Synergistic Incorporation of Boron Nitride Nanosheets and Fluoropolymers to Amplify Anti-Corrosion Attributes of Waterborne Epoxy Resin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Exfoliation of h-BN
2.3. Fabrication of Fluorinated Nanosilica (FSiO2) and Fluorinated Tannic Acid (FTA)
2.4. Preparation of BNNS/WEP, BFS/WEP and BFT/WEP Composite Coatings
3. Characterization
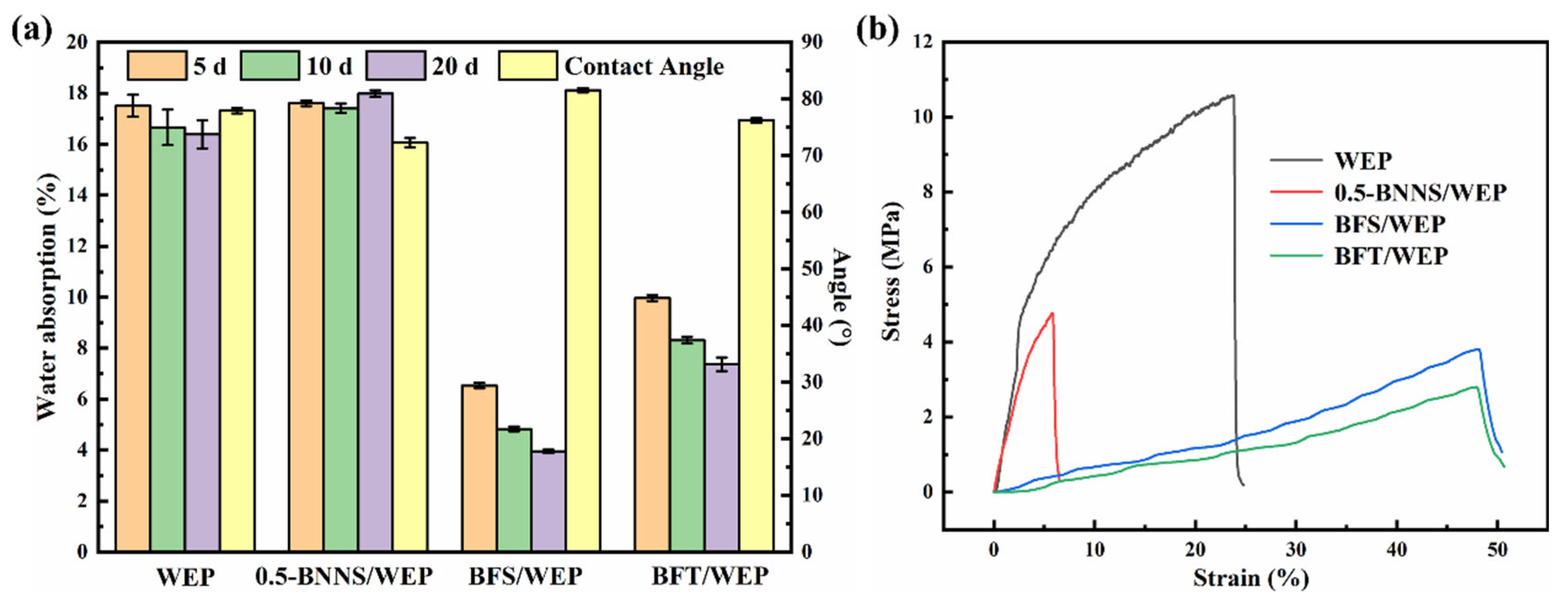
3.1. Evaluation of the Water Absorption Performance of the Composite Coating
3.2. Mechanical Property Testing of the Composite Coating
3.3. Evaluate the Corrosion Resistance Characteristics of the Composite Coating
4. Results and Discussions
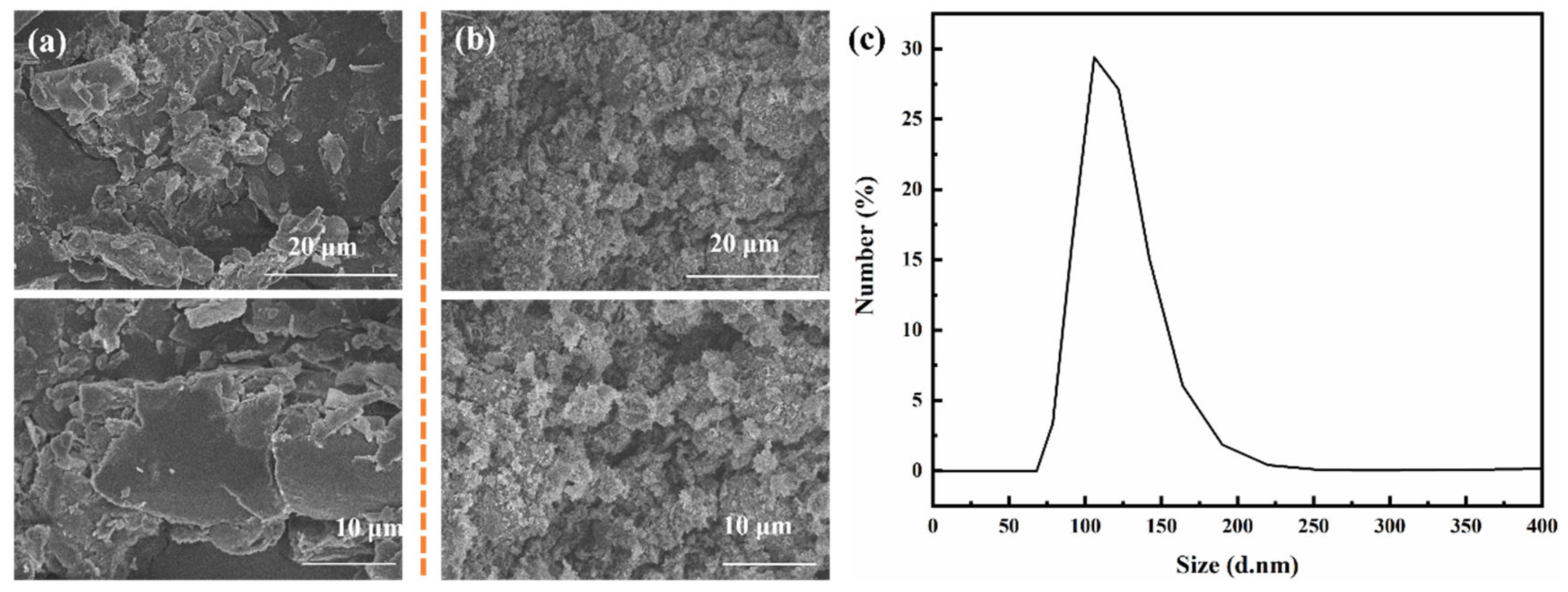
4.1. Morphology of h-BN and BNNS and Particle Size Analysis of BNNS
4.2. FT-IR Spectra and XRD Analysis of h-BN Before and After Exfoliation
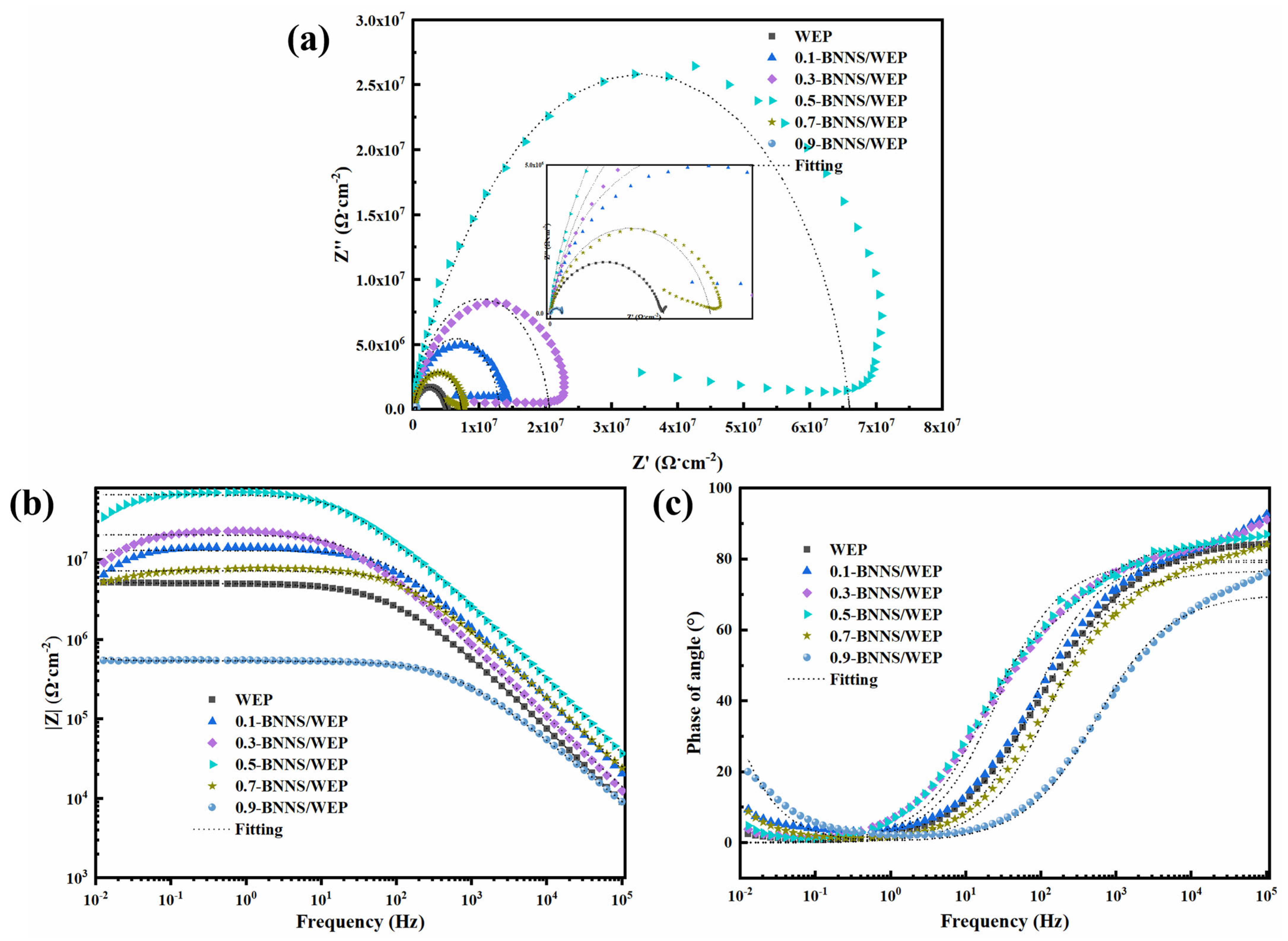
4.3. Electrochemical Performance of Pristine WEP Coatings Versus BNNS/WEP Composite Coatings
4.4. Cross-Section Morphology of Pristine WEP, 0.5-BNNS/WEP, BFS/WEP and BFT/WEP Composite Coatings
4.5. Hydrophobic and Mechanical Properties of Pristine WEP, 0.5-BNNS/WEP, BFS/WEP and BFT/WEP Composite Coatings
4.6. Electrochemical Performance of Pristine WEP Coatings, 0.1-FSiO2/WEP, 4.9-FTA/WEP, 0.5-BNNS/WEP, BFS/WEP, and BFT/WEP Composite Coatings
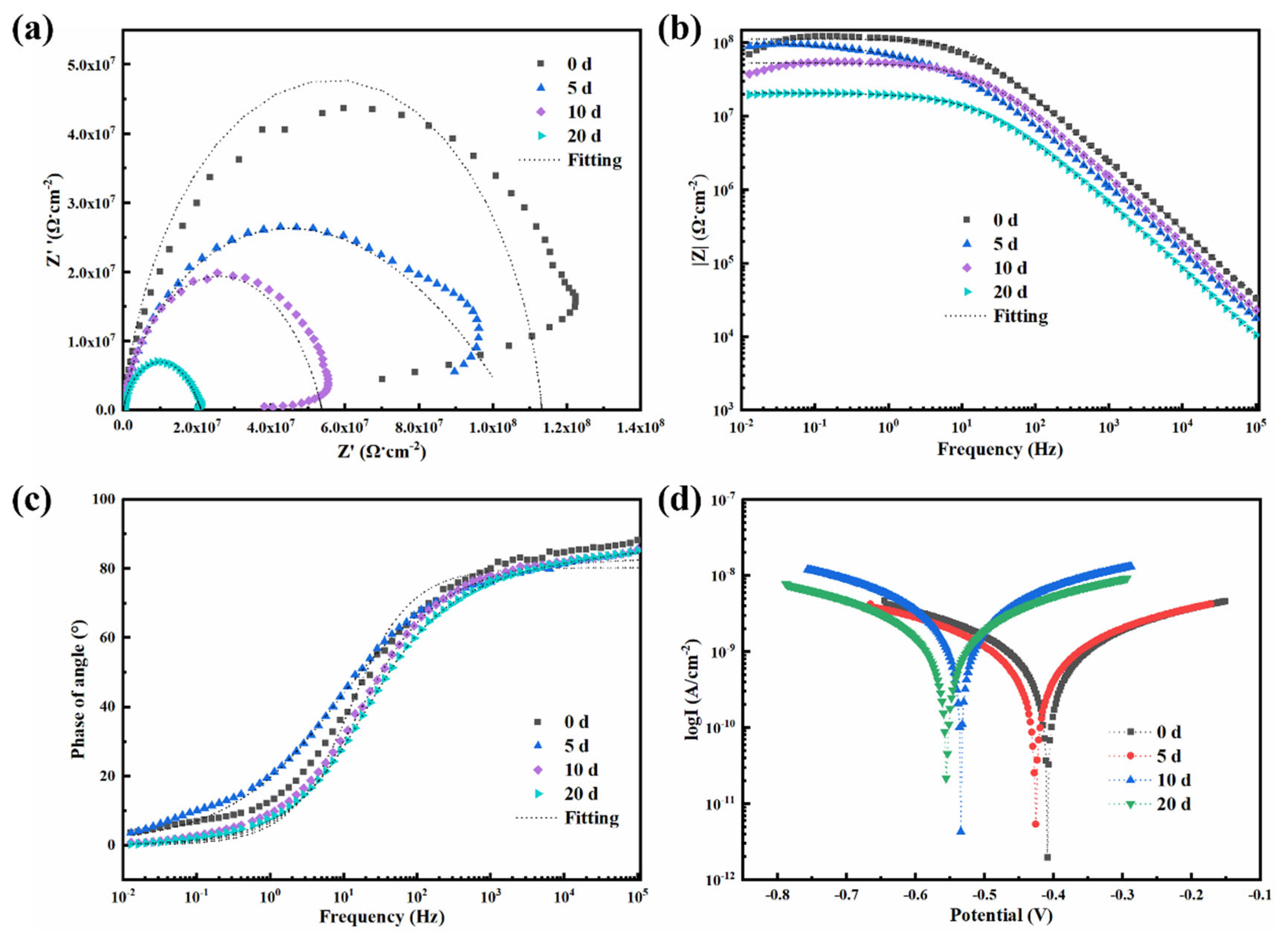
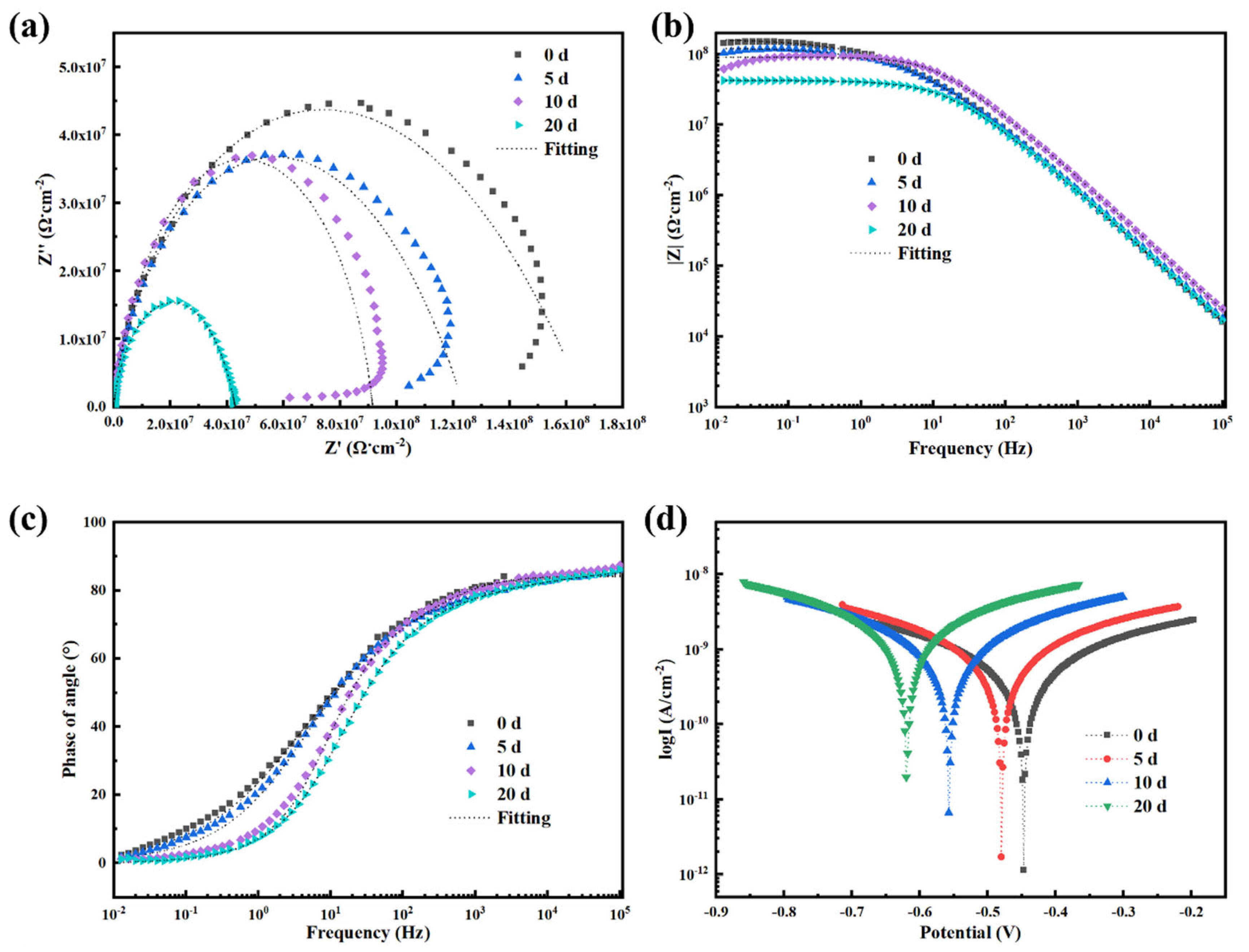
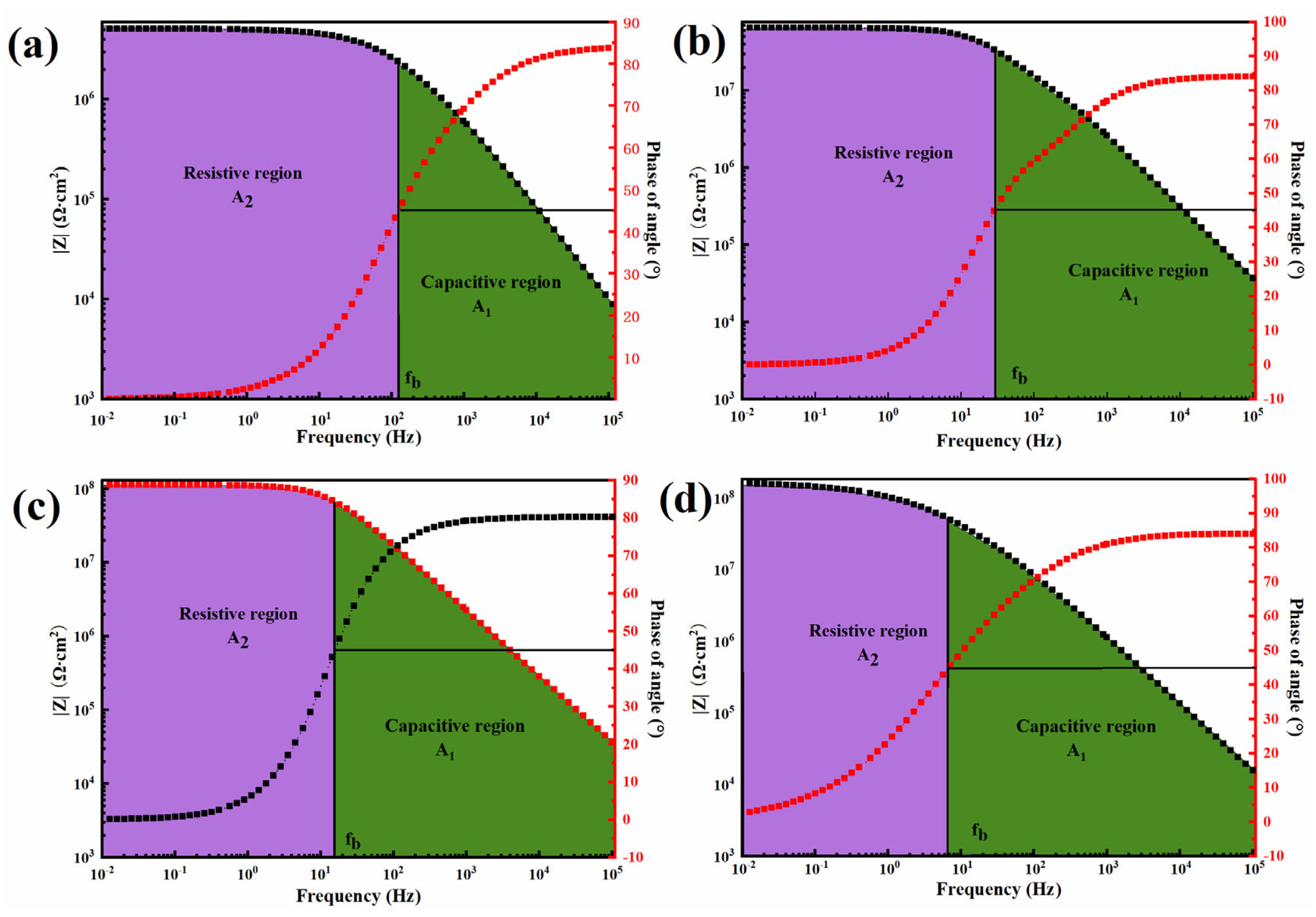
4.7. Electrochemical Performance of 0.5-BNNS/WEP, BFS/WEP, and BFT/WEP Composite Coatings After Salt Spraying
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ren, S.; Cui, M.; Chen, X.; Mei, S.; Qiang, Y. Comparative study on corrosion inhibition of N doped and N,S codoped carbon dots for carbon steel in strong acidic solution. J. Colloid Interface Sci. 2022, 628, 384–397. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Xu, F.; Lin, D.; Peng, J.; Zhu, Y.; Wang, H. A smart anti-corrosion coating based on triple functional fillers. Chem. Eng. J. 2022, 446, 137078. [Google Scholar] [CrossRef]
- Ren, G.; Zheng, W.; Qiao, Z.; Tuo, Y.; Chen, X.; Zhao, Q.; Bi, Y.; Du, F.; Gao, X.; Li, S. Thermally conductive superhydrophobic composite coatings with anti-corrosion property. Prog. Org. Coat. 2025, 198, 108913. [Google Scholar] [CrossRef]
- Wan, S.; Chen, H.; Liao, B.; Guo, X. Enhanced anti-corrosive capability of waterborne epoxy coating by ATT exfoliated boron nitride nanosheets composite fillers. Prog. Org. Coat. 2024, 186, 108089. [Google Scholar] [CrossRef]
- Qiang, Y.; Zhang, S.; Wang, L. Understanding the adsorption and anticorrosive mechanism of DNA inhibitor for copper in sulfuric acid. Appl. Surf. Sci. 2019, 492, 228–238. [Google Scholar] [CrossRef]
- Bai, W.; Ma, Y.; Meng, M.; Li, Y. The influence of graphene on the cathodic protection performance of zinc-rich epoxy coatings. Prog. Org. Coat. 2021, 161, 106456. [Google Scholar] [CrossRef]
- Faccini, M.; Bautista, L.; Soldi, L.; Escobar, A.M.; Altavilla, M.; Calvet, M.; Domènech, A.; Domínguez, E. Environmentally Friendly Anticorrosive Polymeric Coatings. Appl. Sci. 2021, 11, 3446. [Google Scholar] [CrossRef]
- Sun, J.; Tang, Z.; Meng, G.; Gu, L. Silane functionalized plasma-treated boron nitride nanosheets for anticorrosive reinforcement of waterborne epoxy coatings. Prog. Org. Coat. 2022, 167, 106831. [Google Scholar] [CrossRef]
- An, H.; Jiang, C.; Yin, X.; Liu, K.; Liang, S.; Wang, X.; Xiao, J.; Zhao, X.; Sun, Z. Polyaniline/TiO2/MXene Ternary Composites for Enhancing Corrosion Resistance of Waterborne Epoxy Coatings. ACS Appl. Nano Mater. 2025, 8, 340–350. [Google Scholar] [CrossRef]
- Rahman, F.A.; Basirun, W.J.; Johan, M.R. Synergistic Effect of Sn Doping in TiO2 Nanoparticles as an Additive in Anti-Corrosion Coatings. Mater. Corros. 2025, 76, 295–304. [Google Scholar] [CrossRef]
- Xu, L.; Yang, X.; Fu, X.; Wang, L.; Fan, Y.; Xu, J.; Yin, Y.; Tian, L.; Zhao, J. Fluorinated epoxy based superhydrophobic coating with robust self-healing and anticorrosive performances. Prog. Org. Coat. 2022, 171, 107045. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, G.; Fan, X.; Wang, X.; Hou, B. Intermediate-layer strengthened superhydrophobic coating with stable corrosion resistance, delayed icing and long-term weatherability. J. Ind. Eng. Chem. 2023, 127, 331–342. [Google Scholar] [CrossRef]
- Mohammadpour, Z.; Zare, H.R. Fabrication of a pH-Sensitive Epoxy Nanocomposite Coating Based on a Zn-BTC Metal–Organic Framework Containing Benzotriazole as a Smart Corrosion Inhibitor. Cryst. Growth Des. 2021, 21, 3954–3966. [Google Scholar] [CrossRef]
- Li, W.; Liu, H. Rational design and facile preparation of hybrid superhydrophobic epoxy coatings modified by fluorinated silsesquioxane-based giant molecules via photo-initiated thiol-ene click reaction with potential applications. Chem. Eng. J. 2024, 480, 147943. [Google Scholar] [CrossRef]
- Qiang, Y.; Ran, B.; Li, M.; Xu, Q.; Peng, J. GO-functionalized MXene towards superior anti-corrosion coating. J. Colloid Interface Sci. 2023, 642, 595–603. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Liu, H.; Liu, X.; He, R.; Meng, S. Structure, morphology and anti-corrosion performance of polyaniline modified molybdenum sulfide/epoxy composite coating. Colloids Surf. A Physicochem. Eng. Asp. 2022, 639, 128345. [Google Scholar] [CrossRef]
- Shi, N.; Li, Z.; Li, X.; Luo, H.; Dong, S.; Li, H. Bionic modified h-BN loaded CeO2 nanoparticles to improve the anti-corrosion properties of composited epoxy coatings. Colloids Surf. A Physicochem. Eng. Asp. 2024, 694, 134111. [Google Scholar] [CrossRef]
- Shi, N.; Li, Z.; Li, X.; Luo, H.; Jin, J.; Dong, S.; Li, H. H-BN base triple-functional filler enhances the anti-corrosion performance of epoxy coating. Polymer 2024, 300, 126975. [Google Scholar] [CrossRef]
- Wan, S.; Chen, H.; Cai, G.; Liao, B.; Guo, X. Functionalization of h-BN by the exfoliation and modification of carbon dots for enhancing corrosion resistance of waterborne epoxy coating. Prog. Org. Coat. 2022, 165, 106757. [Google Scholar] [CrossRef]
- Wan, S.; Chen, H.; Ma, X.; Chen, L.; Lei, K.; Liao, B.; Dong, Z.; Guo, X. Anticorrosive reinforcement of waterborne epoxy coating on Q235 steel using NZ/BNNS nanocomposites. Prog. Org. Coat. 2021, 159, 106410. [Google Scholar] [CrossRef]
- Qiu, S.; Yu, Y.; Chang, Y.-H.; Shan, L.; Xu, S. Nonconductive layered hexagonal boron nitride preparation via a co-exfoliation strategy and nanohybrid coating construction for corrosion protection. Prog. Org. Coat. 2024, 186, 108052. [Google Scholar] [CrossRef]
- Cui, M.; Ren, S.; Qin, S.; Xue, Q.; Zhao, H.; Wang, L. Non-covalent functionalized hexagonal boron nitride nanoplatelets to improve corrosion and wear resistance of epoxy coatings. RSC Adv. 2017, 7, 44043–44053. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, Y.; Chen, J.; Yan, J.; Huang, J.; Zhang, Q.; Zhao, G.; Liu, Y. Wear and corrosion resistance of fluorocarbon/epoxy blended coatings nanofilled by mechanically peeled few-layer fluorinated graphene. J. Mater. Res. Technol. 2022, 20, 2369–2384. [Google Scholar] [CrossRef]
- Li, C.; Li, Y.; Wang, X.; Yuan, S.; Lin, D.; Zhu, Y.; Wang, H. Synthesis of hydrophobic fluoro-substituted polyaniline filler for the long-term anti-corrosion performance enhancement of epoxy coatings. Corros. Sci. 2021, 178, 109094. [Google Scholar] [CrossRef]
- Zhang, B.; Zhu, S.; Cao, G.; Wang, H.; Di, Y.; Tian, Y.; Shi, K.; Song, N.; Yao, H.; Guan, S. Fabrication of low-temperature curing PES coatings with excellent anti-corrosion properties by introducing the crosslinking agent. Corros. Sci. 2019, 157, 481–486. [Google Scholar] [CrossRef]
- Shabalina, A.V.; Kozlov, V.A.; Popov, I.A.; Gudkov, S.V. A Review on Recently Developed Antibacterial Composites of Inorganic Nanoparticles and Non-Hydrogel Polymers for Biomedical Applications. Nanomaterials 2024, 14, 1753. [Google Scholar] [CrossRef]
- Liu, X.; Han, X.; Wang, M.; Wang, D.; Fei, G.; Chen, A.; Lai, X. Improvement of the anti-corrosion coating properties of waterborne epoxy resin with the incorporation of fluoropolymer-modified silica nanofillers. Prog. Org. Coat. 2024, 191, 108407. [Google Scholar] [CrossRef]
- Zhao, Y.; Jiang, F.; Chen, Y.-Q.; Hu, J.-M. Coatings embedded with GO/MOFs nanocontainers having both active and passive protecting properties. Corros. Sci. 2020, 168, 108563. [Google Scholar] [CrossRef]
- GB/T 10125-2021; Corrosion Tests in Artificial Atmospheres—Salt Spray Tests. State Administration for Market Regulation; Standardization Administration of PRC: Beijing, China, 2021.
- Huang, H.; Huang, X.; Xie, Y.; Tian, Y.; Jiang, X.; Zhang, X. Fabrication of h-BN-rGO@PDA nanohybrids for composite coatings with enhanced anticorrosion performance. Prog. Org. Coat. 2019, 130, 124–131. [Google Scholar] [CrossRef]
- Zou, Q.; Xiong, S.-w.; Jiang, M.-y.; Chen, L.-y.; Zheng, K.; Fu, P.-g.; Gai, J.-g. Highly thermally conductive and eco-friendly OH-h-BN/chitosan nanocomposites by constructing a honeycomb thermal network. Carbohydr. Polym. 2021, 266, 118127. [Google Scholar] [CrossRef]
- Xie, B.-H.; Huang, X.; Zhang, G.-J. High thermal conductive polyvinyl alcohol composites with hexagonal boron nitride microplatelets as fillers. Compos. Sci. Technol. 2013, 85, 98–103. [Google Scholar] [CrossRef]
- Wu, J.; Wang, H.; Li, R.; Guo, L. Anticorrosive waterborne polyurethane coating with Gemini surfactant-assisted exfoliation of hexagonal boron nitride nanosheets. J. Appl. Polym. Sci. 2023, 140, e54008. [Google Scholar] [CrossRef]
- Abdi, S.; Dorranian, D. Effect of CTAB concentration on the properties of ZnO nanoparticles produced by laser ablation method in CTAB solution. Opt. Laser Technol. 2018, 108, 372–377. [Google Scholar] [CrossRef]
- Wu, Y.; Yu, J.; Zhao, W.; Wang, C.; Wu, B.; Lu, G. Investigating the anti-corrosion behaviors of the waterborne epoxy composite coatings with barrier and inhibition roles on mild steel. Prog. Org. Coat. 2019, 133, 8–18. [Google Scholar] [CrossRef]
- Cui, M.; Ren, S.; Chen, J.; Liu, S.; Zhang, G.; Zhao, H.; Wang, L.; Xue, Q. Anticorrosive performance of waterborne epoxy coatings containing water-dispersible hexagonal boron nitride (h-BN) nanosheets. Appl. Surf. Sci. 2017, 397, 77–86. [Google Scholar] [CrossRef]
- Wu, N.; Yang, W.; Che, S.; Sun, L.; Li, H.; Ma, G.; Sun, Y.; Liu, H.; Wang, X.; Li, Y. Green preparation of high-yield and large-size hydrophilic boron nitride nanosheets by tannic acid-assisted aqueous ball milling for thermal management. Compos. Part A Appl. Sci. Manuf. 2023, 164, 107266. [Google Scholar] [CrossRef]
- Meng, L.; Li, Z.; Wu, W.; Li, B.; Niu, Z.; Wang, P.; Zhang, J.; Tang, C.; Xue, Y. Highly Anti-Corrosive Performance of Poly(Vinylidene Fluoride-Hexafluoropropylene)/Boron Nitride Nanosheet/Polyimide Composite Coating. Macromol. Chem. Phys. 2024, 225, 2400233. [Google Scholar] [CrossRef]
- Ammar, S.; Ramesh, K.; Ma, I.A.W.; Farah, Z.; Vengadaesvaran, B.; Ramesh, S.; Arof, A.K. Studies on SiO2-hybrid polymeric nanocomposite coatings with superior corrosion protection and hydrophobicity. Surf. Coat. Technol. 2017, 324, 536–545. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Niroumandrad, S.; Ahmadi, A.; Mahdavian, M.; Moghadam, M.H.M. Enhancement of barrier and corrosion protection performance of an epoxy coating through wet transfer of amino functionalized graphene oxide. Corros. Sci. 2016, 103, 283–304. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, H.; Liu, X.; Han, X.; Yang, R.; Liu, Z.; Lv, J. Synergistic Incorporation of Boron Nitride Nanosheets and Fluoropolymers to Amplify Anti-Corrosion Attributes of Waterborne Epoxy Resin. Polymers 2025, 17, 1020. https://doi.org/10.3390/polym17081020
Ma H, Liu X, Han X, Yang R, Liu Z, Lv J. Synergistic Incorporation of Boron Nitride Nanosheets and Fluoropolymers to Amplify Anti-Corrosion Attributes of Waterborne Epoxy Resin. Polymers. 2025; 17(8):1020. https://doi.org/10.3390/polym17081020
Chicago/Turabian StyleMa, Hui, Xuan Liu, Xiaofeng Han, Rui Yang, Zhaotie Liu, and Jian Lv. 2025. "Synergistic Incorporation of Boron Nitride Nanosheets and Fluoropolymers to Amplify Anti-Corrosion Attributes of Waterborne Epoxy Resin" Polymers 17, no. 8: 1020. https://doi.org/10.3390/polym17081020
APA StyleMa, H., Liu, X., Han, X., Yang, R., Liu, Z., & Lv, J. (2025). Synergistic Incorporation of Boron Nitride Nanosheets and Fluoropolymers to Amplify Anti-Corrosion Attributes of Waterborne Epoxy Resin. Polymers, 17(8), 1020. https://doi.org/10.3390/polym17081020





