Ionizing Radiation and Its Effects on Thermoplastic Polymers: An Overview
Abstract
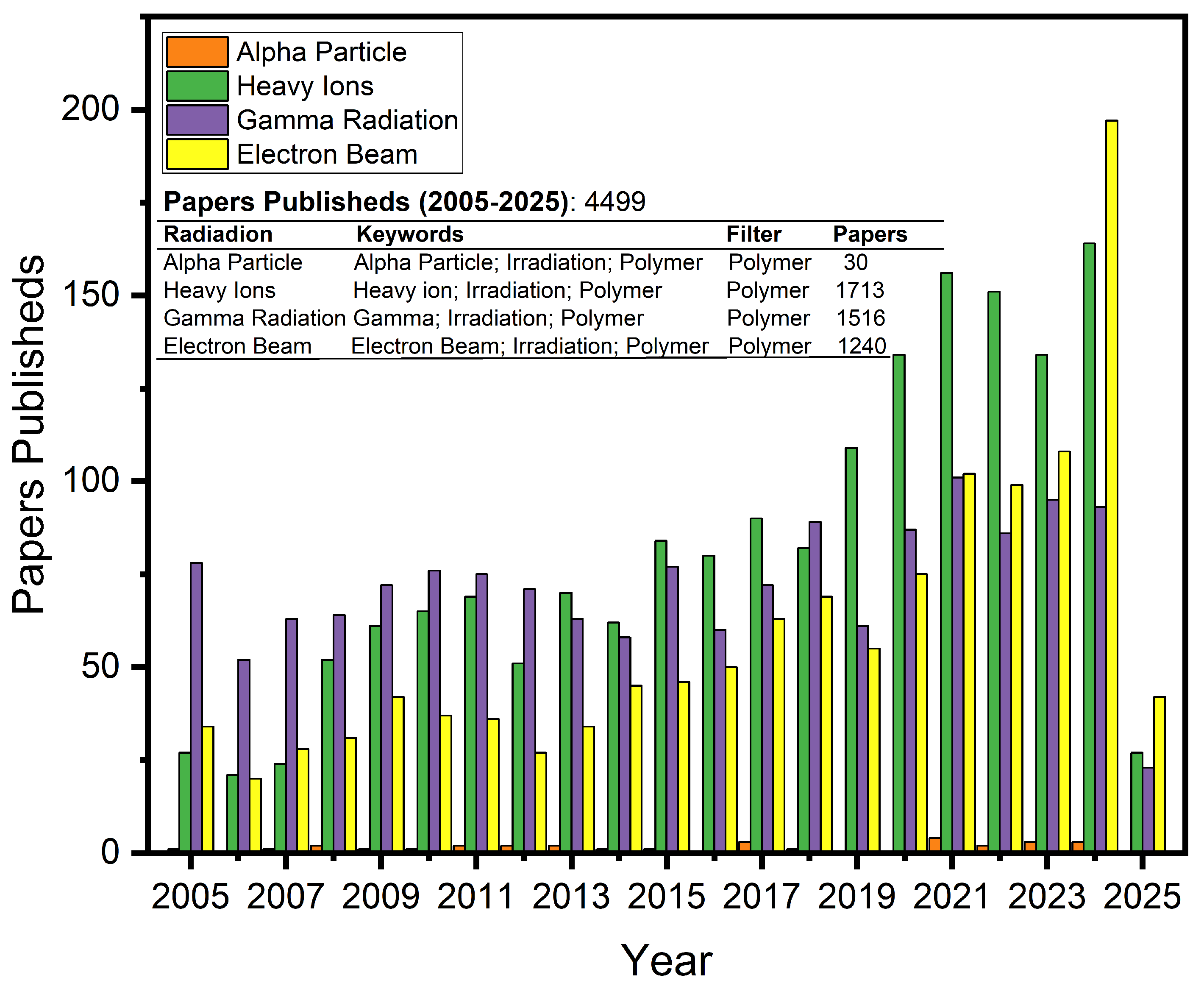
1. Introduction
2. Theoretical Basis
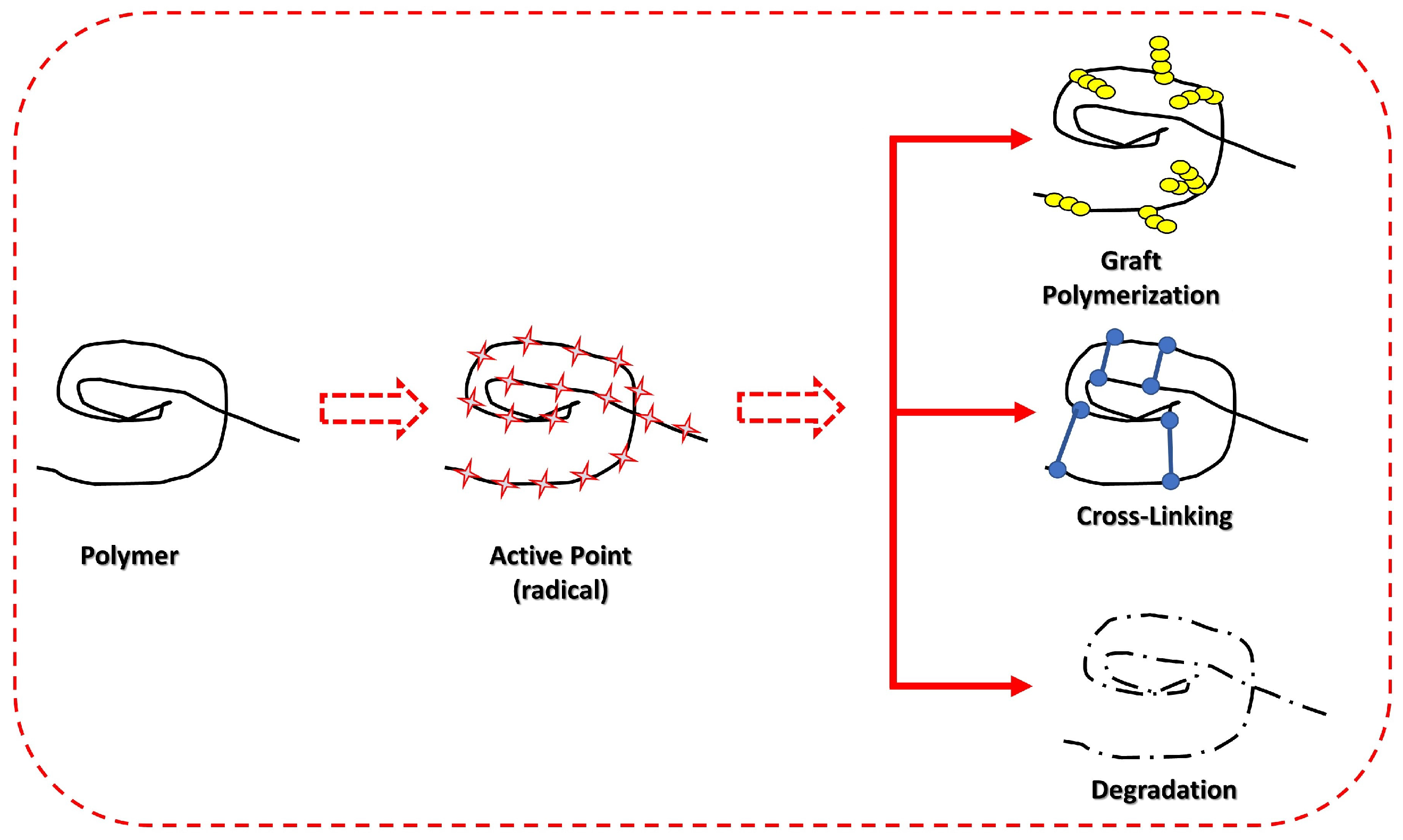
2.1. Basic Effects of Polymer Irradiation
- Excitation;
- Charge recombination;
- Trapped electrons and holes;
- Charge transfer;
- Formation of radicals.
2.1.1. Excitation
2.1.2. Charge Recombination
2.1.3. Trapped Electrons and Holes
2.1.4. Energy Transfer
2.1.5. Formation of Free Radicals
2.2. Irradiation Conditions
- Chemical structure;
- Temperature of irradiation;
- Radiation type;
- Radiation absorbed doses;
- Atmosphere of irradiation.
2.2.1. Influence of Chemical Structure
2.2.2. Influence of Temperature on Polymers
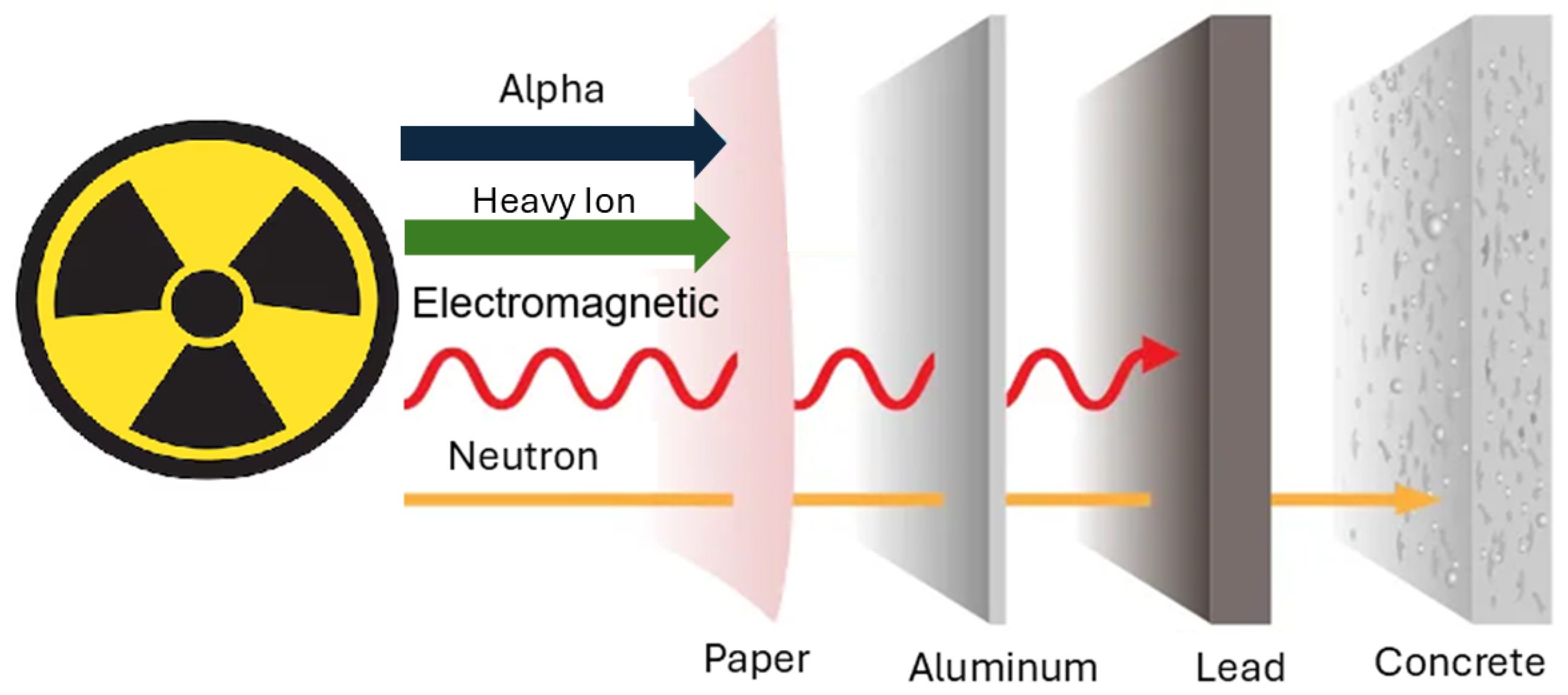
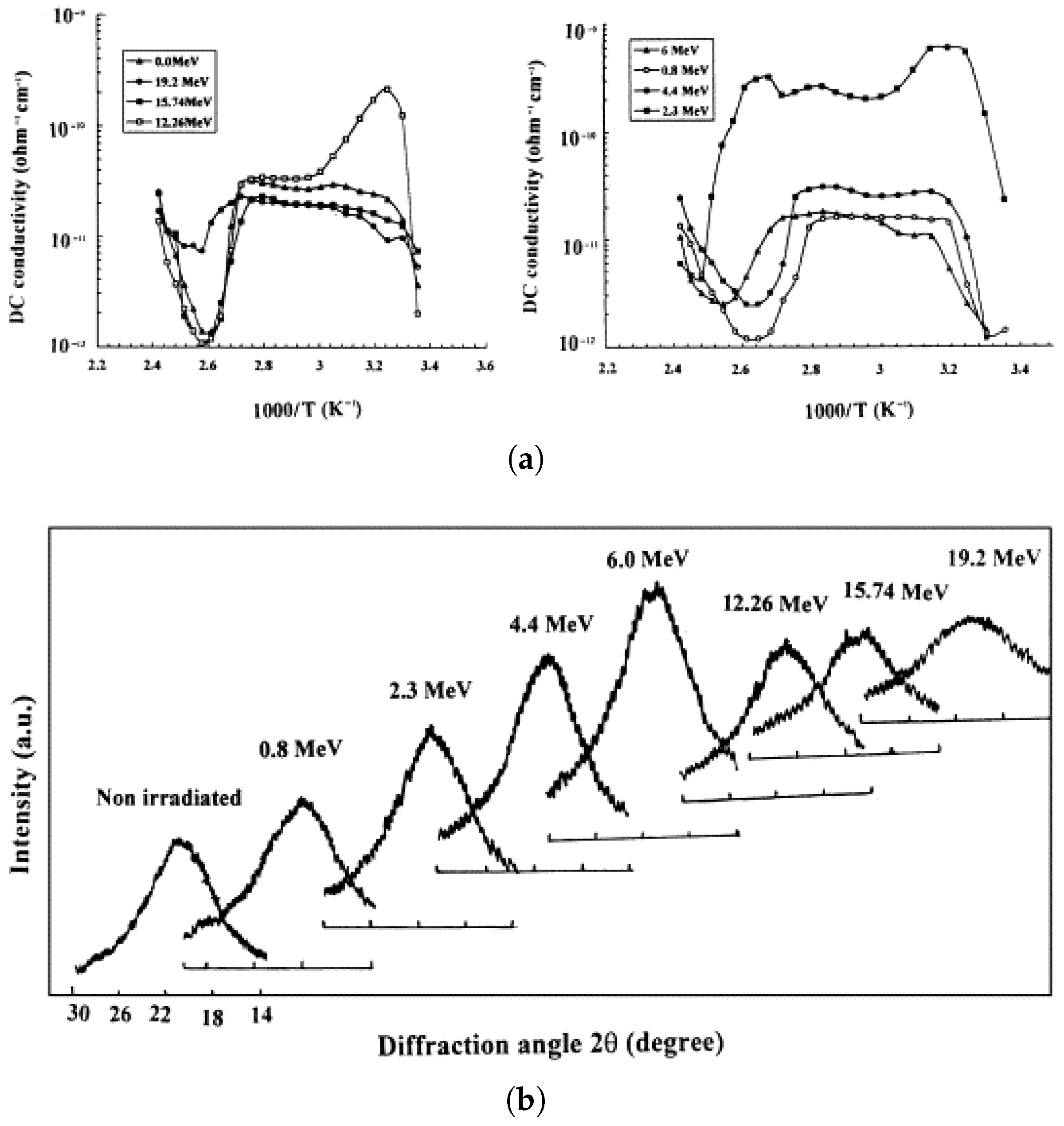
2.2.3. Influence of Radiation Type
2.2.4. Influence of the Absorbed Dose
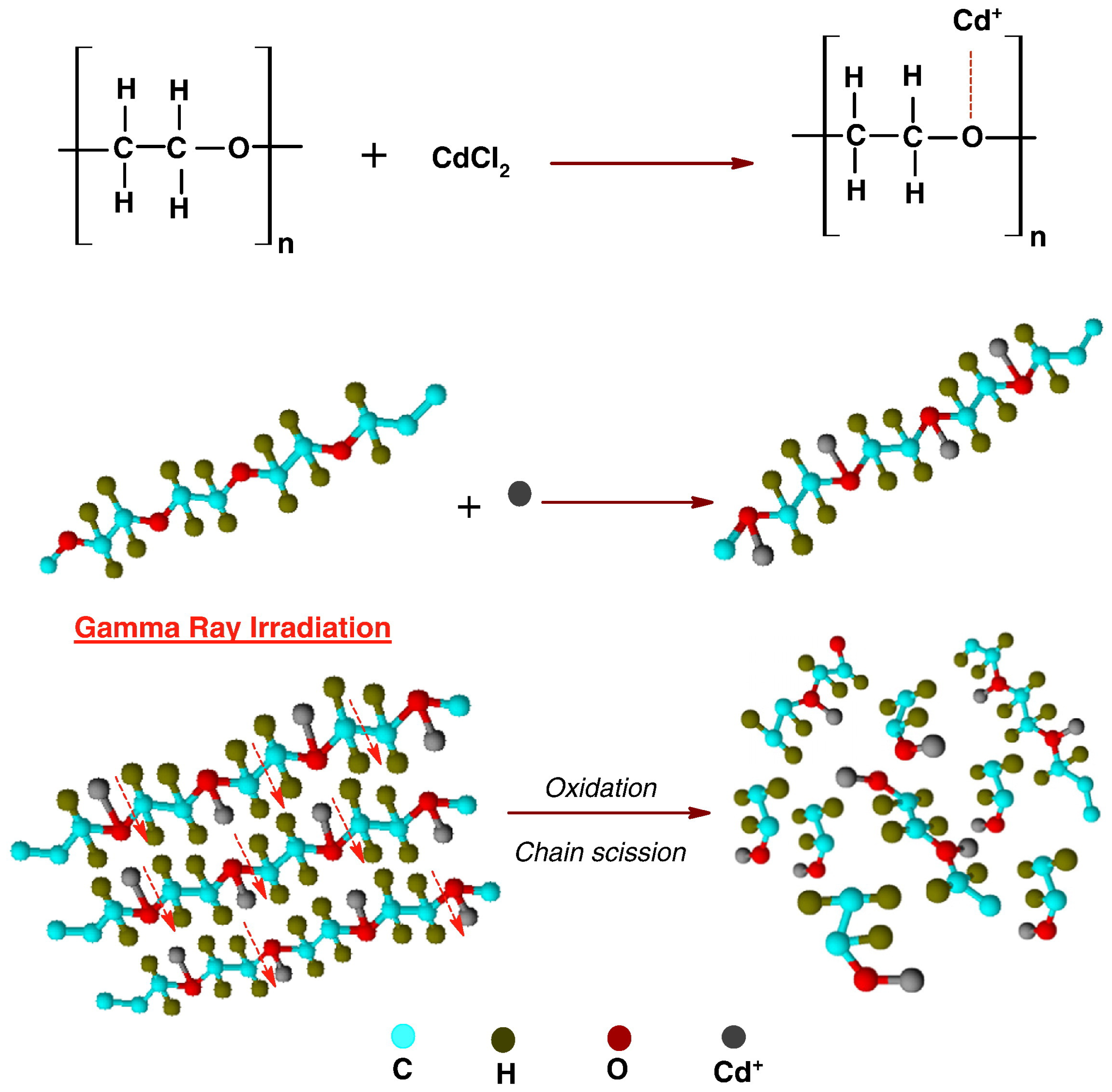
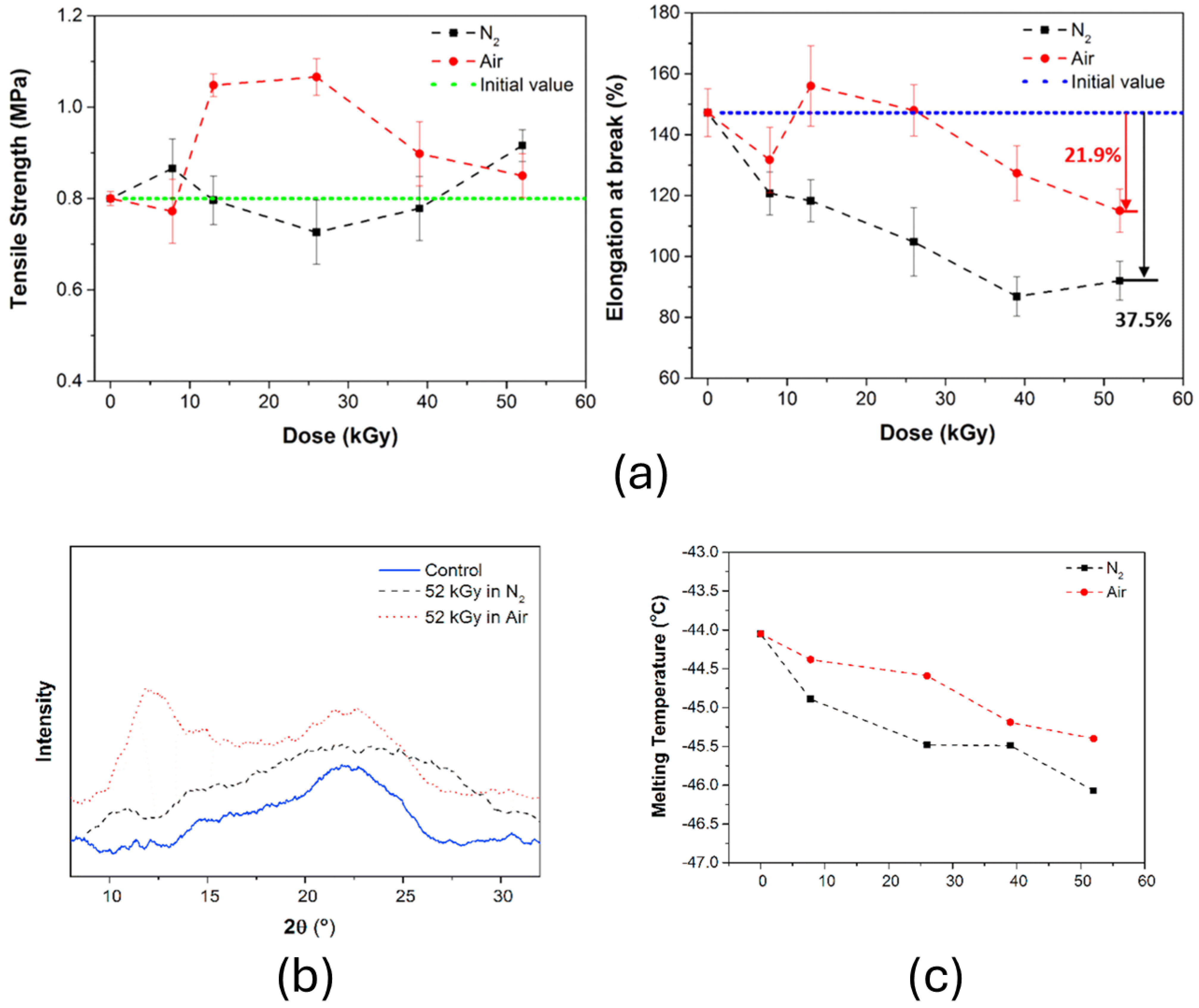
2.2.5. Influence of Atmosphere on Irradiation
3. Applications of Irradiated Thermoplastic Polymers
3.1. Classification Based on Polymer Type
3.1.1. Polyethylene
3.1.2. Polypropylene
3.1.3. Polyamide
3.1.4. Acrylonitrile–Butadiene–Styrene
3.1.5. Polycarbonate
3.2. Application of Irradiated Thermoplastic Polymers
4. Final Remarks and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABS | Acrylonitrile–Butadiene–Styrene |
| CdCl2 | Cadmium Chloride |
| COC | Cyclic Olefin Copolymer |
| CPG | Chitosan@PVA@rGO |
| CR-39 | Polyallyldiglycol Carbonate |
| CS | Chitosan |
| DAP | Polydiallyl Phthalate |
| DSC | Differential Scanning Calorimetry |
| EIS | Electrochemical Impedance Spectroscopy |
| FEP | Fluorinated Ethylene Propylene |
| FP | Fluoropolymer |
| FT-IR | Fourier Transform Infrared Spectroscopy |
| HA | Hydroxyapatite |
| HIPS | High-Impact Polystyrene |
| LCP | Liquid Crystal Polymer |
| NIST | National Institute of Standards and Technology |
| PA6 | Polyamide 6 |
| PA9T | Poly(nonamethylene terephthalamide) |
| PA11 | Polyamide 11 |
| PA12 | Polyamide 12 |
| PA46 | Polyamide 46 |
| PA66 | Polyamide 66 |
| PBT | Poly(butylene terephthalate) |
| PC | Polycarbonate |
| PE | Polyethylene |
| PEI | Polyetherimide |
| PEO | Polyethylene Oxide |
| PEEK | Poly(ether-ether-ketone) |
| PES | Polyethersulfone |
| PET | Polyethylene Terephthalate |
| PI | Polyimide |
| PLA | Polylactic Acid |
| PMMA | Poly(methyl methacrylate) |
| PMP | Polymethylpentene |
| PP | Polypropylene |
| PPS | Polyphenylene Sulfide |
| PSC | Polymer Solar Cell |
| PS | Polystyrene |
| PTFE | Polytetrafluoroethylene |
| PVC | Polyvinyl Chloride |
| PVF | Poly(vinyl fluoride) |
| rGo | Reduced Graphene Oxide |
| Rct | Charge-Transfer Resistance |
| RTV-5370 | Siloxane Rubber |
| SAN | Styrene–Acrylonitrile |
| Glass Transition Temperature | |
| TGA | Thermogravimetric Analysis |
| TPA | Thermoplastic Amide |
| TPC | Thermoplastic Copolyester |
| TPU | Thermoplastic Polyurethane |
| TPO | Thermoplastic Polyolefin |
| TPS | Thermoplastic Styrene |
| TPV | Thermoplastic Vulcanizate |
| UHMWPE | Ultra-High-Molecular-Weight Polyethylene |
| XLPE | Crosslinked Polyethylene |
| XRD | X-ray Diffraction |
References
- Gueven, O. An overview of current developments in applied radiation chemistry of polymers. In Advances in Radiation Chemistry of Polymers; IAEATECDOC-1420; IAEA: Vienna, Austria, 2004; pp. 33–39. [Google Scholar]
- Clough, R. High-energy radiation and polymers: A review of commercial processes and emerging applications. Nucl. Instruments Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2001, 185, 8–33. [Google Scholar] [CrossRef]
- Bhattacharya, A. Radiation and industrial polymers. Prog. Polym. Sci. 2000, 25, 371–401. [Google Scholar] [CrossRef]
- Blackburn, R.; Charlesby, A.; Woods, R. A comparison of the effects of α and γ irradiation on the crystallinity of polyethylene. Eur. Polym. J. 1965, 1, 161–172. [Google Scholar] [CrossRef]
- Parkinson, W.; Kirkland, W. The Effect of Air on the Radiation-Induced Degradation of Polytetrafluoroethylene (Teflon); Technical Report; Oak Ridge National Lab. (ORNL): Oak Ridge, TN, USA, 1967. [Google Scholar]
- Ferry, M.; Ngono-Ravache, Y.; Aymes-Chodur, C.; Clochard, M.; Coqueret, X.; Cortella, L.; Pellizzi, E.; Rouif, S.; Esnouf, S. Ionizing radiation effects in polymers. Ref. Modul. Mater. Sci. Mater. Eng. 2016. [Google Scholar] [CrossRef]
- Drobny, J.G. Radiation Technology for Polymers; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]
- Medeiros, P.R.d.; Araujo, P.L.; Aquino, K.A.; Araujo, E.S. Green Polyethylene in Harsh Environments: Gamma-irradiation Effects. Mater. Res. 2022, 25, e20220186. [Google Scholar] [CrossRef]
- Ivanov, V.S. Radiation Chemistry of Polymers; CRC Press: Boca Raton, FL, USA, 2023. [Google Scholar]
- Cleland, M.; Parks, L.; Cheng, S. Applications for radiation processing of materials. Nucl. Instruments Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2003, 208, 66–73. [Google Scholar] [CrossRef]
- Makuuchi, K.; Cheng, S. Radiation Processing of Polymer Materials and Its Industrial Applications; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Fifield, L.S.; Pharr, M.; Staack, D.; Pillai, S.D.; Nichols, L.; McCoy, J.; Faucette, T.; Bisel, T.T.; Huang, M.; Hasan, M.K.; et al. Direct comparison of gamma, electron beam and X-ray irradiation effects on single-use blood collection devices with plastic components. Radiat. Phys. Chem. 2021, 180, 109282. [Google Scholar] [CrossRef]
- Duarte-Pena, L.; López-Saucedo, F.; Concheiro, A.; Alvarez-Lorenzo, C.; Bucio, E. Modification of indwelling PVC catheters by ionizing radiation with temperature-and pH-responsive polymers for antibiotic delivery. Radiat. Phys. Chem. 2022, 193, 110005. [Google Scholar] [CrossRef]
- Fintzou, A.T.; Badeka, A.V.; Kontominas, M.G.; Riganakos, K.A. Changes in physicochemical and mechanical properties of γ-irradiated polypropylene syringes as a function of irradiation dose. Radiat. Phys. Chem. 2006, 75, 87–97. [Google Scholar] [CrossRef]
- Sedlacek, O.; Kucka, J.; Monnery, B.D.; Slouf, M.; Vetrik, M.; Hoogenboom, R.; Hruby, M. The effect of ionizing radiation on biocompatible polymers: From sterilization to radiolysis and hydrogel formation. Polym. Degrad. Stab. 2017, 137, 1–10. [Google Scholar] [CrossRef]
- Fathy, E.; Ibrahim, S.; Elnaggar, M.Y.; Fahmy, H.; Lotfy, S. Polypropylene based bio-composites for packaging materials: Physico-mechanical impacts of prepared hyper-branched polyamidoamine and gamma-irradiation. J. Thermoplast. Compos. Mater. 2024, 37, 66–83. [Google Scholar] [CrossRef]
- Sarmast, E.; Shankar, S.; Salmieri, S.; Rahmouni, S.A.; Mahmud, J.; Lacroix, M. Cross-linked gelatin–riboflavin-based film incorporated with essential oils and silver nanoparticle by gamma-irradiation: A novel approach for extending the shelf life of meat. Food Hydrocoll. 2024, 147, 109330. [Google Scholar] [CrossRef]
- Irimia, A.; Grigoraș, V.C.; Popescu, C.M. Active Cellulose-Based Food Packaging and Its Use on Foodstuff. Polymers 2024, 16, 389. [Google Scholar] [CrossRef] [PubMed]
- Naikwadi, A.T.; Sharma, B.K.; Bhatt, K.D.; Mahanwar, P.A. Gamma radiation processed polymeric materials for high performance applications: A review. Front. Chem. 2022, 10, 837111. [Google Scholar] [CrossRef]
- Maléchaux, A.; Colombani, J.; Amat, S.; Marque, S.R.; Dupuy, N. Influence of gamma irradiation on electric cables models: Study of additive effects by mid-infrared spectroscopy. Polymers 2021, 13, 1451. [Google Scholar] [CrossRef]
- Wang, T.Y.; Mao, J.; Zhang, B.; Zhang, G.X.; Dang, Z.M. Polymeric insulating materials characteristics for high-voltage applications. Nat. Rev. Electr. Eng. 2024, 1, 516–528. [Google Scholar] [CrossRef]
- Kosińska, A.; Jagielski, J.; Wilczopolska, M.; Bieliński, D.; Okraska, M.; Jóźwik, I.; Kurpaska, Ł.; Nowakowska-Langier, K. Study of the electrical properties of ion irradiated polymer materials. Surf. Coat. Technol. 2020, 388, 125562. [Google Scholar] [CrossRef]
- Cota, S.; Vasconcelos, V.d.; Senne Jr, M.; Carvalho, L.; Rezende, D.; Côrrea, R. Changes in mechanical properties due to gamma irradiation of high-density polyethylene (HDPE). Braz. J. Chem. Eng. 2007, 24, 259–265. [Google Scholar] [CrossRef]
- White, I.; Von, G.; Tandon, R.; Serna, L.M.; Celina, M.C.; Bernstein, R. An Overview of Basic Radiation Effects on Polymers and Glasses. 2013. Available online: https://www.osti.gov/servlets/purl/1671997 (accessed on 15 April 2025).
- Tamada, M. Radiation processing of polymers and its applications. In Radiation Applications; Springer: Singapore, 2018; pp. 63–80. [Google Scholar]
- Little, K. Irradiation of linear high polymers. Nature 1952, 170, 1075–1076. [Google Scholar] [CrossRef]
- Mousa, E.; Taha, E.O.; Lotfy, S.; Anwar, A. Influence of Gamma irradiation on shape memory polymer nano-composite for satellite deployment mechanism. Sci. Rep. 2024, 14, 23917. [Google Scholar] [CrossRef]
- Vasilca, S.; Virgolici, M.; Cutrubinis, M.; Moise, V.; Mereuta, P.; Sirbu, R.; Medvedovici, A.V. Wood consolidation through an epoxy-acrylic gamma-crosslinked three-dimensional system. Polym. Adv. Technol. 2024, 35, e6381. [Google Scholar] [CrossRef]
- Xia, W.; Stapleton, S.E.; Schmidt, D.F. Effect of an unsaturated-chain extender on the response of epoxy resins to radiation post-curing. Polym. Degrad. Stab. 2024, 225, 110793. [Google Scholar] [CrossRef]
- Ashfaq, A.; Clochard, M.C.; Coqueret, X.; Dispenza, C.; Driscoll, M.S.; Ulański, P.; Al-Sheikhly, M. Polymerization Reactions and Modifications of Polymers by Ionizing Radiation. Polymers 2020, 12, 2877. [Google Scholar] [CrossRef]
- Yongxia Sun, A.G.C. Controlling of Degradation Effects in Radiation Processing of Polymers; International Atomic Energy Agency: Vienna, Austria, 2009; p. 67. [Google Scholar]
- Spadaro, G.; Alessi, S.; Dispenza, C. Ionizing Radiation-Induced Crosslinking and Degradation of Polymers; Institute of Nuclear Chemistry and Technology: Warszawa, Poland, 2017; pp. 167–182. [Google Scholar]
- Proctor, C.M.; Kuik, M.; Nguyen, T.Q. Charge carrier recombination in organic solar cells. Prog. Polym. Sci. 2013, 38, 1941–1960. [Google Scholar] [CrossRef]
- March, J. Advanced Organic Chemistry: Reactions, Mechanisms, and Structure; McGraw-Hill: New York, NY, USA, 1977. [Google Scholar]
- Grigoriev, E.I.; Trakhtenberg, L.I. Radiation-Chemical Processes in Solid Phase: Theory and Application; CRC Press: Boca Raton, FL, USA, 1996. [Google Scholar]
- An, Z.; Zheng, C.; Tao, Y.; Chen, R.; Shi, H.; Chen, T.; Wang, Z.; Li, H.; Deng, R.; Liu, X.; et al. Stabilizing triplet excited states for ultralong organic phosphorescence. Nat. Mater. 2015, 14, 685–690. [Google Scholar] [CrossRef]
- Wang, L.; Yu, H.; Ullah, R.S.; Haroon, M.; Fahad, S.; Li, J.; Elshaarani, T.; Khan, R.U.; Nazir, A. Recent progress in the electron paramagnetic resonance study of polymers. Polym. Chem. 2018, 9, 3306–3335. [Google Scholar] [CrossRef]
- Fedin, M.V.; Veber, S.L.; Maryunina, K.Y.; Romanenko, G.V.; Suturina, E.A.; Gritsan, N.P.; Sagdeev, R.Z.; Ovcharenko, V.I.; Bagryanskaya, E.G. Intercluster exchange pathways in polymer-chain molecular magnets Cu (hfac) 2LR unveiled by electron paramagnetic resonance. J. Am. Chem. Soc. 2010, 132, 13886–13891. [Google Scholar] [CrossRef] [PubMed]
- Kabachii, Y.A.; Kochev, S.Y.; Valetski, P. Dithioesters in atom-transfer radical polymerization. Polym. Sci. Ser. B 2006, 48, 32–36. [Google Scholar] [CrossRef]
- Andrew, T.L.; Swager, T.M. Structure—Property relationships for exciton transfer in conjugated polymers. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 476–498. [Google Scholar] [CrossRef]
- Barford, W. Exciton transfer integrals between polymer chains. J. Chem. Phys. 2007, 126, 134905. [Google Scholar] [CrossRef]
- Hallermann, M.; Kriegel, I.; Da Como, E.; Berger, J.M.; Von Hauff, E.; Feldmann, J. Charge transfer excitons in polymer/fullerene blends: The role of morphology and polymer chain conformation. Adv. Funct. Mater. 2009, 19, 3662–3668. [Google Scholar] [CrossRef]
- Cheng, C.; Yu, J.; Xu, D.; Wang, L.; Liang, G.; Zhang, L.; Jaroniec, M. In-situ formatting donor-acceptor polymer with giant dipole moment and ultrafast exciton separation. Nat. Commun. 2024, 15, 1313. [Google Scholar] [CrossRef] [PubMed]
- Bauch, F.; Dong, C.D.; Schumacher, S. Dynamics of Electron–Hole Coulomb Attractive Energy and Dipole Moment of Hot Excitons in Donor–Acceptor Polymers. J. Phys. Chem. C 2024, 128, 3525–3532. [Google Scholar] [CrossRef]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. IJBS 2008, 4, 89. [Google Scholar] [CrossRef]
- Kornacka, E.M. Radiation-induced oxidation of polymers. Appl. Ioniz. Radiat. Mater. Process. 2017, 1, 183–192. [Google Scholar]
- Ferry, M.; Roma, G.; Cochin, F.; Esnouf, S.; Dauvois, V.; Nizeyimana, F.; Gervais, B.; Ngono-Ravache, Y. Polymers in the Nuclear Power Industry. In Comprehensive Nuclear Materials, 2nd ed.; Konings, R.J., Stoller, R.E., Eds.; Elsevier: Oxford, UK, 2020; pp. 545–580. [Google Scholar] [CrossRef]
- Colombo, P.; Kukacka, L.; Fontana, J.; Chapman, R.; Steinberg, M. Co60 γ-radiation-induced copolymerization of ethylene and carbon monoxide. J. Polym. Sci. Part A-1 Polym. Chem. 1966, 4, 29–57. [Google Scholar] [CrossRef]
- Rabek, J.F. Photodegradation of Polymers: Physical Characteristics and Applications; Springer Science & Business Media: New York, NY, USA, 2012. [Google Scholar]
- Altintas, O.; Barner-Kowollik, C. Single chain folding of synthetic polymers by covalent and non-covalent interactions: Current status and future perspectives. Macromol. Rapid Commun. 2012, 33, 958–971. [Google Scholar] [CrossRef]
- Tamura, T.; Kawano, M.; Hamachi, I. Targeted Covalent Modification Strategies for Drugging the Undruggable Targets. Chem. Rev. 2025, 125, 1191–1253. [Google Scholar] [CrossRef] [PubMed]
- Yevale, P.; Kumar, S.S.; Agarkar, P.; Yashwantrao, G.; Tripathi, A.; Badani, P.; Rao, A.; Saha, S. Novel diamide covalent organic polymers (COPs) for arresting actinides U (VI), Pu (IV) and Am (III) from highly acidic nuclear stream. Sep. Purif. Technol. 2025, 363, 132181. [Google Scholar] [CrossRef]
- Farinha, A.F.; Oliveira, G.N.; Araújo, J.P.; Santos, L.M.; Costa, J.C. Giant Growth of Crystalline Films of 1, 3, 5-Tris (N-carbazolyl) benzene (TCB) and 1, 3, 5-Tris (diphenylamino) benzene (TDAB) on Engineered Shapes of Ionic Liquid in Vacuum. Cryst. Growth Des. 2025, 25, 612–623. [Google Scholar] [CrossRef]
- Jabbarova, L.Y.; Mustafaev, I.I.; Ibadov, N.A.; Guliyeva, N.G.; Mirzayeva, A.S. Study of Post-Radiation Effects in Gasolines. BIO Web Conf. 2025, 151, 04024. [Google Scholar] [CrossRef]
- Prokhorenko, E.; Lytvynenko, V.; Zakharchenko, A.; Khazhmuradov, M.; Prokhorenko, T.; Ben, A. Brief overview on research and development of radiation shielding protective composite materials based on polystyrene. Probl. At. Sci. Technol. 2024, 107–114. [Google Scholar] [CrossRef]
- Buchalla, R.; Schüttler, C.; Bögl, K.W. Effects of ionizing radiation on plastic food packaging materials: A review. J. Food Prot. 1993, 56, 998–1005. [Google Scholar] [CrossRef]
- Głuszewski, W. GC investigation of post-irradiation oxidation phenomena on polypropylene. Nukleonika 2021, 66, 187–192. [Google Scholar] [CrossRef]
- Tao, Z.; Hu, X.; He, L.; Zhang, H. Direct complexation of citric acid to synthesize high-efficiency bismuth vanadate through molten polymerization route for the degradation of tetracycline hydrochloride under visible light irradiation. New J. Chem. 2022, 46, 8901–8911. [Google Scholar] [CrossRef]
- Krause, B.; Stephan, M.; Volkland, S.; Voigt, D.; Häußler, L.; Dorschner, H. Long-chain branching of polypropylene by electron-beam irradiation in the molten state. J. Appl. Polym. Sci. 2006, 99, 260–265. [Google Scholar] [CrossRef]
- Negaresh, M.; Karbalaei-Bagher, M.; Jahani, Y.; Forouzan, A. A pragmatic approach to analyze the ability of ethylene-octene copolymer in the long chain branching of polypropylene in molten and solid state. SPE Polym. 2024, 5, 151–168. [Google Scholar] [CrossRef]
- Seguchi, T. New trend of radiation application to polymer modification—Irradiation in oxygen free atmosphere and at elevated temperature. Radiat. Phys. Chem. 2000, 57, 367–371. [Google Scholar] [CrossRef]
- Hogstrom, K.R.; Mills, M.D.; Almond, P.R. Electron beam dose calculations. Phys. Med. Biol. 1981, 26, 445. [Google Scholar] [CrossRef]
- Sabharwal, S. Electron beam irradiation applications. In Proceedings of the 25th North American Particle Accelerator Conference, Pasadena, CA, USA, 29 September–4 October 2013; pp. 745–748. [Google Scholar]
- Tretinnikov, O.N.; Ogata, S.; Ikada, Y. Surface crosslinking of polyethylene by electron beam irradiation in air. Polymer 1998, 39, 6115–6120. [Google Scholar] [CrossRef]
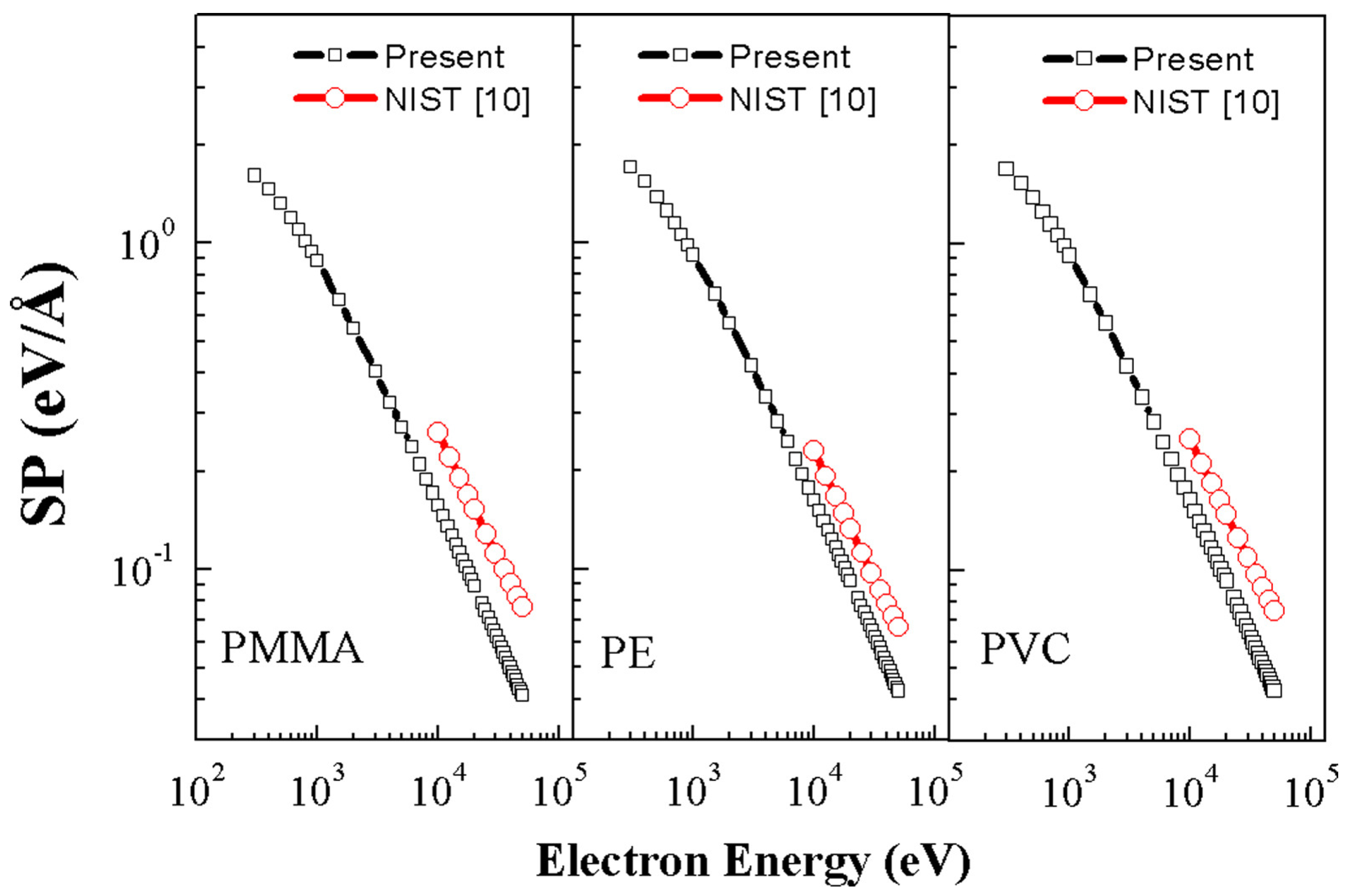
- Tahir, D.; Suarga; Sari, N.H.; Yulianti. Stopping powers and inelastic mean free path of 200eV–50keV electrons in polymer PMMA, PE, and PVC. Appl. Radiat. Isot. 2015, 95, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Apel, P.Y. Intersections of pore channels in track-etched polymer templates and membranes. Mater. Chem. Phys. 2025, 339, 130681. [Google Scholar] [CrossRef]
- Coffey, T.; Urquhart, S.; Ade, H. Characterization of the effects of soft X-ray irradiation on polymers. J. Electron Spectrosc. Relat. Phenom. 2002, 122, 65–78. [Google Scholar] [CrossRef]
- Meng, Q.; Hu, J. A review of shape memory polymer composites and blends. Compos. Part A Appl. Sci. Manuf. 2009, 40, 1661–1672. [Google Scholar] [CrossRef]
- Li, F.; Liu, Y.; Leng, J. Progress of shape memory polymers and their composites in aerospace applications. Smart Mater. Struct. 2019, 28, 103003. [Google Scholar] [CrossRef]
- Phillips, D. Effects of radiation on polymers. Mater. Sci. Technol. 1988, 4, 85–91. [Google Scholar] [CrossRef]
- Manaila, E.; Craciun, G.; Stelescu, M.D.; Ighigeanu, D.; Ficai, M. Radiation vulcanization of natural rubber with polyfunctional monomers. Polym. Bull. 2014, 71, 57–82. [Google Scholar] [CrossRef]
- Jayalath, S.; Herath, M.; Epaarachchi, J.; Trifoni, E.; Gdoutos, E.E.; Fang, L. Durability and long-term behaviour of shape memory polymers and composites for the space industry-A review of current status and future perspectives. Polym. Degrad. Stab. 2023, 211, 110297. [Google Scholar] [CrossRef]
- Barsbay, M.; Guven, O. Nanostructuring of polymers by controlling of ionizing radiation-induced free radical polymerization, copolymerization, grafting and crosslinking by RAFT mechanism. Radiat. Phys. Chem. 2020, 169, 107816. [Google Scholar] [CrossRef]
- Bracco, P.; Costa, L.; Luda, M.P.; Billingham, N. A review of experimental studies of the role of free-radicals in polyethylene oxidation. Polym. Degrad. Stab. 2018, 155, 67–83. [Google Scholar] [CrossRef]
- S, R.; K, A.; C, S.; S, G.; H, D. The physical and chemical properties of gamma ray irradiated polymer electrolyte films. J. Non-Cryst. Solids 2015, 426, 55–62. [Google Scholar] [CrossRef]
- Liu, B.; Wang, P.C.; Ao, Y.Y.; Zhao, Y.; An, Y.; Chen, H.B.; Huang, W. Effects of combined neutron and gamma irradiation upon silicone foam. Radiat. Phys. Chem. 2016, 133, 31–36. [Google Scholar] [CrossRef]
- Sasuga, T.; Hayakawa, N.; Yoshida, K.; Hagiwara, M. Degradation in tensile properties of aromatic polymers by electron beam irradiation. Polymer 1985, 26, 1039–1045. [Google Scholar] [CrossRef]
- Żenkiewicz, M. Effects of electron-beam irradiation on some mechanical properties of polymer films. Radiat. Phys. Chem. 2004, 69, 373–378. [Google Scholar] [CrossRef]
- Oshima, A.; Shiraki, F.; Fujita, H.; Washio, M. Surface modification of polymeric materials using ultra low energy electron beam irradiation. Radiat. Phys. Chem. 2011, 80, 196–200. [Google Scholar] [CrossRef]
- Faltermeier, A.; Behr, M.; Reicheneder, C.; Proff, P.; Römer, P. Electron-beam irradiation of polymer bracket materials. Am. J. Orthod. Dentofac. Orthop. 2011, 139, e7–e11. [Google Scholar] [CrossRef]
- Cairns, M.L.; Sykes, A.; Dickson, G.R.; Orr, J.F.; Farrar, D.; Dumba, A.; Buchanan, F.J. Through-thickness control of polymer bioresorption via electron beam irradiation. Acta Biomater. 2011, 7, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Fares, S. Influence of gamma-ray irradiation on optical and thermal degradation of poly (ethyl-methacrylate)(PEMA) polymer. Nat. Sci. 2012, 4, 499–507. [Google Scholar] [CrossRef]
- Mathakari, N.; Bhoraskar, V.; Dhole, S. A comparative study on the effects of Co-60 gamma radiation on polypropylene and polyimide. Radiat. Eff. Defects Solids 2014, 169, 779–790. [Google Scholar] [CrossRef]
- Assink, R.A.; Gillen, K.T.; Bernstein, R. Nuclear Energy Plant Optimization (NEPO) Final Report on Aging and Condition Monitoring of Low-Voltage Cable Materials; Technical Report; Sandia National Laboratories (SNL): Albuquerque, NM, USA; Livermore, CA, USA, 2005. [Google Scholar]
- Gillen, K.T.; Clough, R.L. Aging Predictions in Nuclear Power Plants: Crosslinked Polyolefin and EPR Cable Insulation Materials; Technical Report; Sandia National Labs.: Albuquerque, NM, USA, 1991. [Google Scholar]
- Svoboda, P.; Trivedi, K.; Stoklasa, K.; Svobodova, D.; Ougizawa, T. Study of crystallization behaviour of electron beam irradiated polypropylene and high-density polyethylene. R. Soc. Open Sci. 2021, 8, 202250. [Google Scholar] [CrossRef]
- Sirin, M.; Zeybek, M.S.; Sirin, K.; Abali, Y. Effect of gamma irradiation on the thermal and mechanical behaviour of polypropylene and polyethylene blends. Radiat. Phys. Chem. 2022, 194, 110034. [Google Scholar] [CrossRef]
- Carswell-Pomerantz, T.; Babanalbandi, A.; Dong, L.; Hill, D.; Perera, M.; Pomery, P.; Saadat, G.; Whittaker, A. Stability and Stabilization of Polymers Under Irradiation; IAEA: Vienna, Austria, 1999; p. 111. [Google Scholar]
- Liu, B.; Gao, Y.; Li, J.; Guo, C.; Gao, J.; Chen, Y.; Du, B. Aging behavior of polypropylene as cable insulation under gamma-ray irradiation. IEEE Trans. Dielectr. Electr. Insul. 2023, 31, 956–964. [Google Scholar] [CrossRef]
- Alariqi, S.A.; Kumar, A.P.; Rao, B.; Singh, R. Effect of γ-dose rate on crystallinity and morphological changes of γ-sterilized biomedical polypropylene. Polym. Degrad. Stab. 2009, 94, 272–277. [Google Scholar] [CrossRef]
- Kagiya, T.; Nishimoto, S.; Watanabe, Y.; Kato, M. Importance of the amorphous fraction of polypropylene in the resistance to radiation-induced oxidative degradation. Polym. Degrad. Stab. 1985, 12, 261–275. [Google Scholar] [CrossRef]
- Perera, R.; Albano, C.; Gonzalez, J.; Silva, P.; Ichazo, M. The effect of gamma radiation on the properties of polypropylene blends with styrene–butadiene–styrene copolymers. Polym. Degrad. Stab. 2004, 85, 741–750. [Google Scholar] [CrossRef]
- Pajuste, E.; Reinholds, I.; Vaivars, G.; Antuzevičs, A.; Avotiņa, L.; Sprūḡis, E.; Mikko, R.; Heikki, K.; Meri, R.; Kaparkalējs, R. Evaluation of radiation stability of electron beam irradiated Nafion® and sulfonated poly (ether ether ketone) membranes. Polym. Degrad. Stab. 2022, 200, 109970. [Google Scholar] [CrossRef]
- Nakamura, H.; Nakamura, T.; Noguchi, T.; Imagawa, K. Photodegradation of PEEK sheets under tensile stress. Polym. Degrad. Stab. 2006, 91, 740–746. [Google Scholar] [CrossRef]
- Li, H.; Fouracre, R.; Given, M.; Banford, H.; Wysocki, S.; Karolczak, S. The effects on polyetheretherketone and polyethersulfone of electron and/spl gamma/irradiation. IEEE Trans. Dielectr. Electr. Insul. 1999, 6, 295–303. [Google Scholar] [CrossRef]
- Birkinshaw, C.; Buggy, M.; Daly, S. The effect of sterilising radiation on the properties of Nylon 66. Mater. Chem. Phys. 1987, 17, 239–248. [Google Scholar] [CrossRef]
- Suarez, J.C.M.; da Costa Monteiro, E.E.; Mano, E.B. Study of the effect of gamma irradiation on polyolefins—Low-density polyethylene. Polym. Degrad. Stab. 2002, 75, 143–151. [Google Scholar] [CrossRef]
- Bludenko, A.; Ponomarev, A.; Kholodkova, E.; Khusyainova, D.; Shapagin, A. Influence of Irradiation and Postradiation Storage on the Adhesion of Polyolefins. High Energy Chem. 2022, 56, 258–263. [Google Scholar] [CrossRef]
- Deshmukh, D.; Kulkarni, H.; Srivats, D.S.; Bhanushali, S.; More, A.P. Recycling of acrylonitrile butadiene styrene (ABS): A review. Polym. Bull. 2024, 81, 1–38. [Google Scholar] [CrossRef]
- Alonso, A.; Lázaro, M.; Lázaro, D.; Alvear, D. Thermal characterization of acrylonitrile butadiene styrene-ABS obtained with different manufacturing processes. J. Therm. Anal. Calorim. 2023, 148, 10557–10572. [Google Scholar] [CrossRef]
- Gupta, N.K.; Pandey, P.; Mehta, S.; Swati, S.; Mishra, S.K.; Tom, K.J. Characterization of ABS material in hybrid composites: A review. In Advances in Engineering Design: Select Proceedings of FLAME 2018; Springer: Singapore, 2019; pp. 619–630. [Google Scholar]
- Massey, L. Acrylonitrile-butadiene-styrene. In The Effects of UV Light and Weather on Plastics and Elastomers; William Andrew: Norwich, NY, USA, 2007; pp. 13–32. [Google Scholar]
- McTaggart, R.; Rankouhi, B.; Letcher, T. Effects of gamma irradiation on mechanical properties of 3D-printed carbon fiber-reinforced ABS. Int. J. Adv. Manuf. Technol. 2020, 111, 1917–1927. [Google Scholar] [CrossRef]
- Hassan, M.M. Mechanical, thermal, and morphological behavior of the polyamide 6/acrylonitrile–butadiene–styrene blends irradiated with gamma rays. Polym. Eng. Sci. 2008, 48, 373–380. [Google Scholar] [CrossRef]
- de Brouwer, H. Comparison of the effects of X-ray and gamma irradiation on engineering thermoplastics. Radiat. Phys. Chem. 2022, 193, 109999. [Google Scholar] [CrossRef]
- Pickett, J.; Gibson, D.; Gardner, M. Effects of irradiation conditions on the weathering of engineering thermoplastics. Polym. Degrad. Stab. 2008, 93, 1597–1606. [Google Scholar] [CrossRef]
- Antonakou, E.; Achilias, D. Recent advances in polycarbonate recycling: A review of degradation methods and their mechanisms. Waste Biomass Valorization 2013, 4, 9–21. [Google Scholar] [CrossRef]
- Chen, J.; Czayka, M.; Uribe, R.M. Effects of electron beam irradiations on the structure and mechanical properties of polycarbonate. Radiat. Phys. Chem. 2005, 74, 31–35. [Google Scholar] [CrossRef]
- de Melo, N.S.; Weber, R.P.; Suarez, J.C.M. Toughness behavior of gamma-irradiated polycarbonate. Polym. Test. 2007, 26, 315–322. [Google Scholar] [CrossRef]
- Wady, P.; Wasilewski, A.; Brock, L.; Edge, R.; Baidak, A.; McBride, C.; Leay, L.; Griffiths, A.; Valles, C. Effect of ionising radiation on the mechanical and structural properties of 3D printed plastics. Addit. Manuf. 2020, 31, 100907. [Google Scholar] [CrossRef]
- El-Farahaty, K.; Sadik, A.; Hezma, A. γ-Irradiation effects on opto-thermal and-mechanical properties of PET and PETG fibers. Int. J. Polym. Mater. 2009, 58, 366–383. [Google Scholar] [CrossRef]
- Shaffer, S.; Yang, K.; Vargas, J.; Di Prima, M.A.; Voit, W. On reducing anisotropy in 3D printed polymers via ionizing radiation. Polymer 2014, 55, 5969–5979. [Google Scholar] [CrossRef]
- West, C.; McTaggart, R.; Letcher, T.; Raynie, D.; Roy, R. Effects of gamma irradiation upon the mechanical and chemical properties of 3D-printed samples of polylactic acid. J. Manuf. Sci. Eng. 2019, 141, 041002. [Google Scholar] [CrossRef]
- Cressall, S.; Phillips, C.O.; Al-Shatty, W.; Deganello, D. The effect of high-intensity gamma radiation on PETG and ASA polymer-based fused deposition modelled 3D printed parts. J. Mater. Sci. 2024, 59, 1768–1782. [Google Scholar] [CrossRef]
- Amza, C.G.; Zapciu, A.; Baciu, F.; Vasile, M.I.; Popescu, D. Aging of 3D printed polymers under sterilizing UV-C radiation. Polymers 2021, 13, 4467. [Google Scholar] [CrossRef]
- Yogeshwaran, V.; Chandradass, J.; Chinnapandi, M.; Velmurugan, R. Effect of Gamma Irradiation on Mechanical and Thermal Properties of 3D-Printed PLAs. In Proceedings of the International Symposium on Plasticity and Impact Mechanics, Chennai, India, 21–26 August 2022; Springer: Singapore, 2022; pp. 335–348. [Google Scholar] [CrossRef]
- Rankouhi, B.; Javadpour, S.; Delfanian, F.; McTaggart, R.; Letcher, T. Experimental investigation of mechanical performance and printability of gamma-irradiated additively manufactured ABS. J. Mater. Eng. Perform. 2018, 27, 3643–3654. [Google Scholar] [CrossRef]
- Mastalerz, C.; Vroman, I.; Coqueret, X.; Alix, S. Effects of electron beam irradiation on 3D-printed biopolymers for bone tissue engineering. J. Compos. Sci. 2021, 5, 182. [Google Scholar] [CrossRef]
- Behl, M.; Lendlein, A. Shape-memory polymers. Mater. Today 2007, 10, 20–28. [Google Scholar] [CrossRef]
- Mather, P.T.; Luo, X.; Rousseau, I.A. Shape memory polymer research. Annu. Rev. Mater. Res. 2009, 39, 445–471. [Google Scholar] [CrossRef]
- Voit, W.; Ware, T.; Gall, K. Radiation crosslinked shape-memory polymers. Polymer 2010, 51, 3551–3559. [Google Scholar] [CrossRef]
- Sharma, V.; Mahajan, J.; Bhattacharyya, P. Electron beam (EB) crosslinking of PVC insulation in presence of sensitiser additives. Radiat. Phys. Chem. 1995, 45, 695–701. [Google Scholar] [CrossRef]
- Shi, J.; Lu, H.; Zheng, T.; Fu, Y.Q. A dynamic phase separation model for glass transition behavior in water-triggered shape memory polymer towards programmable recovery onset. J. Phys. D Appl. Phys. 2024, 57, 305301. [Google Scholar] [CrossRef]
- Xie, F.; Liu, L.; Gong, X.; Huang, L.; Leng, J.; Liu, Y. Effects of accelerated aging on thermal, mechanical and shape memory properties of cyanate-based shape memory polymer: I vacuum ultraviolet radiation. Polym. Degrad. Stab. 2017, 138, 91–97. [Google Scholar] [CrossRef]
- Xu, T.; Li, G.; Pang, S.S. Effects of ultraviolet radiation on morphology and thermo-mechanical properties of shape memory polymer based syntactic foam. Compos. Part A Appl. Sci. Manuf. 2011, 42, 1525–1533. [Google Scholar] [CrossRef]
- Zhu, G.; Liang, G.; Xu, Q.; Yu, Q. Shape-memory effects of radiation crosslinked poly (ϵ-caprolactone). J. Appl. Polym. Sci. 2003, 90, 1589–1595. [Google Scholar] [CrossRef]
- Alfayyadh, A.A.; Lotfy, S.; Basfar, A.; Khalil, M.I. Influences of poly (vinyl alcohol) molecular weight and carbon nanotubes on radiation crosslinking shape memory polymers. Prog. Nat. Sci. Mater. Int. 2017, 27, 316–325. [Google Scholar] [CrossRef]
- Leng, J.; Xie, F.; Wu, X.; Liu, Y. Effect of the γ-radiation on the properties of epoxy-based shape memory polymers. J. Intell. Mater. Syst. Struct. 2014, 25, 1256–1263. [Google Scholar] [CrossRef]
- Clough, R.L.; Gillen, K.T. Combined environment aging effects: Radiation-thermal degradation of polyvinylchloride and polyethylene. J. Polym. Sci. Polym. Chem. Ed. 1981, 19, 2041–2051. [Google Scholar] [CrossRef]
- Bartoníček, B.; Hnát, V.; Plaček, V. Life-assessment technique for nuclear power plant cables. Radiat. Phys. Chem. 1998, 52, 639–642. [Google Scholar] [CrossRef]
- Shimada, A.; Sugimoto, M.; Kudoh, H.; Tamura, K.; Seguchi, T. Radiation ageing technique for cable life evaluation of nuclear power plant. IEEE Trans. Dielectr. Electr. Insul. 2012, 19, 1768–1773. [Google Scholar] [CrossRef]
- Cataldo, F.; Prata, M. New composites for neutron radiation shielding. J. Radioanal. Nucl. Chem. 2019, 320, 831–839. [Google Scholar] [CrossRef]
- Nagaraja, N.; Manjunatha, H.; Seenappa, L.; Sridhar, K.; Ramalingam, H. Radiation shielding properties of silicon polymers. Radiat. Phys. Chem. 2020, 171, 108723. [Google Scholar] [CrossRef]
- Nambiar, S.; Yeow, J.T. Polymer-composite materials for radiation protection. ACS Appl. Mater. Interfaces 2012, 4, 5717–5726. [Google Scholar] [CrossRef] [PubMed]
- Kaçal, M.; Akman, F.; Sayyed, M. Evaluation of gamma-ray and neutron attenuation properties of some polymers. Nucl. Eng. Technol. 2019, 51, 818–824. [Google Scholar] [CrossRef]
- Chmielewski, A.G.; Haji-Saeid, M.; Ahmed, S. Progress in radiation processing of polymers. Nucl. Instruments Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2005, 236, 44–54. [Google Scholar] [CrossRef]
- Chang, Z.; LaVerne, J.A. The γ-radiolysis of nylons: Molecular rearrangement and gas production. J. Phys. Chem. B 2002, 106, 508–514. [Google Scholar] [CrossRef]
- Seguchi, T. Mechanisms and kinetics of hydrogen yield from polymers by irradiation. Nucl. Instruments Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2001, 185, 43–49. [Google Scholar] [CrossRef]
- Darwis, D.; Erizal; Abbas, B.; Nurlidar, F.; Putra, D.P. Radiation processing of polymers for medical and pharmaceutical applications. Macromol. Symp. 2015, 353, 15–23. [Google Scholar] [CrossRef]
- Islam, A.; Yasin, T.; ur Rehman, I. Synthesis of hybrid polymer networks of irradiated chitosan/poly (vinyl alcohol) for biomedical applications. Radiat. Phys. Chem. 2014, 96, 115–119. [Google Scholar] [CrossRef]
- Sintzel, M.B.; Merkli, A.; Tabatabay, C.; Gurny, R. Influence of irradiation sterilization on polymers used as drug carriers—A review. Drug Dev. Ind. Pharm. 1997, 23, 857–878. [Google Scholar] [CrossRef]
- Bruck, S.D.; Mueller, E.P. Radiation sterilization of polymeric implant materials. J. Biomed. Mater. Res. 1988, 22, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Chouirfa, H.; Migonney, V.; Falentin-Daudré, C. Grafting bioactive polymers onto titanium implants by UV irradiation. RSC Adv. 2016, 6, 13766–13771. [Google Scholar] [CrossRef]
- Zhu, X.; Punia, A.; Skomski, D.; Su, Y.; Shultz, C.S.; Giles, M.B.; Rudd, N.D.; Raman, N.; Koynov, A.; Lamm, M.S. Insights into Factors Affecting Ethylene-Vinyl Acetate Copolymer Crystallinity in Islatravir Implant. Mol. Pharm. 2024, 21, 1933–1941. [Google Scholar] [CrossRef] [PubMed]
- Ulery, B.D.; Nair, L.S.; Laurencin, C.T. Biomedical applications of biodegradable polymers. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 832–864. [Google Scholar] [CrossRef]
- Lekkala, S.; Inverardi, N.; Grindy, S.C.; Hugard, S.; Muratoglu, O.K.; Oral, E. Irradiation behavior of analgesic and nonsteroidal anti-inflammatory drug-loaded UHMWPE for joint replacement. Biomacromolecules 2024, 25, 2312–2322. [Google Scholar] [CrossRef]
- Lin, J.; Lin, Z.; Liu, L.; Lin, W.; Xie, X.; Zhang, X. Enhancing glioma-specific drug delivery through self-assembly of macrophage membrane and targeted polymer assisted by low-frequency ultrasound irradiation. Mater. Today Bio. 2024, 26, 101067. [Google Scholar] [CrossRef]
- Herczeg, C.K.; Song, J. Sterilization of Polymeric Implants: Challenges and Opportunities. ACS Appl. Bio Mater. 2022, 5, 5077–5088. [Google Scholar] [CrossRef] [PubMed]
- Premnath, V.; Harris, W.; Jasty, M.; Merrill, E. Gamma sterilization of UHMWPE articular implants: An analysis of the oxidation problem. Biomaterials 1996, 17, 1741–1753. [Google Scholar] [CrossRef]
- Zhang, F.; Mao, X.; Lei, H.; Guo, F.; Shen, R.; Xing, Z.; Wu, G. Investigation on discoloration mechanism of cyclic olefin copolymer under ionizing irradiation sterilization. Polym. Degrad. Stab. 2024, 221, 110676. [Google Scholar] [CrossRef]
- Havlickova, K.; Kuzelova Kostakova, E.; Lisnenko, M.; Hauzerova, S.; Stuchlik, M.; Vrchovecka, S.; Vistejnova, L.; Molacek, J.; Lukas, D.; Prochazkova, R.; et al. The Impacts of the Sterilization Method and the Electrospinning Conditions of Nanofibrous Biodegradable Layers on Their Degradation and Hemocompatibility Behavior. Polymers 2024, 16, 1029. [Google Scholar] [CrossRef] [PubMed]
- Coquen, A.D.; Bondzior, B.; Petit, L.; Kellomäki, M.; Pauthe, E.; Boissière, M.; Massera, J. Evaluation of the sterilization effect on biphasic scaffold based on bioactive glass and polymer honeycomb membrane. J. Am. Ceram. Soc. 2024, 107, 154–165. [Google Scholar] [CrossRef]
- Karl, D.M. Plastics-irradiated-etched: The Nuclepore® filter turns 45 years old. Limnol. Oceanogr. Bull. 2007, 16, 49–54. [Google Scholar] [CrossRef]
- Sergeev, A.; Khataibe, E.; Berezkin, V.; Nechaev, A.; Chikhacheva, I.; Zubov, V.; Mchedlishvili, B. Modification of the surfaces of polymer films irradiated by heavy ions and nucleopore nanofilters by XeF 2 vapors. Colloid J. 2003, 65, 84–88. [Google Scholar] [CrossRef]
- Yang, B.; Yang, W. Photografting modification of PET nucleopore membranes. J. Macromol. Sci. Part A 2003, 40, 309–320. [Google Scholar] [CrossRef]
- Wang, Y.; Bao, Z.; Ding, S.; Jia, J.; Dai, Z.; Li, Y.; Shen, S.; Chu, S.; Yin, Y.; Li, X. γ-Ray Irradiation Significantly Enhances Capacitive Energy Storage Performance of Polymer Dielectric Films. Adv. Mater. 2024, 36, 2308597. [Google Scholar] [CrossRef]
- Atta, A.; Alotaibi, B.; Waly, S.; Reheem, A.A.; Al-Yousef, H.A. Influence of argon irradiation on electrical properties of PVA/NaI polymer composites. Surf. Rev. Lett. 2024, 31, 2450029. [Google Scholar] [CrossRef]
- Altuijri, R.; Abdelhamied, M.; Atta, A.; Abdel-Hamid, H.; Henaish, A.; El-Aassar, M. Impacts of Low Energy Oxygen Irradiation on the Dielectric Properties of PVA/TiO2 Nanocomposite Films. ECS J. Solid State Sci. Technol. 2024, 13, 043005. [Google Scholar] [CrossRef]
- Guo, C.; Sun, Y.; Wang, L.; Liu, C.; Chen, C.; Cheng, J.; Xia, W.; Gan, Z.; Zhou, J.; Chen, Z.; et al. Light-induced quinone conformation of polymer donors toward 19.9% efficiency organic solar cells. Energy Environ. Sci. 2024, 17, 2492–2499. [Google Scholar] [CrossRef]
- Coghi, P.; Coluccini, C. Literature Review on Conjugated Polymers as Light-Sensitive Materials for Photovoltaic and Light-Emitting Devices in Photonic Biomaterial Applications. Polymers 2024, 16, 1407. [Google Scholar] [CrossRef]
- Bibi, A.; Shakoor, A.; Niaz, N.A.; Raffi, M.; Salman, M.; Usman, Z. Enhanced solar-driven photocatalytic and photovoltaic performance of polymer composite containing carbon black and calcium titanate nanoparticles. Polym. Bull. 2024, 81, 8359–8382. [Google Scholar] [CrossRef]
- Haji-Saeid, M.; Sampa, M.H.O.; Chmielewski, A.G. Radiation treatment for sterilization of packaging materials. Radiat. Phys. Chem. 2007, 76, 1535–1541. [Google Scholar] [CrossRef]
- Welle, F.; Mauer, A.; Franz, R. Migration and sensory changes of packaging materials caused by ionising radiation. Radiat. Phys. Chem. 2002, 63, 841–844. [Google Scholar] [CrossRef]
- Abou Elmaaty, T.; Okubayashi, S.; Elsisi, H.; Abouelenin, S. Electron beam irradiation treatment of textiles materials: A review. J. Polym. Res. 2022, 29, 117. [Google Scholar] [CrossRef]
- Nouh, S.; Barakat, M.; El-Nabarawy, H.A.; Benthami, K.; Elhalawany, N. Color changes in some UV irradiated polymer nanocomposite materials for the application in textile industry. Fibers Polym. 2021, 22, 1711–1717. [Google Scholar] [CrossRef]
- Silva, D.; Rocha, R.; Silva, C.J.; Barroso, H.; Botelho, J.; Machado, V.; Mendes, J.J.; Oliveira, J.; Loureiro, M.V.; Marques, A.C.; et al. Gamma radiation for sterilization of textile based materials for personal protective equipment. Polym. Degrad. Stab. 2021, 194, 109750. [Google Scholar] [CrossRef]
- An, X.; Shao, Y.; Ma, L.; Wang, H.; Xu, G.; Luo, M. Research Progress of Electron Beam Radiation Grafting Modification of Textiles. Radiat. Phys. Chem. 2024, 222, 111817. [Google Scholar] [CrossRef]
- Walsh, W.; Bittencourt, E. Radiation processes for textiles. Radiat. Phys. Chem. 1977, 9, 289–305. [Google Scholar] [CrossRef]
- Drábková, K.; Ďurovič, M.; Kučerová, I. Influence of gamma radiation on properties of paper and textile fibres during disinfection. Radiat. Phys. Chem. 2018, 152, 75–80. [Google Scholar] [CrossRef]
- Lachance, J.; Coia, C.; Fozza, A.; Czeremuszkin, G.; Houdayer, A.; Wertheimer, M.R. Radiation-induced degradation of polymeric spacecraft materials under protective oxide coatings. Nucl. Instruments Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2001, 185, 328–335. [Google Scholar] [CrossRef]
- Bhowmik, S.; Bonin, H.; Bui, V.; Weir, R. Modification of high-performance polymer composite through high-energy radiation and low-pressure plasma for aerospace and space applications. J. Appl. Polym. Sci. 2006, 102, 1959–1967. [Google Scholar] [CrossRef]
- Chen, J.; Ding, N.; Li, Z.; Wang, W. Organic polymer materials in the space environment. Prog. Aerosp. Sci. 2016, 83, 37–56. [Google Scholar] [CrossRef]
- de Groh, K.K.; Banks, B.A.; Miller, S.K.; Dever, J.A. Degradation of spacecraft materials. In Handbook of Environmental Degradation of Materials; Elsevier: Amsterdam, The Netherlands, 2018; pp. 601–645. [Google Scholar] [CrossRef]
- Kleiman, J.; Iskanderova, Z.; Perez, F.; Tennyson, R. Protective coatings for LEO environments in spacecraft applications. Surf. Coat. Technol. 1995, 76, 827–834. [Google Scholar] [CrossRef]
- Grossman, E.; Gouzman, I. Space environment effects on polymers in low earth orbit. Nucl. Instruments Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2003, 208, 48–57. [Google Scholar] [CrossRef]
- Kleiman, J. Surface modification technologies for durable space polymers. MRS Bull. 2010, 35, 55–65. [Google Scholar] [CrossRef]
- Kalev, K.; Manov, L. A Preliminary Study of Photon Radiation Attenuation from Ballistic Protection Materials. In Proceedings of the ENVIRONMENT. TECHNOLOGIES. RESOURCES. Proceedings of the International Scientific and Practical Conference, Rezekne, Latvia, 27–28 June 2024. [CrossRef]
- Qi, C.; Zhao, Y.; Wang, Z. Improving ballistic limits of UHMWPE laminates by gamma-ray irradiation induced enhancement of interfacial bonding. Polym. Compos. 2024, 45, 12709–12722. [Google Scholar] [CrossRef]
- Nurazzi, N.M.; Asyraf, M.; Khalina, A.; Abdullah, N.; Aisyah, H.; Rafiqah, S.A.; Sabaruddin, F.; Kamarudin, S.; Norrrahim, M.; Ilyas, R.; et al. A review on natural fiber reinforced polymer composite for bullet proof and ballistic applications. Polymers 2021, 13, 646. [Google Scholar] [CrossRef]
- Wang, T.; Hou, Z.; Yang, H.; Hu, J. A PEGylated PVDF Antifouling Membrane Prepared by Grafting of Methoxypolyethylene Glycol Acrylate in Gama-Irradiated Homogeneous Solution. Materials 2024, 17, 873. [Google Scholar] [CrossRef]
- Du, X.; Li, L.; Li, J.; Yang, C.; Frenkel, N.; Welle, A.; Heissler, S.; Nefedov, A.; Grunze, M.; Levkin, P.A. UV-triggered dopamine polymerization: Control of polymerization, surface coating, and photopatterning. Adv. Mater. 2014, 26, 8029–8033. [Google Scholar] [CrossRef]
- Ward, J.H.; Bashir, R.; Peppas, N.A. Micropatterning of biomedical polymer surfaces by novel UV polymerization techniques. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2001, 56, 351–360. [Google Scholar] [CrossRef]
- Mellott, M.B.; Searcy, K.; Pishko, M.V. Release of protein from highly cross-linked hydrogels of poly (ethylene glycol) diacrylate fabricated by UV polymerization. Biomaterials 2001, 22, 929–941. [Google Scholar] [CrossRef] [PubMed]
- Mendes-Felipe, C.; Oliveira, J.; Etxebarria, I.; Vilas-Vilela, J.L.; Lanceros-Mendez, S. State-of-the-art and future challenges of UV curable polymer-based smart materials for printing technologies. Adv. Mater. Technol. 2019, 4, 1800618. [Google Scholar] [CrossRef]
- Motegi, T.; Omichi, M.; Maekawa, Y.; Seko, N. Direct observation of radiation-induced graft polymerization on a polyethylene film. Radiat. Phys. Chem. 2024, 214, 111281. [Google Scholar] [CrossRef]
- Sadiq, A.; Saleem, F.; Mumtaz, S.; Nasir, A.; Yasin, T. Synthesis and Study of Free Radical Graft Polymerization of Glycidyl Methacrylate on Gamma-Irradiated Graphene Oxide. Mater. Today Commun. 2024, 40, 109433. [Google Scholar] [CrossRef]
- Chapiro, A. Radiation induced grafting. Radiat. Phys. Chem. 1977, 9, 55–67. [Google Scholar] [CrossRef]
- El-Nemr, K.F.; Khalil, A.M.; Fathy, E. Thermoplastic elastomers based on waste rubber and expanded polystyrene: Role of devulcanization and ionizing radiation. Int. J. Polym. Anal. Charact. 2018, 23, 58–69. [Google Scholar] [CrossRef]
- Stelescu, M.D.; Manaila, E.; Craciun, G. Vulcanization of ethylene-propylene–terpolymer-based rubber mixtures by radiation processing. J. Appl. Polym. Sci. 2013, 128, 2325–2336. [Google Scholar] [CrossRef]
- Yasin, T.; Ahmed, S.; Yoshii, F.; Makuuchi, K. Radiation vulcanization of acrylonitrile–butadiene rubber with polyfunctional monomers. React. Funct. Polym. 2002, 53, 173–181. [Google Scholar] [CrossRef]
- Coran, A.Y. Vulcanization. In Science and Technology of Rubber; Elsevier: Amsterdam, The Netherlands, 1994; pp. 339–385. [Google Scholar] [CrossRef]
- Haque, M.; Dafader, N.; Akhtar, F.; Ahmad, M. Radiation dose required for the vulcanization of natural rubber latex. Radiat. Phys. Chem. 1996, 48, 505–510. [Google Scholar] [CrossRef]
- Antonietto, H.P.; de Oliveira, M.J.A.; Araujo, M.S.; Otubo, L.; Castanho, S.R.M.; Vasquez, P.A.S. Effects of ionizing radiation on artistic paints and pigments used in the restoration of works of art. Braz. J. Radiat. Sci. 2024, 12, e2684. [Google Scholar] [CrossRef]
- Mestric, V.; Pucic, I.; Desnica, V.; Satovic, D.; Marusic, K. Impact of gamma irradiation on protein-based binders in historical painting media. Dyes Pigments 2025, 235, 112606. [Google Scholar] [CrossRef]
- Manea, M.M.; Negut, C.; Stanculescu, I.R.; Ponta, C. Irradiation effects on canvas oil painting: Spectroscopic observations. Radiat. Phys. Chem. 2012, 81, 1595–1599. [Google Scholar] [CrossRef]
- Müller, K.; Szikszai, Z.; Csepregi, Á.; Huszánk, R.; Kertész, Z.; Reiche, I. Proton beam irradiation induces invisible modifications under the surface of painted parchment. Sci. Rep. 2022, 12, 113. [Google Scholar] [CrossRef]
- Burillo, G.; Clough, R.L.; Czvikovszky, T.; Guven, O.; Le Moel, A.; Liu, W.; Singh, A.; Yang, J.; Zaharescu, T. Polymer recycling: Potential application of radiation technology. Radiat. Phys. Chem. 2002, 64, 41–51. [Google Scholar] [CrossRef]
- Dutta, K.; Gohil, J.M. Polymer Recycling by Radiation. In Applications of High Energy Radiations: Synthesis and Processing of Polymeric Materials; Springer: Singapore, 2023; pp. 347–372. [Google Scholar] [CrossRef]
- Zaharuddin, I.M.; Zaid, M.H.M.; Harun, M.H.; Yaakob, Y.; Ismail, N.B. Polyethylene Terephthalate (PET) Recycling: Gamma irradiation impact on crystallinity, chemical structure and thermal stability. Appl. Phys. A 2025, 131, 171. [Google Scholar] [CrossRef]
- Krizsma, S.; Mészáros, L.; Kovács, N.K.; Suplicz, A. Expanding the applicability of material jetting–printed photopolymer prototype injection moulds by gamma irradiation post-treatment. J. Manuf. Process. 2025, 134, 135–145. [Google Scholar] [CrossRef]
- Nouh, S.; Abdel-Salam, M.; Morsy, A.A. Electrical, optical and structural behaviour of fast neutron-irradiation-induced CR-39 SSNTD. Radiat. Meas. 2002, 37, 25–29. [Google Scholar] [CrossRef]
- Fisher, G.L.; Lakis, R.E.; Davis, C.C.; Szakal, C.; Swadener, J.G.; Wetteland, C.J.; Winograd, N. Mechanical properties and the evolution of matrix molecules in PTFE upon irradiation with MeV alpha particles. Appl. Surf. Sci. 2006, 253, 1330–1342. [Google Scholar] [CrossRef]
- Rahaman, M.; Periyasami, G.; Aldalbahi, A. Effect of different gamma dose and chemical etching on pre-and post-alpha-irradiated PM-355 polymer. Int. J. Polym. Sci. 2021, 2021, 8825079. [Google Scholar] [CrossRef]
- Roxby, P.; Michel, H.; Huart, C.; Dorey, S. Effect of Gamma and X-ray Irradiation on Polymers Commonly Used in Healthcare Products. Biomed. Instrum. Technol. 2024, 58, 7–17. [Google Scholar] [CrossRef]
- Sebak, M.; Aladim, A.; Mostafa, M.; Abdelhamid Shahat, M. Improving the efficiency of polymer solar cells based on chitosan@PVA@rGO composites via gamma-irradiated treatment of rGO nanoparticles. Solid State Sci. 2025, 159, 107773. [Google Scholar] [CrossRef]
- Manas, D.; Ovsik, M.; Mizera, A.; Manas, M.; Hylova, L.; Bednarik, M.; Stanek, M. The effect of irradiation on mechanical and thermal properties of selected types of polymers. Polymers 2018, 10, 158. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, A.B.H.d.S.; Vital, H.d.C.; Weber, R.P.; Lima Júnior, É.P.; Rodrigues, J.G.P.; Aguilera, L.d.S.; Biasi, R.S.d. Ballistic tests of alumina-UHMWPE composites submitted to gamma radiation. Mater. Res. 2019, 22, e20190251. [Google Scholar] [CrossRef]
- Saleh, B.A.A.; Kraishan, A.; Elimat, Z.M.; Karaki, I.A.; Alzubi, R.I.; Juwhari, H.K. Effect of gamma radiation on the optical properties of PMMA composites with varying Al concentrations. Radiat. Phys. Chem. 2025, 226, 112342. [Google Scholar] [CrossRef]
- Goncalves Bardi, M.A.; Leite Munhoz, M.d.M.; Oliveira, H.A.d.; Auras, R.; Machado, L.D.B. Behavior of UV-cured print inks on LDPE and PBAT/TPS blend substrates during curing, postcuring, and accelerated degradation. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Jelčić, Ž.; Ranogajec, F. Radiation modified high impact polystyrene. Radiat. Phys. Chem. 2012, 81, 1366–1369. [Google Scholar] [CrossRef]


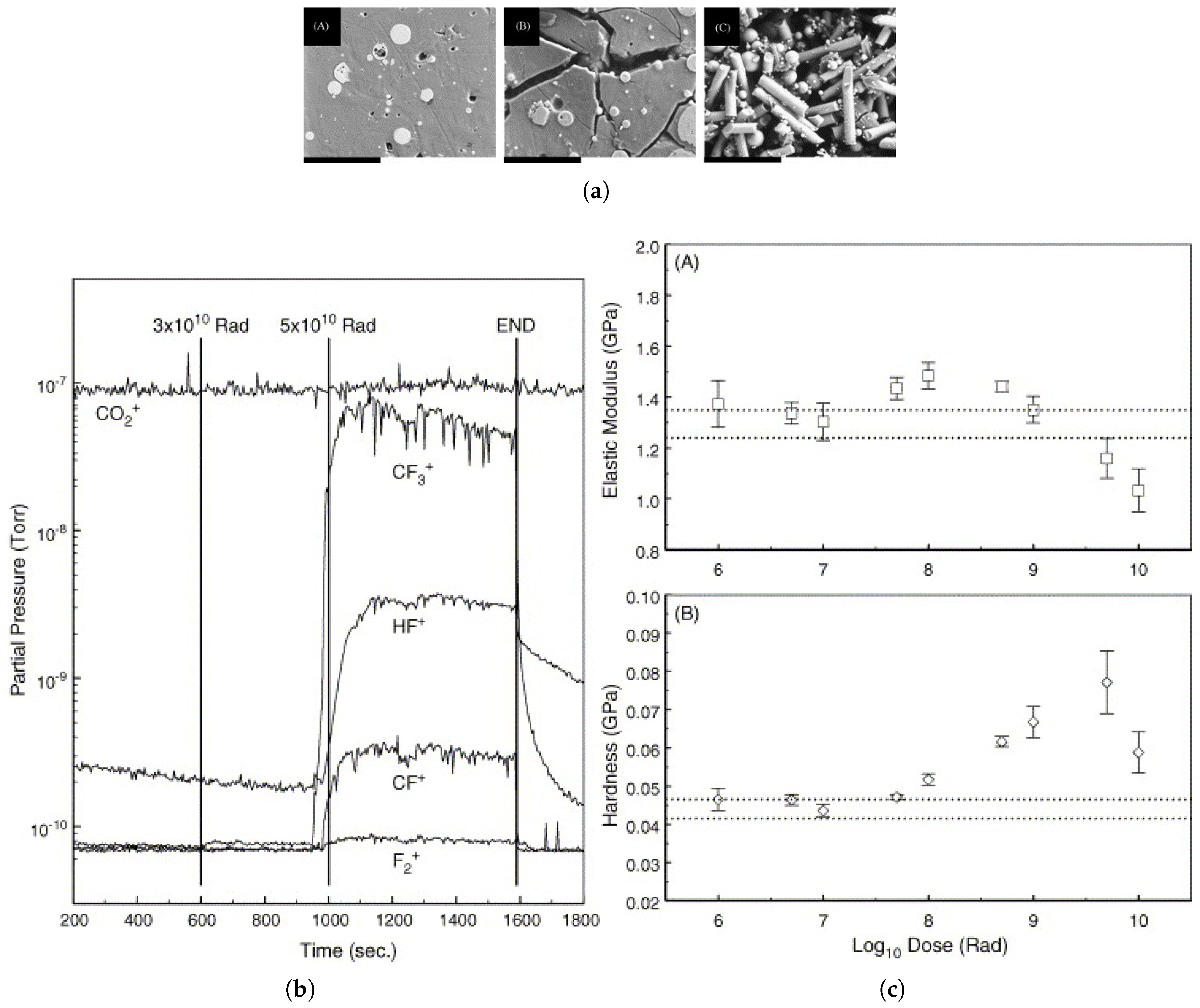
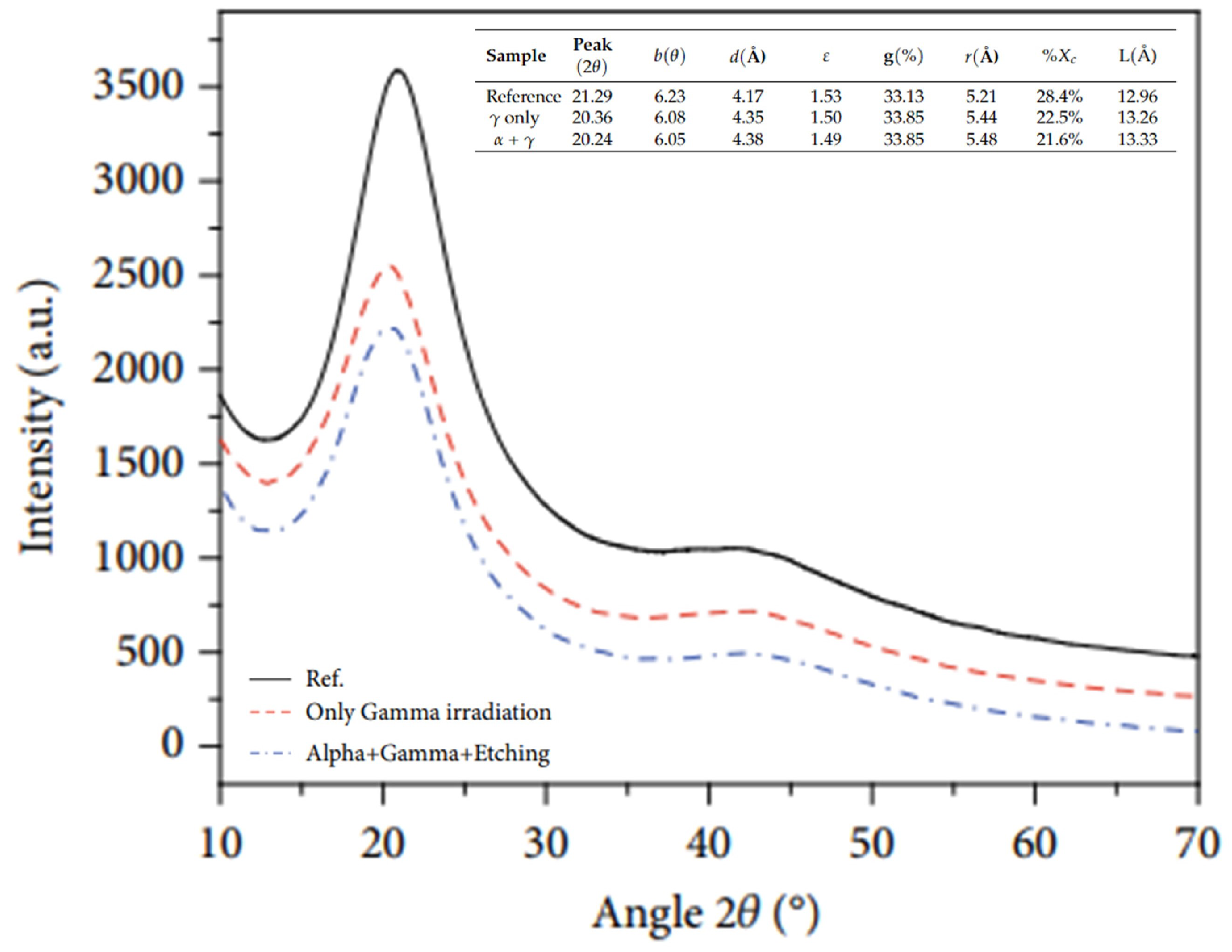

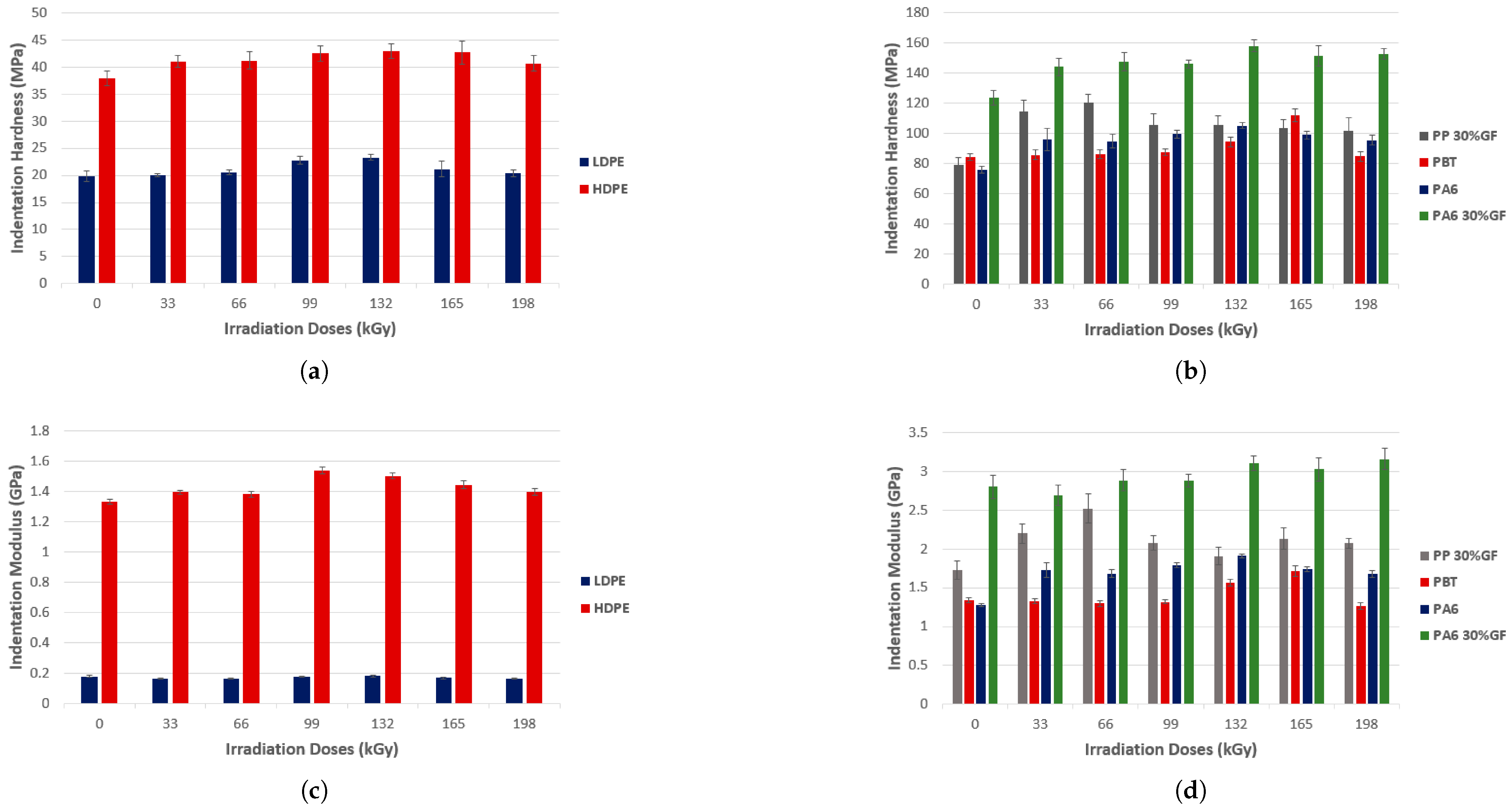



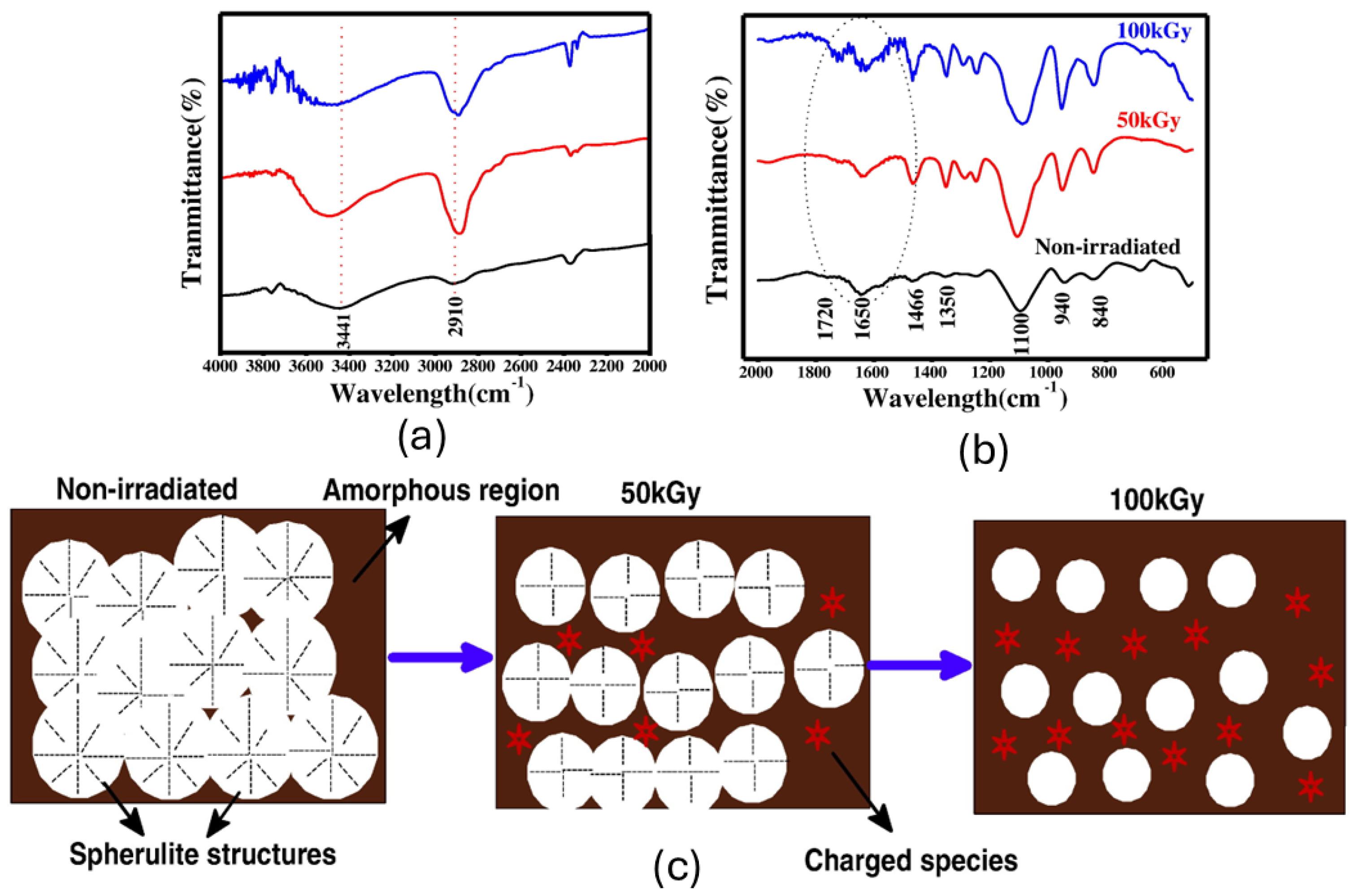
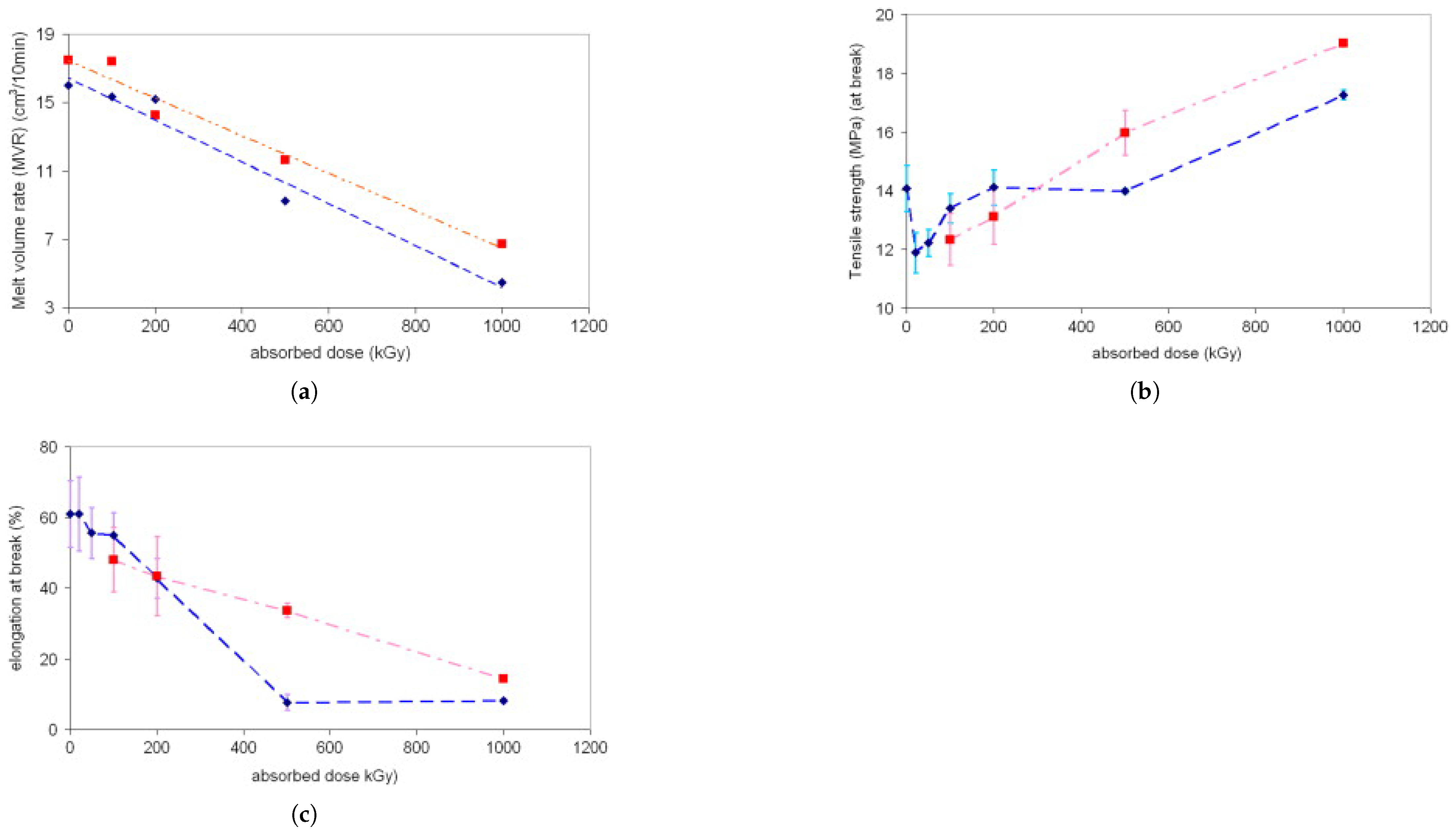
| Polymer | Absorbed Dose | Effects | Relevant Characteristics |
|---|---|---|---|
| PE | 50 kGy | Low degradation up to absorbed doses close to 50 kGy; mechanical performance preserved. | High radiation resistance; used in nuclear cables. |
| PP | 40 kGy | Acceptable performance; 40% property loss at absorbed doses above 40 kGy. | High resistance; used in medical devices sterilized by irradiation. |
| Butyl Rubber | 10 kGy | Stress and elongation gradually decrease; hardening at higher absorbed doses (200 kGy). | Used in sealing and wires; maintains basic properties at moderate absorbed doses. |
| Nylon 6 | 25 kGy | 5% loss in tensile modulus; reduced resistance at higher absorbed doses. | Applications in textiles and rigid plastics. |
| ABS | 50 kGy | Became more brittle (40% reduction in elongation); no resistance loss up to 50 kGy. | Moderate radiation resistance; used in automotive exteriors. |
| PC | 100 kGy | Minimal degradation up to 20 kGy; elongation reduced at higher absorbed doses. | High heat and flame resistance; used in electronics. |
| RTV-5370 | 500 kGy | Hardening and increase in storage modulus at high doses (>500 kGy). | Excellent resistance in gasket and cable applications. |
| PTFE | 10 kGy | Significant elongation loss; 40% reduction in tensile strength at high absorbed doses (80 kGy). | Used in chemically resistant environments. |
| FEP | 10 kGy | 10% reduction in tensile strength after 10 kGy. | Amorphous material, more processable than PTFE. |
| PEEK | 600 kGy | Dielectric alterations observed; mechanical properties maintained at high absorbed doses. | Used in high-performance applications. |
| DAP | 10 kGy | Resistance maintained; used in radiation tracking experiments. | Insulating properties and high rigidity. |
| Vespel SP-1 | 100 MGy | 8% loss in flexural strength at high absorbed doses; no change in thermal properties. | High thermal resistance; used in nuclear and aerospace applications. |
| Torlon 4203 | 100 Gy | Mechanical and thermal properties unchanged at low absorbed doses. | Similar resistance to Vespel SP-1; still limited in technical data. |
| Section | Application | Mechanism | Ref. |
|---|---|---|---|
| Advanced Manufacturing | Additive Manufacturing | Radiation-induced crosslinking enhances mechanical integrity. | [32,103,110,111,112,113,114,115,116,117,118] |
| Shape Memory Materials | Enables recovery of original form under specific stimuli. | [68,69,72,119,120,121,122,123,124,125,126,127,128] | |
| Nuclear Industry | Cable Coatings | Enhanced thermal and radiation resistance for reactor cables. | [2,3,47,129,130,131] |
| Radiation Shielding | Polymers that attenuate neutron radiation. | [132,133,134,135] | |
| Hydrogen Confinement | Specialized polymers prevent leakage in nuclear facilities. | [2,10,136,137,138] | |
| Biomedical Applications | Medical Implants | Improved wear resistance and biocompatibility. | [139,140,141,142,143,144] |
| Drug Delivery Systems | Enables controlled drug release via grafting. | [145,146,147] | |
| Sterilization | Ensures safety of medical devices without compromising properties. | [148,149,150,151,152] | |
| Environmental and Energy Applications | Water Filtration | Enhanced membranes for desalination and wastewater treatment. | [153,154,155] |
| Energy Storage | Polymers improve battery and supercapacitor performance. | [156,157,158] | |
| Photovoltaics | Optimized encapsulation materials for solar panels. | [159,160,161] | |
| Consumer Goods | Packaging Materials | Improved toughness and thermal resistance for food packaging. | [2,162,163] |
| Textiles | Enhanced fibers with increased strength and abrasion resistance. | [164,165,166,167,168,169] | |
| Military and Aerospace | Spacecraft Components | Radiation-resistant polymers withstand extreme space conditions. | [170,171,172,173] |
| Protective Coatings | Durable coatings for harsh environmental conditions. | [174,175,176] | |
| Ballistic Protection | Irradiation induces grafting, modifying chemical and mechanical properties, and enhancing polymer functionality. | [177,178,179] | |
| Grafting | Monomers | Grafting monomers create new copolymers with properties tailored to the desired use. | [180,181,182,183,184] |
| Molecules | The grafting of specific molecules can create polymeric compounds with advanced functionalities. | [48,185,186,187] | |
| Vulcanization | Combines sulfur grafting with crosslinking, offering significant structural improvements. | [71,188,189,190,191,192] | |
| Surface Coating | Ink pigmentation | Ionizing radiation can degrade organic pigments and alter oxidation states of inorganic pigments. | [193,194,195,196] |
| Sustainability | Recycling | Ionizing radiation causes degradation and polymer chain scission, reducing molecular weight and facilitating chemical or mechanical recycling. | [197,198,199] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azevedo, A.M.d.; da Silveira, P.H.P.M.; Lopes, T.J.; da Costa, O.L.B.; Monteiro, S.N.; Veiga-Júnior, V.F.; Silveira, P.C.R.; Cardoso, D.D.; Figueiredo, A.B.-H.d.S. Ionizing Radiation and Its Effects on Thermoplastic Polymers: An Overview. Polymers 2025, 17, 1110. https://doi.org/10.3390/polym17081110
Azevedo AMd, da Silveira PHPM, Lopes TJ, da Costa OLB, Monteiro SN, Veiga-Júnior VF, Silveira PCR, Cardoso DD, Figueiredo AB-HdS. Ionizing Radiation and Its Effects on Thermoplastic Polymers: An Overview. Polymers. 2025; 17(8):1110. https://doi.org/10.3390/polym17081110
Chicago/Turabian StyleAzevedo, Ary Machado de, Pedro Henrique Poubel Mendonça da Silveira, Thomaz Jacintho Lopes, Odilon Leite Barbosa da Costa, Sergio Neves Monteiro, Valdir Florêncio Veiga-Júnior, Paulo Cezar Rocha Silveira, Domingos D’Oliveira Cardoso, and André Ben-Hur da Silva Figueiredo. 2025. "Ionizing Radiation and Its Effects on Thermoplastic Polymers: An Overview" Polymers 17, no. 8: 1110. https://doi.org/10.3390/polym17081110
APA StyleAzevedo, A. M. d., da Silveira, P. H. P. M., Lopes, T. J., da Costa, O. L. B., Monteiro, S. N., Veiga-Júnior, V. F., Silveira, P. C. R., Cardoso, D. D., & Figueiredo, A. B.-H. d. S. (2025). Ionizing Radiation and Its Effects on Thermoplastic Polymers: An Overview. Polymers, 17(8), 1110. https://doi.org/10.3390/polym17081110










