Enhanced Vitamin D3 Adsorption Through Novel Hydrophobic Halloysite–Alginate Biopolymer Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Hexadecyl Alginate Ester
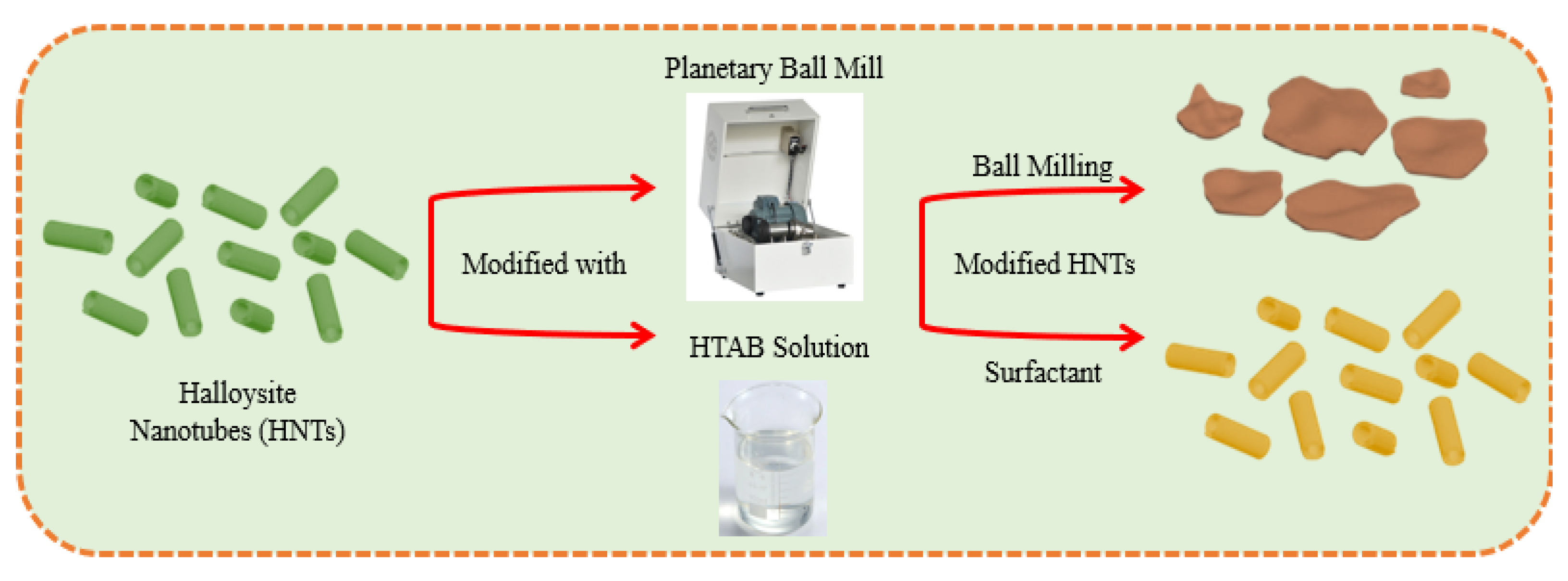
2.3. Mechanical Activation of HNT Nanotubes via Ball Milling
2.4. Surfactant Treatment of HNT Nanotubes
2.5. Hydrophobic Halloysite–Alginate Biocomposites
2.6. Adsorption Experiments
2.7. Statistical Evaluation of Adsorption Isotherm Models
2.8. Desorption Experiments
2.9. Characterization Studies
3. Results and Discussion
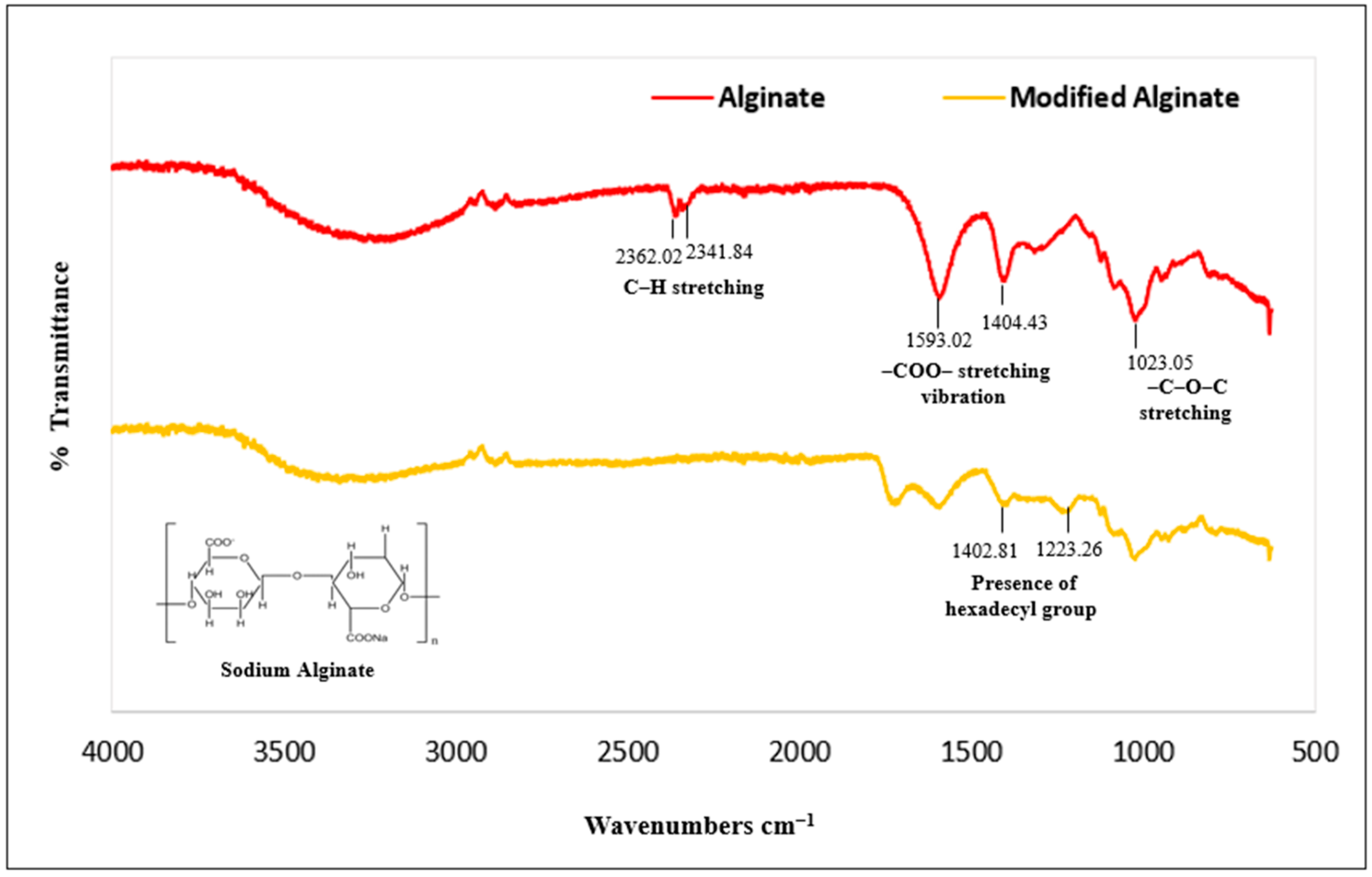
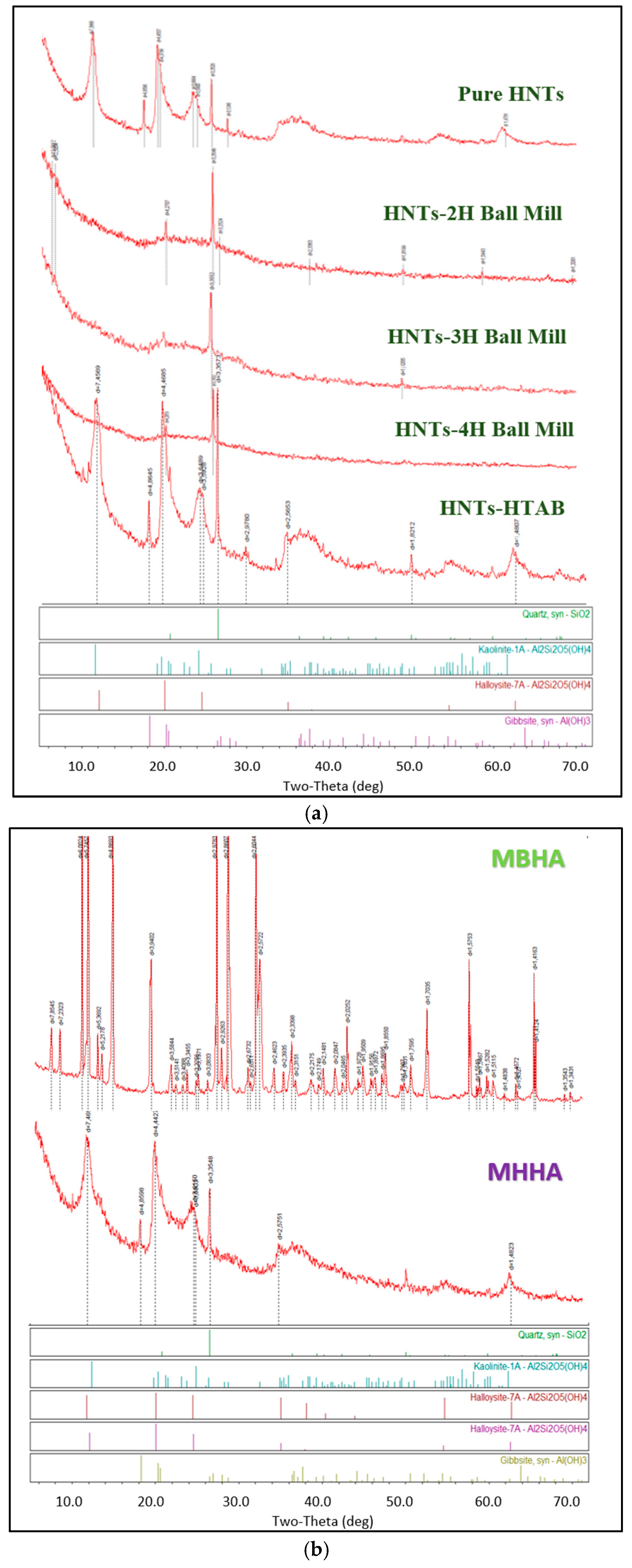
3.1. Structural Characterization of Biocomposites
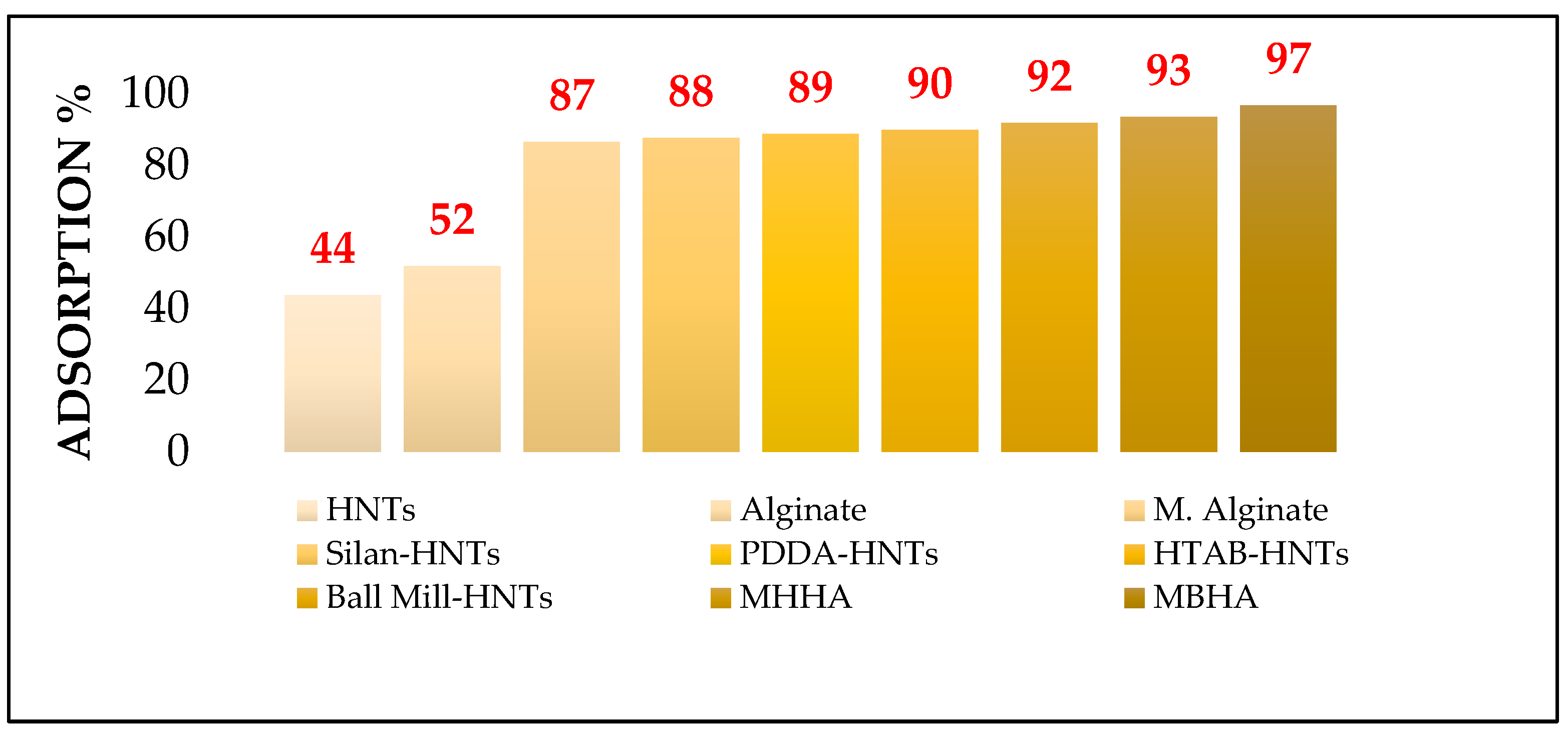
3.2. Adsorption Studies of Vitamin D3 Solutions on Biocomposites
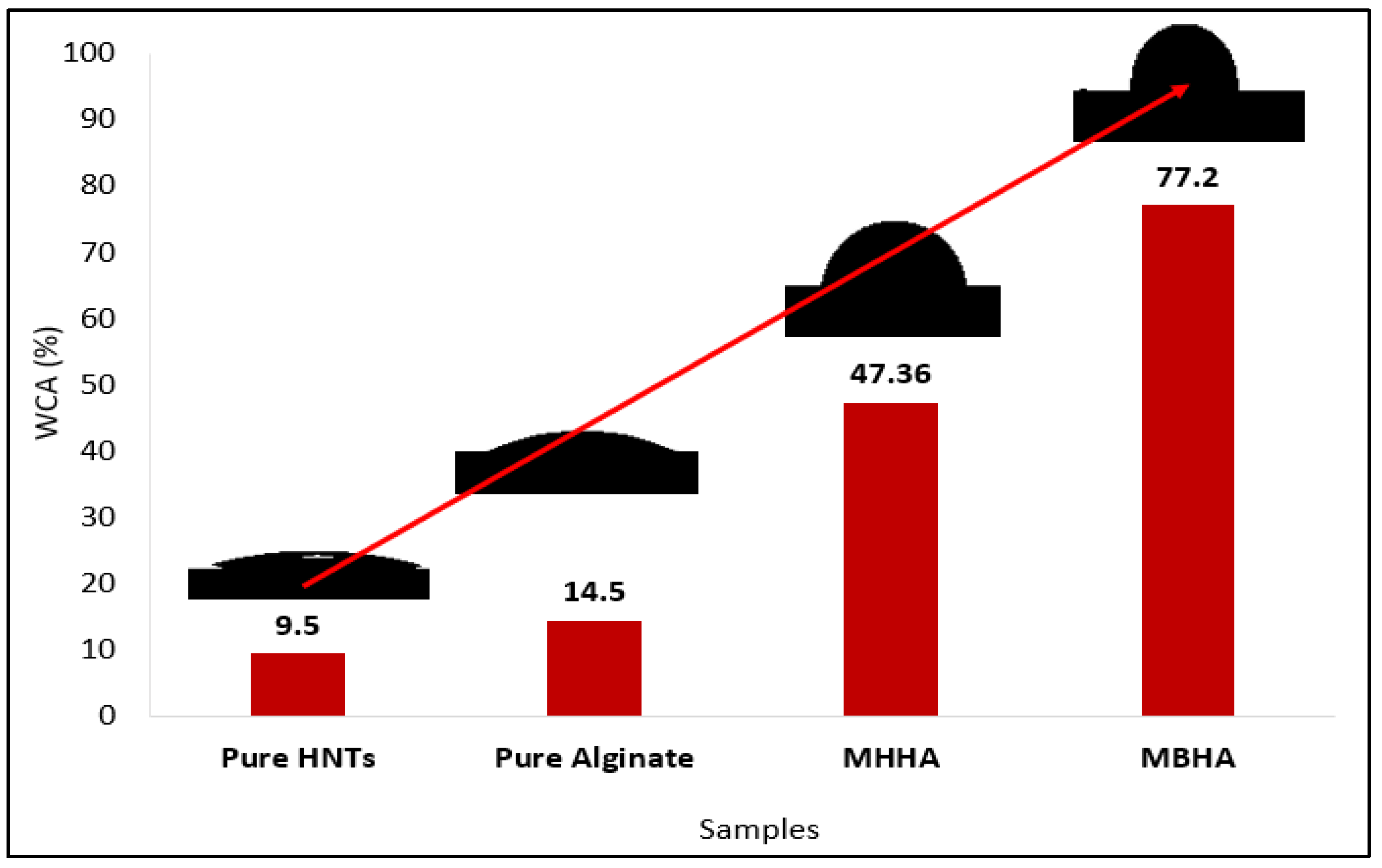
3.3. Hydrophobic Performance of Biocomposites
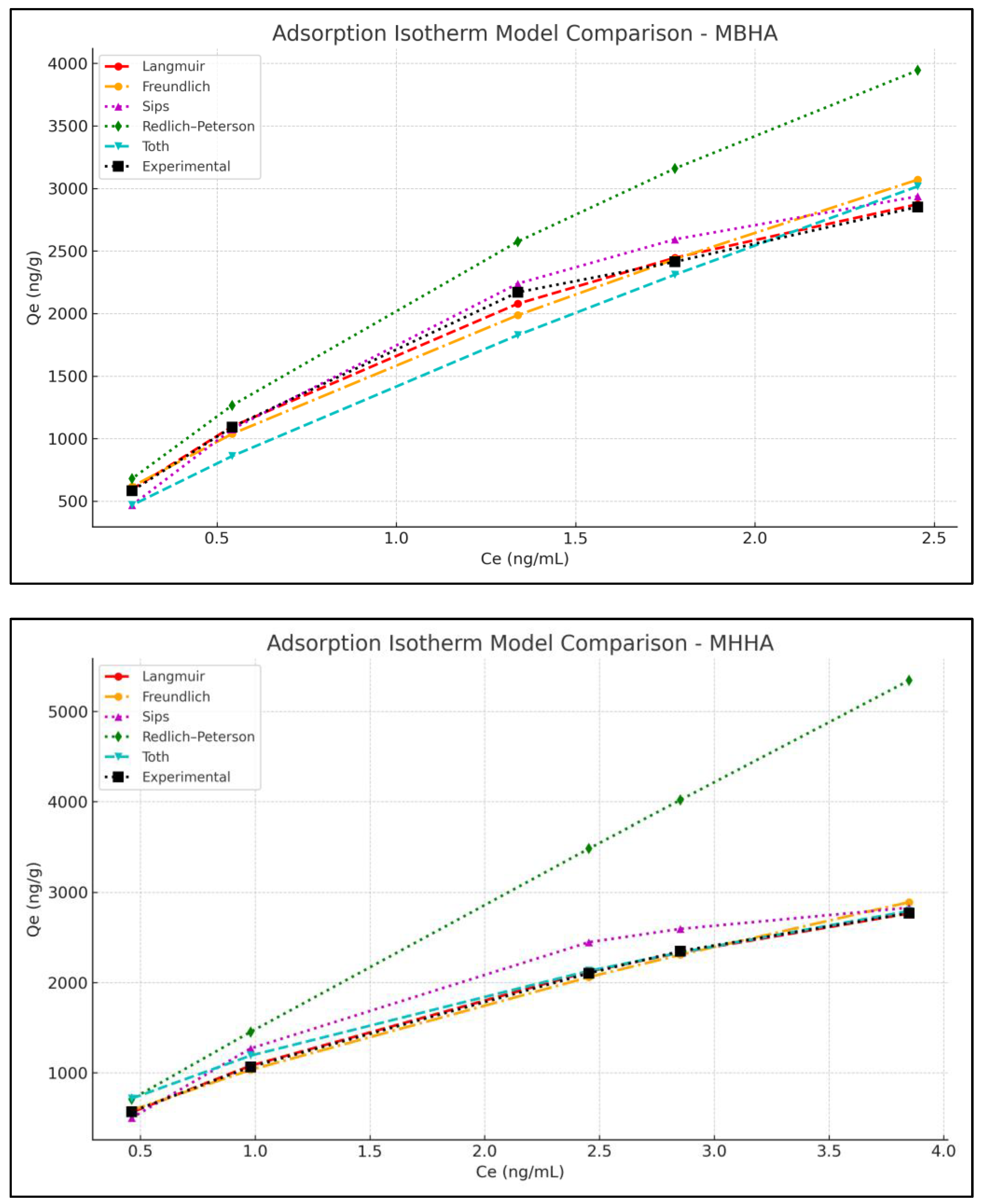
3.4. Adsorption Isotherm Studies
3.5. Adsorption Kinetics Studies
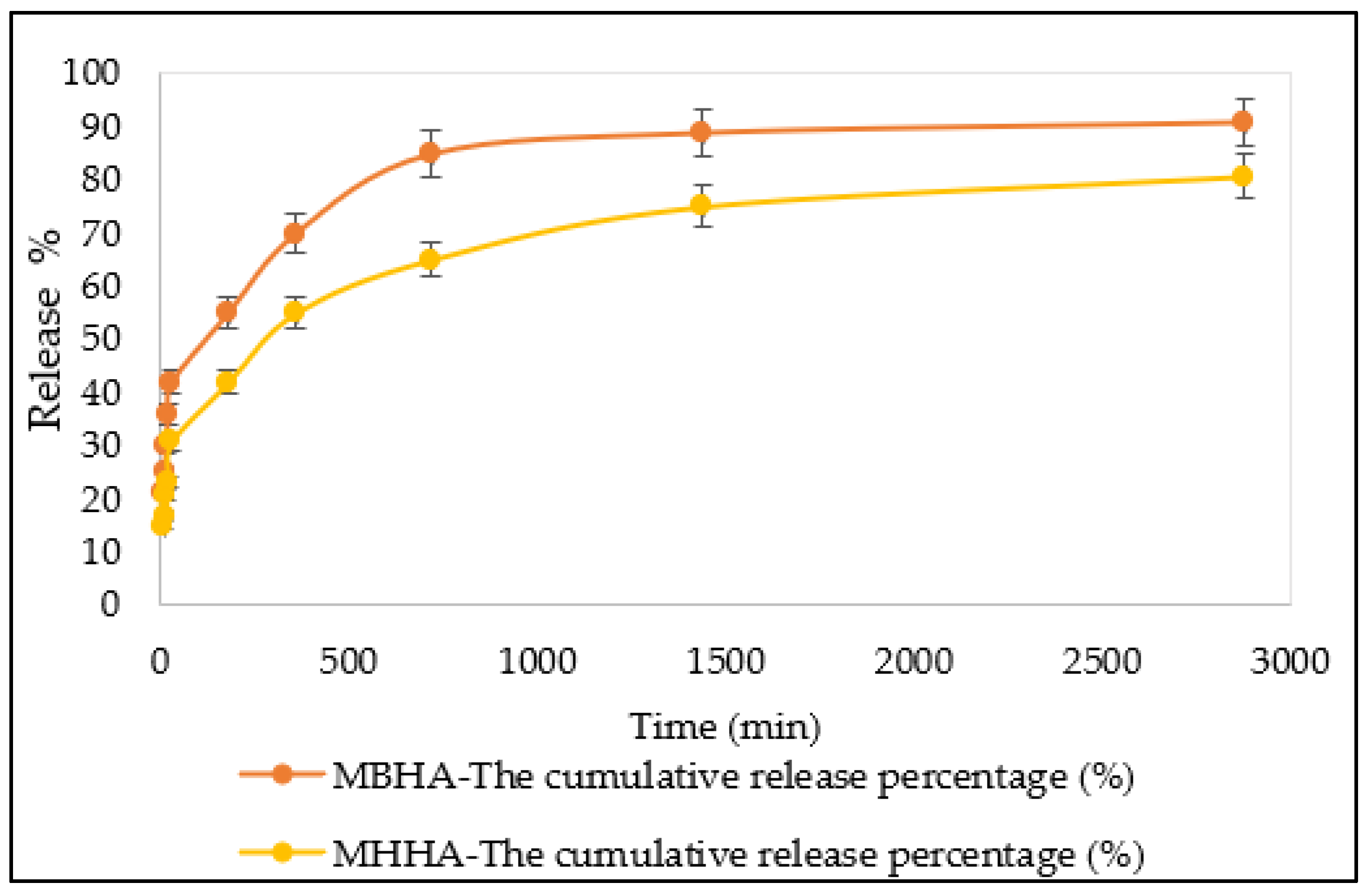
3.6. Desorption Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EEs | Encapsulation efficiency |
| FTIR | Fourier transform infrared spectroscopy |
| HTAB | Hexadecyltrimethylammonium bromide |
| HNTs | Halloysite nanotube nanoparticles |
| NPsR2 | Correlation coefficient |
| UVB | Ultraviolet B |
| UV-Vis | UV–visible spectrophotometer |
| PDDA | Poly(diallyldimethylammonium) chloride |
| SEM | Scanning electron microscopy |
| SPEX | Small capacity mills |
| Vitamin D | D (1α25 (OH)2D) (1,25 dihydroxyvitamin D) |
References
- Avramović, N.; Mandić, B.; Savić-Radojević, A.; Simić, T. Polymeric nanocarriers of drug delivery systems in cancer therapy. Pharmaceutics 2020, 12, 298. [Google Scholar] [CrossRef] [PubMed]
- Karabasz, A.; Bzowska, M.; Szczepanowicz, K. Biomedical applications of multifunctional polymeric nanocarriers: A review of current literature. Int. J. Nanomed. 2020, 15, 8673–8696. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Tao, Z.; Zhu, B.; Zhang, W.; Liu, C.; Chen, S.; Song, M. Targeted delivery of drugs and genes using polymer nanocarriers for cancer therapy. Int. J. Mol. Sci. 2021, 22, 9118. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhong, W.; Meng, X.; Yang, X.; Zhang, W.; Tian, Y.; Li, Y. Polymeric nanocarriers delivery systems in ischemic stroke for targeted therapeutic strategies. J. Nanobiotechnol. 2024, 22, 424. [Google Scholar] [CrossRef]
- Ismail, M.; Wang, Y.; Li, Y.; Liu, J.; Zheng, M.; Zou, Y. Stimuli-responsive polymeric nanocarriers accelerate on-demand drug release to combat glioblastoma. Biomacromolecules 2024, 25, 6250–6282. [Google Scholar] [CrossRef]
- Chia, J.J.; Shameli, K.; Yusefi, M.; Ali, R.R.; Balasundram, V.; Teow, S.Y. Preparation and application of cross-linked alginate nanoparticles as drug carrier: A review. J. Res. Nanosci. Nanotechnol. 2022, 5, 1–11. [Google Scholar] [CrossRef]
- Dodero, A.; Alberti, S.; Gaggero, G.; Ferretti, M.; Botter, R.; Vicini, S.; Castellano, M. An up-to-date review on alginate nanoparticles and nanofibers for biomedical and pharmaceutical applications. Adv. Mater. Interfaces 2021, 8, 2100809. [Google Scholar] [CrossRef]
- Niculescu, A.G.; Grumezescu, A.M. Applications of chitosan-alginate-based nanoparticles—An up-to-date review. Nanomaterials 2022, 12, 186. [Google Scholar] [CrossRef]
- Lv, Y.; Shi, G.; Ji, Z.; Shi, C.; Luo, Y.; Hong, H.; Tan, Y.; Yang, X. Development and application of visual fish freshness indicator film incorporated with anthocyanins encapsulation by whey protein-propylene glycol alginate nanoparticles. Int. J. Biol. Macromol. 2024, 282, 137054. [Google Scholar] [CrossRef]
- Singh, S.; Rastogi, H.; Deva, V.; Dixit, R.; Gupta, T.; Tyagi, M. Alginate based nanoparticles and its application in drug delivery systems. J. Pharm. Negat. Results 2022, 13, 1463–1469. [Google Scholar] [CrossRef]
- Maurya, K.L.; Swain, G.; Sonwani, R.K.; Verma, A.; Singh, R.S. Biodegradation of Congo red dye using polyurethane foam-based biocarrier combined with activated carbon and sodium alginate: Batch and continuous study. Bioresour. Technol. 2022, 351, 126999. [Google Scholar] [CrossRef]
- Abdullayev, E.; Price, R.; Shchukin, D.; Lvov, Y. Halloysite tubes as nanocontainers for anticorrosion coating with benzotriazole. ACS Appl. Mater. Interfaces 2009, 1, 1437–1443. [Google Scholar] [CrossRef] [PubMed]
- Abdullayev, E.; Joshi, A.; Wei, W.; Zhao, Y.; Lvov, Y. Enlargement of halloysite clay nanotube lumen by selective etching of aluminum oxide. ACS Nano 2012, 6, 7216–7226. [Google Scholar] [CrossRef]
- Boraei, S.B.A.; Eşghabadi, F.; Hüseyinpour, R.; Zare, Y.; Münir, T.M.; Rhee, Y.R. Halloysite nanotubes in biomedical applications. Prog. Mater. Sci. 2024, 253, 107346. [Google Scholar] [CrossRef]
- Brindley, G.W. Kaolin, serpentine and kindred minerals. In The X-Ray Identification and Crystal Structures of Clay Minerals; Brown, G., Ed.; Mineralogical Society: London, UK, 1961; pp. 51–131. [Google Scholar]
- Cheng, C.; Song, W.; Zhao, Q.; Zhang, H. Halloysite nanotubes in polymer science: Purification, characterization, modification and applications. Nanotechnol. Rev. 2020, 9, 323–344. [Google Scholar] [CrossRef]
- Durgut, E. Enrichment of Halloysite Ore and Investigation of Its Industrial Applications. Ph.D. Thesis, Istanbul University-Cerrahpasa, Istanbul, Turkey, 2022. [Google Scholar]
- Fakhruddin, K.; Hassan, R.; Khan, M.U.A.; Allisha, S.N.; Razak, S.I.A.; Zreaqat, M.H.; Latip, H.F.M.; Jamaludin, M.N.; Hassan, A. Halloysite nanotubes and halloysite-based composites for biomedical applications. Prog. Nat. Sci. Mater. Int. 2021, 31, 542–554. [Google Scholar] [CrossRef]
- Garcia-Garcia, D.; Ferri, J.M.; Ripoll, L.; Hidalgo, M.; Lopez-Martinez, J.; Balart, R. Characterization of selectively etched halloysite nanotubes by acid treatment. Appl. Surf. Sci. 2017, 422, 616–625. [Google Scholar] [CrossRef]
- Jenkins, R.; Snyder, R.L. Introduction to X-Ray Powder Diffractometry; Wiley-Interscience: New York, NY, USA, 1996. [Google Scholar] [CrossRef]
- Liu, M.; Jia, Z.; Jia, D.; Zhou, C. Recent advances in research on halloysite nanotubes-polymer nanocomposites. Prog. Polym. Sci. 2014, 39, 1498–1525. [Google Scholar] [CrossRef]
- Luo, P.; Zhao, Y.; Zhang, B.; Liu, J.; Yang, Y.; Liu, J. Study on the adsorption of Neutral Red from aqueous solution onto halloysite nanotubes. Water Res. 2009, 259, 120–127. [Google Scholar] [CrossRef]
- Lvov, Y.M.; Shchukin, D.G.; Mohwald, H.; Price, R.R. Halloysite clay nanotubes for controlled release of protective agents. ACS Nano 2008, 2, 814–820. [Google Scholar] [CrossRef]
- Sandri, G.; Aguzzi, C.; Rossi, S.; Bonferoni, M.C.; Bruni, G.; Boselli, C.; Cornaglia, A.I.; Riva, F.; Viseras, C.; Caramella, C.; et al. Halloysite and chitosan oligosaccharide nanocomposite for wound healing. Int. J. Pharm. 2017, 523, 246–258. [Google Scholar] [CrossRef] [PubMed]
- Santhosh, G.; Madhukar, B.S.; Nayaka, G.P.; Hemaraju, B.C.; Siddaramaiah, B. Influence of gamma radiation on optical properties of Halloysite nanotubes incorporated polycarbonate nanocomposites. Radiat. Eff. Defects Solids 2018, 173, 489–503. [Google Scholar] [CrossRef]
- Kirazoğlu, M.; Benli, B. Recent Point of Care (PoC) Electrochemical Testing Trends of New Diagnostics Platforms for Vitamin D. ChemistrySelect 2023, 8, e202301600. [Google Scholar] [CrossRef]
- Liu, M.; Dai, L.; Shi, H.; Xiong, S.; Zhou, C. In vitro evaluation of alginate/halloysite nanotube composite scaffolds for tissue engineering. Mater. Sci. Eng. C 2015, 49, 700–712. [Google Scholar] [CrossRef]
- Kirazoğlu, M.; Benli, B. D vitamini ve tespitine yönelik geliştirilen elektrokimyasal biyosensörler. NÖHÜ Müh. Bilim. Derg. 2023, 12, 525–537. [Google Scholar] [CrossRef]
- Han, J.H.; Ginde, A.A.; Brown, S.M.; Baughman, A.; Collar, E.M.; Ely, E.W.; Gong, M.N.; Hope, A.A.; Hou, P.C.; Hough, C.L.; et al. Treatment Network Investigators. Effect of early high-dose vitamin D3 repletion on cognitive outcomes in critically ill adults. Chest 2021, 160, 909–918. [Google Scholar] [CrossRef]
- Bendik, I.; Friedel, A.; Roos, F.F.; Weber, P.; Eggersdorfer, M. Vitamin D: A critical and essential micronutrient for human health. Front. Physiol. 2014, 5, 248. [Google Scholar] [CrossRef]
- van Schoor, N.; de Jongh, R.; Lips, P. Worldwide Vitamin D Status. In Feldman and Pike’s Vitamin D; Feldman, D., Pike, J.W., Eds.; Academic Press: London, UK, 2024; pp. 47–75. [Google Scholar] [CrossRef]
- Ramalho, M.J.; Coelho, M.A.; Pereira, M.C. Nanoparticles for delivery of vitamin D: Challenges and opportunities. In A Critical Evaluation of Vitamin D: Clinical Overview; Intech Open: Rijeka, Croatia, 2017; Volume 11, pp. 231–249. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Y.; Liu, Z.; Pei, Y.; Xu, P.; Chong, W.; Hai, Y.; He, L.; He, Y.; Yu, J.; et al. Association between vitamin D supplementation and cancer mortality: A systematic review and meta-analysis. Cancers 2022, 14, 3717. [Google Scholar] [CrossRef]
- Dini, I.; Laneri, S. Nutricosmetics: A brief overview. Phytother. Res. 2019, 33, 3054–3063. [Google Scholar] [CrossRef]
- Christakos, S.; Ajibade, D.V.; Dhawan, P.; Fechner, A.J.; Mady, L.J. Vitamin D: Metabolism. Rheum. Dis. Clin. 2012, 38, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Reboul, E.; Goncalves, A.; Comera, C.; Bott, R.; Nowicki, M.; Landrier, J.F.; Jourdheuil-Rahmani, D.; Dufour, C.; Collet, X.; Borel, P. Vitamin D intestinal absorption is not a simple passive diffusion: Evidence for involvement of cholesterol transporters. Mol. Nutr. Food Res. 2011, 55, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, C.G.; Huang, Z.H.; Xue, F.F. Preparation and characterization of nanoparticles based on hydrophobic alginate derivative as carriers for sustained release of vitamin D3. J. Agric. Food Chem. 2011, 59, 1962–1967. [Google Scholar] [CrossRef] [PubMed]
- Aburto, J.; Alric, I.; Borredon, E. Preparation of long-chain esters of starch using fatty acid chlorides in the absence of an organic solvent. Starch 1999, 51, 132–135. [Google Scholar] [CrossRef]
- Vu, A.A.; Bose, S. Effects of vitamin D3 release from 3D printed calcium phosphate scaffolds on osteoblast and osteoclast cell proliferation for bone tissue engineering. RSC Adv. 2019, 9, 34847–34853. [Google Scholar] [CrossRef]
- Burmeister, C.F.; Kwade, A. Process engineering with planetary ball mills. Chem. Soc. Rev. 2013, 42, 7660–7667. [Google Scholar] [CrossRef]
- Tan, C.; Zhao, P.; Zhou, Y.; Liu, M. Hydrophobic halloysite nanotubes via ball milling for stable Pickering emulsions: Implications for food preservation. ACS Appl. Nano Mater. 2022, 5, 11289–11301. [Google Scholar] [CrossRef]
- Kuziora, P.; Wyszyńska, M.; Polanski, M.; Bystrzycki, J. Why the ball to powder ratio (BPR) is insufficient for describing the mechanical ball milling process. Int. J. Hydrogen Energy 2014, 39, 9883–9887. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Yue, X.; Yang, X.; Li, H.; Zhang, R.; Qin, D. Quaternary Ammonium Compounds-Modified Halloysite and Its Antifungal Performance. In Physics and Techniques of Ceramic and Polymeric Materials, Proceedings of the 19th Chinese Materials Conference 2018, Xiamen, China, 12–16 July 2018; Springer: Singapore, 2019; pp. 121–131. [Google Scholar] [CrossRef]
- Cavallaro, G.; Lazzara, G.; Milioto, S.; Parisi, F.; Evtugyn, V.; Rozhina, E.; Fakhrullin, R. Nanohydrogel formation within the halloysite lumen for triggered and sustained release. ACS Appl. Mater. Interfaces 2018, 10, 8265–8273. [Google Scholar] [CrossRef]
- Kaygusuz, H.; Uysal, M.; Adımcılar, V.; Erim, F.B. Natural alginate biopolymer montmorillonite clay composites for vitamin B2 delivery. J. Bioact. Compat. Polym. 2015, 30, 48–56. [Google Scholar] [CrossRef]
- Hethnawi, A.; Rajabi, R.; Badran, A.M.; Tomazei, Y. Novel synthesis and application of surface decorated vitamin D3 in starch-based nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2023, 674, 131854. [Google Scholar] [CrossRef]
- Liu, Y. Some consideration on the Langmuir isotherm equation. Colloids Surf. A Physicochem. Eng. Asp. 2006, 274, 34–36. [Google Scholar] [CrossRef]
- Proctor, A.; Toro-Vazquez, J.F. The Freundlich isotherm in studying adsorption in oil processing. J. Am. Oil Chem. Soc. 1996, 73, 1627–1633. [Google Scholar] [CrossRef]
- Tran, H.N.; Lima, E.C.; Juang, R.S.; Bollinger, J.C.; Chao, H.P. Thermodynamic parameters of liquid–phase adsorption process calculated from different equilibrium constants related to adsorption isotherms: A comparison study. J. Environ. Chem. Eng. 2021, 9, 106674. [Google Scholar] [CrossRef]
- Wu, F.C.; Liu, B.L.; Wu, K.T.; Tseng, R.L. A new linear form analysis of Redlich–Peterson isotherm equation for the adsorptions of dyes. Chem. Eng. J. 2010, 162, 21–27. [Google Scholar] [CrossRef]
- Özkaya, B. Adsorption and desorption of phenol on activated carbon and a comparison of isotherm models. J. Hazard. Mater. 2006, 129, 158–163. [Google Scholar] [CrossRef]
- Benli, B. Effect of borax addition on the structural modification of bentonite in biodegradable alginate-based biocomposites. J. Appl. Polym. Sci. 2013, 128, 4172–4180. [Google Scholar] [CrossRef]
- Deng, S.; Zhang, J.; Ye, L. Halloysite–epoxy nanocomposites with improved particle dispersion through ball mill homogenisation and chemical treatments. Compos. Sci. Technol. 2009, 69, 2497–2505. [Google Scholar] [CrossRef]
- Tomiczek, B.; Dobrzański, L.A.; Adamiak, M.; Labisz, K. Effect of milling conditions on microstructure and properties of AA6061/halloysite composites. Procedia Manuf. 2015, 2, 402–407. [Google Scholar] [CrossRef]
- Alsaqr, A.; Rasoully, M.; Musteata, F.M. Investigating transdermal delivery of vitamin D3. AAPS PharmSciTech 2015, 16, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Hitzky, E.; Van Meerbeek, A. Clay mineral– and organoclay–polymer nanocomposite. Dev. Clay Sci. 2006, 1, 583–621. [Google Scholar] [CrossRef]
- Bagheri, H.; Al Lawati, H.A.J.; Keshavarz, M.; Zare, E.N. A novel amino-functionalized magnetic metal–organic framework@ layered double hydroxide composite as an efficient adsorbent for vitamin D3 enrichment and delivery. J. Environ. Chem. Eng. 2023, 1, 109098. [Google Scholar] [CrossRef]
- Esfahani, M.R.K.; Ghiyasi, S. Effect of hydroxyapatite (HA) nanoparticles on adsorption of vitamin D3 on polyamide-6 electrospun nanofiber scaffold. Adv. Pharm. Bull. 2020, 2, 287–293. [Google Scholar] [CrossRef]
- Sereshti, H.; Toloutehrani, T. Determination of cholecalciferol (vitamin D3) in bovine milk by DLLME-SFO followed by GC–MS using magnetic graphene/sporopollenin as a novel sorbent. J. Sep. Sci. 2019, 24, 3702–3709. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 5, 451–465. [Google Scholar] [CrossRef]



| Adsorbent System | Adsorbed Compound | Adsorption Capacity (mg/g) | Reference |
|---|---|---|---|
| This study: Hydrophobically modified alginate–HNT composite | Vitamin D3 | 3.0 mg/g | — |
| IRMOF-3@MLDH (magnetic MOF-LDH composite) | Vitamin D3 | 126 mg/g | [58] |
| Polyamide-6 nanofiber scaffold with hydroxyapatite | Vitamin D3 | 0.0103 mg/g (steady state) | [59] |
| Magnetic graphene–sporopollenin sorbent | Vitamin D3 | Not specified (recovery: 71.8–113.3%) | [60] |
| (a) | Langmuir Model | Freundlich Model | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| qmax | KL | R2 | KF | n | R2 | |||||||
| MBHA | 5318.108 | 0.48 | 0.9916 | 1611.169 | 1.4 | 0.9891 | ||||||
| MHHA | 5935.263 | 0.23 | 0.9980 | 1048.538 | 1.33 | 0.9975 | ||||||
| (b) | Sips Model | Redlich–Peterson Model | Toth Model | |||||||||
| qms | as | n | R2 | KR | aR | g | R2 | qmt | KT | T | R2 | |
| MBHA | 3795 | 0.949 | 0.7 | 0.86 | 3100 | 0.5 | 0.69 | 0.98 | 8540 | 0.31 | 0.22 | 0.95 |
| MHHA | 3290 | 0.65 | 0.6 | 0.79 | 2270 | 0.1 | 0.87 | 0.95 | 3500 | 0.45 | 0.5 | 0.98 |
| qmax | KL | R2 | RSS | AIC | BIC | Chi-Square | |
|---|---|---|---|---|---|---|---|
| MBHA | 5318.108 | 0.48 | 0.9916 | 5632.43 | 52.3 | 52.51 | 2.39 |
| MHHA | 5935.263 | 0.23 | 0.998 | 2071.06 | 47.89 | 48.1 | 0.92 |
| First-Order Model | Second-Order Model | |||||
|---|---|---|---|---|---|---|
| qe | K1 | R2 | qe | K2 | R2 | |
| MBHA | 889.5 | 0.011285 | 0.9733 | 1815.049 | 0.000587 | 0.999 |
| MHHA | 1130 | 0.018885 | 0.94 | 1855.893 | 0.000276 | 0.9969 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirazoğlu, M.; Benli, B. Enhanced Vitamin D3 Adsorption Through Novel Hydrophobic Halloysite–Alginate Biopolymer Composites. Polymers 2025, 17, 1083. https://doi.org/10.3390/polym17081083
Kirazoğlu M, Benli B. Enhanced Vitamin D3 Adsorption Through Novel Hydrophobic Halloysite–Alginate Biopolymer Composites. Polymers. 2025; 17(8):1083. https://doi.org/10.3390/polym17081083
Chicago/Turabian StyleKirazoğlu, Mervenur, and Birgül Benli. 2025. "Enhanced Vitamin D3 Adsorption Through Novel Hydrophobic Halloysite–Alginate Biopolymer Composites" Polymers 17, no. 8: 1083. https://doi.org/10.3390/polym17081083
APA StyleKirazoğlu, M., & Benli, B. (2025). Enhanced Vitamin D3 Adsorption Through Novel Hydrophobic Halloysite–Alginate Biopolymer Composites. Polymers, 17(8), 1083. https://doi.org/10.3390/polym17081083







