Development and Characterization of Polymeric Films Loaded with Terbinafine for Fungal Infection Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Phosphate Buffer pH 7.4
2.2.2. Preparation of Oxidized Pullulan (T-OP)
2.2.3. Preparation of Films
2.3. The Physical–Chemistry Analysis of Polymer Films
2.3.1. Development of a Spectrophotometric Method
2.3.2. Solubility Study
2.3.3. Estimation of Drug Loading and Entrapment Efficiency
- EE—entrapment efficiency, %;
- —amount of TH loaded into the polymeric film; mg;
- —amount of TH that was added into the polymeric film; mg.
- LC—loading capacity of TH in polymeric films;
- —amount of TH loaded into the polymeric film; mg;
- mpolymeric film—mass of the polymeric film; mg.
2.3.4. Attenuated Total Reflection Fourier Transform IR (ATR-FTIR) Spectroscopy
2.3.5. Zeta-Potential Measurements
- —viscosity;
- —dielectric constant.
2.3.6. Energy-Dispersive X-Ray (EDX)
2.3.7. Mechanical Tests
2.3.8. Contact Angle Determination
2.3.9. Dynamic Vapors Sorption Measurements
2.3.10. Bioadhesive Properties
2.3.11. In Vitro Drug Release
- cr—concentration of the released drug, µg/mL;
- cl—concentration of the loaded drug, µg/mL.
2.3.12. Analysis of In Vitro Drug Release Kinetics
- Ft—amount of active substance released at t moment,
- F0—the initial amount of drug substance in the polymer film,
- K0—constant of zero order release rate,
- K—constant of first order release rate,
- KH—constant of Higuchi model release rate,
- KP—constant of Korsmeyer–Peppas model release rate,
- n—exponential coefficient, an indicator of release mechanism of active substance,
- Kw—constant of Weibull model,
- β—shape parameter,
- F∞—the maximum amount of substance that can be released from the polymer film,
- t—time.
2.3.13. Antifungal Activity of Polymeric Films
3. Results and Discussion
3.1. Solubility Study
3.2. Estimation of Drug Loading and Entrapment Efficiency
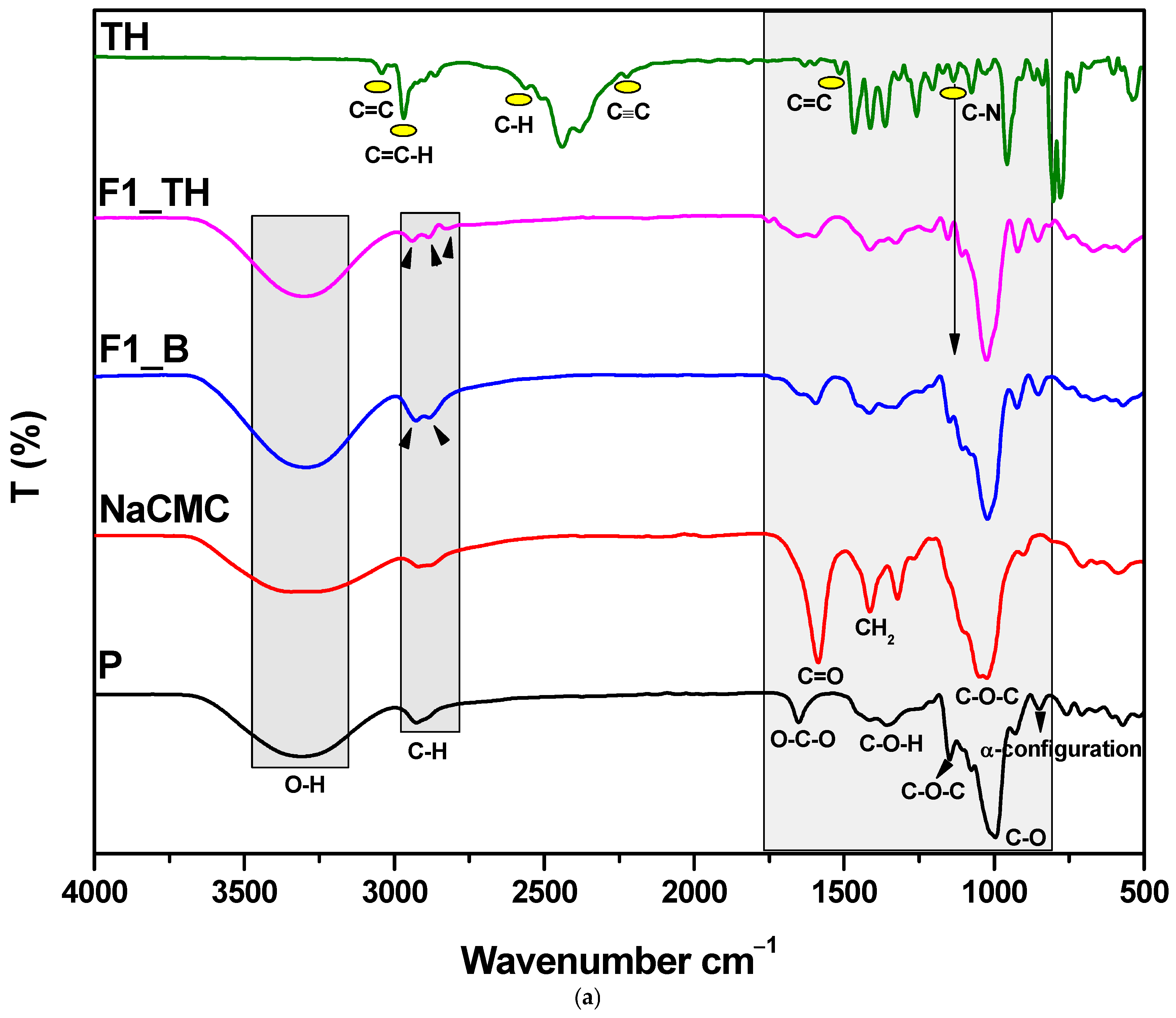
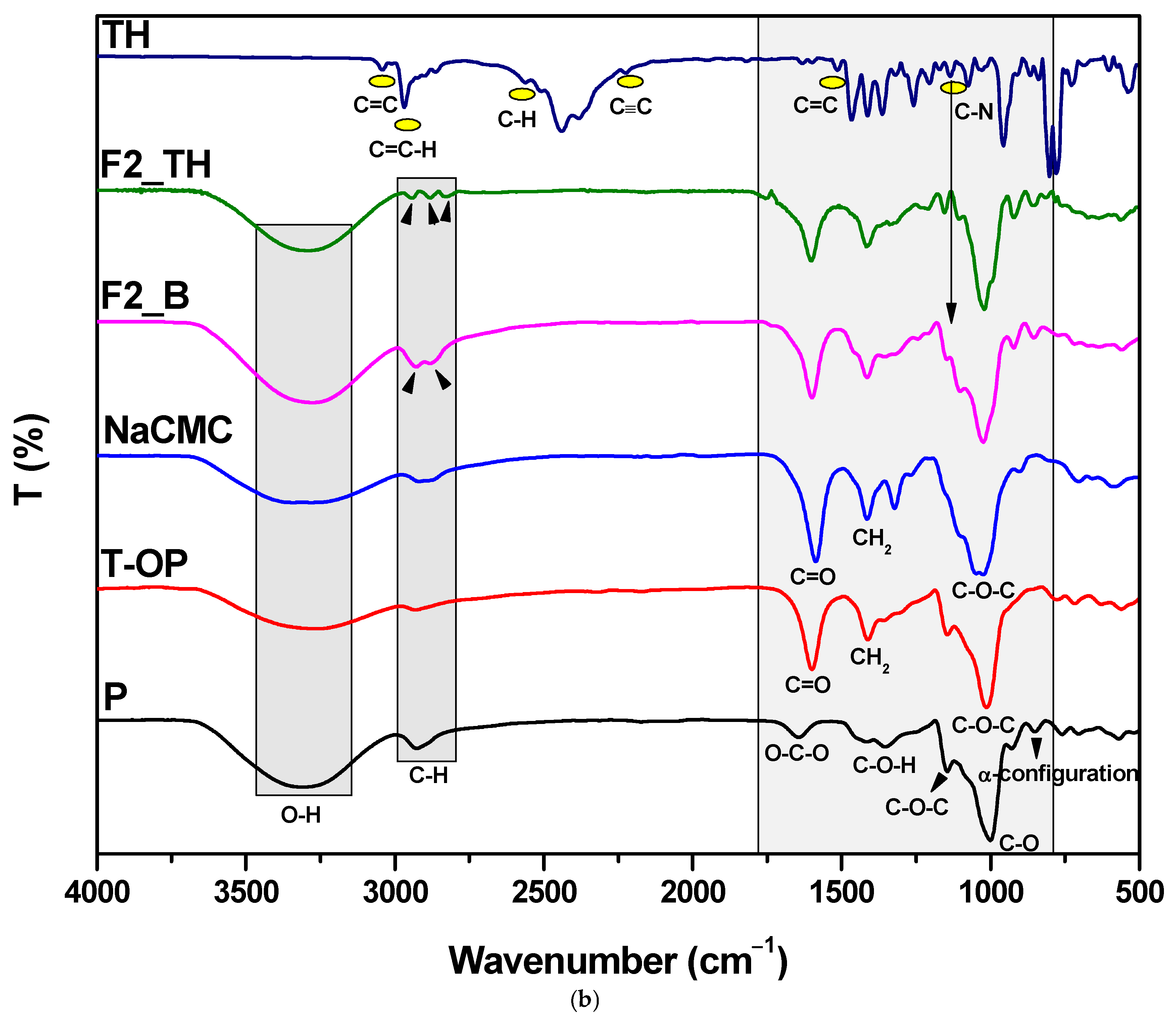
3.3. Structural Characterization by FTIR Spectroscopy
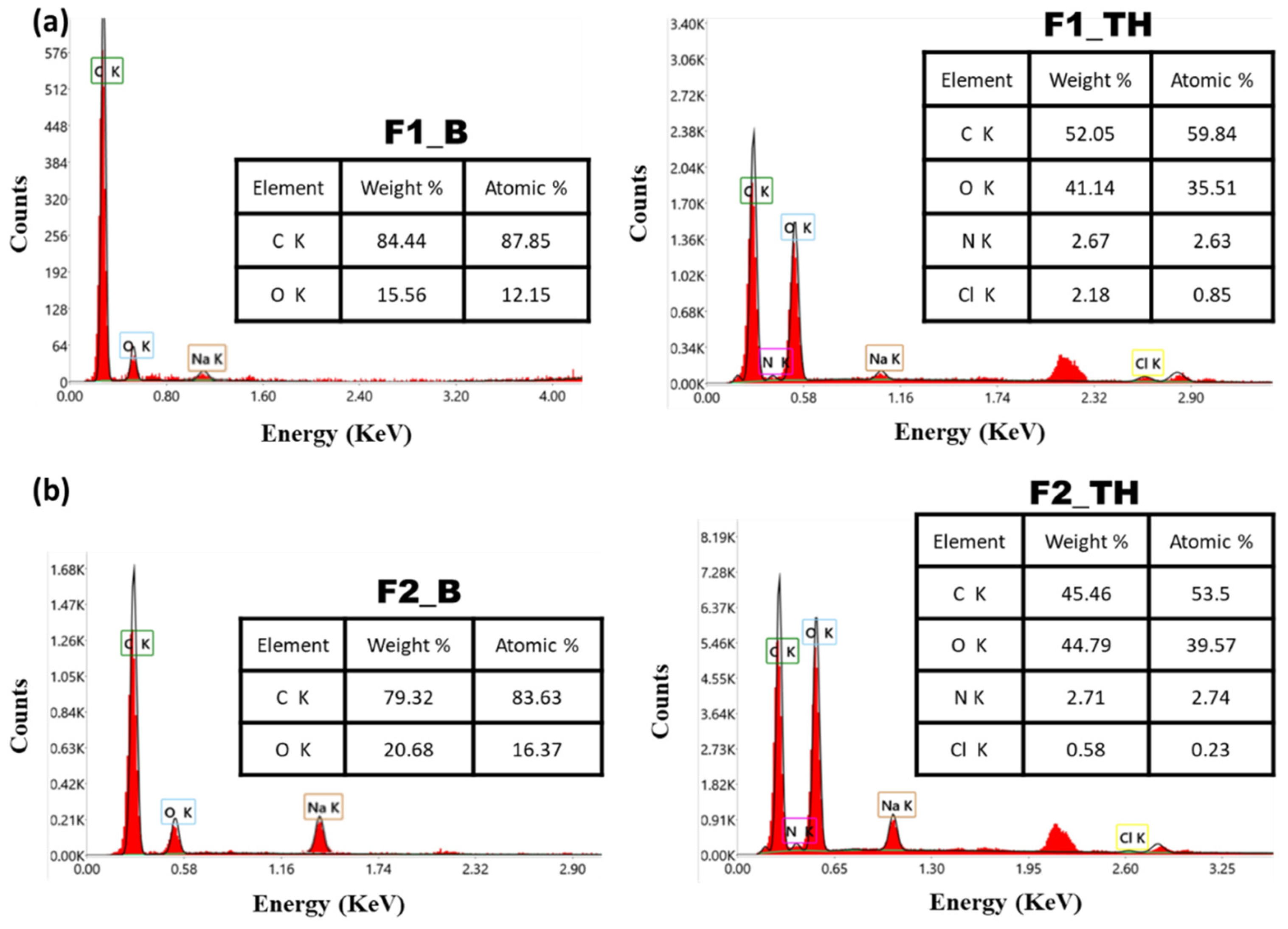
3.4. Energy-Dispersive X-Ray (EDX)
3.5. Mechanical Tests
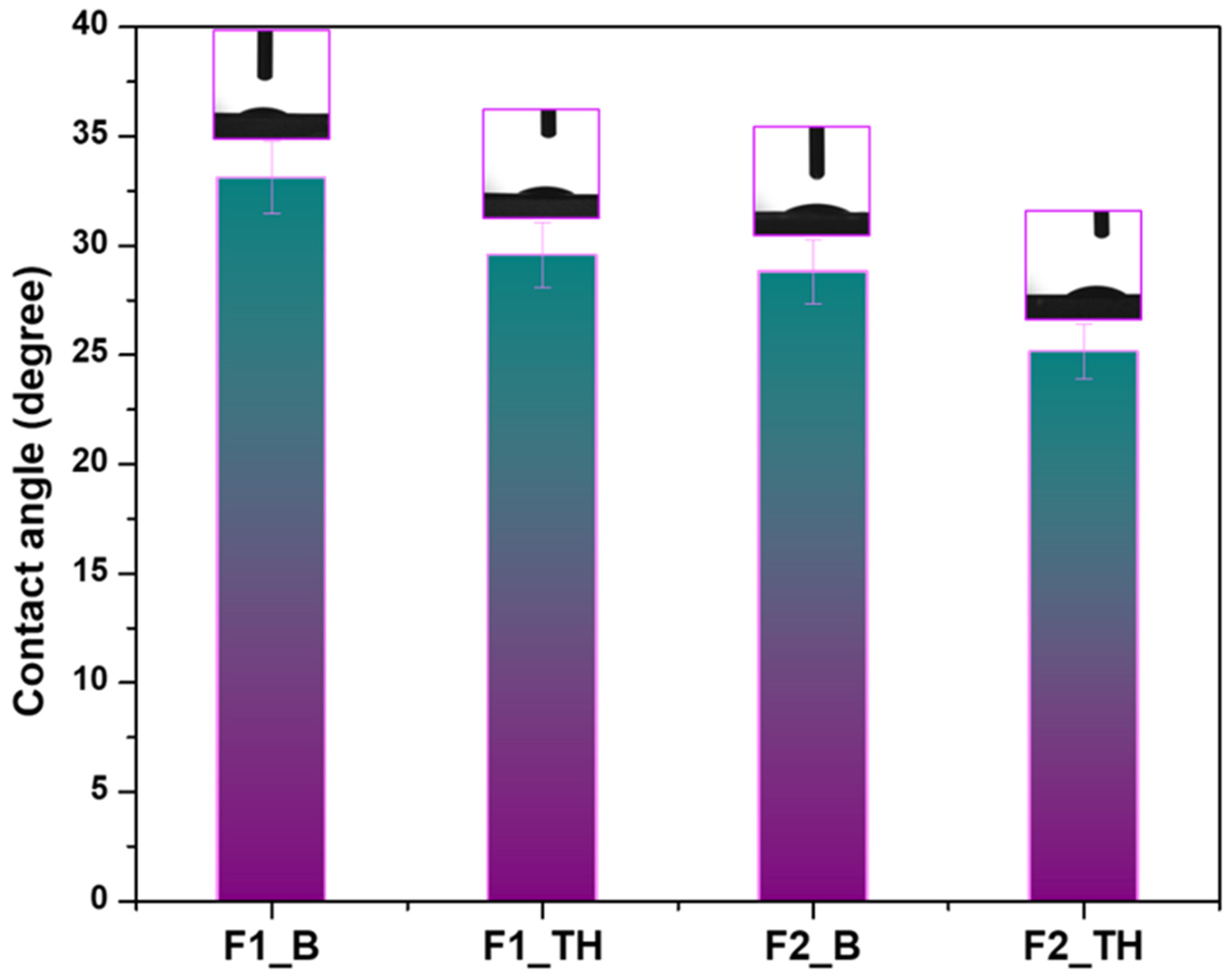
3.6. Contact Angle Determination
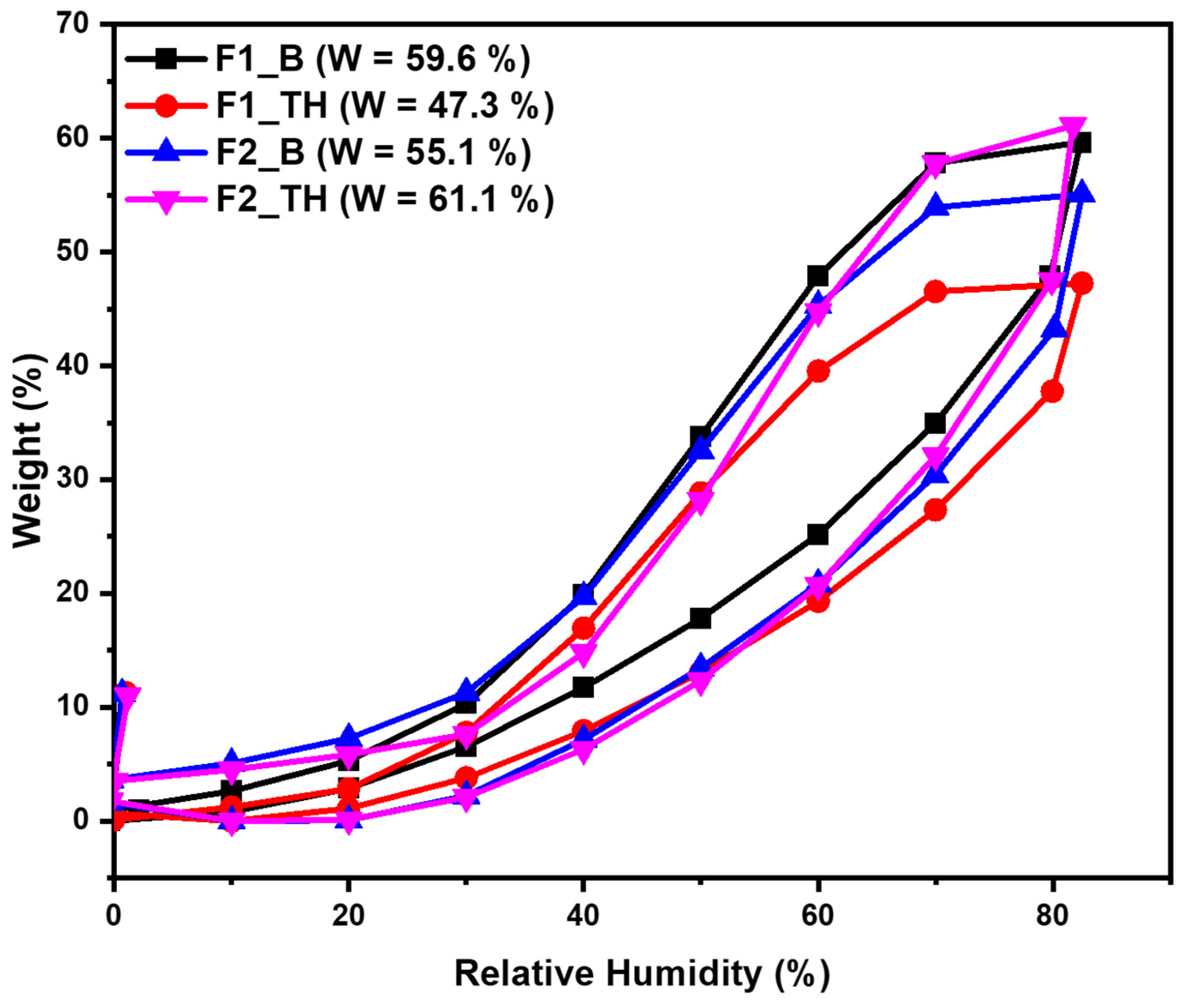
3.7. Dynamic Water Vapors Sorption Measurements
3.8. Bioadhesive Properties
3.9. In Vitro Release Study
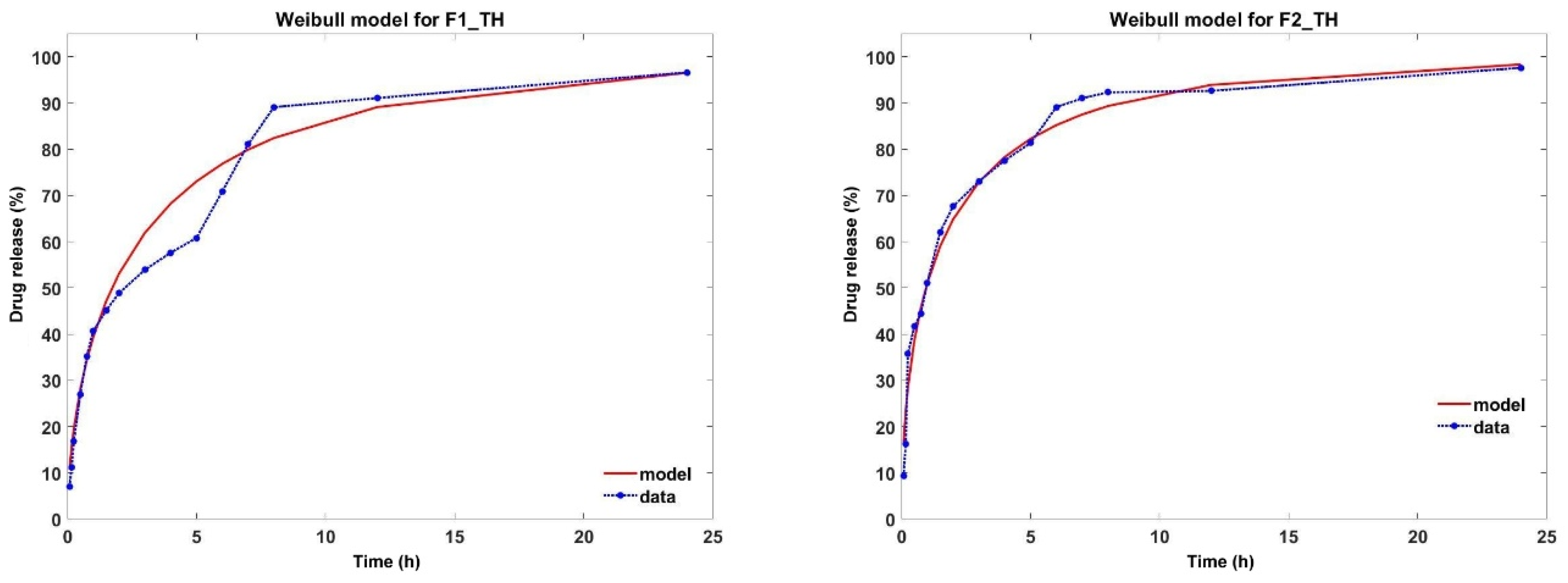
3.10. Analysis of In Vitro Drug Release Kinetics
3.11. Antimicrobial Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Shoaiba, S.; Kaurb, G.; Yusufc, K.; Yusuf, N. Herbal medicines and skin disorders. In Herbal Medicines; Sarwat, M., Siddique, H., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 307–328. ISBN 9780323905725. [Google Scholar]
- Havlickova, B.; Czaika, V.A.; Friedrich, M. Epidemiological trends in skin mycoses worldwide. Mycoses 2008, 51, 2–15. [Google Scholar] [PubMed]
- Gaba, B.; Fazil, M.; Khan, S.; Ali, A.; Baboota, S.; Ali, J. Nanostructured lipid carrier system for topical delivery of terbinafine hydrochloride. Bull. Fac. Pharm. Cairo Univ. 2015, 53, 147–159. [Google Scholar] [CrossRef]
- Maurya, V.K.; Kachhwaha, D.; Bora, A.; Khatri, P.K.; Rathore, L. Determination of antifungal minimum inhibitory concentration and its clinical correlation among treatment failure cases of dermatophytosis. J. Family Med. Prim. Care 2019, 8, 2577–2581. [Google Scholar] [CrossRef]
- Hassan, S.U.; Khalid, I.; Hussain, L.; Imam, M.T.; Shahid, I. Topical Delivery of Terbinafine HCL Using Nanogels: A New Approach to Superficial Fungal Infection Treatment. Gels 2023, 9, 841. [Google Scholar] [CrossRef]
- Kondoros, B.A.; Jójárt-Laczkovich, O.; Berkesi, O.; Szabó-Révész, P.; Csóka, I.; Ambrus, R.; Aigner, Z. Development of Solvent-Free Co-Ground Method to Produce Terbinafine Hydrochlorid Cyclodextrin Binary Systems; Structural and In Vitro Characterizations. Pharmaceutics 2022, 14, 744. [Google Scholar] [CrossRef]
- Pervaiz, F.; Mushtaq, R.; Noreen, S. Formulation and optimization of terbinafine HCl loaded chitosan/xanthan gum nanoparticles containing gel: Ex-vivo permeation and in-vivo antifungal studies. J. Drug Deliv. Sci. Technol. 2021, 66, 102935. [Google Scholar] [CrossRef]
- Tundisi, L.L.; Ataide, J.A.; da Fonseca, J.H.L.; Silvério, L.A.L.; Lancellotti, M.; Paiva-Santos, A.C.; d’Ávila, M.A.; Kohane, D.S.; Mazzola, P.G. Terbinafine Nanohybrid: Proposing a Hydrogel Carrying Nanoparticles for Topical Release. Pharmaceutics 2023, 15, 841. [Google Scholar] [CrossRef]
- Rangel-Yagui, C.O.; Pessoa-Jr, A.; Tavares, L.C. Micellar solubilization of drugs. J. Pharm. Pharm. Sci. 2005, 8, 147–163. [Google Scholar]
- Khan, K.U.; Minhas, M.U.; Badshah, S.F.; Suhail, M.; Ahmad, A.; Ijaz, S. Overview of nanoparticulate strategies for solubility enhancement of poorly soluble drugs. Life Sci. 2022, 291, 120301. [Google Scholar] [CrossRef]
- Ravichandran, V.; Lee, M.; Cao, T.G.N.; Shim, M.S. Polysorbate-Based Drug Formulations for Brain-Targeted Drug Delivery and Anticancer Therapy. Appl. Sci. 2021, 11, 9336. [Google Scholar] [CrossRef]
- Ainurofiq, A.; Putro, D.S.; Ramadhani, D.A.; Putra, G.M.; Do Espirito Santo, L.D.C. A review on solubility enhancement methods for poorly water-soluble drugs. J. Rep. Pharm. Sci. 2021, 10, 137–147. [Google Scholar] [CrossRef]
- Hmingthansanga, V.; Singh, N.; Banerjee, S.; Manickam, S.; Velayutham, R.; Natesan, S. Improved Topical Drug Delivery: Role of Permeation Enhancers and Advanced Approaches. Pharmaceutics 2022, 14, 2818. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Sharma, G.S.; Goyal, A.K.; Ghosh, G.; Si, C.S.; Rath, G. Recent advances in topical carriers of anti-fungal agents. Heliyon 2020, 6, e04663. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Beck-Broichsitter, M.; Banga, A.K. Design and Evaluation of a Poly(Lactide-co-Glycolide)-Based In Situ Film-Forming System for Topical Delivery of Trolamine Salicylate. Pharmaceutics 2019, 11, 409. [Google Scholar] [CrossRef]
- Conte, J.; Saatkamp, R.H.; Sanches, M.P.; Argenta, D.F.; Monte Machado, G.R.; Kretzer, I.F.; Parizeb, A.L.; Caon, T. Development of biopolymer films loaded with fluconazole and thymol for resistant vaginal candidiasis. Int. J. Biol. Macromol. 2024, 275, 133356. [Google Scholar] [CrossRef]
- Kraisit, P.; Yonemochi, E.; Furuishi, T.; Mahadlek, J.; Limmatvapirat, S. Chitosan film containing antifungal agent-loaded SLNs for the treatment of candidiasis using a Box-Behnken design. Carbohydr. Polym. 2022, 283, 119178. [Google Scholar] [CrossRef]
- Real, D.A.; Martinez, M.V.; Frattini, A.; Soazo, M.; Luque, A.G.; Marisa, S.; Biasoli, M.S.; Salomon, C.J.; Olivieri, A.C.; Leonardi, D. Design, Characterization, and In Vitro Evaluation of Antifungal Polymeric Films. AAPS PharmSciTech 2012, 14, 1. [Google Scholar] [CrossRef]
- Salama, M.; Mahdy, M.A.; Mohamed, A.; Mohamed, A.T.; Keleb, E.I.; Omar, A.A.; Elmarzugi, N.A. Formulation and Evaluation of Ketoconazole Polymeric Films for Topical Application. J. Appl. Pharm. Sci. 2015, 5, 28–32. [Google Scholar] [CrossRef]
- Xin, Y.; Quan, L.; Zhang, H.; Ao, Q. Emerging Polymer-Based Nanosystem Strategies in the Delivery of Antifungal Drugs. Pharmaceutics 2023, 15, 1866. [Google Scholar] [CrossRef]
- Aquinas, N.; Chithra, C.H.; Bhat, M.R. Progress in bioproduction, characterization and applications of pullulan: A review. Polym. Bull. 2024, 81, 12347–12382. [Google Scholar] [CrossRef]
- Baron, R.I.; Coseri, S. Preparation of water-soluble cellulose derivatives using TEMPO radical-mediated oxidation at extended reaction time. React. Funct. Polym. 2020, 157, 104768. [Google Scholar]
- Karuppiah, S.; Rahman, S.S.A.; Ponnusami, V. Microbial pullulan: Properties, bioprocess engineering, and applications. In Pullulan: Processing, Properties, and Applications; Ahmed, S., Soundararajan, A., Eds.; Jenny Stanford Publishing: New York, NY, USA, 2020; pp. 167–207. [Google Scholar]
- Thomas, N.; Puluhulawa, L.E.; Cindana Mo’o, F.R.; Rusdin, A.; Gazzali, A.M.; Budiman, A. Potential of Pullulan-Based Polymeric Nanoparticles for Improving Drug Physicochemical Properties and Effectiveness. Polymers 2024, 16, 2151. [Google Scholar] [CrossRef] [PubMed]
- Henni-Silhadi, W.; Deyme, M.; Boissonnade, M.M.; Appel, M.; Le Cerf, D.; Picton, L.; Rosilio, V. Enhancement of the solubility and efficacy of poorly water-soluble drugs by hydrophobically-modified polysaccharide derivatives. Pharm. Res. 2007, 24, 2317–2326. [Google Scholar] [CrossRef]
- Spera, M.B.; Taketa, T.B.; Beppu, M.M. Roughness dynamic in surface growth: Layer-by-layer thin films of carboxymethyl cellulose/chitosan for biomedical applications. Biointerphases 2017, 12, 04E401-6. [Google Scholar]
- Rahman, M.S.; Hasan, M.S.; Nitai, A.S.; Nam, S.; Karmakar, A.K.; Ahsan, M.S.; Shiddiky, M.J.A.; Ahmed, M.B. Recent Developments of Carboxymethyl Cellulose. Polymers 2021, 13, 1345. [Google Scholar] [CrossRef]
- Palmer, D.; Levina, M.; Nokhodchi, A.; Douroumis, D.; Farrell, T.; Rajabi-Siahboomi, A. The Influence of Sodium Carboxymethylcellulose on Drug Release from Polyethylene Oxide Extended Release Matrices. AAPS PharmSciTech 2011, 12, 862–871. [Google Scholar] [CrossRef]
- European Parliament. 4.1.3. Buffer Solutions; Council of Europe: Strasbourg, France, 2008; Volume 6, p. 512. [Google Scholar]
- Luki´c, M.; Panteli´c, I.; Savi´c, S.D. Towards Optimal pH of the Skin and Topical Formulations: From the Current State of the Art to Tailored Products. Cosmetics 2021, 8, 69. [Google Scholar] [CrossRef]
- Committee for Medicinal Products for Human Use. ICH Q2(R2) Guideline on Validation of Analytical Procedures. EMA/CHMP/ICH/82072/2006; 14 December 2023. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-q2r2-guideline-validation-analytical-procedures-step-5-revision-1_en.pdf (accessed on 3 April 2025).
- Kanakapura, B.; Penmatsa, V.K. Analytical methods for determination of terbinafine hydrochloride in pharmaceuticals and biological materials. J. Pharm. Anal. 2016, 6, 137–149. [Google Scholar] [CrossRef]
- Khalbas, A.H.; Albayati, T.M.; Ali, N.S.; Salih, I.K. Drug loading methods and kinetic release using of mesoporous silica nanoparticles as a drug delivery system: A review. S. Afr. J. Chem. Eng 2024, 50, 261–280. [Google Scholar]
- Al-Bazali, T. On the stability of shale: The role of zeta potential (ζ) and Debye Hückel length (κ−1) on shale swelling. Pet. Sci. Technol. 2021, 39, 1–19. [Google Scholar] [CrossRef]
- Morariu, S.; Bercea, M.; Gradinaru, L.M.; Rosca, I.; Avadanei, M. Versatile poly(vinyl alcohol)/clay physical hydrogels with tailorable structure as potential candidates for wound healing applications. Mater. Sci. Eng. C 2020, 109, 110395. [Google Scholar] [CrossRef]
- Ciarfaglia, N.; Pepe, A.; Piccirillo, G.; Laezza, A.; Daum, R.; Schenke-Layland, K.; Bochicchio, B. Nanocellulose and elastin act as plasticizers of electrospun bioinspired scaffolds ACS. Appl. Polym. Mater. 2020, 2, 4836–4847. [Google Scholar] [CrossRef]
- Nistor, A.; Stiubianu, G.; Racles, C.; Cazacu, M. Evaluation of the Water Sorption Capacity of Some Polymeric Materials by Dynamic Vapour Sorption. Mater. Plast. 2011, 48, 33–48. [Google Scholar]
- Abobakr, F.E.; Fayez, S.M.; Elwazzan, V.S.; Sakran, W. Formulation and optimization of Terbinafine HCl solid lipid nanoparticles for topical antifungal activity. Int. J. Pharm. Pharm. Sci. 2019, 11, 16–25. [Google Scholar] [CrossRef]
- Baron, R.I.; Culica, M.E.; Biliuta, G.; Bercea, M.; Gherman, S.; Zavastin, D.; Ochiuz, L.; Avadanei, M.; Coseri, S. Physical Hydrogels of Oxidized Polysaccharides and Poly(Vinyl Alcohol) for Wound Dressing Applications. Materials 2019, 12, 1569. [Google Scholar] [CrossRef]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic Modeling on Drug Release from Controlled Drug Delivery Systems. Acta Pol. Pharm. 2010, 67, 217–223. [Google Scholar]
- Paolino, D.; Tudose, A.; Celia, C.; Di Marzio, L.; Cilurzo, F.; Mircioiu, C. Mathematical models as tools to predict the release kinetic of fluorescein from lyotropic colloidal liquid crystals. Materials 2019, 12, 693. [Google Scholar] [CrossRef]
- Ata, S.; Rasool, A.; Islam, A.; Bibi, I.; Rizwan, M.; Azeem, M.K.; Qureshi, A.R.; Iqbal, M. Loading of Cefixime to pH sensitive chitosan based hydrogel and investigation of controlled release kinetics. Int. J. Biol. Macromol. 2020, 155, 1236–1244. [Google Scholar]
- Caccavo, D.; Cascone, S.; Lamberti, G.; Barba, A.A. Controlled drug release from hydrogel-based matrices: Experiments and modeling. Int. J. Pharm. 2015, 486, 144–152. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 34th ed.; CLSI Supplement M100; Clinical and Laboratory Standards Institute: Berwyn, PA, USA, 2024. [Google Scholar]
- Clinical and Laboratory Standard Institute. Method for Antifungal Disk Diffusion Susceptibility Testing of Yeasts, 2nd ed.; Approved Guideline; CLSI: Wayne, PA, USA, 2009. [Google Scholar]
- Spatareanu, A.; Bercea, M.; Budtova, T.; Harabagiu, V.; Sacarescu, L.; Coseri, S. Synthesis, characterization and solution behaviour of oxidized pullulan. Carbohydr. Polym. 2014, 111, 63–71. [Google Scholar]
- Michel, C.; Purmann, T.; Mentrup, E.; Seiller, E.; Kreuter, J. Effect of liposomes on percutaneous penetration of lipophilic materials. Int. J. Pharm. 1992, 84, 93–105. [Google Scholar]
- Wang, C.; Yanga, Y.; Cui, X.; Ding, Z.; Chena, Z. Three different types of solubilization of thymol in Tween 80: Micelles, solutions, and emulsions- a mechanism study of micellar solubilization. J. Mol. Liq. 2020, 306, 112901. [Google Scholar] [CrossRef]
- Chouhan, P.; Saini, T.R. Hydroxypropyl-β-cyclodextrin: A novel transungual permeation enhancer for development of topical drug delivery system for onychomycosis. J. Drug Deliv. 2014, 2014, 950358. [Google Scholar] [CrossRef] [PubMed]
- Vejnovic, I.; Huonder, C.; Betz, G. Permeation studies of novel terbinafine formulations containing hydrophobins through human nails in vitro. Int. J. Pharm. 2010, 397, 67–76. [Google Scholar]
- Arpa, M.D.; Ünükür, M.Z.; Erim, U.C. Formulation, characterization and in vitro release studies of terbinafine hydrochloride loaded buccal films. J. Res. Pharm. 2021, 25, 667–680. [Google Scholar] [CrossRef]
- Committee for Medicinal Products for Human Use (CHMP). Information for the Package Leaflet Regarding Polysorbates Used as Excipients in Medicinal Products for Human Use. EMA/CHMP/190743/2016, 19 November 2018. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/draft-information-package-leaflet-regarding-polysorbates-used-excipients-medicinal-products-human-use_en.pdf (accessed on 3 April 2025).
- Delgado, R. Misuse of Beer–Lambert Law and other calibration curves. R. Soc. Open Sci. 2021, 9, 211103. [Google Scholar] [CrossRef]
- Kačuráková, M.; Capek, P.; Sasinková, V.; Wellner, N.; Ebringerová, A. FT-IR study of plant cell wall model compounds: Pectic polysaccharides and hemicelluloses. Carbohydr. Polym. 2000, 43, 195–203. [Google Scholar]
- Singh, R.S.; Saini, G.K.; Kennedy, J.F. Pullulan production in stirred tank reactor by a colour-variant strain of Aureobasidium pullulans FB-1. Carbohydr. Polym. Technol. Appl. 2021, 2, 100086. [Google Scholar]
- Saito, T.; Hirota, M.; Tamura, N.; Kimura, S.; Fukuzumi, H.; Heux, L.; Isogai, A. Individualization of nano-sized plant cellulose fibrils by direct surface carboxylation using TEMPO catalyst under neutral conditions. Biomacromolecules 2009, 10, 1992–1996. [Google Scholar] [CrossRef]
- Cuba-Chiem, L.T.; Huynh, L.; Ralston, J.; Beattie, D.A. In Situ Particle Film ATR FTIR Spectroscopy of Carboxymethyl Cellulose Adsorption on Talc: Binding Mechanism, pH Effects, and Adsorption Kinetics. Langmuir 2008, 24, 8036–8044. [Google Scholar] [CrossRef]
- Iizhar, S.A.; Syed, I.A.; Satar, R.; Ansari, S.A. In vitro assessment of pharmaceutical potential of ethosomes entrapped with terbinafine hydrochloride. J. Adv. Res. 2016, 7, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Scimeca, M.; Bischetti, S.; Lamsira, H.K.; Bonfiglio, R.; Bonanno, E. Energy Dispersive X-ray (EDX) microanalysis: A powerful tool in biomedical research and diagnosis. Eur. J. Histochem. 2018, 62, 2841. [Google Scholar] [PubMed]
- Niu, B.; Shao, P.; Chen, H.; Sun, P. Structural and physiochemical characterization of novel hydrophobic packaging films based on pullulan derivatives for fruits preservation. Carbohydr. Polym. 2019, 208, 276–284. [Google Scholar]
- Rossi, D.; Pittia, P.; Realdon, N. Contact Angle Measurements and Applications in Pharmaceuticals and Foods: A Critical Review. Rev. Adhes. Adhes. 2018, 6, 202–252. [Google Scholar] [CrossRef]
- Rouquerol, J.; Avnir, D.; Fairbridge, C.W.; Everett, D.H.; Haynes, J.M.; Pernicone, N.; Ramsay, J.D.F.; Sing, K.S.W.; Unger, K.K. Recommendations for the Characterization Of Porous Solids. Pure Appl. Chem. 1994, 66, 1739–1758. [Google Scholar]
- Dewan, F.; Islam, N. Pullulan—Based films: Unveiling its multifaceted versatily for sustainability. Adv. Polym. Tech. 2024, 2024, 2633384. [Google Scholar] [CrossRef]
- Heredia, N.S.; Vizuete, K.; Flores-Calero, M.; Pazmiño, K.; Pilaquinga, F.; Kumar, B.; Debut, A. Comparative statistical analysis of the release kinetics models for nanoprecipitated drug delivery systems based on poly(lactic-co-glycolic acid). PLoS ONE 2022, 17, e0264825. [Google Scholar] [CrossRef]
- Kosmidis, K.; Macheras, P. On the dilemma of fractal or fractional kinetics in drug release studies: A comparison between Weibull and Mittag-Leffler functions. Int. J. Pharm. 2018, 543, 269–273. [Google Scholar] [CrossRef]
- Martín-Camacho, U.J.; Rodríguez-Barajas, N.; Sánchez-Burgos, J.A.; Pérez-Larios, A. Weibull β value for the discernment of drug release mechanism of PLGA particles. Int. J. Pharm. 2023, 640, 123017. [Google Scholar] [CrossRef]
- Corsaro, C.; Neri, G.; Mezzasalma, A.M.; Fazio, E. Weibull Modeling of Controlled Drug Release from Ag-PMA Nanosystems. Polymers 2021, 13, 2897. [Google Scholar] [CrossRef]
- Roy, S.; Halder, M.; Ramprasad, P.; Dasgupta, S.; Singh, Y.; Pal, D. Oxidized pullulan exhibits potent antibacterial activity against S. aureus by disrupting its membrane integrity. Int. J. Biol. Macromol. 2023, 249, 126049. [Google Scholar] [CrossRef]
- Farooq, U.; Rasul, A.; Sher, M.; Qadir, M.I.; Nazir, I.; Mehmood, Y.; Riaz, H.; Shah, P.A.; Jamil, Q.A.; Khan, B.A. Development, characterization and evaluation of anti-fungal activity of miconazole based nanogel prepared from biodegradable polymer. Pak. J. Pharm. Sci. 2020, 33, 449–457. [Google Scholar]



| Samples | Tween 80 (g) | 2% Tween 80 Solution of TH (g) | P (g) | T-OP (g) | NaCMC (g) | Glycerol (g) |
|---|---|---|---|---|---|---|
| F1_B | 10 | - | 0.4 | - | 0.16 | 0.4 |
| F1_TH | - | 10 | 0.4 | - | 0.16 | 0.4 |
| F2_B | 10 | - | - | 0.4 | 0.16 | 0.4 |
| F2_TH | - | 10 | - | 0.4 | 0.16 | 0.4 |
| Concentration of Tween-80 g% | Solubility (g% ± SD) | Increasing Solubility Toward Water ± SD |
|---|---|---|
| Water (0% Tween-80) | 7.38 × 10−5 | - |
| 2 | 0.6596 ± 2.08 | 893.77 ± 2.17 |
| 4 | 0.8133 ± 1.95 | 1102.03 ± 2.06 |
| 6 | 0.8653 ± 1.67 | 1172.49 ± 1.98 |
| 8 | 0.9444 ± 1.14 | 1279.67 ± 1.53 |
| 10 | 1 ± 0.87 | 1355.01 ± 1.12 |
| Polymeric Films | LC ± SD% | EE ± SD% | mTH/cm2 mg/cm2 |
|---|---|---|---|
| F1_TH | 5.95 ± 0.006 | 96.40 ± 0.04 | 1.55 |
| F2_TH | 6.04 ± 0.005 | 95.25 ±0.03 | 1.83 |
| Sample Code | Tensile Strength (kPa) | Young’s Modulus (kPa) | Elongation at Break (%) |
|---|---|---|---|
| F1_B | 27.30 | 5.77 | 489.95 |
| F2_B | 19.98 | 5.70 | 272.51 |
| Kinetic Model | Parameters | Sample | |
|---|---|---|---|
| F1_TH | F2_TH | ||
| Zero order | K0 | 3.748 | 3.202 |
| R2 | 0.658 | 0.498 | |
| AIC | 138.420 | 144.043 | |
| First order | K | 0.145 | 0.154 |
| R2 | 0.915 | 0.839 | |
| AIC | 6.887 | 20.342 | |
| Higuchi | KH | 26.613 | 30.568 |
| R2 | 0.883 | 0.775 | |
| AIC | 126.649 | 144.365 | |
| Korsmeyer–Peppas | KP | 35.991 | 48.097 |
| n | 0.360 | 0.290 | |
| R2 | 0.938 | 0.894 | |
| AIC | 108.589 | 118.123 | |
| Weibull | KW | 0.499 | 0.713 |
| β | 0.600 | 0.550 | |
| R2 | 0.950 | 0.970 | |
| AIC | −5.150 | −7.252 | |
| Baker Lonsdale | KB | 0.019 | 0.023 |
| R2 | 0.885 | 0.775 | |
| AIC | −54.089 | −33.577 | |
| Sample/Quality Control | Diameter of Inhibition Zones (d, mm) | |||
|---|---|---|---|---|
| Concentration of Drug mg/Sample | C. albicans ATCC 10231 | C. albicans 4746 | C. albicans 4763 | |
| F1_B | - | 0 | 0 | 0 |
| F2_B | - | 0 | 0 | 0 |
| F1_TH | 1.26 | 17.0 ± 0.00 | 16.0 ± 0.00 | 20.0 ± 0.00 |
| F2_TH | 1.44 | 20.1 ± 0.05 | 21.1 ± 0.05 | 21 ± 0.01 |
| FCA (25 µg/disc) | - | 25.1 ± 0.05 | 25.7 ± 0.06 | 25.7 ± 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biliuta, G.; Gherman, S.P.; Baron, R.I.; Bargan, A.; Ochiuz, L.; Tuchilus, C.G.; Spac, A.F.; Zavastin, D.E. Development and Characterization of Polymeric Films Loaded with Terbinafine for Fungal Infection Treatment. Polymers 2025, 17, 1004. https://doi.org/10.3390/polym17081004
Biliuta G, Gherman SP, Baron RI, Bargan A, Ochiuz L, Tuchilus CG, Spac AF, Zavastin DE. Development and Characterization of Polymeric Films Loaded with Terbinafine for Fungal Infection Treatment. Polymers. 2025; 17(8):1004. https://doi.org/10.3390/polym17081004
Chicago/Turabian StyleBiliuta, Gabriela, Simona Petronela Gherman, Raluca Ioana Baron, Alexandra Bargan, Lacramioara Ochiuz, Cristina Gabriela Tuchilus, Adrian Florin Spac, and Daniela Elena Zavastin. 2025. "Development and Characterization of Polymeric Films Loaded with Terbinafine for Fungal Infection Treatment" Polymers 17, no. 8: 1004. https://doi.org/10.3390/polym17081004
APA StyleBiliuta, G., Gherman, S. P., Baron, R. I., Bargan, A., Ochiuz, L., Tuchilus, C. G., Spac, A. F., & Zavastin, D. E. (2025). Development and Characterization of Polymeric Films Loaded with Terbinafine for Fungal Infection Treatment. Polymers, 17(8), 1004. https://doi.org/10.3390/polym17081004









