barros, L.; Glamoclija, J.; Sokovic, M.; Peralta, R.M.; Bracht, A.; Ferreira, I.C.F.R. Bioactive formulations prepared from fruiting bodies and submerged culture mycelia of the Brazilian edible mushroom Pleurotus ostreatoroseus Singer. Food Funct. 2015, 6, 2155–2164. [Google Scholar] [CrossRef]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C. AIN-93 Purified Diets for Laboratory Rodents: Final Report of the American Institute of Nutrition Ad Hoc Writing Committee on the Reformulation of the AIN-76A Rodent Diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef]
- Hajb, A.; Salehpour, Z.; Aghaei, R.; Najafian, A.; Mahmoodi, M.; Latifi, M.; Fallahi, S. The Effect of Palm Pollen and Black Seed Pollen on Male Sex Hormones and Sperm Quality: A Single-Blind, Placebo-Controlled Clinical Trial Study. Int. J. Fertil. Steril. 2023, 17, 75–79. [Google Scholar] [CrossRef]
- Balistreri, W.F.; Shaw, L.M. Liver function. In Fundamentals of Clinical Chemistry, 3rd ed.; Tietz, N.W., Ed.; WB Saunders: Philadelphia, PA, USA, 1987; pp. 729–761. [Google Scholar]
- Bessey, O.A.; Lowery, O.H.; Brock, M.J. A method for the rapid determination of alkaline phosphatase with five millimetres of serum. J. Biol. Chem. 1946, 164, 321–329. [Google Scholar] [CrossRef]
- Reitman, S.; Frankel, S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 1957, 28, 56–63. [Google Scholar] [CrossRef]
- Szasz, G. A kinetic photometric method for serum γ-glutamyl transpeptidase. Clin. Chem. 1969, 15, 124–136. [Google Scholar] [CrossRef]
- Doumas, B.T.; Watson, W.A.; Biggs, H.G. Albumin standards and the measurement of serum albumin with bromcresol green. Clin. Chim. Acta 1997, 258, 21–30. [Google Scholar] [CrossRef]
- Larsen, K. Creatinine assay by a reaction-kinetic principle. Clin. Chim. Acta 1972, 41, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, J.K.; Scott, J.E. A rapid and precise method for the determination of Urea. J. Clin. Pathol. 1960, 13, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, H.J.; Weinstein, H.G. Lactic dehydrogenase activity in human serum. J. Lab. Clin. Med. 1956, 48, 607–616. [Google Scholar]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar]
- Montgomery, H.A.C.; Dymock, J.F. The determination of nitrite in water. Analyst 1961, 86, 414–416. [Google Scholar]
- Nishikimi, M.; Rao, N.A.; Yagi, K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 1972, 46, 849–854. [Google Scholar] [CrossRef]
- Bancroft, J.D.; Cook, H.C. Manual of Histological Techniques; Churchill Livingstone: Edinburgh, UK; London, UK; Melbourne, Australia; New York, NY, USA, 1984; Volume 145, pp. 355–356. [Google Scholar]
- Guamán-Balcázar, M.C.; Montero, M.; Celi, A.; Montes, A.; Carrera, C.; Pereyra, C.; Meneses, M.Á. Encapsulation of Phenolic Compounds Extracted from Beet By-Products: Analysis of Physical and Chemical Properties. Foods 2024, 13, 2859. [Google Scholar] [CrossRef]
- Li, X.; Li, L.; Wang, D.; Zhang, J.; Yi, K.; Su, Y.; Luo, J.; Deng, X.; Deng, F. Fabrication of polymeric microspheres for biomedical applications. Mater. Horiz. 2024, 11, 2820–2855. [Google Scholar] [CrossRef]
- Arshad, A.; Arshad, S.; Alamgeer Mahmood, A.; Hussain Asim, M.; Ijaz, M.; Muhammad Irfan, H.; Rubab, M.; Ali, S.; Raza Hashmi, A. Zeta potential changing self-nanoemulsifying drug delivery systems: A newfangled approach for enhancing oral bioavailability of poorly soluble drugs. Int. J. Pharm. 2024, 655, 123998. [Google Scholar] [CrossRef] [PubMed]
- Silverio, G.B.; Sakanaka, L.S.; Alvim, I.D.; Shirai, M.A.; Grosso, C.R.F. Production and characterization of alginate microparticles obtained by ionic gelation and electrostatic adsorption of concentrated soy protein. Ciênc. Rural. 2018, 48, e20180637. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Ramirez, M.J.; Orrego, C.E.; Teixeira, J.A.; Mussatto, S.I. Encapsulation of antioxidant phenolic compounds extracted from spent coffee grounds by freeze-drying and spray-drying using different coating materials. Food Chem. 2017, 237, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.; Azad, A.K.; Sulaiman, W.M.A.W.; Kumarasamy, V.; Subramaniyan, V.; Alshehade, S.A. Alginate-Based Encapsulation Fabrication Technique for Drug Delivery: An Updated Review of Particle Type, Formulation Technique, Pharmaceutical Ingredient, and Targeted Delivery System. Pharmaceutics 2024, 16, 370. [Google Scholar] [CrossRef]
- El-Kholy, W.M.; Soliman, T.N.; Darwish, A.M.G. Evaluation of date palm pollen (Phoenix dactylifera L.) encapsulation, impact on the nutritional and functional properties of fortified yoghurt. PLoS ONE 2019, 14, e0222789. [Google Scholar] [CrossRef]
- Almehdi, A.M.; Maraqa, M.; Abdulkhalik, S. Aerobiological studies and low allerginicity of Date-Palm pollen in the UAE. Int. J. Environ. Health Res. 2005, 15, 217–224. [Google Scholar] [CrossRef]
- Zhou, B.; Zhang, W.; Wu, Y.; Yang, Y.; Wang, N.; Li, J.; Fu, T.; Wang, L.; Di, L. Improved efficacy of Panax notoginseng saponin loaded into BSP/alginate microspheres for the treatment of alcoholic gastric ulcers. Int. J. Pharm. 2021, 596, 120218. [Google Scholar] [CrossRef]
- Reddy, O.S.; Subha, M.; Jithendra, T.; Madhavi, C.; Rao, K.C. Fabrication and characterization of smart karaya gum/sodium alginate semi-IPN microbeads for controlled release of D-penicillamine drug. Polym. Polym. Compos. 2020, 29, 163–175. [Google Scholar] [CrossRef]
- Abdallah, W.E.; Awad, H.M.; AbdelMohsen, M.M. Phytochemical Composition, Antioxidant and Antitumor Activities of Some Date Palm Pollen Extracts. Egypt. J. Chem. 2023, 66, 425–434. [Google Scholar] [CrossRef]
- Abou Zeid, H.M.; Shiha, M.A.; Shehata, A.A. Comparative Study of Pollen Grains Morphology and Phytochemical Constituents of Some Saudi Arabian Date Palm (Phoenix dactylifera L.) Cultivars. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 2800–2809. [Google Scholar] [CrossRef]
- Daoud, A.; Malika, D.; Bakari, S.; Hfaiedh, N.; Mnafgui, K.; Kadri, A.; Gharsallah, N. Assessment of Polyphenol Composition, Antioxidant and Antimicrobial Properties of Various Extracts of Date Palm Pollen (DPP) from Two Tunisian Cultivars. Arab. J. Chem. 2019, 12, 3075–3086. [Google Scholar] [CrossRef]
- Benouamane, O.; Vergara-Barberan, M.; Benaziza, A.; Garcia-Alvarez-Coque, M.C.; Simó-Alfonso, E.; China, B.; Lerma-Garcia, M.J. Characterization of Different Cultivars of Algerian Date Palm (Phoenix dactylifera L.) Leaves and Pollen by Comprehensive Two-Dimensional Liquid Chromatography of Phenolic Compounds Extracted with Different Solvents. Microchem. J. 2022, 182, 107874. [Google Scholar] [CrossRef]
- Chebout, A.; Ydjedd, S.; Chaalal, M.; Himed, L. Optimization of Malabar Nut (Justicia adhatoda L.) Leaves’ Phenolic Compounds by Alginate Emulsion-gelation Approach using Response Surface Methodology. Curr. Bioact. Comp. 2025, 21, e120324227913. [Google Scholar] [CrossRef]
- Sebii, H.; Karra, S.; Bchir, B.; Ghribi, A.M.; Danthine, S.M.; Blecker, C.; Attia, H.; Besbes, S. Physico-chemical, surface and thermal properties of date palm pollen as a novel nutritive ingredient. Adv. Food Technol. Nutr. Sci. 2019, 5, 84–91. [Google Scholar] [CrossRef]
- Biswas, M.; Haldar, P.K.; Ghosh, A.K. Antioxidant and free-radical-scavenging effects of fruits of Dregea volubilis. J. Nat. Sci. Biol. Med. 2010, 1, 29–34. [Google Scholar] [CrossRef]
- Zeb, A. Concept, mechanism, and applications of phenolic antioxidants in foods. J. Food Biochem. 2020, 44, e13394. [Google Scholar] [CrossRef]
- Pérez-Pérez, V.; Jiménez-Martínez, C.; González-Escobar, J.L.; Corzo-Ríos, L.J. Exploring the impact of encapsulation on the stability and bioactivity of peptides extracted from botanical sources: Trends and opportunities. Front. Chem. 2024, 12, 1423500. [Google Scholar] [CrossRef]
- Machado, A.R.; Silva, P.M.P.; Vicente, A.A.; Souza-Soares, L.A.; Pinheiro, A.C.; Cerqueira, M.A. Alginate Particles for Encapsulation of Phenolic Extract from Spirulina sp. LEB-18: Physicochemical Characterization and Assessment of In Vitro Gastrointestinal Behavior. Polymers 2022, 14, 4759. [Google Scholar] [CrossRef]
- Gorbunova, N.; Bannikova, A.; Evteev, A.; Evdokimov, I.; Kasapis, S. Alginate-based encapsulation of extracts from beta Vulgaris cv. beet greens: Stability and controlled release under simulated gastrointestinal conditions. LWT 2018, 93, 442–449. [Google Scholar] [CrossRef]
- Harris, H.; Lecumberri, E.; Mateos-Aparicio, I.; Mengibar, M.; Heras, A. Chitosan nanoparticles and microspheres for the encapsulation of natural antioxidants extracted from Ilex paraguariensis. Carbohydr. Polym. 2011, 84, 803–808. [Google Scholar] [CrossRef]
- Fuenmayor, C.A.; Cosio, M.S. Encapsulation of antioxidant phenolic compounds in zein ultra-thin fibers via electrospinning. Rev. EIA 2016, 12, E13–E26. [Google Scholar] [CrossRef]
- Kobroob, A.; Peerapanyasut, W.; Chattipakorn, N.; Wongmekiat, O. Damaging effects of bisphenol a on the kidney and the protection by melatonin: Emerging evidences from in vivo and in vitro studies. Oxidative Med. Cell. Longev. 2018, 2018, 3082438. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, N.A.; Ahmed, O.M.; Hozayen, W.G.; Ahmed, M.A. Ameliorative effects of bee pollen and date palm pollen on the glycemic state and male sexual dysfunctions in streptozotocin-Induced diabetic wistar rats. Biomed. Pharmacother. 2018, 97, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Wisniewski, P.; Romano, R.M.; Kizys, M.M.L.; Oliveira, K.C.; Kasamatsu, T.; Giannocco, G.; Chiamolera, M.I.; Dias-da-Silva, M.R.; Romano, M.A. Adult exposure to bisphenol A (BPA) in Wistar rats reduces sperm quality with disruption of the hypothalamic–pituitary–testicular axis. Toxicology 2015, 329, 1–9. [Google Scholar] [CrossRef]
- El-Kashlan, A.M.; Nooh, M.M.; Hassan, W.A.; Rizk, S.M. Therapeutic Potential of Date Palm Pollen for Testicular Dysfunction Induced by Thyroid Disorders in Male Rats. PLoS ONE 2015, 10, e0139493. [Google Scholar] [CrossRef]
- Wu, Z.; Shangguan, D.; Huang, Q.; Wang, Y.K. Drug metabolism and transport mediated the hepatotoxicity of Pleuropterus multiflorus root: A review. Drug Metab. Rev. 2024, 56, 349–358. [Google Scholar] [CrossRef]
- Elblehi, S.S.; El-Sayed, Y.S.; Soliman, M.M.; Shukry, M. Date Palm Pollen Extract Avert Doxorubicin-Induced Cardiomyopathy Fibrosis and Associated Oxidative/Nitrosative Stress, Inflammatory Cascade, and Apoptosis-Targeting Bax/Bcl-2 and Caspase-3 Signaling Pathways. Animals 2021, 11, 886. [Google Scholar] [CrossRef]
- Li, J.; Chen, Y.; Zhang, S.; Zhao, Y.; Gao, D.; Xing, J.; Cao, Y.; Xu, G. Purslane (Portulaca oleracea L.) polysaccharide attenuates carbon tetrachloride-induced acute liver injury by modulating the gut microbiota in mice. Genomics 2025, 117, 110983. [Google Scholar] [CrossRef]
- Banerjee, O.; Singh, S.; Paul, T.; Maji, B.K.; Mukherjee, S. Centella asiatica mitigates the detrimental effects of Bisphenol-A (BPA) on pancreatic islets. Sci. Rep. 2024, 14, 8043. [Google Scholar] [CrossRef]
- Dallio, M.; Romeo, M.; Cipullo, M.; Ventriglia, L.; Scognamiglio, F.; Vaia, P.; Iadanza, G.; Coppola, A.; Federico, A. Systemic Oxidative Balance Reflects the Liver Disease Progression Status for Primary Biliary Cholangitis (Pbc): The Narcissus Fountain. Antioxidants 2024, 13, 387. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef] [PubMed]
- Maćczak, A.; Cyrkler, M.; Bukowska, B.; Michałowicz, J. Bisphenol A, bisphenol S, bisphenol F and bisphenol AF induce different oxidative stress and damage in human red blood cells (in vitro study). Toxicol. In Vitro 2017, 41, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Galleano, M.; Pechanova, O.; Fraga, C.G. Hypertension, Nitric Oxide, Oxidants, and Dietary Plant Polyphenols. Curr. Pharm. Biotechnol. 2010, 11, 837–848. [Google Scholar] [CrossRef]
- Yang, S.Y.; Pyo, M.C.; Nam, M.H.; Lee, K.W. ERK/Nrf2 Pathway Activation by Caffeic Acid in HepG2 Cells Alleviates Its Hepatocellular Damage Caused by t-Butylhydroperoxide-Induced Oxidative Stress. BMC Complement. Altern. Med. 2019, 19, 139. [Google Scholar] [CrossRef]
- Ma, N.; Liu, X.; Zhao, L.; Liu, Y.; Peng, X.; Ma, D.; Ma, L.; Kiyama, R.; Dong, S. Bisphenol P induces increased oxidative stress in renal tissues of C57BL/6 mice and human renal cortical proximal tubular epithelial cells, resulting in kidney injury. Sci. Total Environ. 2024, 949, 175159. [Google Scholar] [CrossRef]
- Priego, A.R.; Parra, E.G.; Mas, S.; Morgado-Pascual, J.L.; Ruiz-Ortega, M.; Rayego-Mateos, S. Bisphenol A Modulates Autophagy and Exacerbates Chronic Kidney Damage in Mice. Int. J. Mol. Sci. 2021, 22, 7189. [Google Scholar] [CrossRef]
- Saleh, S.M.M.; Mahmoud, A.B.; Al-Salahy, M.B.; Mohamed Moustafa, F.A. Morphological, immunohistochemical, and biochemical study on the ameliorative effect of gallic acid against bisphenol A-induced nephrotoxicity in male albino rats. Sci. Rep. 2023, 13, 1732. [Google Scholar] [CrossRef]




| Conc. (µg/g Extract) | |
|---|---|
| Gallic acid | 390.54 |
| Chlorogenic acid | 51.35 |
| Catechin | 172.15 |
| Methyl gallate | 576.00 |
| Coffeic acid | 82.34 |
| Syringic acid | 50.93 |
| Pyro catechol | 59.26 |
| Rutin | 23.83 |
| Ellagic acid | 30.07 |
| Coumaric acid | 7.91 |
| Vanillin | 83.29 |
| Ferulic acid | 9.05 |
| Naringenin | 302.93 |
| Rosmarinic acid | 36.43 |
| Daidzein | 1.38 |
| Querectin | 57.14 |
| Cinnamic acid | 2.00 |
| Hesperetin | 15.91 |
| Total phenolic content (mg GAE/g extract) | 66.48 ± 0.22 |
| Total flavonoids content (mg CE/g extract) | 15.30 ± 0.07 |
| DPPH scavenging activity (EC50 value, mg mL−1) | 17.33 ± 0.11 |
| TBARS inhibition (EC50 value, mg mL−1) | 9.30 ± 0.17 |
| pH | 1 h | 2 h | 3 h |
|---|---|---|---|
| 2 | 46.56 | 57.56 | 59.43 |
| 7.4 | 83.26 | 86.29 | 89.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fouda, K.; Mohamed, R.S. Alginate Beads with Encapsulated Date Palm Pollen Extract: Development, Characterization and Their Potential Role in Hepato-Protection and Fertility-Stimulating Hormones Improvement in Bisphenol A-Treated Rats. Polymers 2025, 17, 912. https://doi.org/10.3390/polym17070912
Fouda K, Mohamed RS. Alginate Beads with Encapsulated Date Palm Pollen Extract: Development, Characterization and Their Potential Role in Hepato-Protection and Fertility-Stimulating Hormones Improvement in Bisphenol A-Treated Rats. Polymers. 2025; 17(7):912. https://doi.org/10.3390/polym17070912
Chicago/Turabian StyleFouda, Karem, and Rasha S. Mohamed. 2025. "Alginate Beads with Encapsulated Date Palm Pollen Extract: Development, Characterization and Their Potential Role in Hepato-Protection and Fertility-Stimulating Hormones Improvement in Bisphenol A-Treated Rats" Polymers 17, no. 7: 912. https://doi.org/10.3390/polym17070912
APA StyleFouda, K., & Mohamed, R. S. (2025). Alginate Beads with Encapsulated Date Palm Pollen Extract: Development, Characterization and Their Potential Role in Hepato-Protection and Fertility-Stimulating Hormones Improvement in Bisphenol A-Treated Rats. Polymers, 17(7), 912. https://doi.org/10.3390/polym17070912







