Development of Stimuli-Responsive Polymeric Nanomedicines in Hypoxic Tumors and Their Therapeutic Promise in Oral Cancer
Abstract
:1. Introduction
1.1. Background
1.1.1. Mechanisms of Hypoxic Tumor Formation and Their Implications for Treatment
1.1.2. Stimuli-Responsive Polymeric Nanomedicines and Their Potential in Tumor Therapy
- i.
- Biodegradable polymer backbones (e.g., chitosan) functionalized with stimulus-sensitive moieties (e.g., sulfonamide groups for pH responsiveness)
- ii.
- Enzyme-cleavable linkages in natural polymers (e.g., hyaluronidase-degradable hyaluronic acid carriers)
1.2. Specificities of Oral Cancer
1.2.1. Oral Cancer and Clinical Treatment Challenges
1.2.2. Treatment of Oral Cancer and the Anoxic Environment
2. Design Concepts for Stimuli-Responsive Polymer Nanomedicines for Hypoxic Tumors
2.1. Introduction to Amphiphilic Polymers
2.2. Targeting of Hypoxic Tumor-Related Nanomedicines
2.2.1. Passive Targeting of Nanomedicines
2.2.2. Active Targeting Based on the Hypoxia-Associated Proteins
2.3. Microenvironmental Responses Associated with Hypoxic Tumors
2.3.1. Tumor-Related pH Response to Hypoxia
2.3.2. Tumor-Associated Redox Response in Relation to Hypoxic Tumors
2.3.3. Enzymatic Responses Associated with a Tumor of Hypoxia
2.3.4. Light Response Associated with a Tumor of Hypoxia
2.4. Realization of Anti-Desiccated Tumor Effects
2.4.1. Pharmacodynamic Optimization of Conventional Chemotherapeutic Agents
2.4.2. Photothermal, Photodynamic and Optical Immunotherapy
2.4.3. Cytotoxicity Achieved by Other Means
3. Typical Case Studies of Stimuli-Responsive Polymeric Nanomedicines in Oxygen-Depleted Tumor Therapy
3.1. Major Research Advances and Results for Hypoxic Tumors
3.2. Stimuli-Responsive Polymeric Nanomedicines with Potential for Clinical Translation
3.2.1. Drug Release Based on Hypoxic Activation
3.2.2. Therapeutic Mechanisms Utilizing Hypoxia-Inducible Enzymes
3.3. Typical Study Case Studies
3.3.1. Key Research Advances and Results for Hypoxic Tumors
3.3.2. Polymeric Nanomedicines with Clinical Translational Potential
4. Prospects for Stimuli-Responsive Polymeric Nanomedicines in Oral Cancer Therapy
4.1. Special Needs for Oral Cancer Treatment
4.1.1. Impact of a Hypoxic Environment in the Treatment of Oral Cancer
4.1.2. Importance of Local Delivery and Targeted Therapeutic Strategies
4.2. Potential Advantages of Stimuli-Responsive Polymeric Nanomedicines
4.2.1. Enhancement of Drug Permeability and Absorption

4.2.2. Reduction in Toxic Side Effects of Treatment on Normal Tissues
4.3. Innovative Technologies and Future Directions
4.3.1. Multifunctional Combined Nanomedicine-Based Therapeutic Programs
4.3.2. Future Directions in the Development of Novel Stimulus Responsive Materials
5. Existing Problems and Challenges
5.1. Bottlenecks in the Design Optimization of Stimuli-Responsive Nanomedicines—The Balance Between Drug Loading, Release Efficiency and Targeting
5.2. Limitations of Animal Models and Clinical Translation
5.2.1. Inadequate Methods for Modeling and Assessing the Hypoxia Microenvironments
5.2.2. Challenges in the Transition from Laboratory Research to Clinical Application
5.3. Toxicity and Safety of Polymeric Nanomedicines
5.4. Research on Specific Applicability to Oral Cancer Is Still Pending
6. Conclusions and Outlook
6.1. Summary of the Potential for Stimuli-Responsive Polymer Nanomedicines
6.2. Prospective Forecasts in the Field of Treatment of Hypoxic Tumors and Oral Cancer
6.3. Suggested Directions for Future Research
6.3.1. The Need for Interdisciplinary Co-Operation
6.3.2. Augmented Emphasis on Clinical Translational Research
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| HIF | hypoxia-inducible factor |
| MMPs | matrix metalloproteinases |
| VEGF | vascular endothelial growth factor |
| PLGA | poly (lactic acid-glycolic acid) |
| EPR | enhanced permeability and retention |
| PLA | polylactic acid |
| PEG | polyethylene glycol |
| HA | hyaluronic acid |
| DOX | doxorubicin |
| NO | nitric oxide |
| ROS | reactive oxygen species |
| NIR-II | near-infrared II |
| PACT | photoacoustic computed tomography |
| WHO | World Health Organization |
| PLL | polylysine |
| MTX | methotrexate |
| HIFs | hypoxia-inducible factors |
| TNBC | triple-negative breast cancer |
| TME | tumor microenvironment |
| shHIF-1α | ShRNA targeting HIF-1α |
| MIPs | molecularly imprinted polymer nanoparticles |
| QDs | quantum dots |
| CAIX | carbonic anhydrase IX |
| PEG-b-PDMT | poly (ethylene glycol)-b-poly (dithiothiol methacrylate) |
| PTT | photothermal therapy |
| DSN | DiR-loaded self-assembled nanoparticles |
| NPs | nanoparticles |
| MSCs | mesenchymal stem cells |
| SOR-NP | single-linear oxygen-responsive nanoparticles |
| BDP | dipyrrolylmethylene boron |
| PDT | photodynamic therapy |
| Br | bromelain |
| STBs | shear-thinning biomaterials |
| BBB | blood–brain barrier |
| RNAi | RNA interference |
| HIF-1 | hypoxia-inducible factor 1 |
| HR-NPs | hypoxia-responsive polymer nanoparticles |
| TMEs | tumor microenvironments |
| CESs | carboxylesterases |
| CYPs | cytochrome P450 enzymes |
| pNGs | peptide-crosslinked nanogels |
| HRM NPs | hypoxia-responsive micelle nanoparticles |
| PAP NPs | PTX prodrug nanoparticle |
| HAPs | hypoxia-activated prodrugs |
| EGFR | Epidermal Growth Factor Receptor |
| SRPNs | stimuli-responsive polymer nanomedicines |
| ATLP | acid-triggered ligand presentation |
| iPDOX | IRGD-modified adriamycin polymer prodrug |
| NIR | near-infrared |
| ROS | reactive oxygen species |
| LA | lactobionic acid |
| OA | oleic acid |
| mPEG | methoxy poly (ethylene glycol) |
| CPT | camptothecin |
| SPNs | stimuli-responsive polymeric nanoparticles |
| PDT | photodynamic therapy |
| GMP | Good Manufacturing Practices |
| PDC | polymer–drug conjugate |
| NTR | nitroreductase |
References
- Krohn, K.A.; Link, J.M.; Mason, R.P. Molecular Imaging of Hypoxia. J. Nucl. Med. 2008, 49, 129S–148S. [Google Scholar] [CrossRef] [PubMed]
- Hlushchuk, R.; Barré, S.; Djonov, V. Morphological Aspects of Tumor Angiogenesis. Methods Mol. Biol. 2016, 1464, 13–24. [Google Scholar] [CrossRef]
- Gao, Y.; Zhou, H.; Liu, G.; Wu, J.; Yuan, Y. Tumor Microenvironment: Lactic Acid Promotes Tumor Development. J. Immunol. Res. 2022, 2022, 1–8. [Google Scholar] [CrossRef]
- Telarovic, I.; Wenger, R.H.; Pruschy, M. Interfering with Tumor Hypoxia for Radiotherapy Optimization. J. Exp. Clin. Cancer Res. 2021, 40, 197–226. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Shi, Z.; Li, M.; Mi, J. Hypoxia-induced miR-424 decreases tumor sensitivity to chemotherapy by inhibiting apoptosis. Cell Death Dis. 2014, 5, e1301. [Google Scholar] [CrossRef]
- Arvindam, U.; Kennedy, P.; Ettestad, B.; Steinert, E.M.; Moore, K.M.; Miller, J.S. Hypoxia profoundly alters Natural Killer cell phenotype and function: Implications for immunotherapy within the solid tumour microenvironment. J. Immunol. 2021, 206, 56.17. [Google Scholar] [CrossRef]
- Khoei, S.G.; Sadeghi, H.; Dermani, F.K. Targeting the SPHK1/HIF1 Pathway to Inhibit Colorectal Cancer Stem Cells Niche. J. Gastrointest. Cancer 2020, 51, 716–717. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, H.; Qian, W.; Cheng, L.; Yan, B.; Han, L.; Xu, Q.; Ma, Q.; Ma, J. Hyperglycemia aggravates microenvironment hypoxia and promotes the metastatic ability of pancreatic cancer. Comput. Struct. Biotechnol. J. 2018, 16, 479–487. [Google Scholar] [CrossRef]
- Xie, Z.; Guo, W.; Guo, N.; Huangfu, M.; Liu, H.; Lin, M.; Xu, W.; Chen, J.; Wang, T.; Wei, Q.; et al. Targeting tumor hypoxia with stimulus-responsive nanocarriers in overcoming drug resistance and monitoring anticancer efficacy. Acta Biomater. 2018, 71, 351–362. [Google Scholar] [CrossRef]
- Luo, S.; Lv, Z.; Yang, Q.; Chang, R.; Wu, J. Research Progress on Stimulus-Responsive Polymer Nanocarriers for Cancer Treatment. Pharmaceutics 2023, 15, 1928. [Google Scholar] [CrossRef]
- Wu, M.; Xue, Y.; Li, N.; Zhao, H.; Lei, B.; Wang, M.; Wang, J.; Luo, M.; Zhang, C.; Du, Y.; et al. Tumor-Microenvironment-Induced Degradation of Ultrathin Gadolinium Oxide Nanoscrolls for Magnetic-Resonance-Imaging-Monitored, Activatable Cancer Chemotherapy. Angew. Chem. Int. Ed. 2019, 58, 6880–6885. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Yang, Y.; Zhang, J.; Bai, Z.; Zhang, X.; Li, K.; Shi, M.; Liu, Z.; Gao, L.; Wang, J.; et al. Multilayer nanodrug delivery system with spatiotemporal drug release improves tumour microenvironment for synergistic anticancer therapy. Biofabrication 2024, 16, 25012. [Google Scholar] [CrossRef] [PubMed]
- Mundel, R.; Thakur, T.; Chatterjee, M. Emerging uses of PLA-PEG copolymer in cancer drug delivery. 3 Biotech 2022, 12, 41. [Google Scholar] [CrossRef]
- Toro-Córdova, A.; Sanz, B.; Goya, G. A Concise Review of Nanomaterials for Drug Delivery and Release. Curr. Nanosci. 2020, 16, 399–412. [Google Scholar] [CrossRef]
- Su, Y.; Jin, G.; Zhou, H.; Yang, Z.; Wang, L.; Mei, Z.; Jin, Q.; Lv, S.; Chen, X. Development of stimuli responsive polymeric nanomedicines modulating tumour microenvironment for improved cancer therapy. Med. Rev. 2023, 3, 4–30. [Google Scholar] [CrossRef]
- Kaur, N.; Gautam, P.; Nanda, D.; Meena, A.S.; Shanavas, A.; Prasad, R. Lipid Nanoparticles for Brain Tumor Theranostics: Challenges and Status. Bioconjug. Chem. 2024, 35, 1283–1299. [Google Scholar] [CrossRef]
- Chis, A.A.; Arseniu, A.M.; Morgovan, C.; Dobrea, C.M.; Frum, A.; Juncan, A.M.; Butuca, A.; Ghibu, S.; Gligor, F.G.; Rus, L.L. Biopolymeric Prodrug Systems as Potential Antineoplastic Therapy. Pharmaceutics 2022, 14, 1773. [Google Scholar] [CrossRef]
- Han, W.; Liu, F.; Li, Y.; Liu, G.; Li, H.; Xu, Y.; Sun, S. Advances in Natural Polymer-Based Transdermal Drug Delivery Systems for Tumor Therapy. Small 2023, 19, e2301670. [Google Scholar] [CrossRef]
- Yu, H.; Liu, H.; Shen, Y.; Ao, Q. Synthetic biodegradable polymer materials in the repair of tumor-associated bone defects. Front. Bioeng. Biotechnol. 2023, 11, 1096525. [Google Scholar] [CrossRef]
- Chakraborty, D.D.; Chakraborty, P.; Mondal, A. An insight into cancer nanomedicine based on polysaccharides. Int. J. Biol. Macromol. 2025, 290, 138678. [Google Scholar] [CrossRef]
- Song, J.; Lee, E.; Lee, E.S. pH-dependent swellable hyaluronate nanogels targeting acidic tumors. Polym. Adv. Technol. 2023, 34, 3485–3492. [Google Scholar] [CrossRef]
- Wang, K.; Jiang, M.; Zhou, J.; Liu, Y.; Zong, Q.; Yuan, Y. Tumor-acidity and bioorthogonal chemistry-mediated on-site size transformation clustered nanosystem to overcome hypoxic resistance and enhance chemoimmunotherapy. ACS Nano 2022, 16, 721–735. [Google Scholar] [CrossRef]
- Tang, D.; Yu, Y.; Zhang, J.; Dong, X.; Liu, C.; Xiao, H. Self-sacrificially degradable pseudo-semiconducting polymer nanoparticles that integrate NIR-II fluorescence bioimaging, photodynamic immunotherapy, and photo-activated chemotherapy. Adv. Mater. 2022, 34, e2203820. [Google Scholar] [CrossRef]
- Huang, F.; Yang, S.; Wang, H.; Zhao, P.; Zhou, B.; Cheng, B.; Dong, S.; Yang, J.; Li, B.; Wang, X. pH-responsive PLGA/gelatin porous microspheres containing paclitaxel used for inhibition of cancer cell proliferation. J. Drug Deliv. Sci. Technol. 2023, 86, 104735. [Google Scholar] [CrossRef]
- Wang, X.; Pan, J.; Shi, H.; Liang, N.; Sun, S. Biotin-modified acid-sensitive micelles for enhancing antitumor effect of paclitaxel. J. Drug Deliv. Sci. Technol. 2023, 84, 104538. [Google Scholar] [CrossRef]
- Song, S.; Li, X.; Ji, Y. GSH/pH dual-responsive and HA-targeting nano-carriers for effective drug delivery and controlled release. J. Drug Deliv. Sci. Technol. 2021, 62, 102327. [Google Scholar] [CrossRef]
- Han, X. Paclitaxel-Paclitaxel Prodrug Nanoassembly as a Versatile Nanoplatform for Combinational Cancer Therapy. ACS Appl. Mater. Interfaces 2016, 8, 33506–33513. [Google Scholar] [CrossRef]
- Hua, D.; Tang, L.; Wang, W.; Tang, S.; Yu, L.; Zhou, X.; Wang, Q.; Sun, C.; Shi, C.; Luo, W.; et al. Improved Antiglioblastoma Activity and BBB Permeability by Conjugation of Paclitaxel to a Cell-Penetrative MMP-2-Cleavable Peptide. Adv. Sci. 2020, 8, 2001960. [Google Scholar] [CrossRef]
- Li, J.; Ke, W.; Wang, L.; Huang, M.; Yin, W.; Zhang, P.; Chen, Q.; Ge, Z. Self-sufficing H2O2-responsive nanocarriers through tumour-specific H2O2 production for synergistic oxidation-chemotherapy. J. Control. Release 2016, 225, 64–74. [Google Scholar] [CrossRef]
- Xu, C.; Feng, Q.; Yang, H.; Wang, G.; Huang, L.; Bai, Q.; Zhang, C.; Wang, Y.; Chen, Y.; Cheng, Q.; et al. A Light-Triggered Mesenchymal Stem Cell Delivery System for Photoacoustic Imaging and Chemo-Photothermal Therapy of Triple Negative Breast Cancer. Adv. Sci. 2018, 24, 1800382. [Google Scholar] [CrossRef]
- Yang, D.C.; Wang, S.; Weng, X.L. Singlet Oxygen-Responsive Polymeric Nanomedicine for Light-Controlled Drug Release and Image-Guided Photodynamic-Chemo Combination Therapy. ACS Appl. Mater. Interfaces 2021, 13, 33905–33914. [Google Scholar] [CrossRef] [PubMed]
- Fuller, E.G.; Sun, H.; Dhavalikar, R.D. Externally Triggered Heat and Drug Release from Magnetically Controlled Nanocarriers. ACS Appl. Polym. Mater. 2019, 1, 211–220. [Google Scholar] [CrossRef]
- Wang, X.; Xu, J.; Xu, X. pH-sensitive bromelain nanoparticles by ortho ester crosslinkage for enhanced doxorubicin penetration in solid tumour. Mater. Sci. Eng. C-Mater. Biol. Appl. 2020, 113, 111004. [Google Scholar] [CrossRef]
- Lee, J.; Wang, Y.; Xue, C. pH-Responsive Doxorubicin Delivery Using Shear-Thinning Biomaterials for Localised Melanoma Treatment. Nanoscale 2022, 14, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Guedes, G.; Wang, S.; Fontana, F. Dual-Crosslinked Dynamic Hydrogel Incorporating Mo154 with pH and NIR Responsiveness for Chemo-Photothermal Therapy. Adv. Mater. 2021, 33, 2007761. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, X.; Du, J. Redox-responsive polymer micelles co-encapsulating immune checkpoint inhibitors and chemotherapeutic agents for glioblastoma therapy. Nat. Commun. 2024, 15, 1118. [Google Scholar] [CrossRef]
- Li, Y.; Lu, A.; Long, M.; Cui, L.; Chen, Z.; Zhu, L. Nitroimidazole derivative incorporated liposomes for hypoxia-triggered drug delivery and enhanced therapeutic efficacy in patient-derived tumour xenografts. Acta Biomater. 2019, 83, 334–348. [Google Scholar] [CrossRef]
- Thambi, T.; Deepagan, V.G.; Yoon, H.Y. Hypoxia-responsive polymeric nanoparticles for tumor-targeted drug delivery. J. Control. Release 2014, 35, 1735–1743. [Google Scholar] [CrossRef]
- Long, M.; Liu, X.; Huang, X. Alendronate-functionalized hypoxia-responsive polymeric micelles for targeted therapy of bone metastatic prostate cancer. J. Control. Release 2021, 334, 303–317. [Google Scholar] [CrossRef]
- Zhang, Q.; Jin, C.; Yu, J.; Lu, W. Synthesis of New Branched 2-Nitroimidazole as a Hypoxia Sensitive Linker for Ligand-Targeted Drugs of Paclitaxel. ACS Omega 2018, 3, 8813–8818. [Google Scholar] [CrossRef]
- Zhou, Z.H.; Liang, S.Y.; Zhao, T.C. Overcoming chemotherapy resistance using pH-sensitive hollow MnO2 nanoshells that target the hypoxic tumour microenvironment of metastasised oral squamous cell carcinoma. J. Nanobiotechnol. 2021, 19, 157. [Google Scholar] [CrossRef]
- Zhang, P.; Yang, H.; Shen, W.; Liu, W.; Chen, L.; Xiao, C. Hypoxia-Responsive Polypeptide Nanoparticles Loaded with Doxorubicin for Breast Cancer Therapy. ACS Biomater. Sci. Eng. 2020, 6, 2167–2174. [Google Scholar] [CrossRef]
- Jang, E.H.; Shim, M.K.; Kim, G.L.; Kim, S.; Kang, H.; Kim, J.H. Hypoxia-responsive folic acid conjugated glycol chitosan nanoparticle for enhanced tumour targeting treatment. Int. J. Pharm. 2020, 580, 119237. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Zhang, X.; Tang, D.; Liu, T.; Xiao, H. Biodegradable NIR-II Pseudo Conjugate Polymeric Nanoparticles Amplify Photodynamic Immunotherapy via Alleviation of Tumor Hypoxia and Tumor-Associated Macrophage Reprogramming. Adv. Mater. 2023, 35, e2209799. [Google Scholar] [CrossRef]
- Hu, C.; Cai, L.; Liu, S.; Liu, Y.; Zhou, Y.; Pang, M. Copper-Doped Nanoscale Covalent Organic Polymer for Augmented Photo/Chemodynamic Synergistic Therapy and Immunotherapy. Bioconjug. Chem. 2020, 31, 1661–1670. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; He, H.; Liu, Y. Cancer-Selective Bioreductive Chemotherapy Mediated by Dual Hypoxia-Responsive Nanomedicine upon Photodynamic Therapy-Induced Hypoxia Aggravation. Biomacromolecules 2019, 20, 2649–2656. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, P.; González, R.D.; Carvalho, A.T.P. Insights into the degradation of polymer-drug conjugates by an overexpressed enzyme in cancer cells. J. Med. Chem. 2023, 66, 2761–2772. [Google Scholar] [CrossRef]
- Zhu, M.; Ren, G.; Guo, J. Hypoxia-Responsive Nanomicelle Based on 2-Nitroimidazole for Tumor Treatment Through Chemotherapy and Modulation of the Tumor Redox Microenvironment. J. Control. Release 2023, 339, 98–108. [Google Scholar] [CrossRef]
- Xiao, H.; Li, X.; Liang, S. Dual-Responsive Nanomedicine Activates Programmed Antitumor Immunity through Targeting Lymphatic System. ACS Nano 2024, 17, 18–25. [Google Scholar] [CrossRef]
- Xu, L.; Liang, H.-W.; Yang, Y.; Yu, S.-H. Stability and Reactivity: Positive and Negative Aspects for Nanoparticle Processing. Chem. Rev. 2018, 118, 3209–3250. [Google Scholar] [CrossRef]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Warnakulasuriya, S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009, 45, 309–316. [Google Scholar] [CrossRef]
- Rana, M.; Luhano, M.K.; Khan, I.; Noor, M.; Zahid, N. Late diagnosis in oral squamous cell carcinoma, the role of psychological and cognitive variables. Pak. J. Med. Health Sci. 2022, 16, 580–584. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Li, X.; Qu, X.; Han, N. Decreased CSTA expression promotes lymphatic metastasis and predicts poor survival in oral squamous cell carcinoma. Arch. Oral Biol. 2021, 126, 105116. [Google Scholar] [CrossRef]
- Ord, R.A.; Blanchaert, R.H. Current management of oral cancer: A multidisciplinary approach. J. Am. Dent. Assoc. 2001, 132, 19S–23S. [Google Scholar] [CrossRef] [PubMed]
- Goker, F.; Beretta, P.; Baj, A.; Bolzoni, A.R.; Maiorana, C. Oral rehabilitation of oncology patients with dental implants after reconstruction surgery with autogenous flaps. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 7809–7816. [Google Scholar] [CrossRef]
- Shi, F.; Luo, D.; Zhou, X.; Sun, Q.; Shen, P. Combined effects of hyperthermia and chemotherapy on the regulate autophagy of oral squamous cell carcinoma cells under a hypoxic microenvironment. Cell Death Discov. 2021, 7, 227. [Google Scholar] [CrossRef]
- Markovic-Vasiljkovic, B.; Antic, S.; Jelovac, D. Comparative Assessment of the Depth of Invasion of Early-Stage Oral Cavity Carcinomas Based on Intraoral Ultrasound and Computerised Tomography Findings. Vojnosanit. Pregl. 2023, 80, 921–926. [Google Scholar] [CrossRef]
- Chaudhary, A.; Bag, S.; Arora, N.; Radhakrishnan, V.S.; Mishra, D.; Mukherjee, G. Hypoxic Transformation of Immune Cell Metabolism Within the Microenvironment of Oral Cancers. Front. Oral Health 2020, 1, 585710. [Google Scholar] [CrossRef]
- Noorlag, R.; de Bree, R.; Witjes, M.J.H. Image-Guided Surgery in Oral Cancer: Toward Improved Margin Control. Curr. Opin. Oncol. 2022, 34, 170–176. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, Y.; Wang, X.; Zhang, H.; Yang, X. An Acid-Responsive Iron-Based Nanocomposite for OSCC Treatment. J. Dent. Res. 2024, 103, 612–621. [Google Scholar] [CrossRef] [PubMed]
- Marunganathan, V.; Nivetha, S.; Sabarinathan, D.; Kumar, P.S. Marine-Derived κ-Carrageenan-Coated Zinc Oxide Nanoparticles for Targeted Drug Delivery and Apoptosis Induction in Oral Cancer. Mol. Biol. Rep. 2024, 51, 89. [Google Scholar] [CrossRef] [PubMed]
- Pei, Z.; Lei, H.; Cheng, L. Bioactive inorganic nanomaterials for cancer theranostics. Chem. Soc. Rev. 2023, 52, 2031–2081. [Google Scholar] [CrossRef]
- Chen, Z.; Yue, Z.; Yang, K.; Shen, C.; Cheng, Z.; Zhou, X.; Li, S. Four Ounces Can Move a Thousand Pounds: The Enormous Value of Nanomaterials in Tumor Immunotherapy. Adv. Healthc. Mater. 2023, 12, e2300882. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.; Shi, X.; Tian, Y.; Gao, F. pH-Responsive Polymer Nanomaterials for Tumor Therapy. Front. Oncol. 2022, 12, 855019. [Google Scholar] [CrossRef]
- Lin, Z.P.; Nguyen, L.N.M.; Ouyang, B.; MacMillan, P.; Ngai, J.; Kingston, B.R.; Mladjenovic, S.M.; Chan, W.C.W. Macrophages Actively Transport Nanoparticles in Tumors After Extravasation. ACS Nano 2022, 16, 6080–6092. [Google Scholar] [CrossRef]
- Wang, L.; Li, S.Y.; Jiang, W.; Liu, H.; Dou, J.X.; Li, X.Q.; Wang, Y.C. Polyphosphoestered Nanomedicines with Tunable Surface Hydrophilicity for Cancer Drug Delivery. ACS Appl. Mater. Interfaces 2020, 12, 32312–32320. [Google Scholar] [CrossRef]
- Bistrović Popov, A.; Melle, F.; Linnane, E.; González-López, C.; Ahmed, I. Size-tuneable and immunocompatible polymer nanocarriers for drug delivery in pancreatic cancer. Nanoscale 2022, 14, 6656–6666. [Google Scholar] [CrossRef]
- van Rees, D.J.; Bouti, P.; Klein, B.; Verkuijlen, P.J.; Van Houdt, M.; Schornagel, K.; Tool, A.T.; Venet, D.; Sotiriou, C.; El-Abed, S.; et al. Cancer cells resist antibody-mediated destruction by neutrophils through activation of the exocyst complex. J. Immunother. Cancer 2022, 10, e004820. [Google Scholar] [CrossRef]
- Yu, B.; Wang, X.; Ding, L.; Han, M.; Guo, Y. Hydrophilic Natural Polylysine as Drug Nanocarrier for Preparation of Helical Delivery System. Pharmaceutics 2022, 14, 2512. [Google Scholar] [CrossRef]
- Liu, X.; Sun, J.; Gu, J.; Weng, L.; Wang, X.; Zhu, L.; Luo, Q. Effective drug and shRNA delivery for synergistic treatment of triple-negative breast cancer by sequentially targeting tumour hypoxia. Chem. Eng. J. 2023, 470, 144271. [Google Scholar] [CrossRef]
- Cecchini, A.; Raffa, V.; Canfarotta, F.; Signore, G.; Piletsky, S.; MacDonald, M.P.; Cuschieri, A. In vivo recognition of human vascular endothelial growth factor by molecularly imprinted polymers. Nano Lett. 2017, 17, 2307–2312. [Google Scholar] [CrossRef]
- Huang, H.; Guo, H.; Liu, J.; Ni, C.; Xia, L.; Cao, X.; Xia, J.; Shi, X.; Guo, R. Dendrimer/metal-phenolic nanocomplexes encapsulating CuO2 for targeted magnetic resonance imaging and enhanced ferroptosis/cuproptosis/chemodynamic therapy by regulating the tumour microenvironment. Acta Biomater. 2024, 183, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Yook, S.; Yhee, J.Y.; Yoon, H.Y.; Kim, M.-G.; Ku, S.H.; Kim, S.H.; Park, J.H.; Jeong, J.H.; Kwon, I.C.; et al. Co-delivery of VEGF and Bcl-2 dual-targeted siRNA polymer using a single nanoparticle for synergistic anti-cancer effects in vivo. J. Control. Release 2015, 220 Pt B, 631–641. [Google Scholar] [CrossRef]
- Ayatollahi, S.; Salmasi, Z.; Hashemi, M.; Askarian, S.; Oskuee, R.K.; Abnous, K.; Ramezani, M. Aptamer-targeted delivery of Bcl-xL shRNA using alkyl modified PAMAM dendrimers into lung cancer cells. Int. J. Biochem. Cell Biol. 2017, 92, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-G.; Zhu, W.-J.; Liu, Y.; Yuan, Z.-Q.; Yang, S.-D.; Chen, W.-L.; Li, J.-Z.; Zhou, X.-F.; Liu, C.; Zhang, X.-N. Novel polymer micelle mediated co delivery of doxorubicin and P-glycoprotein siRNA for reversal of multidrug resistance and synergistic tumor therapy. Sci. Rep. 2016, 6, 23859. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, T.; Sun, Y.; Wang, S.; Wang, W.; Kuang, Z.; Duan, M.; Du, T.; Liu, M.; Wu, L.; et al. Carbon-spaced tandem-disulfide bond bridge design addresses limitations of homodimer prodrug nanoassemblies: Enhancing both stability and activatability. J. Am. Chem. Soc. 2024, 146, 22675–22688. [Google Scholar] [CrossRef] [PubMed]
- Londoño-Berrío, M.; Pérez-Buitrago, S.; Ortiz-Trujillo, I.C. Cytotoxicity and Genotoxicity of Azobenzene-Based Polymeric Nanocarriers for Phototriggered Drug Release and Biomedical Applications. Polymers 2022, 14, 3199. [Google Scholar] [CrossRef]
- Koide, H.; Yamaguchi, K.; Sato, K. Engineering Temperature-Responsive Polymer Nanoparticles that Load and Release Paclitaxel, a Low-Molecular-Weight Anticancer Drug. J. Control. Release 2023, 9, 1011–1019. [Google Scholar] [CrossRef]
- Jin, Y.; Zhang, B.; Li, J.; Guo, Z.; Zhang, C.; Chen, X.; Ma, L.; Wang, Z.; Yang, H.; Li, Y.; et al. Bioengineered protein nanocarrier facilitating siRNA escape from lysosomes for targeted RNAi therapy in glioblastoma. Sci. Adv. 2025, 11, 9266. [Google Scholar] [CrossRef]
- Semenza, G.L. Involvement of Hypoxia-Inducible Factor 1 in Human Cancer. Intern. Med. 2002, 41, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Han, F.; Du, Y.; Shi, H.; Zhou, W. Hypoxic microenvironment in cancer: Molecular mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2023, 8, 70. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Park, D.H.; Jeon, Y. Eupatilin inhibits angiogenesis-mediated human hepatocellular metastasis by reducing MMP-2 and VEGF signaling. Bioorg. Med. Chem. Lett. 2018, 28, 3150–3154. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, W.; Wang, C. Nanozyme-activating prodrug therapies: A review. Chin. Chem. Lett. 2024, 35, 138–150. [Google Scholar] [CrossRef]
- Saraiva, C.; Praça, C.; Ferreira, R.; Santos, T.; Ferreira, L.; Bernardino, L. Nanoparticle-mediated brain drug delivery: Overcoming blood–brain barrier to treat neurodegenerative diseases. J. Control. Release 2016, 235, 34–47. [Google Scholar] [CrossRef]
- Nagel, G.; Sousa-Herves, A.; Wedepohl, S.; Calderón, M. Matrix Metalloproteinase-sensitive Multistage Nanogels Promote Drug Transport in 3D Tumor Model. Theranostics 2020, 10, 91–108. [Google Scholar] [CrossRef]
- Hao, D.; Meng, Q.; Jiang, B. Hypoxia-Activated PEGylated Paclitaxel Prodrug Nanoparticles for Potentiated Chemotherapy. J. Mater. Chem. B 2023, 11, 1076–1087. [Google Scholar] [CrossRef]
- He, Q.; Chen, J.; Yan, J. Tumor Microenvironment Responsive Drug Delivery Systems. Asian J. Pharm. Sci. 2020, 15, 416–448. [Google Scholar] [CrossRef]
- Lu, X.J.; Guo, X.H.; Wan, D. Progress in dual-responsive nanocarriers based on acid sensitivity for anticancer drug. Chin. J. Biotechnol. 2020, 36, 1723–1731. [Google Scholar]
- Ahmad, Z.; Lv, S.X.; Tang, Z.H.; Shah, A.; Chen, X.S. Methoxy poly (ethylene glycol)-block-poly (glutamic acid)-graft-6-(2-nitroimidazole) hexyl amine nanoparticles for potential hypoxia-responsive delivery of doxorubicin. J. Biomater. Sci. Polym. Ed. 2016, 27, 40–54. [Google Scholar]
- Wu, H.; Du, X.; Xu, J. Multifunctional Biomimetic Nanoplatform Based on Photodynamic Therapy and DNA Repair Intervention for the Synergistic Treatment of Breast Cancer. Acta Biomater. 2023, 157, 551–565. [Google Scholar] [CrossRef] [PubMed]
- Lacerda, Q.; Falatah, H.; Liu, J.B. Improved Tumor Control Following Radiosensitisation with Ultrasound-Sensitive Oxygen Microbubbles and Tumor Mitochondrial Respiration Inhibitors in a Preclinical Model of Head and Neck Cancer. Pharmaceutics 2023, 15, 1302. [Google Scholar] [CrossRef]
- Clavo, B.; Robaina, F.; Fiuza, D. Predictive value of hypoxia in advanced head and neck cancer after treatment with hyperfractionated radio-chemotherapy and hypoxia modification. Clin. Transl. Oncol. 2017, 19, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.; Itoo, A.M.; Ghosh, B.; Biswas, S. Hypoxia alleviating platinum(IV)/chlorin e6-based combination chemotherapeutic-photodynamic nanomedicine for oropharyngeal carcinoma. J. Photochem. Photobiol. B 2022, 238, 112627. [Google Scholar] [CrossRef]
- Krishnamachary, B.; Berg-Dixon, S.; Kelly, B. Regulation of colon carcinoma cell invasion by hypoxia-inducible factor 1. Cancer Res. 2003, 63, 1138–1143. [Google Scholar]
- Lorenz, N.I.; Sittig, A.C.M.; Urban, H. Activating transcription factor 4 mediates adaptation of human glioblastoma cells to hypoxia and temozolomide. Sci. Rep. 2021, 11, 14161. [Google Scholar] [CrossRef]
- Goenka, A.; Tiek, D.; Song, X.; Huang, T.; Hu, B.; Cheng, S.Y. The Many Facets of Therapy Resistance and Tumor Recurrence in Glioblastoma. Cells 2021, 10, 484. [Google Scholar] [CrossRef]
- Wu, Q.; You, L.; Nepovimova, E.; Heger, Z.; Wu, W.; Kuca, K.; Adam, V. Hypoxia-inducible factors: Master regulators of hypoxic tumor immune escape. J. Hematol. Oncol. 2022, 15, 77. [Google Scholar] [CrossRef]
- Lequeux, A.; Noman, M.Z.; Xiao, M. Targeting HIF-1 alpha transcriptional activity drives cytotoxic immune effector cells into melanoma and improves combination immunotherapy. Oncogene 2021, 40, 4725–4735. [Google Scholar] [CrossRef]
- Farhadi, P.; Yarani, R.; Kiani, S.; Mansouri, K. Perfluorocarbon as an adjuvant for tumour anti-angiogenic therapy: Relevance to hypoxia and HIF-1. Med. Hypotheses 2020, 146, 110357. [Google Scholar] [CrossRef]
- Fan, P.; Zhang, N.; Candi, E. Alleviating hypoxia to improve cancer immunotherapy. Oncogene 2023, 42, 3591–3604. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Miao, K.; Sun, H.; Deng, C.X. Tumor heterogeneity reshapes the tumour microenvironment to influence drug resistance. Int. J. Biol. Sci. 2022, 18, 3019–3033. [Google Scholar] [CrossRef]
- Filipczak, N.; Joshi, U.; Attia, S.A.; Berger Fridman, I.; Cohen, S.; Konry, T.; Torchilin, V. Hypoxia-sensitive drug delivery to tumors. J. Control. Release 2022, 341, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Yu, B.; Cong, H.; Shen, Y. Delivery process and effective design of vectors for cancer therapy. J. Mater. Chem. B 2022, 10, 6896–6921. [Google Scholar] [CrossRef]
- Yang, D.C.; Wen, L.F.; Du, L. A Hypoxia-Activated Prodrug Conjugated with a BODIPY-Based Photothermal Agent for Imaging-Guided Chemo-Photothermal Combination Therapy. ACS Appl. Mater. Interfaces 2022, 14, 40546–40558. [Google Scholar] [CrossRef] [PubMed]
- Apetoh, L. Chemoimmunotherapy combinations: Translating basic knowledge into clinical successes. Genes Immun. 2024, 25, 99–101. [Google Scholar] [CrossRef]
- Arias, J.L.; Clares, B.; Morales, M.E.; Gallardo, V.; Ruiz, M.A. Lipid-based drug delivery systems for cancer treatment. Curr. Drug Targets 2011, 12, 1151–1165. [Google Scholar]
- Vohra, T.; Kaur, I.; Heer, H.; Murthy, R.R. Nanolipid carrier-based thermoreversible gel for localised delivery of docetaxel to breast cancer. Cancer Nanotechnol. 2013, 4, 1–12. [Google Scholar] [CrossRef]
- Miyabe, S.; Fujinaga, Y.; Tsuchiya, H.; Fujimoto, S. TiO2 nanotubes with customised diameters for local drug delivery systems. J. Biomed. Mater. Res. B Appl. Biomater. 2024, 112, e35445. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Liang, A. Current research progress of local drug delivery systems based on biodegradable polymers in treating chronic osteomyelitis. Front. Bioeng. Biotechnol. 2022, 10, 1042128. [Google Scholar] [CrossRef]
- Ohnishi, Y.; Yasui, H.; Nozaki, M.; Nakajima, M. Molecularly-targeted therapy for the oral cancer stem cells. Jpn. Dent. Sci. Rev. 2018, 54, 88–103. [Google Scholar] [CrossRef] [PubMed]
- Grimm, M.; Iftner, T.; Altaki, H. Detection of mutation-specific epidermal growth factor receptor (E746-A750del) and lack of detection of human papillomavirus in oral squamous cell carcinoma. Int. J. Oral Maxillofac. Surg. 2014, 43, 1199–1205. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Du, Y.; Zheng, J.; Shi, L.; Li, T. G-Quadruplex-Programmed Versatile Nanorobot Combined with Chemotherapy and Gene Therapy for Synergistic Targeted Therapy. Small 2024, 20, 40. [Google Scholar] [CrossRef] [PubMed]
- Saito, A.; Kitayama, J.; Nagai, R.; Aizawa, K. Anatomical Targeting of Anticancer Drugs to Solid Tumors Using Specific Administration Routes: Review. Pharmaceutics 2023, 15, 61664. [Google Scholar] [CrossRef] [PubMed]
- Hajebi, S.; Rabiee, N.; Bagherzadeh, M.; Ahmadi, S.; Rabiee, M.; Roghani-Mamaqani, H.; Tahriri, M.; Tayebi, L.; Hamblin, M.R. Stimulus-responsive polymeric nanogels as smart drug delivery systems. Acta Biomater. 2019, 92, 1–18. [Google Scholar] [CrossRef]
- Liyanage, P.Y.; Hettiarachchi, S.D.; Zhou, Y.; Ouhtit, A.; Seven, E.S.; Oztan, C.Y.; Celik, E.; Leblanc, R.M. Nanoparticle-mediated targeted drug delivery for breast cancer treatment. Biochim. Biophys. Acta Rev. Cancer 2019, 1871, 419–433. [Google Scholar] [CrossRef] [PubMed]
- Tong, W.Y.; Tan, W.N.; Kamarul Azizi, M.A.; Leong, C.R.; El Azab, I.H.; Lim, J.W.; Mahmoud, M.H.H.; Dailin, D.J.; Ibrahim, M.M.; Chuah, L.F. Nanoparticle-laden contact lens for controlled release of vancomycin with enhanced antibiotic efficacy. Chemosphere 2023, 338, 139492. [Google Scholar] [CrossRef] [PubMed]
- Suwa, T.; Kobayashi, M.; Nam, J.M.; Harada, H. Tumor microenvironment and radioresistance. Exp. Mol. Med. 2021, 53, 1029–1035. [Google Scholar] [CrossRef]
- Tan, S.; Xia, L.; Yi, P.; Han, Y.; Tang, L.; Pan, Q.; Tian, Y.; Rao, S.; Oyang, L.; Liang, J.; et al. Exosomal miRNAs in tumor microenvironment. J. Exp. Clin. Cancer Res. 2020, 39, 67. [Google Scholar] [CrossRef]
- Ghaemi, B.; Javad Hajipour, M. Tumor acidic environment directs nanoparticle impacts on cancer cells. J. Colloid Interface Sci. 2023, 634, 684–692. [Google Scholar] [CrossRef]
- Bertsch, P.; Schneider, L.; Bovone, G.; Tibbitt, M.W.; Fischer, P.; Gstöhl, S. Injectable Biocompatible Hydrogels from Cellulose Nanocrystals for Locally Targeted Sustained Drug Release. ACS Appl. Mater. Interfaces 2019, 11, 38578–38585. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, D.; Liu, J.; Feng, B.; Zhou, F.; Zhang, H.; Zhou, L.; Yin, Q.; Zhang, Z.; Cao, Z.; et al. Acidity-Triggered Ligand-Presenting Nanoparticles to Overcome Sequential Drug Delivery Barriers To Tumors. Nano Lett. 2017, 17, 5429–5436. [Google Scholar] [CrossRef]
- Ovais, M.; Mukherjee, S.; Pramanik, A.; Das, D.; Mukherjee, A.; Raza, A.; Chen, C. Designing Stimuli-Responsive Upconversion Nanoparticles that Exploit the Tumor Microenvironment. Adv. Mater. 2020, 32, e2000055. [Google Scholar] [CrossRef]
- Reinertsen, R.J.E.; Kewalramani, S.; Jiménez-Ángeles, F.; Weigand, S.J.; Bedzyk, M.J.; Olvera de la Cruz, M. Reexpansion of Charged Nanoparticle Assemblies in Concentrated Electrolytes. Proc. Natl. Acad. Sci. USA 2024, 121, e2316537121. [Google Scholar] [CrossRef] [PubMed]
- Samui, A.; Pal, K.; Karmakar, P.; Sahu, S.K. In Situ Synthesized Lactobionic Acid Conjugated NMOFs, a Smart Material for Imaging and Targeted Drug Delivery in Hepatocellular Carcinoma. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 98, 772–781. [Google Scholar] [CrossRef]
- Zelepukin, I.V.; Griaznova, O.Y.; Shevchenko, K.G.; Ivanov, A.V.; Baidyuk, E.V.; Serejnikova, N.B.; Volovetskiy, A.B.; Deyev, S.M.; Zvyagin, A.V. Flash Drug Release from Nanoparticles Accumulated in the Targeted Blood Vessels Facilitates the Tumour Treatment. Nat. Commun. 2022, 13, 6910. [Google Scholar] [CrossRef] [PubMed]
- Alejo, T.; Uson, L.; Arruebo, M. Reversible Stimuli-Responsive Nanomaterials with On-Off Switching Ability for Biomedical Applications. J. Control. Release 2019, 314, 162–176. [Google Scholar] [CrossRef]
- Li, J.; Zheng, H.; Xu, E.Y.; Moehwald, M.; Chen, L.; Zhang, X.; Mao, S. Inhalable PLGA Microspheres: Tunable Lung Retention and Systemic Exposure via Polyethylene Glycol Modification. Acta Biomater. 2021, 123, 325–334. [Google Scholar] [CrossRef]
- Paredes, A.J.; Volpe-Zanutto, F.; Vora, L.K.; Tekko, I.A.; Permana, A.D.; Picco, C.J.; McCarthy, H.O.; Donnelly, R.F. Systemic Delivery of Tenofovir Alafenamide Using Dissolving and Implantable Microneedle Patches. Mater. Today Bio 2022, 13, 100217. [Google Scholar] [CrossRef]
- Wang, J.J.; Liu, X.X.; Zhu, C.C.; Wang, T.Z.; Wang, S.Y.; Liu, Y.; Pan, X.Y.; Liu, M.H.; Chen, D.; Li, L.L.; et al. Improving Ocular Bioavailability of Hydrophilic Drugs through Dynamic Covalent Complexation. J. Control. Release 2023, 355, 395–405. [Google Scholar] [CrossRef]
- Tao, R.; Gao, M.; Liu, F.; Guo, X.; Fan, A.; Ding, D.; Kong, D.; Wang, Z.; Zhao, Y. Alleviating the Liver Toxicity of Chemotherapy via pH-Responsive Hepatoprotective Prodrug Micelles. ACS Appl. Mater. Interfaces 2018, 10, 21836–21846. [Google Scholar] [CrossRef] [PubMed]
- Hao, Q.; Wang, Z.; Zhao, W.; Wen, L.; Wang, W.; Lu, S.; Xing, D.; Zhan, M.; Hu, X. Dual-Responsive Polyprodrug Nanoparticles with Cascade-Enhanced Magnetic Resonance Signals for Deep-Penetration Drug Release in Tumor Therapy. ACS Appl. Mater. Interfaces 2020, 12, 49489–49501. [Google Scholar] [CrossRef] [PubMed]
- Yeap, Y.Y.; Lock, J.; Lerkvikarn, S.; Semin, T.; Nguyen, N.; Carrier, R.L. Intestinal Mucus Is Capable of Stabilising Supersaturation of Poorly Water-Soluble Drugs. J. Control. Release 2019, 296, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Choi, M.; Kim, H.E.; Jin, M.; Jeon, W.J.; Jung, M.; Yoo, H.; Won, J.H.; Na, Y.G.; Lee, J.Y.; et al. Mannosylated Poly(Acrylic Acid)-Coated Mesoporous Silica Nanoparticles for Anticancer Therapy. J. Control. Release 2022, 349, 241–253. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, M.; Zhang, Q.; Mohammadniaei, M.; Shen, J.; Sun, Y. Brain-Targeted Antigen-Generating Nanoparticles Improve Glioblastoma Prognosis. J. Control. Release 2022, 352, 399–410. [Google Scholar] [CrossRef]
- Li, M.; Yue, X.; Wang, Y.; Zhang, J.; Kan, L.; Jing, Z. Remodeling the Tumor Microenvironment to Improve Drug Permeation and Antitumor Effects by Co-Delivering Quercetin and Doxorubicin. J. Mater. Chem. B 2019, 7, 7619–7626. [Google Scholar] [CrossRef]
- Loria, R.; Giliberti, C.; Bedini, A.; Palomba, R.; Caracciolo, G.; Ceci, P.; Falvo, E.; Marconi, R.; Falcioni, R.; Bossi, G.; et al. Very Low Intensity Ultrasounds as a New Strategy to Improve Selective Delivery of Nanoparticles-Complexes in Cancer Cells. J. Exp. Clin. Cancer Res. 2019, 38, 1–11. [Google Scholar] [CrossRef]
- Amarsy, I.; Papot, S.; Gasser, G. Stimuli-Responsive Metal Complexes for Biomedical Applications. Angew. Chem. Int. Ed. Engl. 2022, 61, e202205900. [Google Scholar] [CrossRef]
- Neher, S.B.; Bradshaw, N.; Floor, S.N.; Gross, J.D.; Walter, P. SRP RNA Controls a Conformational Switch Regulating the SRP-SRP Receptor Interaction. Nat. Struct. Mol. Biol. 2008, 15, 916–923. [Google Scholar] [CrossRef]
- Bastiancich, C.; Malfanti, A.; Préat, V.; Rahman, R. Rationally Designed Drug Delivery Systems for the Local Treatment of Resected Glioblastoma. Adv. Drug Deliv. Rev. 2021, 177, 113951. [Google Scholar] [CrossRef]
- Wu, L.; Wen, X.; Wang, X.; Wang, C.; Sun, X.; Wang, K.; Zhang, H.; Williams, T.; Stacy, A.J.; Chen, J.; et al. Local Intratracheal Delivery of Perfluorocarbon Nanoparticles to Lung Cancer Demonstrated with Magnetic Resonance Multimodal Imaging. Theranostics 2018, 8, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Talluri, S.; Zhao, J.; Liao, C.; Potluri, L.B.; Buon, L.; Mu, S.; Shi, J.; Chakraborty, C.; Tai, Y.T.; et al. ABL1 Kinase Plays an Important Role in Spontaneous and Chemotherapy-Induced Genomic Instability in Multiple Myeloma. Blood 2024, 143, 996–1005. [Google Scholar] [CrossRef] [PubMed]
- Grotwinkel, J.T.; Wild, K.; Segnitz, B.; Sinning, I. SRP RNA Remodeling by SRP68 Explains Its Role in Protein Translocation. Science 2014, 344, 101–104. [Google Scholar] [CrossRef]
- Cao, X.; Lu, X.; Wang, D.; Jia, F.; Tan, X.; Corley, M.; Chen, X.; Zhang, K. Modulating the Cellular Immune Response of Oligonucleotides by Brush Polymer-Assisted Compaction. Small 2017, 13, 1701432. [Google Scholar] [CrossRef]
- Becker, M.M.; Lapouge, K.; Segnitz, B.; Wild, K.; Sinning, I. Structures of Human SRP72 Complexes Provide Insights into SRP RNA Remodeling and Ribosome Interaction. Nucleic Acids Res. 2017, 45, 470–481. [Google Scholar] [CrossRef]
- Ren, Q.; Ma, J.; Li, X.; Meng, Q.; Wu, S.; Xie, Y.; Qi, Y.; Liu, S.; Chen, R. Intestinal Toxicity of Metal Nanoparticles: Silver Nanoparticles Disorder the Intestinal Immune Microenvironment. ACS Appl. Mater. Interfaces 2023, 15, 27774–27788. [Google Scholar] [CrossRef]
- Marques, C.; Hajipour, M.J.; Marets, C.; Oudot, A.; Safavi-Sohi, R.; Guillemin, M.; Borchard, G.; Jordan, O.; Saviot, L.; Maurizi, L. Identification of the Proteins Determining the Blood Circulation Time of Nanoparticles. ACS Nano 2023, 17, 12458–12470. [Google Scholar] [CrossRef]
- Peng, N.; Kang, H.H.; Feng, Y.; Minikes, A.M.; Jiang, X. Autophagy Inhibition Signals Through Senescence to Promote Tumor Suppression. Autophagy 2023, 19, 1764–1780. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, Y.; Zhang, H.; Li, G.; Ding, Z.; Hu, B. Stimulus-Response Patterns: The Key to Giving Generalizability to Text-Based Depression Detection Models. IEEE J. Biomed. Health Inform. 2024, 28, 4925–4936. [Google Scholar] [CrossRef]
- Chen, J.; Qian, C.; Ren, P.; Yu, H.; Kong, X.; Huang, C.; Luo, H.; Chen, G. Light-Responsive Micelles Loaded with Doxorubicin for Osteosarcoma Suppression. Front. Pharmacol. 2021, 12, 679610. [Google Scholar] [CrossRef]
- Liu, D.; Liang, S.; Ma, K.; Meng, Q.F.; Li, X.; Wei, J.; Zhou, M.; Yun, K.; Pan, Y.; Rao, L.; et al. Tumor Microenvironment-Responsive Nanoparticles Amplifying STING Signaling Pathway for Cancer Immunotherapy. Adv. Mater. 2024, 36, e2304845. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Dang, H.; Tian, Y.; Teng, C.; Yin, D.; Yan, L. Macromolecular Conjugated Cyanine Fluorophore Nanoparticles for Tumor-Responsive Photo Nanotheranostics. J. Colloid Interface Sci. 2022, 626, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Simmons, V.N.; Sutton, S.K.; Meltzer, L.R.; Martinez, U.; Palmer, A.M.; Meade, C.D.; Jacobsen, P.B.; McCaffrey, J.C.; Haura, E.B.; Brandon, T.H. Preventing Smoking Relapse in Patients with Cancer: A Randomised Controlled Trial. Cancer 2020, 126, 5165–5172. [Google Scholar] [CrossRef]
- Iyer, R.; Nguyen, T.; Padanilam, D.; Xu, C.; Saha, D.; Nguyen, K.T.; Hong, Y. Glutathione-Responsive Biodegradable Polyurethane Nanoparticles for Lung Cancer Treatment. J. Control. Release 2020, 321, 363–371. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, X.; Fu, K.; Wei, G.; Su, Z. Stimulus-Responsive Nanomaterials Under Physical Regulation for Biomedical Applications. J. Mater. Chem. B 2021, 9, 9642–9657. [Google Scholar] [CrossRef]
- Chan, T.G.; O’Neill, E.; Habjan, C.; Cornelissen, B. Combination Strategies to Improve Targeted Radionuclide Therapy. J. Nucl. Med. 2020, 61, 1544–1552. [Google Scholar] [CrossRef]
- Wang, T.; Sun, G.; Wang, M.; Zhou, B.; Fu, J. Voltage/pH-Driven Mechanized Silica Nanoparticles for the Multimodal Controlled Release of Drugs. ACS Appl. Mater. Interfaces 2015, 7, 21295–21304. [Google Scholar] [CrossRef]
- Hu, J.; Albadawi, H.; Zhang, Z.; Salomao, M.A.; Gunduz, S.; Rehman, S.; D’Amone, L.; Mayer, J.L.; Omenetto, F.; Oklu, R. Silk Embolic Material for Catheter-Directed Endovascular Drug Delivery. Adv. Mater. 2022, 34, e2106865. [Google Scholar] [CrossRef]
- Zhang, J.; Lin, Y.; Lin, Z.; Wei, Q.; Qian, J.; Ruan, R.; Jiang, X.; Hou, L.; Song, J.; Ding, J.; et al. Stimuli-Responsive Nanoparticles for Controlled Drug Delivery in Synergistic Cancer Immunotherapy. Adv. Sci. 2022, 9, e2103444. [Google Scholar] [CrossRef]
- Yang, H.; Liu, R.; Xu, Y.; Qian, L.; Dai, Z. Photosensitizer Nanoparticles Boost Photodynamic Therapy for Pancreatic Cancer Treatment. Nanomicro Lett. 2021, 13, 35. [Google Scholar] [CrossRef]
- Kim, M.; Lee, N.K.; Wang, C.J.; Lim, J.; Byun, M.J.; Kim, T.H.; Park, W.; Park, D.H.; Kim, S.N.; Park, C.G. Reprogramming the Tumor Microenvironment with Biotechnology. Biomater. Res. 2023, 27, 5. [Google Scholar] [CrossRef]
- Jiang, X.; Fan, X.; Zhang, R.; Xu, W.; Wu, H.; Zhao, F.; Xiao, H.; Zhang, C.; Zhao, C.; Wu, G. In Situ Tumor-Triggered Subcellular Precise Delivery of Multi-Drugs for Enhanced Chemo-Photothermal-Starvation Combination Antitumor Therapy. Theranostics 2020, 10, 12158–12173. [Google Scholar] [CrossRef]
- Xu, C.; Song, R.; Lu, P.; Chen, J.; Zhou, Y.; Shen, G.; Jiang, M.; Zhang, W. A pH-Responsive Charge-Reversal Drug Delivery System with Tumor-Specific Drug Release and ROS Generation for Cancer Therapy. Int. J. Nanomed. 2020, 15, 65–80. [Google Scholar] [CrossRef]
- Thapa, R.K.; Kim, J.O. Nanomedicine-based commercial formulations: Current developments and future prospects. J. Pharm. Investig. 2023, 53, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Shen, Z.; Zou, J.; Ye, Q.; Ge, C.; Zhao, Y.; Wang, T.; Chen, Y. Therapeutic Approaches of Dual-targeted Nanomedicines for Tumor Multidrug Resistance. Curr. Drug Deliv. 2024, 21, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Han, X.; Nie, G. Responsive and activable nanomedicines for remodeling the tumor microenvironment. Nat. Protoc. 2021, 16, 405–430. [Google Scholar] [CrossRef]
- Cho, H.; Kim, K. Multi-functional nanomedicines for combinational cancer immunotherapy that transform cold tumors to hot tumors. Expert Opin. Drug Deliv. 2024, 21, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Qin, M.; Xia, H.; Xu, W.; Chen, B.; Wang, Y. The spatiotemporal journey of nanomedicines in solid tumors on their therapeutic efficacy. Adv. Drug Deliv. Rev. 2023, 203, 115137. [Google Scholar] [CrossRef]
- Larrosa, C.; Mora, J.; Cheung, N.K. Global Impact of Monoclonal Antibodies (mAbs) in Children: A Focus on Anti-GD2. Cancers 2023, 15, 3729. [Google Scholar] [CrossRef]
- Hyun, E.J.; Hasan, M.N.; Kang, S.H.; Cho, S.; Lee, Y.K. Oral siRNA Delivery Using Dual Transporting Systems to Efficiently Treat Colorectal Liver Metastasis. Int. J. Pharm. 2019, 555, 250–258. [Google Scholar] [CrossRef]
- Gao, X.; Wang, H.F.; Fang, Z.Y.; Lu, X.; Li, Y.M.; Wang, Y.; Sun, Y.H. Salvage Radical Prostatectomy for Radiorecurrent Prostate Cancer: The Chinese Experience. Chin. Med. J. 2013, 126, 4592–4593. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Han, Y.; Jo, H.A.; Lee, J.; Song, Y.S. Non-Coding RNAs Shuttled via Exosomes Reshape the Hypoxic Tumor Microenvironment. J. Hematol. Oncol. 2020, 13, 67. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.C.; Kim, M.; Kim, S.; Seo, H.; An, S.; Jang, E.H.; Han, S.Y.; Kim, M.J.; Kim, N.K.; Cho, S.W.; et al. In Situ Diagnosis and Simultaneous Treatment of Cardiac Diseases Using a Single-Device Platform. Sci. Adv. 2022, 8, 897. [Google Scholar] [CrossRef]
- Byrne, J.D.; Young, C.C.; Chu, J.N.; Pursley, J.; Chen, M.X.; Wentworth, A.J.; Feng, A.; Kirtane, A.R.; Remillard, K.A.; Hancox, C.I.; et al. Personalised Radiation Attenuating Materials for Gastrointestinal Mucosal Protection. Adv. Sci. 2021, 8, 2100510. [Google Scholar] [CrossRef]
- Tao, J.; Tian, Y.; Chen, D.; Lu, W.; Chen, K.; Xu, C.; Bao, L.; Xue, B.; Wang, T.; Teng, Z.; et al. Stiffness-Transformable Nanoplatforms Responsive to the Tumor Microenvironment for Enhanced Tumor Therapeutic Efficacy. Angew. Chem. Int. Ed. Engl. 2023, 62, e202216361. [Google Scholar] [CrossRef]
- Ullah, A.; Mamun, A.A.; Zaidi, M.B.; Roome, T.; Hasan, A. A Calcium Peroxide Incorporated Oxygen Releasing Chitosan-PVA Patch for Diabetic Wound Healing. Biomed. Pharmacother. 2023, 165, 115156. [Google Scholar] [CrossRef]
- Vollertsen, A.R.; de Boer, D.; Dekker, S.; Wesselink, B.A.M.; Haverkate, R.; Rho, H.S.; Boom, R.J.; Skolimowski, M.; Blom, M.; Passier, R.; et al. Modular Operation of Microfluidic Chips for Highly Parallelised Cell Culture and Liquid Dosing via a Fluidic Circuit Board. Microsyst. Nanoeng. 2020, 6, 107. [Google Scholar] [CrossRef]
- Leung, E.; Schneider, C.; Yan, F.; Mohi-El-Din, H.; Kudla, G.; Tuck, A.; Wlotzka, W.; Doronina, V.A.; Bartley, R.; Watkins, N.J.; et al. Integrity of SRP RNA Is Ensured by La and the Nuclear RNA Quality Control Machinery. Nucleic Acids Res. 2014, 42, 10698–10710. [Google Scholar] [CrossRef]
- Xia, K.; Shen, X.; Ang, X.; Hou, B.; Chen, Y.; Zhang, K.; Hao, Z. Surface Modification of Ureteral Stents: Development History, Classification, Function, and Future Developments. Expert Rev. Med. Devices 2023, 20, 401–416. [Google Scholar] [CrossRef]
- Pomykala, K.L.; Hadaschik, B.A.; Sartor, O.; Gillessen, S.; Sweeney, C.J.; Maughan, T.; Hofman, M.S.; Herrmann, K. Next Generation Radiotheranostics. Promoting Precision Medicine. Ann. Oncol. 2023, 34, 507–519. [Google Scholar] [CrossRef]
- Khanal, S.; Adhikari, U.; Rijal, N.P.; Bhattarai, S.R.; Sankar, J.; Bhattarai, N. pH-Responsive PLGA Nanoparticle for Controlled Payload Delivery of Diclofenac Sodium. J. Funct. Biomater. 2016, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- Sabatelle, R.C.; Liu, R.; Hung, Y.P. Ultra-High Drug Loading Improves Nanoparticle Efficacy Against Peritoneal Mesothelioma. Biomaterials 2022, 285, 121534. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, C.; Song, Y.; Ruan, J.; Quan, P.; Fang, L. High Drug-Loading and Controlled-Release Hydroxyphenyl-Polyacrylate Adhesive for Transdermal Patch. J. Control. Release 2023, 353, 475–489. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Cai, Z.; Ai, H. Stimulus-Responsive Nanoparticle Magnetic Resonance Imaging Contrast Agents: Design Considerations and Applications. Adv. Healthc. Mater. 2021, 10, e2001091. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, M.; Yao, B.; Lu, X.; Song, B.; Vasilatos, S.N.; Zhang, X.; Ren, X.; Yao, C.; Bian, W.; et al. Dual pH/ROS-Responsive Nanoplatform with Deep Tumor Penetration and Self-Amplified Drug Release for Enhancing Tumor Chemotherapeutic Efficacy. Small 2020, 16, e2002188. [Google Scholar] [CrossRef]
- DuCote, T.J.; Naughton, K.J.; Skaggs, E.M.; Bocklage, T.J.; Allison, D.B.; Brainson, C.F. Using Artificial Intelligence to Identify Tumor Microenvironment Heterogeneity in Non-Small Cell Lung Cancers. Lab. Investig. 2023, 103, 100176. [Google Scholar] [CrossRef]
- Yue, J.; Pallares, R.M.; Cole, L.E.; Coughlin, E.E.; Mirkin, C.A.; Lee, A.; Odom, T.W. Smaller CpG-Conjugated Gold Nanoconstructs Achieve Higher Targeting Specificity of Immune Activation. ACS Appl. Mater. Interfaces 2018, 10, 21920–21926. [Google Scholar] [CrossRef]
- Luo, J.; Schmaus, J.; Cui, M.; Hörterer, E.; Wilk, U.; Höhn, M.; Däther, M.; Berger, S.; Benli-Hoppe, T.; Peng, L.; et al. Hyaluronate siRNA Nanoparticles with Positive Charge Display Rapid Attachment to Tumor Endothelium and Penetration into Tumors. J. Control. Release 2021, 329, 919–933. [Google Scholar] [CrossRef]
- Zhi, D.; Yang, T.; Yang, J.; Fu, S.; Zhang, S. Targeting Strategies for Superparamagnetic Iron Oxide Nanoparticles in Cancer Therapy. Acta Biomater. 2020, 102, 13–34. [Google Scholar] [CrossRef]
- Ernst, L.M.; Casals, E.; Italiani, P.; Boraschi, D.; Puntes, V. The Interactions Between Nanoparticles and the Innate Immune System from a Nanotechnologist Perspective. Nanomaterials 2021, 11, 2991. [Google Scholar] [CrossRef]
- Machado, M.; Silva, G.A.; Bitoque, D.B.; Ferreira, J.; Pinto, L.A.; Morgado, J.; Ferreira, Q. Self-Assembled Multilayer Films for Time-Controlled Ocular Drug Delivery. ACS Appl. Bio Mater. 2019, 2, 4173–4180. [Google Scholar] [CrossRef]
- Rosenblatt, K.M.; Bunjes, H. Evaluation of the Drug Loading Capacity of Different Lipid Nanoparticle Dispersions by Passive Drug Loading. Eur. J. Pharm. Biopharm. 2017, 117, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Bahuguna, A.; Bharadwaj, S.; Bajpai, V.K.; Shukla, S.; Won, D.W.; Park, I.; Na, M.; Sonwal, S.; Huh, Y.S.; Han, Y.K.; et al. Insights into Cyclooxygenase-2 Inhibition by Isolated Bioactive Compounds 3-Caffeoyl-4-Dihydrocaffeoyl Quinic Acid and Isorhamnetin 3-O-β-D-Glucopyranoside from Salicornia herbacea. Phytomedicine 2021, 90, 153638. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhou, Y.; Li, R.; Chen, Z.; Fan, X. Advanced Drug Delivery System Against Ischemic Stroke. J. Control. Release 2022, 344, 173–201. [Google Scholar] [CrossRef]
- Zatovicova, M.; Kajanova, I.; Barathova, M.; Takacova, M.; Labudova, M.; Csaderova, L.; Jelenska, L.; Svastova, E.; Pastorekova, S.; Harris, A.L.; et al. Novel Humanized Monoclonal Antibodies for Targeting Hypoxic Human Tumors via Two Distinct Extracellular Domains of Carbonic Anhydrase IX. Cancer Metab. 2022, 10, 1–16. [Google Scholar] [CrossRef]
- Ding, D.; Yang, C.; Lv, C.; Li, J.; Tan, W. Improving Tumor Accumulation of Aptamers by Prolonged Blood Circulation. Anal. Chem. 2020, 92, 4108–4114. [Google Scholar] [CrossRef]
- Muccini, A.M.; Tran, N.T.; de Guingand, D.L.; Philip, M.; Della Gatta, P.A.; Galinsky, R.; Sherman, L.S.; Kelleher, M.A.; Palmer, K.R.; Berry, M.J.; et al. Creatine Metabolism in Female Reproduction, Pregnancy and Newborn Health. Nutrients 2021, 13, 490. [Google Scholar] [CrossRef]
- Braun, R.D.; Vistisen, K.S. Modeling Human Choroidal Melanoma Xenograft Growth in Immunocompromised Rodents to Assess Treatment Efficacy. Invest. Ophthalmol. Vis. Sci. 2012, 53, 2693–2701. [Google Scholar] [CrossRef]
- Vilalta, M.; Hughes, N.P.; Von Eyben, R.; Giaccia, A.J.; Graves, E.E. Patterns of Vasculature in Mouse Models of Lung Cancer Are Dependent on Location. Mol. Imaging Biol. 2017, 19, 215–224. [Google Scholar] [CrossRef]
- Elming, P.B.; Wittenborn, T.R.; Busk, M.; Sørensen, B.S.; Thomsen, M.B.H.; Strandgaard, T.; Dyrskjøt, L.; Nielsen, S.; Horsman, M.R. Refinement of an Established Procedure and Its Application to the Identification of Hypoxia in Prostate Cancer Xenografts. Cancers 2021, 13, 2602. [Google Scholar] [CrossRef]
- Matsumoto, S.; Yasui, H.; Mitchell, J.B.; Krishna, M.C. Imaging Cycling Tumour Hypoxia. Cancer Res. 2010, 70, 10019–10023. [Google Scholar] [CrossRef] [PubMed]
- Gouel, P.; Decazes, P.; Vera, P.; Gardin, I.; Thureau, S.; Bohn, P. Advances in PET and MRI Imaging of Tumour Hypoxia. Front. Med. 2023, 10, 1055062. [Google Scholar] [CrossRef] [PubMed]
- Thureau, S.; Piton, N.; Gouel, P.; Modzelewski, R.; Dujon, A.; Baste, J.M.; Melki, J.; Rinieri, P.; Peillon, C.; Rastelli, O.; et al. First Comparison Between [18F]-FMISO and [18F]-Faza for Preoperative PET Imaging of Hypoxia in Lung Cancer. Cancers 2021, 13, 4101. [Google Scholar] [CrossRef]
- Foyt, D.A.; Taheem, D.K.; Ferreira, S.A.; Norman, M.D.A.; Petzold, J.; Jell, G.; Grigoriadis, A.E.; Gentleman, E. Hypoxia Impacts Human MSC Response to Substrate Stiffness During Chondrogenic Differentiation. Acta Biomater. 2019, 89, 73–83. [Google Scholar] [CrossRef]
- Mathias, T.J.; Chang, K.T.; Martin, S.S.; Vitolo, M.I. Gauging the Impact of Cancer Treatment Modalities on Circulating Tumor Cells (CTCs). Cancers 2020, 12, 743. [Google Scholar] [CrossRef]
- Quesnel, A.; Broughton, A.; Karagiannis, G.S.; Filippou, P.S. Message in the Bottle: Regulation of the Tumour Microenvironment via Exosome-Driven Proteolysis. Cancer Metastasis Rev. 2022, 41, 789–801. [Google Scholar] [CrossRef]
- Triantafyllidis, A.; Polychronidou, E.; Alexiadis, A.; Rocha, C.L.; Oliveira, D.N.; da Silva, A.S.; Freire, A.L.; Macedo, C.; Sousa, I.F.; Werbet, E.; et al. Computerised Decision Support and Machine Learning Applications for the Prevention and Treatment of Childhood Obesity: A Systematic Review of the Literature. Artif. Intell. Med. 2020, 104, 101844. [Google Scholar] [CrossRef]
- Luan, X.; Yuan, H.; Song, Y.; Hu, H.; Wen, B.; He, M.; Zhang, H.; Li, Y.; Li, F.; Shu, P.; et al. Reappraisal of Anticancer Nanomedicine Design Criteria in Three Types of Preclinical Cancer Models for Better Clinical Translation. Biomaterials 2021, 275, 120910. [Google Scholar] [CrossRef]
- Peng, S.; Ouyang, B.; Men, Y.; Du, Y.; Cao, Y.; Xie, R.; Pang, Z.; Shen, S.; Yang, W. Biodegradable Zwitterionic Polymer Membrane Coating Endowing Nanoparticles with Ultra-Long Circulation and Enhanced Tumor Photothermal Therapy. Biomaterials 2021, 231, 119680. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cong, Y.; Cai, M.; Liang, X.; Wang, L.; Zhou, D. Charge-Conversional Click Polyprodrug Nanomedicine for Targeted and Synergistic Cancer Therapy. J. Control. Release 2023, 356, 567–579. [Google Scholar] [CrossRef]
- Chen, Q.; Bai, H.; Wu, W.; Huang, G.; Li, Y.; Wu, M.; Tang, G.; Ping, Y. Bioengineering Bacterial Vesicle-Coated Polymeric Nanomedicine for Enhanced Cancer Immunotherapy and Metastasis Prevention. Nano Lett. 2020, 20, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Watson, M.J.; Vignali, P.D.A.; Mullett, S.J.; Overacre-Delgoffe, A.E.; Peralta, R.M.; Grebinoski, S.; Menk, A.V.; Rittenhouse, N.L.; DePeaux, K.; Whetstone, R.D.; et al. Metabolic Support of Tumour-Infiltrating Regulatory T Cells by Lactic Acid. Nature 2021, 591, 645–651. [Google Scholar] [CrossRef]
- Vito, A.; El-Sayes, N.; Mossman, K. Hypoxia-Driven Immune Escape in the Tumor Microenvironment. Cells 2020, 9, 992. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Li, J.; Gu, P.; Fan, X. The Application of Nanoparticles in Cancer Immunotherapy: Targeting Tumor Microenvironment. Bioact. Mater. 2020, 6, 1973–1987. [Google Scholar] [CrossRef]
- Du, S.S.; Chen, G.W.; Yang, P.; Chen, Y.X.; Hu, Y.; Zhao, Q.Q.; Zhang, Y.; Liu, R.; Zheng, D.X.; Zhou, J.; et al. Radiation Therapy Promotes Hepatocellular Carcinoma Immune Cloaking via PD-L1 Upregulation Induced by cGAS-STING Activation. Int. J. Radiat. Oncol. Biol. Phys. 2022, 112, 1243–1255. [Google Scholar] [CrossRef]
- Silverman, D.A.; Martinez, V.K.; Dougherty, P.M.; Myers, J.N.; Calin, G.A.; Amit, M. Cancer-Associated Neurogenesis and Nerve-Cancer Cross-talk. Cancer Res. 2021, 81, 1431–1440. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Z.; Zhang, Y.; Zhou, W.; Zhang, X.; Peng, C.; Ji, T.; Zou, X.; Zhang, Z.; Ren, Z. Spatial Transcriptomics Reveals that Metabolic Characteristics Define the Tumour Immunosuppression Microenvironment via iCAF Transformation in Oral Squamous Cell Carcinoma. Int. J. Oral Sci. 2024, 16, 9. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, Z.; Xu, W.; Sun, T.; Zhuang, X.; Ding, J.; Chen, X. Spatiotemporally Targeted Nanomedicine Overcomes Hypoxia-Induced Drug Resistance of Tumor Cells after Disrupting Neovasculature. Nano Lett. 2020, 20, 6191–6198. [Google Scholar] [CrossRef]
- Abou Khouzam, R.; Brodaczewska, K.; Filipiak, A.; Zeinelabdin, N.A.; Buart, S.; Szczylik, C.; Kieda, C.; Chouaib, S. Tumor Hypoxia Regulates Immune Escape/Invasion: Influence on Angiogenesis and Potential Impact of Hypoxic Biomarkers on Cancer Therapies. Front. Immunol. 2021, 11, 613114. [Google Scholar] [CrossRef]
- Cao, M.; Shi, E.; Wang, H.; Mao, L.; Wu, Q.; Li, X.; Liang, Y.; Yang, X.; Wang, Y.; Li, C. Personalized Targeted Therapeutic Strategies against Oral Squamous Cell Carcinoma: An Evidence-Based Review of Literature. Int. J. Nanomed. 2022, 17, 4293–4306. [Google Scholar] [CrossRef]




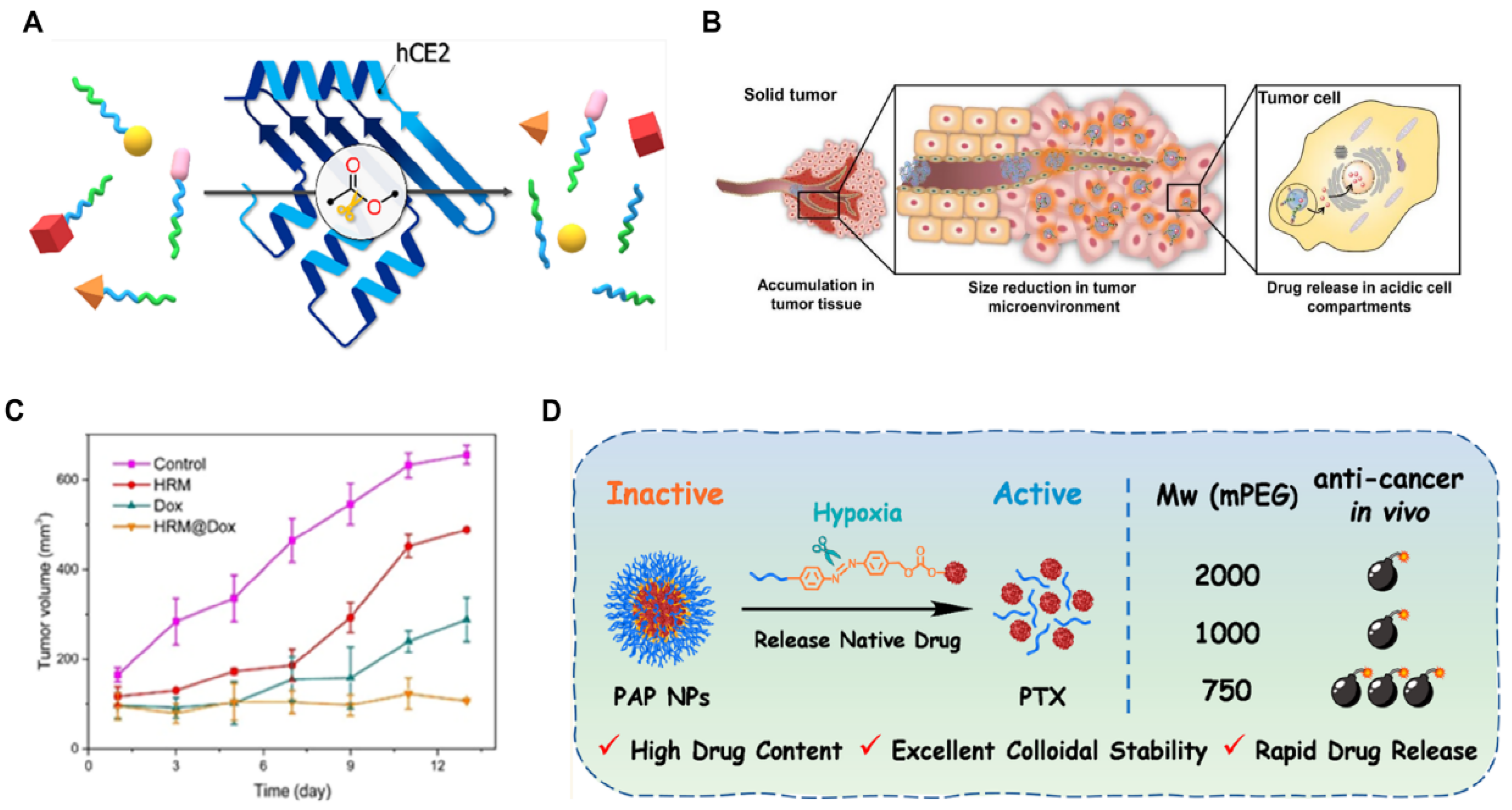
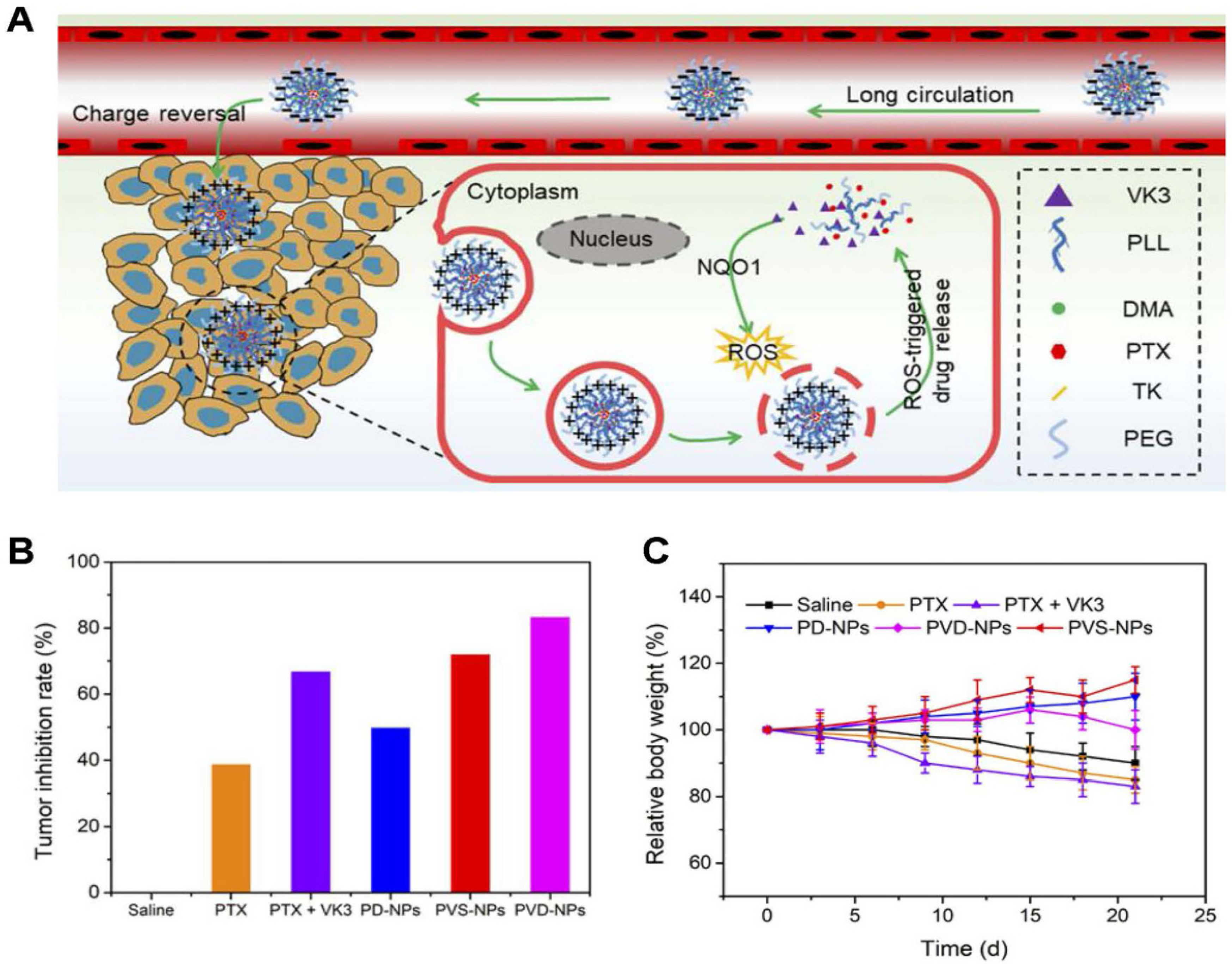
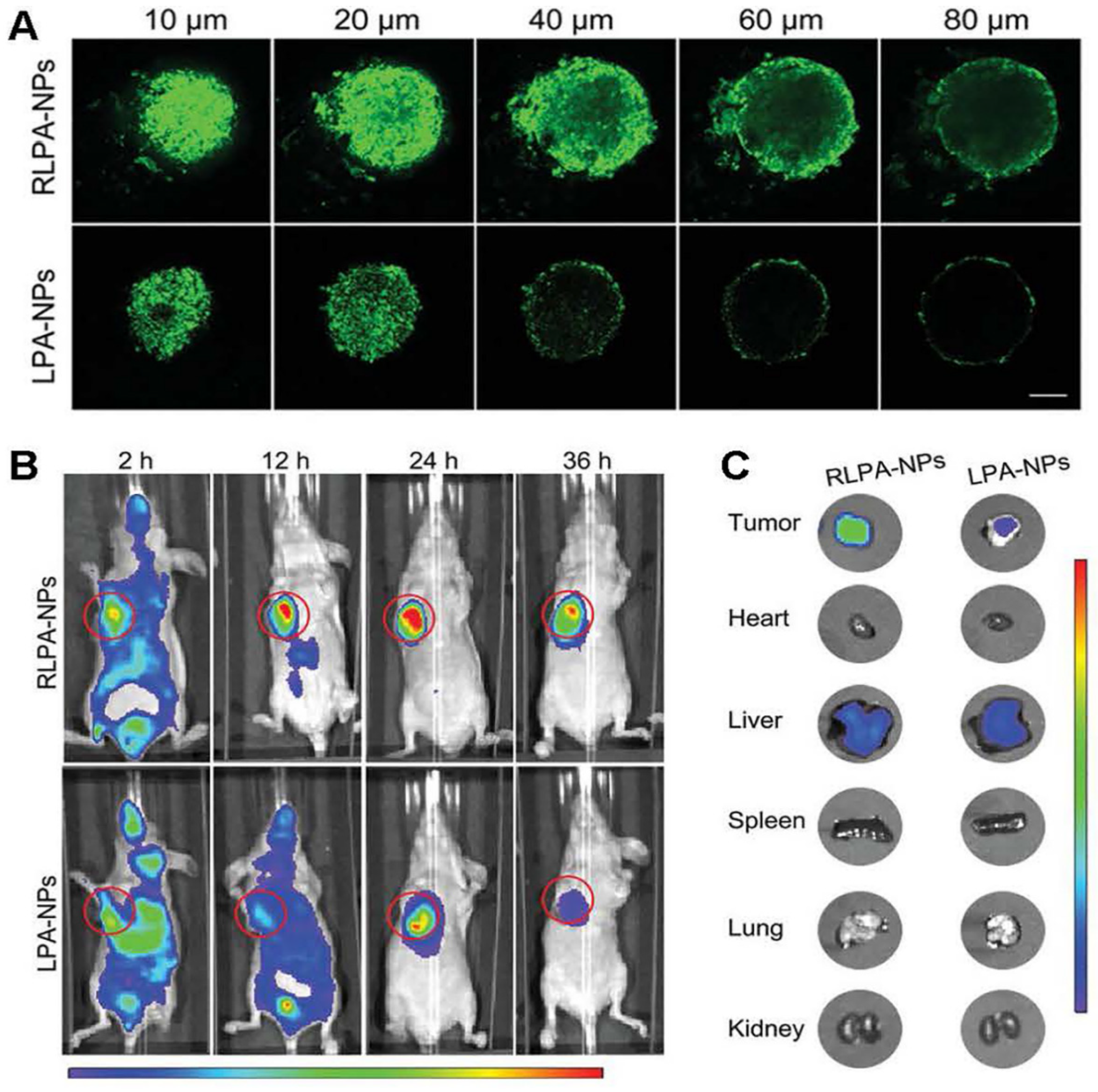
| Nanomedicine | Response Type | Drug Release/Efficacy (Compared to Control Group) | Target Protein | Innovations | Reference |
|---|---|---|---|---|---|
| cHDEA | pH | 3 times | — a | Reversible drug delivery | [21] |
| iCPDN | pH | 5 times | HIF-1α | Overcoming hypoxia-induced chemotherapy resistance | [22] |
| NP@PEDOX/PSP | PDT | 3.3 times | — a | Imaging-guided photodynamic immunotherapy | [23] |
| PTX-PLGA-gel-MS | pH | 1.5 times | — a | Sustained rapid-release drugs | [24] |
| TOS-Ace-PEG-Bio | pH | 2.87 times | CD44 receptor | Rapid drug release and high safety | [25] |
| DDMSNs-DOX HA | Redox/pH | 2 times | — a | High drug-loading capacity Good degradability | [26] |
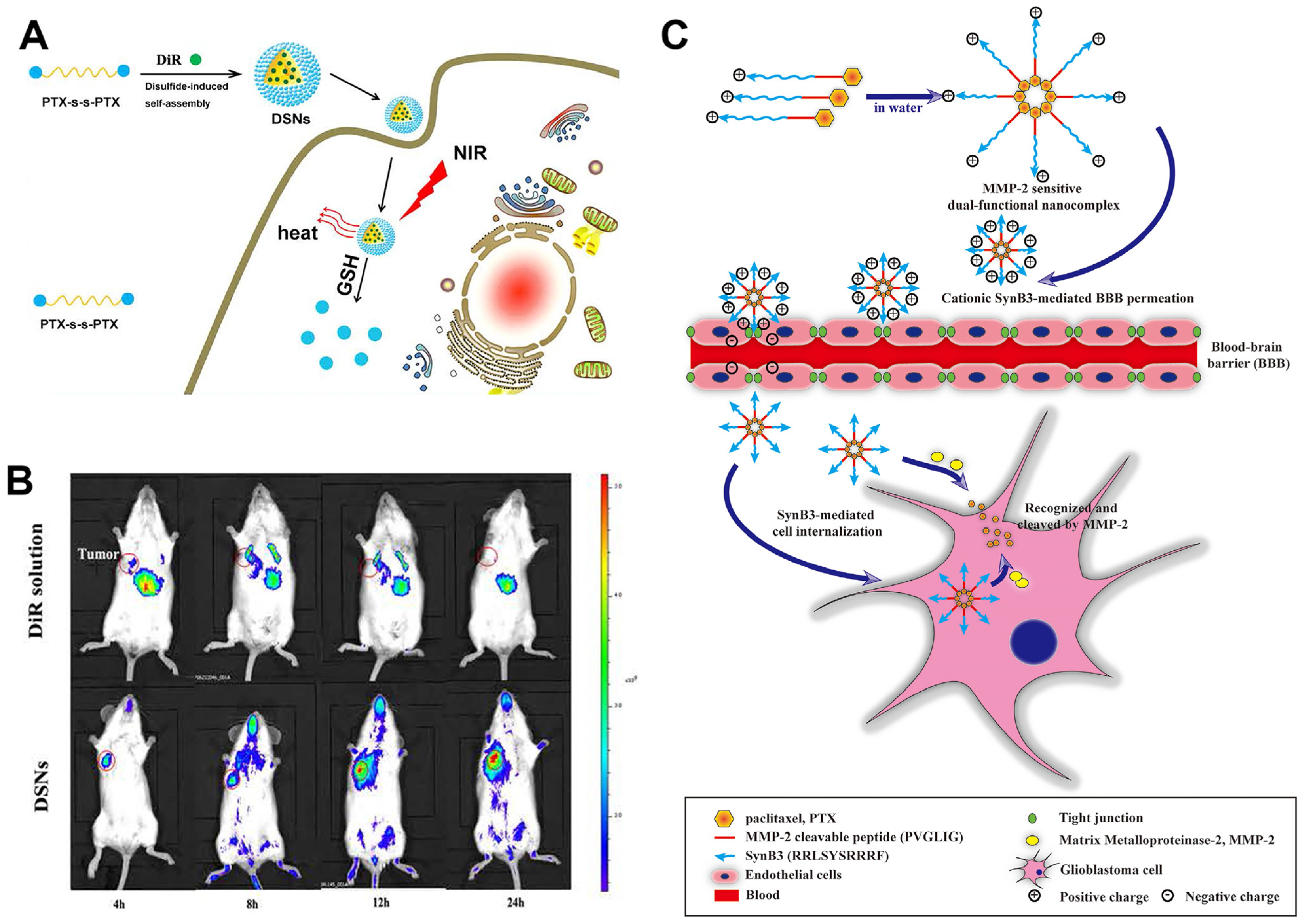
| DSNs | Redox | 5 times | — a | Controlled release of drugs to reduce side effects | [27] |
| SynB3-PVGLIG-PTX | Enzyme | 2.27 times | MMP-2 | MMP-2 targets high permeability with low side effects | [28] |
| PEG-b-PCPTEMA | ROS | 16 times | — a | Active modulation of the tumor microenvironment | [29] |
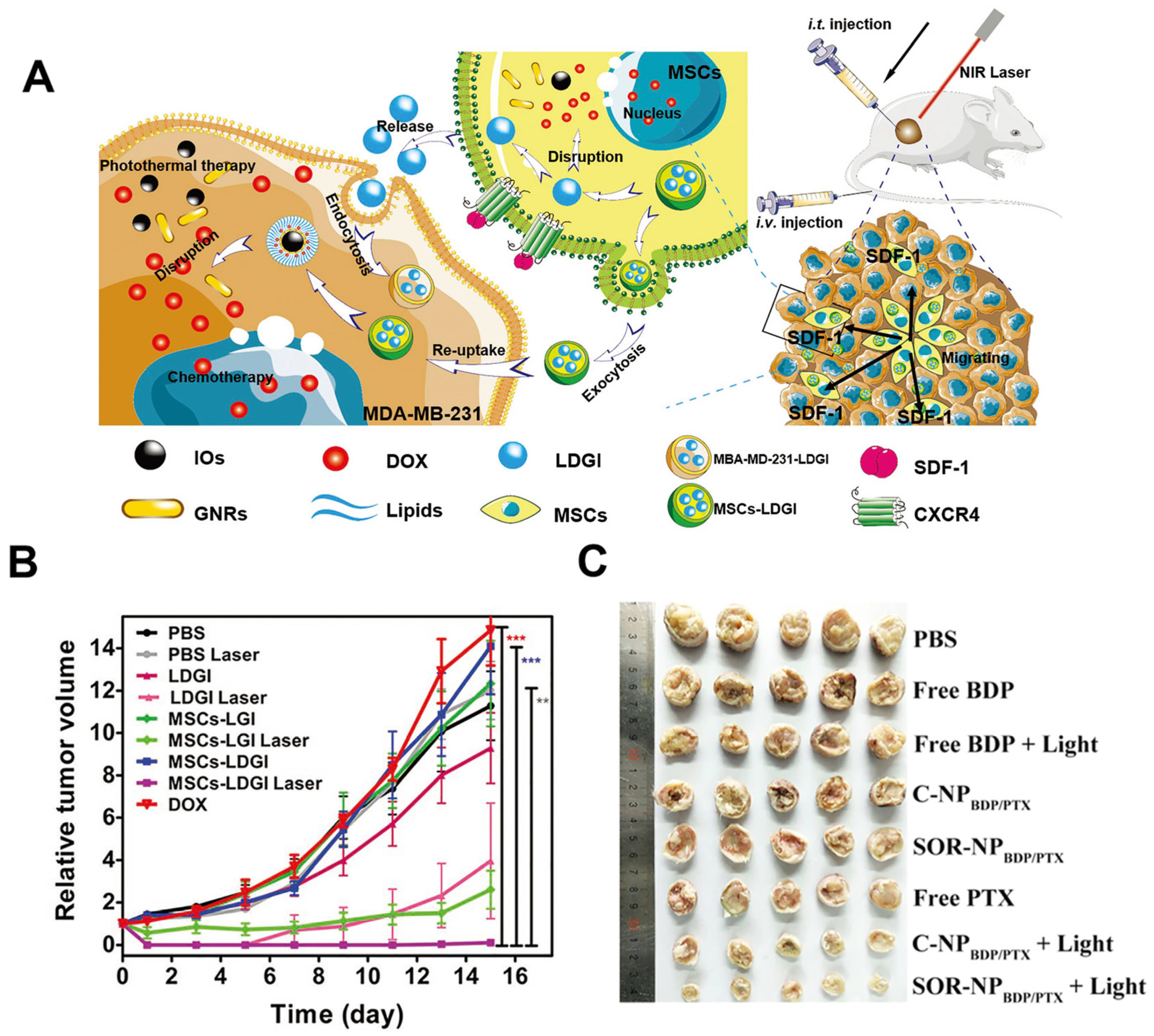
| LDGI | PDT | 6.8 times | — a | Controlled drug release Photothermal therapy | [30] |
| SOR-NPBDP/PTX | ROS/PDT | 2.5 times | — a | Combined photodynamic chemotherapy | [31] |
| MCNC | Magnetic | 3.5 times | — a | Spatiotemporal control of drug release | [32] |
| DOX/Br NP2 | pH | 1.39 times | — a | Improve drug penetration and increase drug concentration | [33] |
| DOX-STB | pH | 2.5 times | — a | Highly controlled and sustained release drugs | [34] |
| Mo154Gel | pH/PDT | 2.27 times | — a | Self-healing injectable hydrogel | [35] |
| A2-APM | redox response | 2.93 times | PDL-1 | Modulating the immunosuppressive tumor microenvironment | [36] |
| DOX-HR-LPs | Hypoxia | 4 times | — a | Enhances anti-tumor efficacy and reduces systemic toxicity | [37] |
| HR-NPs | Hypoxia | 2 times | — a | Reduced systemic toxicity and improved biodistribution facilitated tumor accumulation of nanoparticles | [38] |
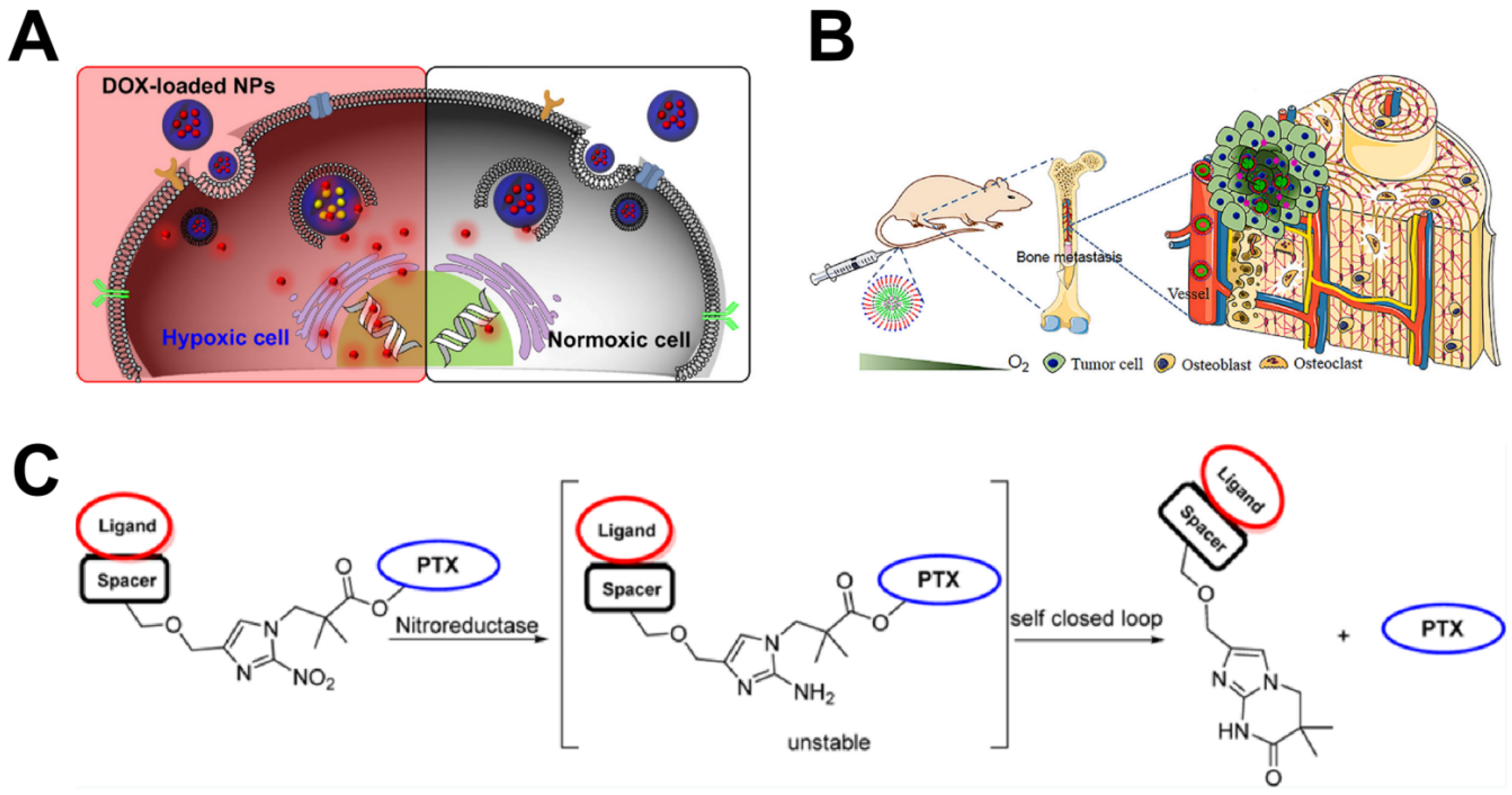
| ALN-HR-PMs/DOX | Hypoxia | 4 times | — a | Inhibition of osteoclast activity and promotion of osteoblast activity effectively inhibit tumor growth and protect bone structure. | [39] |
| GLU-PTX | Hypoxia | 2 times | CA IX | Exhibits good stability under physiological conditions and rapidly releases the active drug in hypoxic environments | [40] |
| H-MnO2-PEG/TP | Hypoxia | — a | HIF-1α | Relief of tumor hypoxia and inhibition of angiogenesis | [41] |
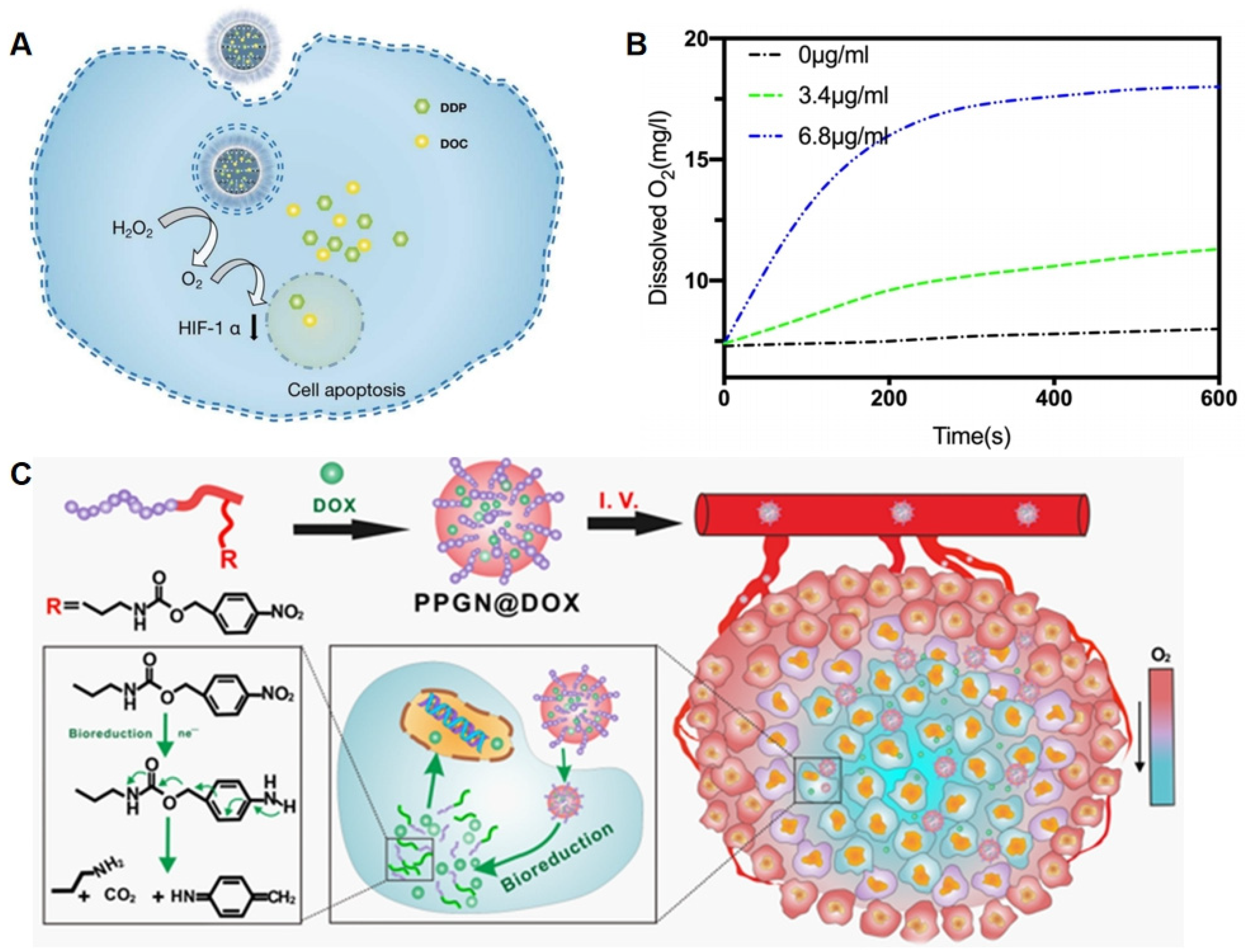
| PPGN@DOX | Hypoxia | — a | — a | Effectively internalizes into the nucleus of the tumor cell and provides superior anti-tumor activity | [42] |
| D@HRGF | Hypoxia | 2.1 times | Folate receptor FR | Low toxicity and good biocompatibility | [43] |
| NP-PDT@Reg | PDT | 4 times | — a | Normalizing blood vessels to overcome tumor hypoxia and reprogramming macrophages | [44] |
| Cu-PPT NPs | PDT | — a | — a | Sustained stimulation of systemic anti-tumor immune responses and reactivation of T cells | [45] |
| DOX-HR-LP | Hypoxia | 3 times | — a | Significantly improves anti-tumor efficacy and significantly reduces DOX toxicity | [37] |
| PA/HA-Ce6@TPZ NPs | PDT/Hypoxi | 6 times | — a | More precise control of efficacy and toxicity of chemotherapy drugs | [46] |
| Polymer–drug conjugate (PDC) | Enzyme | — a | Carboxylesterase hCE2 | Improving the efficacy of anticancer drugs and reducing systemic toxicity | [47] |
| HRM NPs | Hypoxia | 6 times | Nitroreductase (NTR) | Modulation of the redox state of tumor cells enhances the efficiency of chemotherapeutic drug release | [48] |
| PCL@GSK-diABZI/aPD-1 | pH | — a | PD-1 MMP-2 | Creating a positive feedback loop in the cancer immune cycle to enhance anti-tumor immune response | [49] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, J.; Xue, Z.; Chen, Y.; Li, J.; Yue, X.; Zhang, Y.; Gao, J.; Hao, Y.; Shen, J. Development of Stimuli-Responsive Polymeric Nanomedicines in Hypoxic Tumors and Their Therapeutic Promise in Oral Cancer. Polymers 2025, 17, 1010. https://doi.org/10.3390/polym17081010
Hou J, Xue Z, Chen Y, Li J, Yue X, Zhang Y, Gao J, Hao Y, Shen J. Development of Stimuli-Responsive Polymeric Nanomedicines in Hypoxic Tumors and Their Therapeutic Promise in Oral Cancer. Polymers. 2025; 17(8):1010. https://doi.org/10.3390/polym17081010
Chicago/Turabian StyleHou, Jialong, Zhijun Xue, Yao Chen, Jisen Li, Xin Yue, Ying Zhang, Jing Gao, Yonghong Hao, and Jing Shen. 2025. "Development of Stimuli-Responsive Polymeric Nanomedicines in Hypoxic Tumors and Their Therapeutic Promise in Oral Cancer" Polymers 17, no. 8: 1010. https://doi.org/10.3390/polym17081010
APA StyleHou, J., Xue, Z., Chen, Y., Li, J., Yue, X., Zhang, Y., Gao, J., Hao, Y., & Shen, J. (2025). Development of Stimuli-Responsive Polymeric Nanomedicines in Hypoxic Tumors and Their Therapeutic Promise in Oral Cancer. Polymers, 17(8), 1010. https://doi.org/10.3390/polym17081010






