Developing a Novel, Green, and Efficient Synthesis Method for Polycarboxylate Superplasticizers Through Mechanochemical Internal Mixing Polymerization
Abstract
1. Introduction
2. Materials and Experimental
2.1. Materials
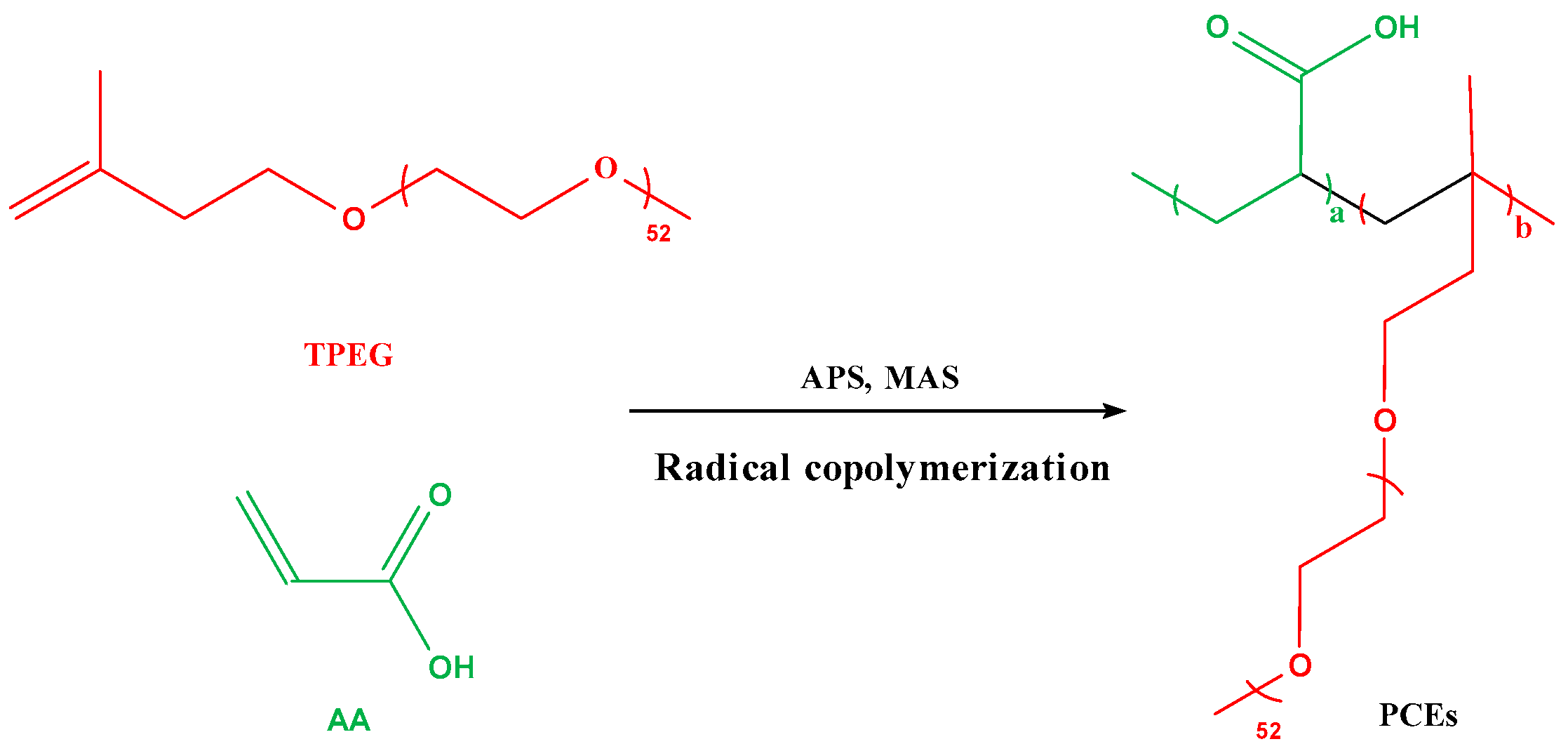
2.2. Synthesis of PCEs
2.2.1. Orthogonal Test
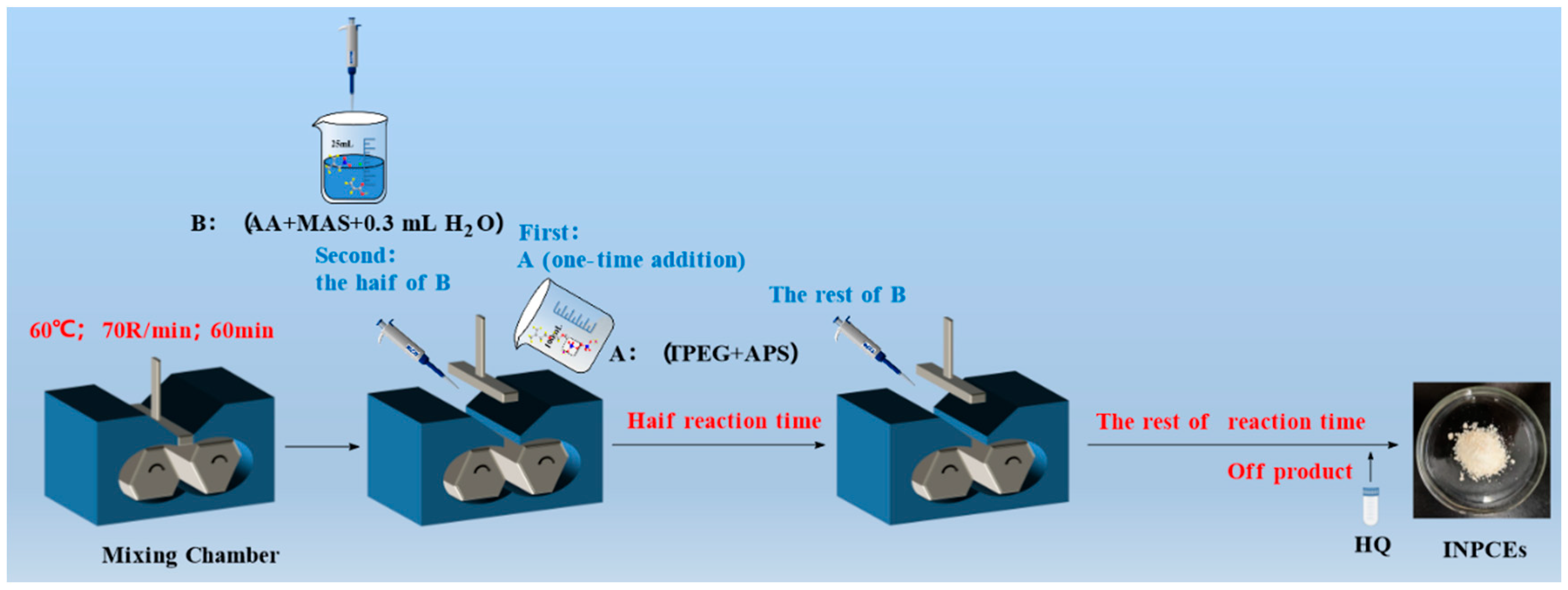
2.2.2. Synthesis of INPCEs
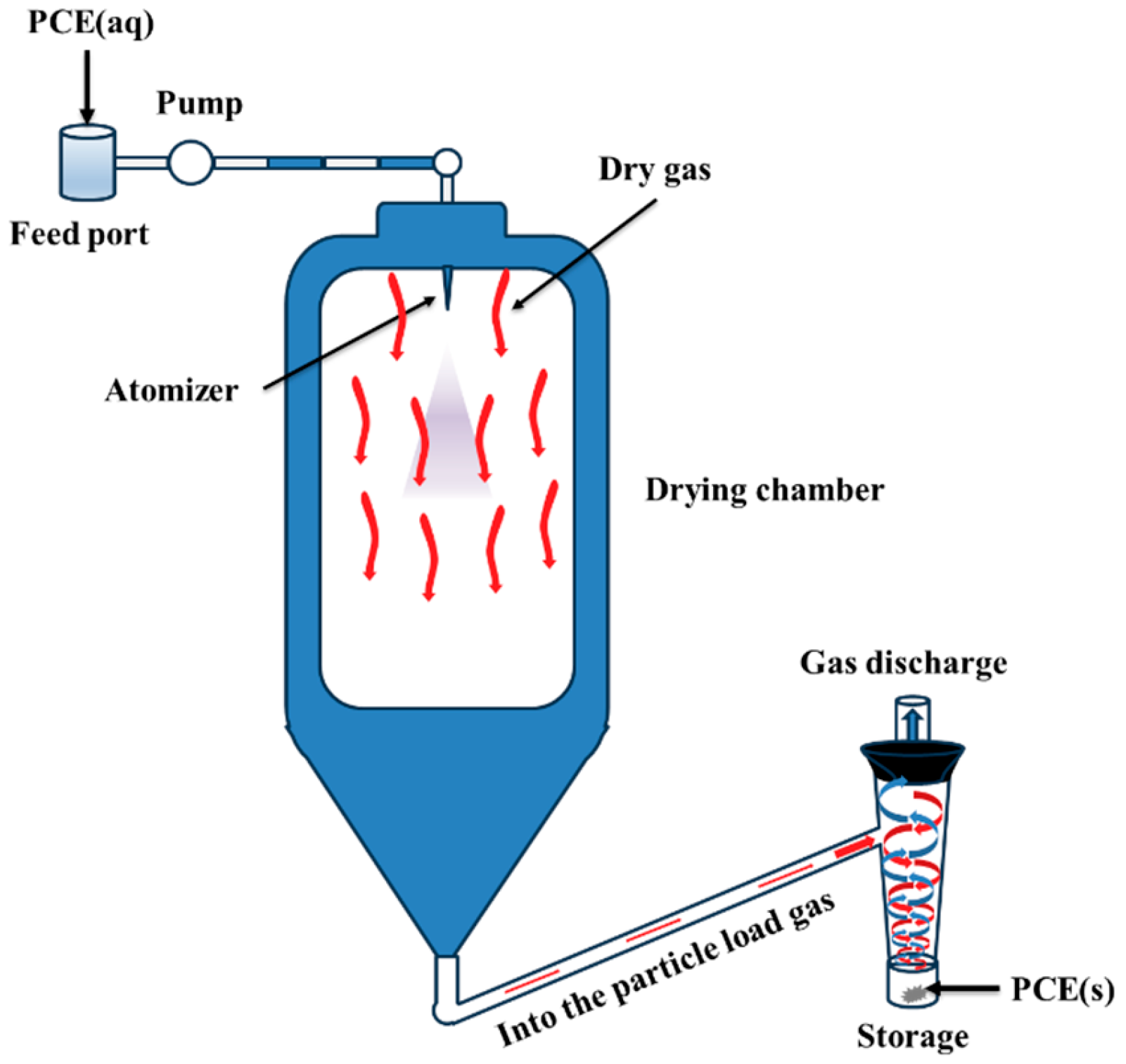
2.3. Synthesis of TPCE
2.4. Purification of PCEs
2.5. Characterization of PCEs
2.5.1. Fourier Transform Infrared Spectroscopy (FT-IR)
2.5.2. Gel Permeation Chromatography (GPC)
2.6. Performance Test of PCEs
2.6.1. Concentration Test
2.6.2. Initial Fluidity
2.6.3. Calculation of Fluidity Loss
2.7. Energy Consumption
3. Results and Discussion
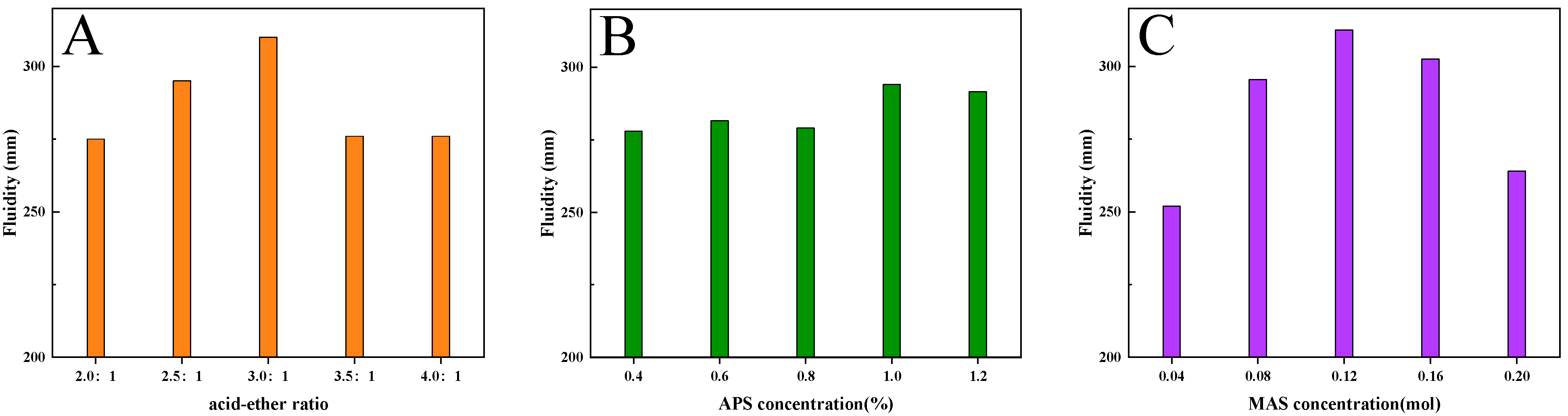
3.1. Orthogonal Test
3.2. Structural Characterization
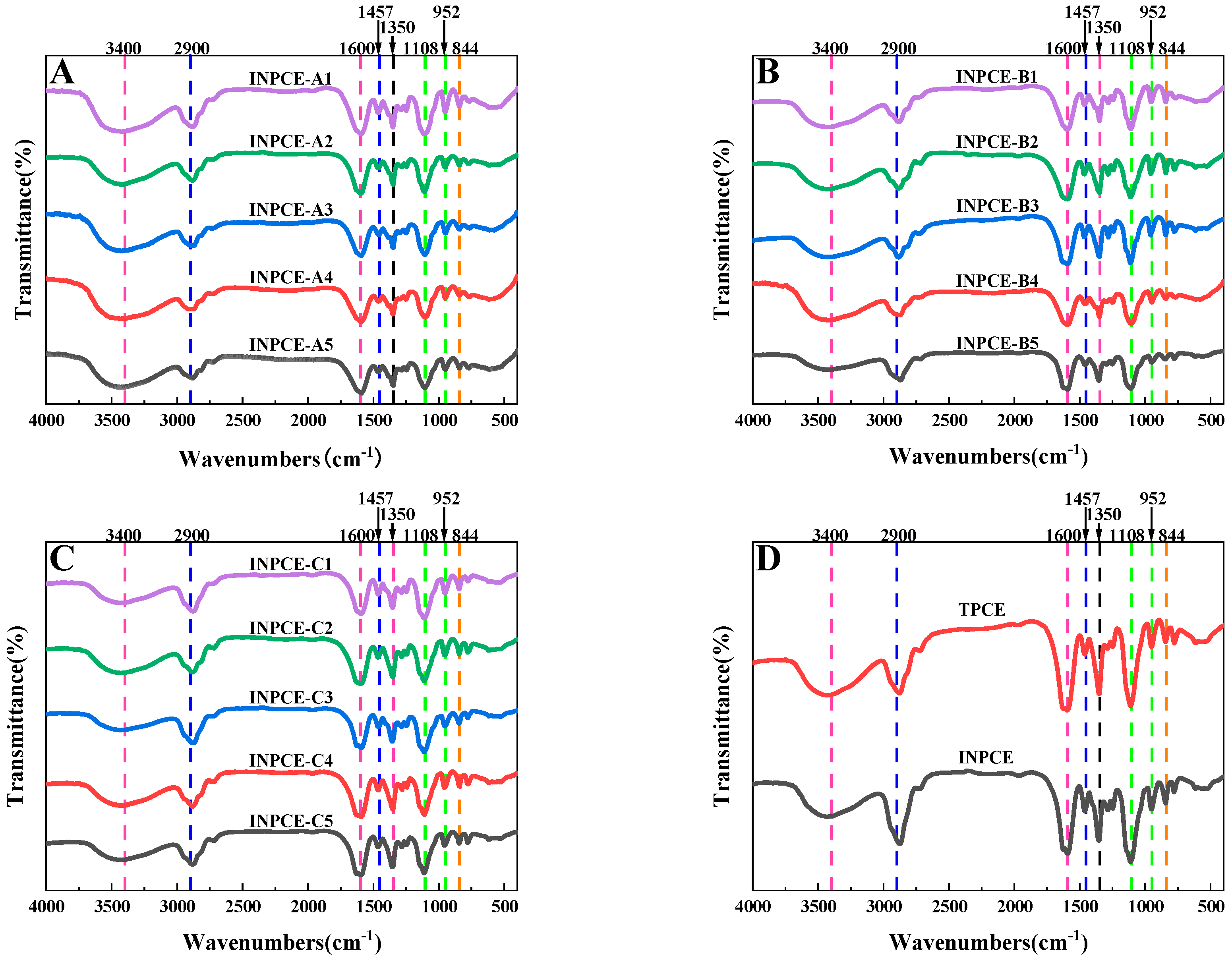
3.2.1. FT-IR
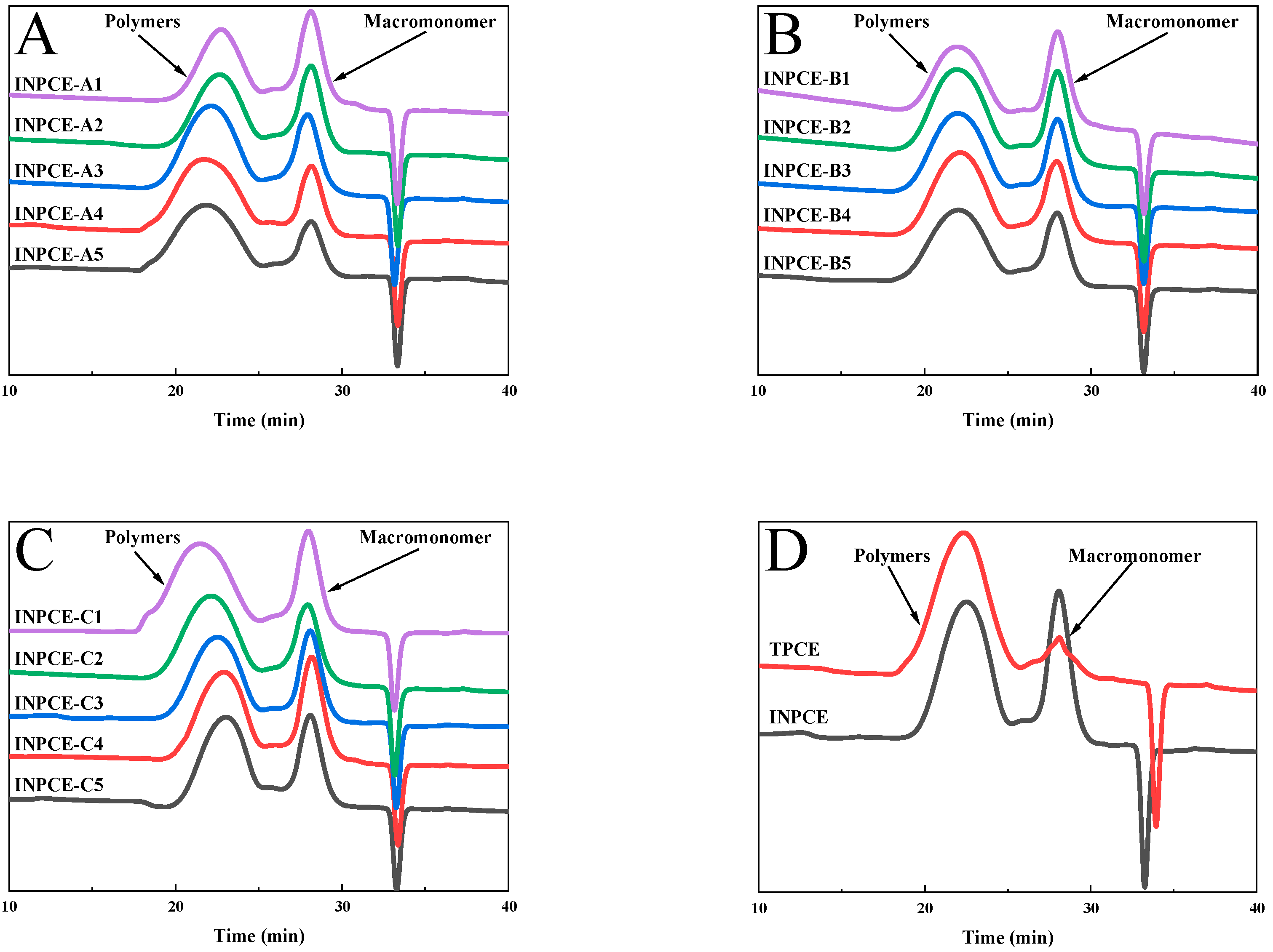
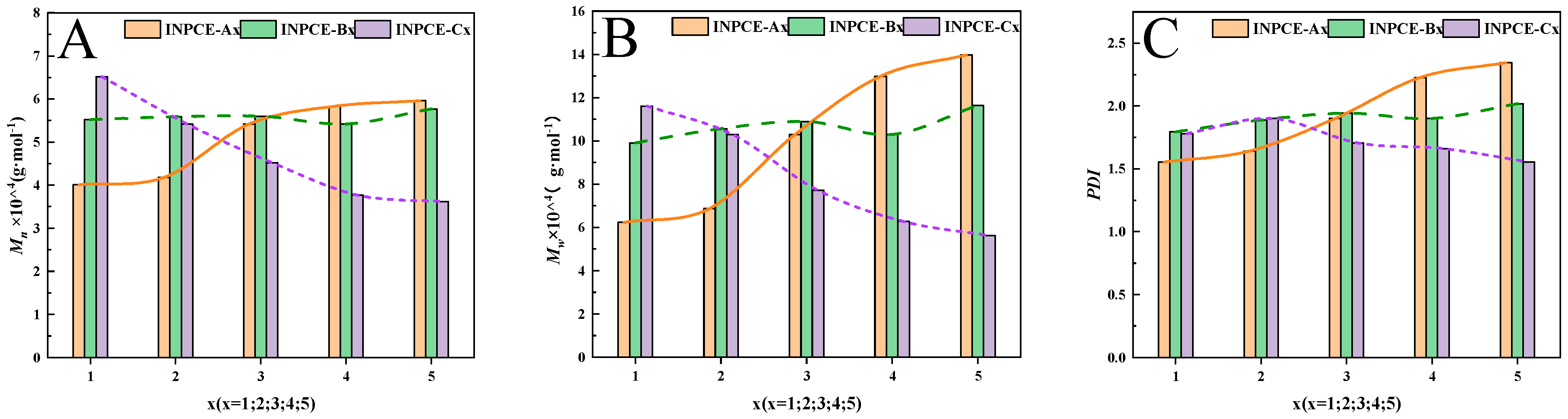
3.2.2. GPC
3.3. Fluidity of Cement Paste with INPCEs
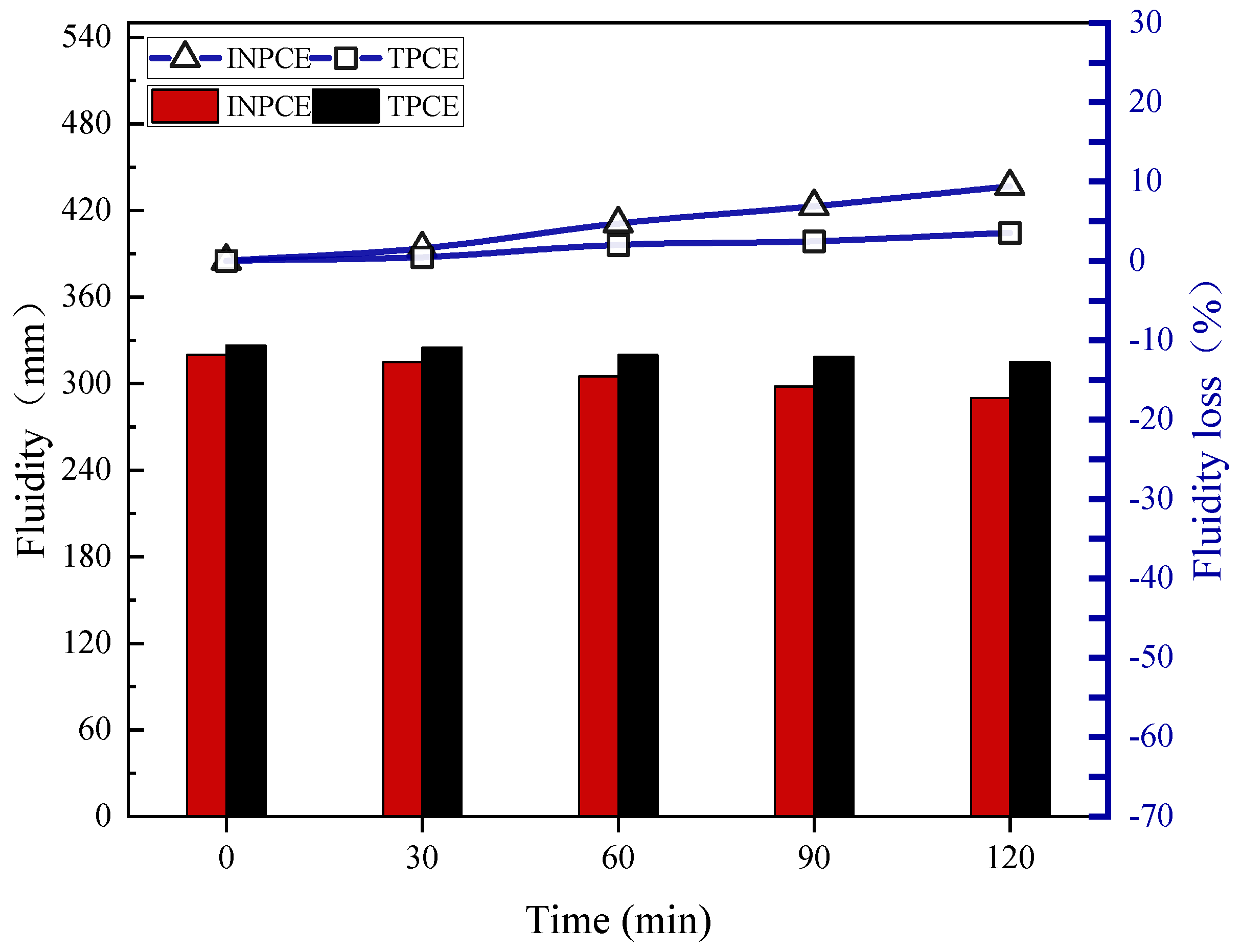
3.4. Effect of Different Synthesis Methods on the Performance of PCEs
3.4.1. Concentration
3.4.2. Fluidity Loss
3.4.3. Energy Consumption
3.5. Reaction Mechanism and Prospects
4. Conclusions
- Varying the acid–ether ratio as well as the concentrations of MAS and APS affects the molecular weight and PDI of INPCEs. These properties are also dependent on the polymerization method used. However, the functional groups are the same regardless of the polymerization method.
- The optimal process parameters were found to be a reaction temperature of 60 °C, a reaction rotating speed of 70 R/min, a reaction time of 60 min, nAA:nTPEG:nMAS = 3.0:1:0.12, and an APS concentration of 1 wt% relative to TPEG.
- The concentrations of all INPCEs were >99.00 wt%, which is much higher than that of TPCE. Further, INPCE showed similar excellent dispersion and dispersion retention properties as TPCE while requiring less energy to synthesize in the laboratory.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Javadi, A.; Jamil, T.; Abouzari-Lotf, E.; Soucek, M.D.; Heinz, H. Working mechanisms and design principles of comb-like polycarboxylate ether superplasticizers in cement hydration: Quantitative insights for a series of well-defined copolymers. ACS Sustain. Chem. Eng. 2021, 9, 8354–8371. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Z.; Zhu, J.; Zheng, Y.; Cui, S.; Lan, M.; Li, H. Synthesis, characterization and performance of a polycarboxylate superplasticizer with amide structure. Colloids Surf. A Physicochem. Eng. Asp. 2014, 448, 119–129. [Google Scholar] [CrossRef]
- Sha, S.; Wang, M.; Shi, C.; Xiao, Y. Influence of the structures of polycarboxylate superplasticizer on its performance in cement-based materials—A review. Constr. Build. Mater. 2020, 233, 117257. [Google Scholar] [CrossRef]
- Feng, H.; Feng, Z.; Mao, Y.; Deng, Z.; Zheng, B. Study on the polymerization process and monomer reactivity of epeg-type polycarboxylate superplasticizer. J. Appl. Polym. Sci. 2022, 139, e52697. [Google Scholar] [CrossRef]
- Xia, Y.; Shi, W.; Xiang, S.; Yang, X.; Yuan, M.; Zhou, H.; Yu, H.; Zheng, T.; Zhang, J.; Jiang, Z.; et al. Synthesis and modification of polycarboxylate superplasticizers—A review. Materials 2024, 17, 1092. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, X.; Jiang, M.; Lai, G.; Li, S.; Wang, Z.; Cui, S. Effect of competitive hydrolysis of diester in polycarboxylate superplasticizer on the fluidity of cement paste. Colloids Surf. A Physicochem. Eng. Asp. 2023, 671, 131691. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, Y.; Wang, J.; Gao, N.; Hu, Z.; Liu, J.; Wang, K. A novel shrinkage-reducing polycarboxylate superplasticizer for cement-based materials: Synthesis, performance and mechanisms. Constr. Build. Mater. 2022, 321, 126342. [Google Scholar] [CrossRef]
- Xiang, S.; Gao, Y.; Shi, C. Progresses in synthesis of polycarboxylate superplasticizer. Adv. Civ. Eng. 2020, 2020, 1–14. [Google Scholar] [CrossRef]
- Chang, Q.; Hu, M.; Cheng, Y.; Zeng, M.; Liu, M.; Pang, J.; Xing, Y.; Guo, J. Preparation of amphoteric polycarboxylate superplasticizer at low temperature and its application in cement-calcined kaolin blended system. J. Clean. Prod. 2024, 435, 140542. [Google Scholar] [CrossRef]
- Wang, H.; Mo, X.; Lin, J. Study on preparation and properties of compound functional polycarboxylic acid superplasticizer. Appl. Chem. Ind. 2024, 53, 511–515. [Google Scholar]
- Dong, Z.; Zhang, J.; Yu, H.; Wang, G.; Jiang, Y. Preparation of polycarboxylic acid superplasticizer with high slump retention by bulk polymerization. Shandong Chem. Ind. 2022, 51, 52–54, 57. [Google Scholar]
- Lin, Y.; Lai, H.Z.; Guo, Y.; Ma, X. Preparation of solid polycarboxylate superlasticizer. IOP Conf. Ser. Mater. Sci. Eng. 2019, 631, 022045. [Google Scholar] [CrossRef]
- Sun, Z.; Guo, E.; Wu, L.; Shui, L.; Xu, Y.; Li, M.; Li, X.; Li, D. Current status of research on synthesis of polycarboxylic acid water reducing agents by propriety polymerization method. China Concr. 2016, 12, 37–40. [Google Scholar]
- Liu, X.; Bai, X.; Xu, Q.; Lei, X.; Lai, G.; Guan, J. Synthesis and application performances of solid polycarboxylate superplasticizers using different initiators. Mater. Sci. Forum 2020, 993, 1367–1372. [Google Scholar] [CrossRef]
- Li, Z.; Sun, Z.; Ji, Y.; Yang, H.; Pang, M.; Ge, H.; Wu, S.; Gu, X.; Zhao, L.; He, Y.; et al. Hazards, mechanisms and influencing factors of mold and mildew in liquid admixtures in summer. Concrete 2021, 06, 80–83, 92. [Google Scholar]
- Liu, X.; Du, Z.; Wen, J.; Wang, S.; Ji, H.; Yang, T. Research on antiseptic of compound polycarboxylate superplasticizer. New Build. Mater. 2021, 48, 104–107, 129. [Google Scholar]
- Lin, Z. Study on the influence of mildew on the structure and composition of polycarboxylic acid superplasticizer. J. Phys. Conf. Ser. 2023, 2539, 012008. [Google Scholar] [CrossRef]
- Lai, H.; Chen, H.; Lin, Z. Study on mildew of polycarboxylic acid water reducing agent. J. Phys. Conf. Ser. 2022, 2174, 12046. [Google Scholar] [CrossRef]
- Yang, M.; Cai, G.; Zhang, J.; Liu, Y.; Lu, T.; Meng, F. Synthesis and properties of hyperbranched solid polycarboxylate superplasticizer. J. Phys. Conf. Ser. 2022, 2393, 12028. [Google Scholar] [CrossRef]
- Gao, R.; Liu, X.; Qian, S.; Zhang, B.; Wang, H.; Gao, C. Preparation methods of solid polycarboxylate superplasticizer: A review. China Powder Sci. Technol. 2023, 29, 61–70. [Google Scholar]
- Pang, L.; Sun, L.; Zhou, Z.; Chang, Q. Research and application of new superplasticized polycarboxylate powder. New Build. Mater. 2021, 48, 151–155. [Google Scholar]
- Wang, J.; Song, A.; Li, S.; Chen, X.; Li, J. Properties of solid polycarboxylate superplasticizer prepared by precipitation method. Bull. Chin. Ceram. Soc. 2018, 37, 1856–1860, 1867. [Google Scholar]
- Li, X. Research status of solid polycarboxylate superplasticizer. China Build. Mater. Sci. Technol. 2019, 28, 51–52, 54. [Google Scholar]
- Zu, X. Experimental Study on the Preparation of Solid Polycarboxylate Superplasticizer by Composite Adsorbent; Shenyang Jianzhu University: Shenyang, China, 2021. [Google Scholar]
- Sun, W.; Pan, L.; Li, J.; Xu, N.; Guo, Z. Enhancing the application of mechanochemistry in the synthesis of high-concentration polycarboxylate superplasticizer: Is aqueous copolymerization needed? J. Dispers. Sci. Technol. 2023, 44, 660–668. [Google Scholar] [CrossRef]
- Zhang, L.; Du, W.; Wang, D.; Wang, F.; Fang, K.; Yu, J.; Sheng, B. Syntheses of polycarboxylate superplasticizers: Microwave induction versus conventional thermal induction. Compos. Part B Eng. 2021, 207, 108560. [Google Scholar] [CrossRef]
- Li, W.; Wang, C.; Bian, H.; Chang, T.; Zhu, L.; Zhang, L. Study on compound properties and energy consumption of tandem mixing and conventional mixing. China Rubber Ind. 2020, 67, 384–387. [Google Scholar]
- Wang, W.; Wu, B.; Hu, J. Analysis of the impact of refiners on energy consumption. Rubber Plast. Technol. Equip. 2024, 50, 40–42. [Google Scholar]
- Wang, C.; Xiao, X.; Xu, M.; Wang, L.; Bian, H.; Wang, Z. Characterization and properties of polycarboxylate. Influence of final-stage mixing temperature of mixer on properties of silica-filled compounds. China Rubber Ind. 2023, 70, 220–224. [Google Scholar]
- Wang, L.; Tian, P. The main contents of the revision of the national standard GB 8076-2008 Concrete Admixtures. Concr. World 2010, 10, 66–70. [Google Scholar]
- Sun, W.; Pan, L.; Guo, Z.; Li, J.; Xu, N.; Pang, S. Characterization and properties of polycarboxylate superplasticizer by mechanochemistry. Concrete 2019, 12, 88–91. [Google Scholar]
- GB/T 8077-2012; Methods for Testing Uniformity of Concrete Admixtures. Standardization Administration of China: Beijing, China, 2012; pp. 19–20.
- Flower, D.J.M.; Sanjayan, J.G. Green house gas emissions due to concrete manufacture. Concr. Manuf. 2007, 5, 282–288. [Google Scholar] [CrossRef]
- Schiefer, C.; Plank, J. CO2 emission of polycarboxylate superplasticizers (PCEs) used in concrete. J. Clean. Prod. 2023, 427, 138785. [Google Scholar] [CrossRef]
- Lucas, E.; Martín, A.J.; Mitchell, S.; Nabera, A.; Santos, L.F.; Pérez-Ramírez, J.; Guillén-Gosálbez, G. The need to integrate mass- and energy-based metrics with life cycle impacts for sustainable chemicals manufacture. Green Chem. 2024, 26, 9300–9309. [Google Scholar] [CrossRef]
- Wang, C.; Kong, F.; Wu, J.; Pan, L. Adjusting concrete resistance to corrosive ions by varying carboxyl contents in chemical additives. Structures 2024, 62, 106168. [Google Scholar] [CrossRef]
- Tammer, M.G. Sokrates: Infrared and Raman characteristic group frequencies: Tables and charts. Colloid Polym. Sci. 2004, 283. [Google Scholar] [CrossRef]
- Plank, J.; Li, H.; Ilg, M.; Pickelmann, J.; Eisenreich, W.; Yao, Y.; Wang, Z. A microstructural analysis of isoprenol ether-based polycarboxylates and the impact of structural motifs on the dispersing effectiveness. Cem. Concr. Res. 2016, 84, 20–29. [Google Scholar] [CrossRef]
- Pan, Z. Polymer Chemistry, 5th ed.; Chemical Industry Press: Beijing, China, 2013; p. 266. [Google Scholar]
- Liu, Q.; Shao, Q.; Song, P.; Pang, L.; Chang, Q.; Fu, P.; Wang, M.; Lang, H. Preparation of early-strength powder polycarboxylic acid superplasticizer by bulk polymerization. New Build. Mater. 2019, 46, 1–4, 12. [Google Scholar]
- Lin, Z.; Zhang, X.; Chen, Z.; Xiao, Y.; Fang, Y. Study on adsorption, rheology and hydration behaviours of polycarboxylate (PCE) superplasticizer synthesized by different acid to ether ratio. J. Phys. Conf. Ser. 2021, 2133, 12007. [Google Scholar] [CrossRef]
- Feng, H.; Feng, Z.; Wang, W.; Deng, Z.; Zheng, B. Impact of polycarboxylate superplasticizers (PCEs) with novel molecular structures on fluidity, rheological behavior and adsorption properties of cement mortar. Constr. Build. Mater. 2021, 292, 123285. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, K.; Deng, L.; Shen, J. Review on development of polycarboxylate superplasticizer. China Build. Mater. Sci. Technol. 2024, 33, 94–98. [Google Scholar] [CrossRef]
- Li, X.; Zheng, G.J.; Bi, Y.; Fu, C.F.; Yuan, M.H.; Wang, S.F. Synthesis and characterization on a kind of polycarboxylate superplasticizer. Adv. Mater. Res. 2014, 989–994, 228–232. [Google Scholar] [CrossRef]
- Shi, H.; Wang, H. Preparation of high slump-preserving polycarboxylate superplasticizers of its influence on concrete properties. J. Funct. Mater. 2022, 53, 8196–8201. [Google Scholar]



| Chemical Composition | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Composition | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | Na2Oeq | f−CaO | Loss | Cl− |
| Cement (wt%) | 20.94 | 4.31 | 3.28 | 63.46 | 2.76 | 2.23 | 0.56 | 0.80 | 2.31 | 0.036 |
| Levels | Factors | |||
|---|---|---|---|---|
| A-Reaction Temperature (°C) | B-Reaction Rotating Speed (R/min) | C-Reaction Time (min) | D-Blank Column * | |
| 1 | 60 | 65 | 60 | 1 |
| 2 | 65 | 70 | 90 | 2 |
| 3 | 70 | 75 | 120 | 3 |
| Samples | nAA (mol) | nTPEG (mol) | nMAS (mol) | mAPS (g) | VH2O (mL) | Reaction Temperature T (°C) | Reaction Rotating Speed (R/min) | Reaction Time (min) | |
|---|---|---|---|---|---|---|---|---|---|
| INPCE-A | INPCE-A1 | 0.1750 | 0.0875 | 0.0070 | 2.10 | 1.5 | 60 | 70 | 60 |
| INPCE-A2 | 0.2188 | 0.0875 | 0.0070 | 2.10 | 1.5 | 60 | 70 | 60 | |
| INPCE-A3 | 0.2625 | 0.0875 | 0.0070 | 2.10 | 1.5 | 60 | 70 | 60 | |
| INPCE-A4 | 0.3063 | 0.0875 | 0.0070 | 2.10 | 1.5 | 60 | 70 | 60 | |
| INPCE-A5 | 0.3500 | 0.0875 | 0.0070 | 2.10 | 1.5 | 60 | 70 | 60 | |
| INPCE-B | INPCE-B1 | 0.2625 | 0.0875 | 0.0070 | 0.84 | 1.5 | 60 | 70 | 60 |
| INPCE-B2 | 0.2625 | 0.0875 | 0.0070 | 1.26 | 1.5 | 60 | 70 | 60 | |
| INPCE-B3 | 0.2625 | 0.0875 | 0.0070 | 1.68 | 1.5 | 60 | 70 | 60 | |
| INPCE-B4 | 0.2625 | 0.0875 | 0.0070 | 2.10 | 1.5 | 60 | 70 | 60 | |
| INPCE-B5 | 0.2625 | 0.0875 | 0.0070 | 2.52 | 1.5 | 60 | 70 | 60 | |
| INPCE-C | INPCE-C1 | 0.2625 | 0.0875 | 0.0035 | 2.10 | 1.5 | 60 | 70 | 60 |
| INPCE-C2 | 0.2625 | 0.0875 | 0.0070 | 2.10 | 1.5 | 60 | 70 | 60 | |
| INPCE-C3 | 0.2625 | 0.0875 | 0.0105 | 2.10 | 1.5 | 60 | 70 | 60 | |
| INPCE-C4 | 0.2625 | 0.0875 | 0.0140 | 2.10 | 1.5 | 60 | 70 | 60 | |
| INPCE-C5 | 0.2625 | 0.0875 | 0.0175 | 2.10 | 1.5 | 60 | 70 | 60 | |
| Samples | nAA (mol) | nTPEG (mol) | nMAS (mol) | mAPS (g) | VH2O (mL) | Reaction Parameters |
|---|---|---|---|---|---|---|
| INPCE | 0.2625 | 0.0875 | 0.0105 | 2.10 | 1.5 | The reaction temperature was 60 °C, the reaction rotating speed was 70 R/min, and the reaction time was 60 min. |
| TPCE | 0.2625 | 0.0875 | 0.0105 | 2.10 | 357.0 | The reaction temperature was 60−75 °C, the stirring speed was 1600 R/min, and the reaction time was 255 min. |
| Samples | Instrument Number | Instruments Names | Power Rating (kW) | Usage Time (h) | |
|---|---|---|---|---|---|
| INPCE | 1 | XSS-300 torque rheometer from Shanghai Kechuang Rubber & Plastic Machinery Equipment Co., Ltd. with a 300mL mixing chamber (Shanghai, China). | 4.00 | 1.25 | |
| TPCE | 1 | DF-101s Collector Type Constant Temperature Heating Magnetic Stirrer from Gongyi Yuhua Instrument Manufacturing Co., Ltd. (Gongyi, China). | Motor power | 0.03 | 0.33 |
| 2 | Heating power | 0.50 | 4.83 | ||
| 3 | DX-204 Low-Temperature Circulator from Beijing Changliu Scientific Instrument Co., Ltd. (Beijing, China). | 0.30 | 4.83 | ||
| 4 | BT100-1L Peristaltic Pump Drive from Baoding Langer Constant Flow Pump Co., Ltd. (Baoding, China). | 0.05 | 2.25 | ||
| 5 | OPD-8 Spray Dryer of Shanghai Dachuan Yuan Drying Equipment Co., Ltd. * (Shanghai, China). | 9.80 | 0.20 | ||
| Experimental Number | A (Reaction Temperature) | B (Reaction Rotating Speed) | C (Reaction Time) | D (Blank Column) | Fluidity (mm) |
|---|---|---|---|---|---|
| 1 | 1 | 1 | 1 | 1 | 237.5 |
| 2 | 1 | 2 | 2 | 2 | 237.5 |
| 3 | 1 | 3 | 3 | 3 | 232.5 |
| 4 | 2 | 1 | 2 | 3 | 210.0 |
| 5 | 2 | 2 | 3 | 1 | 212.0 |
| 6 | 2 | 3 | 1 | 2 | 230.0 |
| 7 | 3 | 1 | 3 | 2 | 228.0 |
| 8 | 3 | 2 | 1 | 3 | 247.0 |
| 9 | 3 | 3 | 2 | 1 | 208.0 |
| k1 | 235.8 | 225.1 | 238.2 | 219.2 | |
| k2 | 217.3 | 232.1 | 218.5 | 231.8 | |
| k3 | 227.7 | 223.5 | 224.2 | 229.8 | |
| R | 18.50 | 8.667 | 19.67 | 12.67 | |
| Primary and secondary order | C > A > B | ||||
| Excellent level | A1 | B2 | C1 | ||
| Excellent combination | A1 B2 C1 | ||||
| Samples | Mn (g·mol−1) | Mw (g·mol−1) | PDI |
|---|---|---|---|
| INPCE | 45,179 | 77,074 | 1.71 |
| TPCE | 46,513 | 99,322 | 2.14 |
| Samples | Mn (g·mol−1) | Mw (g·mol−1) | PDI | |
|---|---|---|---|---|
| INPCE-A | INPCE-A1 | 40,074 | 62,257 | 1.55 |
| INPCE-A2 | 41,845 | 68,699 | 1.64 | |
| INPCE-A3 | 54,188 | 102,996 | 1.90 | |
| INPCE-A4 | 58,314 | 129,939 | 2.23 | |
| INPCE-A5 | 59,575 | 139,677 | 2.34 | |
| INPCE-B | INPCE-B1 | 55,210 | 99,104 | 1.80 |
| INPCE-B2 | 55,925 | 105,609 | 1.89 | |
| INPCE-B3 | 56,015 | 108,856 | 1.94 | |
| INPCE-B4 | 54,188 | 102,996 | 1.90 | |
| INPCE-B5 | 57,681 | 116,409 | 2.02 | |
| INPCE-C | INPCE-C1 | 65,174 | 116,062 | 1.78 |
| INPCE-C2 | 54,188 | 102,996 | 1.90 | |
| INPCE-C3 | 45,179 | 77,074 | 1.71 | |
| INPCE-C4 | 37,741 | 62,701 | 1.66 | |
| INPCE-C5 | 36,151 | 56,187 | 1.55 | |
| Samples | INPCE-A | INPCE-B | INPCE-C | TPEG | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A1 | A2 | A3 | A4 | A5 | B1 | B2 | B3 | B4 | B5 | C1 | C2 | C3 | C4 | C5 | ||
| concentration (wt%) | 99.34 | 99.29 | 99.03 | 99.19 | 99.15 | 99.20 | 99.16 | 99.33 | 99.03 | 99.05 | 99.24 | 99.03 | 99.08 | 99.00 | 99.06 | 39.73 |
| INPCE | TPCE | |||
|---|---|---|---|---|
| Energy (kW·h) | Ea,INPCE | E0,INPCE | Ea,TPCE | E0,TPCE |
| 5.00 | ≈E0,TPCE | 5.95 | ≈E0,INPCE | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.; Li, X.; Pan, L.; Lin, C. Developing a Novel, Green, and Efficient Synthesis Method for Polycarboxylate Superplasticizers Through Mechanochemical Internal Mixing Polymerization. Polymers 2025, 17, 1017. https://doi.org/10.3390/polym17081017
Chen Q, Li X, Pan L, Lin C. Developing a Novel, Green, and Efficient Synthesis Method for Polycarboxylate Superplasticizers Through Mechanochemical Internal Mixing Polymerization. Polymers. 2025; 17(8):1017. https://doi.org/10.3390/polym17081017
Chicago/Turabian StyleChen, Qianqian, Xiaomiao Li, Lisha Pan, and Chang Lin. 2025. "Developing a Novel, Green, and Efficient Synthesis Method for Polycarboxylate Superplasticizers Through Mechanochemical Internal Mixing Polymerization" Polymers 17, no. 8: 1017. https://doi.org/10.3390/polym17081017
APA StyleChen, Q., Li, X., Pan, L., & Lin, C. (2025). Developing a Novel, Green, and Efficient Synthesis Method for Polycarboxylate Superplasticizers Through Mechanochemical Internal Mixing Polymerization. Polymers, 17(8), 1017. https://doi.org/10.3390/polym17081017






