Preparation and Characterization of Low-Molecular-Weight Polyacrylonitrile
Abstract
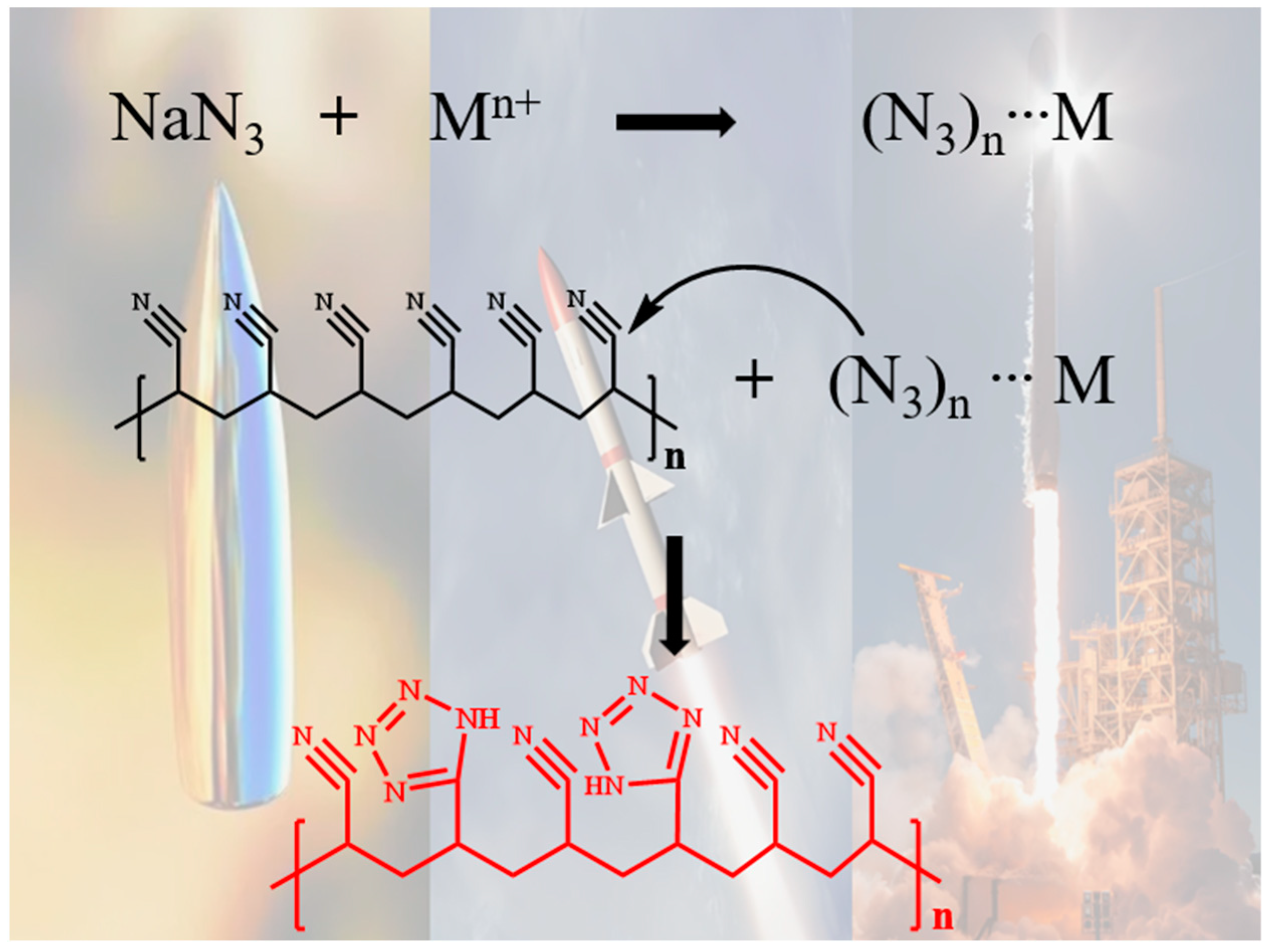
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Low-Molecular-Weight PAN
2.3. Characterization and Testing
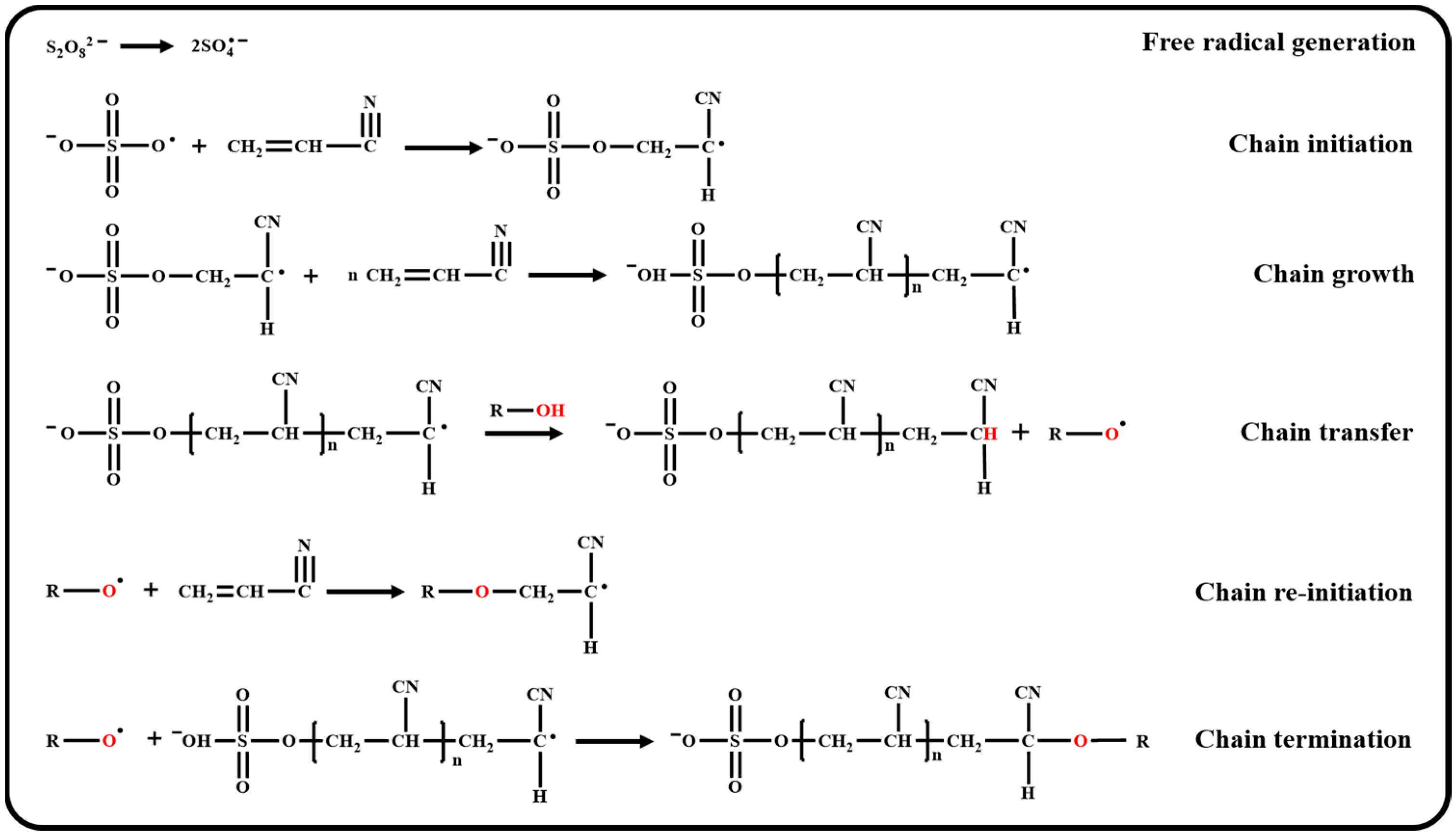
2.3.1. Characterization of Molecular Chain Structure
2.3.2. Molecular Weight and Yield Determination
2.3.3. Morphological Analysis
2.3.4. Thermal Performance Analysis
2.3.5. Solubility Test
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Choi, B.K.; Kim, Y.W.; Gong, M.S.; Ahn, S.H. Characterization of new polyacrylonitrile-co-bis 2-(2-methoxyethoxy)ethyl itaconate based gel polymer electrolytes. Electrochim. Acta 2001, 46, 3475–3479. [Google Scholar] [CrossRef]
- Zhang, L.; Bai, J.; Liang, H.; Guo, L.; Li, C.; Huang, Y. Preparation and Characterization of Polyacrylonitrile and Polyacrylonitrile/β-cyclodextrins Microspheres. J. Macromol. Sci. Part B Phys. 2014, 53, 142–148. [Google Scholar] [CrossRef]
- Lu, M.; Liao, J.; Gulgunje, P.V.; Chang, H.; Arias-Monje, P.J.; Ramachandran, J.; Breedveld, V.; Kumar, S. Rheological behavior and fiber spinning of polyacrylonitrile (PAN)/Carbon nanotube (CNT) dispersions at high CNT loading. Polymer 2020, 215, 123369. [Google Scholar] [CrossRef]
- Darabi, M.; Nikoorazm, M.; Tahmasbi, B.; Ghorbani-Choghamarani, A. Immobilization of Ni(ii) complex on the surface of mesoporous modified-KIT-6 as a new, reusable and highly efficient nanocatalyst for the synthesis of tetrazole and pyranopyrazole derivatives. Rsc Adv. 2023, 13, 12572–12588. [Google Scholar] [CrossRef]
- Furushima, Y.; Kumazawa, R.; Yamaguchi, Y.; Hirota, N.; Sawada, K.; Nakada, M.; Murakami, M. Precursory reaction of thermal cyclization for polyacrylonitrile. Polymer 2021, 226, 123780. [Google Scholar] [CrossRef]
- Xia, Y.; Rajappan, S.C.; Chen, S.; Kraglund, M.R.; Serhiichuk, D.; Pan, D.; Jensen, J.O.; Jannasch, P.; Aili, D. Poly(Arylene Alkylene)s with Tetrazole Pendants for Alkaline Ion-Solvating Polymer Electrolytes. ChemSusChem 2024, 17, e202400844. [Google Scholar] [CrossRef]
- Qiang, Y.; Li, H.; Lan, X. Self-assembling anchored film basing on two tetrazole derivatives for application to protect copper in sulfuric acid environment. J. Mater. Sci. Technol. 2020, 52, 63–71. [Google Scholar] [CrossRef]
- Wang, T.; Lu, Z.; Bu, S.; Kuang, B.; Zhang, L.; Yi, Z.; Wang, K.; Zhu, S.; Zhang, J. Combination of Nitrogen-Rich Skeleton and Coordination Group: Synthesis of a High-Energy Primary Explosive Based on 1H-Tetrazole-5-Carbohydrazide. Def. Technol. 2024, 31, 271–277. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, Q.; Zhong, J.; Chen, M.; Deng, H.; Cao, J.; Wang, L.; Peng, L.; Zhu, J.; Lu, B. 3D Holey Graphene/Polyacrylonitrile Sulfur Composite Architecture for High Loading Lithium Sulfur Batteries. Adv. Energy Mater. 2021, 11, 210048. [Google Scholar] [CrossRef]
- Razzaq, A.A.; Yao, Y.; Shah, R.; Qi, P.; Miao, L.; Chen, M.; Zhao, X.; Peng, Y.; Deng, Z. High-performance lithium sulfur batteries enabled by a synergy between sulfur and carbon nanotubes. Energy Storage Mater. 2019, 16, 194–202. [Google Scholar] [CrossRef]
- Qin, X.-H.; Wan, Y.-Q.; He, J.-H.; Zhang, J.; Yu, J.-Y.; Wang, S.-Y. Effect of LiCl on electrospinning of PAN polymer solution: Theoretical analysis and experimental verification. Polymer 2004, 45, 6409–6413. [Google Scholar] [CrossRef]
- Szepcsik, B.; Pukánszky, B. Separation and kinetic analysis of the thermo-oxidative reactions of polyacrylonitrile upon heat treatment. J. Therm. Anal. Calorim. 2018, 133, 1371–1378. [Google Scholar] [CrossRef]
- Uemura, T.; Kaseda, T.; Sasaki, Y.; Inukai, M.; Toriyama, T.; Takahara, A.; Jinnai, H.; Kitagawa, S. Mixing of immiscible polymers using nanoporous coordination templates. Nat. Commun. 2015, 6, 7473. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Xu, Y.; Liu, C.; Zhai, Y.; Chen, H.; Bai, L.; Yang, H.; Yang, L.; Wang, W.; Niu, Y. Visible light-induced metal-free atom transfer radical polymerization: An efficient approach to polyacrylonitrile. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 1265–1269. [Google Scholar] [CrossRef]
- Liu, H.; Hu, R.; Hu, Z.-Q.; Ji, X.-F. The New Methods for Characterization of Molecular Weight of Supramolecular Polymers. Chin. J. Polym. Sci. 2024, 42, 1403–1413. [Google Scholar] [CrossRef]
- Wang, L.; Yin, Z.; Zhang, Y.; Jiang, Y.; Zhang, L.; Yasin, A. Probing the single pair rupture force of supramolecular quadruply hydrogen bonding modules by nano-adhesion measurement. Rsc Adv. 2018, 8, 21798–21805. [Google Scholar] [CrossRef]
- Liu, N.; Yang, W.; Li, X.; Zhao, P.; Liu, Y.; Guo, L.; Huang, L.; Gao, W. Comparison of characterization and antioxidant activity of different citrus peel pectins. Food Chem. 2022, 386, 132683. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, P.; Zhang, Y.; Zan, X.; Zhu, W.; Jiang, Y.; Zhang, L.; Yasin, A. Improved Ozonation Efficiency for Polymerization Mother Liquid from Polyvinyl Chloride Production Using Tandem Reactors. Molecules 2019, 24, 4436. [Google Scholar] [CrossRef]
- Beltzung, A.; Klaue, A.; Colombo, C.; Wu, H.; Storti, G.; Morbidelli, M. Polyacrylonitrile Nanoparticle-Derived Hierarchical Structure for CO2 Capture. Energy Technol. 2018, 6, 718–727. [Google Scholar] [CrossRef]
- Dey, S.; Aggarwal, M.; Chakraborty, D.; Mukherjee, P.S. Uncovering tetrazoles as building blocks for constructing discrete and polymeric assemblies. Chem. Commun. 2024, 60, 5573–5585. [Google Scholar] [CrossRef]
- Geiselhart, C.M.; Mutlu, H.; Barner-Kowollik, C. Passerini Multicomponent Reactions Enabling Self-Reporting Photosensitive Tetrazole Polymers. ACS Macro Lett. 2021, 10, 5573–5585. [Google Scholar] [CrossRef] [PubMed]
- Ahad, H.; Mostafa, A.; Mehdi, N.-H.; Naeimeh, B.-L. Effect of different chain transfer agents in the coordinative chain transfer oligomerization of dec-1-ene. J. Mol. Struct. 2022, 1263, 133157. [Google Scholar] [CrossRef]
- He, J.-Y.; Lu, M. Photoinduced electron transfer-reversible addition-fragmentation chain transfer (PET-RAFT) polymerization of acrylonitrile in miniemulsion. J. Macromol. Sci. Part A 2019, 56, 443–449. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, L.; Cheng, Z.; Zhu, X. Reversible Addition-Fragmentation Chain Transfer Polymerization of Acrylonitrile Under Irradiation of Blue LED Light. Polymers 2016, 9, 4. [Google Scholar] [CrossRef]
- Kopeć, M.; Krys, P.; Yuan, R.; Matyjaszewski, K. Aqueous RAFT Polymerization of Acrylonitrile. Macromolecules 2016, 49, 5587–5883. [Google Scholar] [CrossRef]
- Zhao, Y.; Liang, J.; Peng, M.; Xu, L.; Shao, Z.; Yang, M. A New Process Based on Mixed-Solvent Precipitation Polymerization to Synthesize High Molecular Weight Polyacrylonitrile Initiated by Ammonium Persulphate. Fibers Polym. 2016, 17, 2162–2166. [Google Scholar] [CrossRef]
- Wallace, M.A.; Burkey, A.A.; Sita, L.R. Phenyl-Terminated Polyolefins via Living Coordinative Chain Transfer Polymerization with ZnPh2 as a Chain Transfer Agent. ACS Catal. 2021, 11, 10710–10718. [Google Scholar] [CrossRef]
- Monteiro, I.S.; Ecoscia, A.C.M.; McKenna, T.F.L. Investigation of the Chain Transfer Agent Effect on the Polymerization of Vinylidene Fluoride. Ind. Eng. Chem. Res. 2019, 58, 20976–20986. [Google Scholar] [CrossRef]
- Cetinkaya, O.; Demirci, G.; Mergo, P. Effect of the different chain transfer agents on molecular weight and optical properties of poly(methyl methacrylate). Opt. Mater. 2017, 70, 25–30. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, L.; Jiang, S.; Li, S.; Lan, T.; Zu, L.; Dong, S. Synthesis of Modified TiO2 Nanoparticles with Polyacrylonitrile and Poly(hydroxyethyl acrylate) via ATRP. ChemistrySelect 2020, 5, 4695–4700. [Google Scholar] [CrossRef]
- Yao, X.-Q.; Wang, Y.-S.; Wang, J. Cp(π-Allyl)Pd-Initiated Polymerization of Diazoacetates: Reaction Development, Kinetic Study, and Chain Transfer with Alcohols. Macromolecules 2021, 54, 10914–10922. [Google Scholar] [CrossRef]
- Zhang, S.; Yin, L.; Wang, J.; Zhang, W.; Zhang, L.; Zhu, X. A Green Platform for Preparation of the Well-Defined Polyacrylonitrile: 60Co γ-ray Irradiation-Initiated RAFT Polymerization at Room Temperature. Polymers 2017, 9, 26. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Ma, H.; Bao, W.; Gao, C.; Ge, M. Polyacrylonitrile/electroconductive TiO2 nanoparticles composite fibers via wet-spinning. Fibers Polym. 2016, 17, 1048–1054. [Google Scholar] [CrossRef]
- Shoubak, W.M.; Hassan, A.; Mahrous, S.; Hassen, A. Controlling the physical properties of polyacrylonitrile by strontium hexaferrite nanoparticles. Polym. Bull. 2023, 81, 697–718. [Google Scholar] [CrossRef]
- Polisetti, V.; Ray, P. Nanoparticles modified Polyacrylonitrile/Polyacrylonitrile-Polyvinylidenefluoride blends as substrate of high flux anti-fouling nanofiltration membranes. J. Appl. Polym. Sci. 2020, 138, 50228. [Google Scholar] [CrossRef]
- Prasher, A.; Hu, H.; Tanaka, J.; Nicewicz, D.A.; You, W. Alcohol mediated degenerate chain transfer controlled cationic polymerisation of para-alkoxystyrene. Polym. Chem. 2019, 10, 4126–4133. [Google Scholar] [CrossRef]
- Ahn, H.R.; Tak, T.M. Optimization grafting conditions and characterization of methyl cellulose grafted polyacrylonitrile copolymer. Macromol. Res. 2014, 22, 318–323. [Google Scholar] [CrossRef]
- Ting-Ting, Z.; Peng, Y.; Zheng-Min, Z.; Zheng-Ying, L.; Ming-Bo, Y.; Wei, Y. Formation of nanosheets-assembled porous polymer microspheres via the combination effect of polymer crystallization and vapor-induced phase separation. Polymer 2021, 231, 124118. [Google Scholar] [CrossRef]
- El-Newehy, M.H.; Alamri, A.; Al-Deyab, S.S. Optimization of amine-terminated polyacrylonitrile synthesis and characterization. Arab. J. Chem. 2014, 7, 235–241. [Google Scholar] [CrossRef]
- Chen, L.-F.; Lu, Y.; Yu, L.; Lou, X.W. Designed formation of hollow particle-based nitrogen-doped carbon nanofibers for high-performance supercapacitors. Energy Environ. Sci. 2017, 10, 1777–1783. [Google Scholar] [CrossRef]
- Vega, I.N.; Sanchez, L.; D’Accorso, N. Synthesis of 1,3-oxazole and benzimidazole pendant groups from polyacrylonitrile hydrolysis products. J. Heterocycl. Chem. 2008, 45, 429–433. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.; Zhang, Y.; Yasin, A.; Zhang, L. Investigating Stability and Tautomerization of Gossypol-A Spectroscopy Study. Molecules 2019, 24, 1286. [Google Scholar] [CrossRef]
- Yue, Z.; Wang, D.X.; Liu, J.Q.; Zhang, J.; Feng, S.Y. Synthesis of polyacrylonitrile-block-polydimethylsiloxane-block-polyacr ylonitrile triblock copolymers via RAFT polymerization. Chin. Chem. Lett. 2012, 23, 989–992. [Google Scholar] [CrossRef]
- Chee, K.K. Estimation of molecular weight averages from intrinsic viscosity. J. Appl. Polym. Sci. 1985, 30, 1359–1363. [Google Scholar] [CrossRef]
- Li, J.; Tian, H. Preparation and characterisation of PE films of carbon nanotube/polyaniline (PAN) composite mixture. J. Exp. Nanosci. 2017, 12, 355–362. [Google Scholar] [CrossRef]
- Wu, Q.-Y.; Liang, H.-Q.; Gu, L.; Yu, Y.; Huang, Y.-Q.; Xu, Z.-K. PVDF/PAN blend separators via thermally induced phase separation for lithium ion batteries. Polymer 2016, 107, 54–60. [Google Scholar] [CrossRef]
- Pripakhaylo, A.V.; Tsypakin, A.A.; Klam, A.A.; Andreichev, A.L.; Timerbaev, A.R.; Shapovalova, O.V.; Magomedov, R.N. Polyacrylonitrile Composites Blended with Asphalt as a Low-Cost Material for Producing Synthetic Fibers: Rheology and Thermal Stability. Materials 2024, 17, 5725. [Google Scholar] [CrossRef]
- Balan, G.S.; Raj, S.A.; Shahar, F.S.; Sultan, M.T.H. Tailoring polymer composites for critical thermal applications: A review on fibers, additives, coatings, aerogels, test methodologies. Polym. Plast. Technol. Mater. 2023, 62, 2221–22254. [Google Scholar] [CrossRef]
- Zhang, S.S. Understanding of Sulfurized Polyacrylonitrile for Superior Performance Lithium/Sulfur Battery. Energies 2014, 7, 4588–4600. [Google Scholar] [CrossRef]
- Zhao, Y.; Guo, Y.; Lu, X.F.; Luan, D.; Gu, X.; Lou, X.W.D. Exposing Single Ni Atoms in Hollow S/N-Doped Carbon Macroporous Fibers for Highly Efficient Electrochemical Oxygen Evolution. Adv. Mater. 2022, 34, 2203442. [Google Scholar] [CrossRef]
- Li, X.; Dang, X. Preparation of plasticized spinning polyacrylonitrile fibers using 1-butyl-3-methylimidazolium chloride: A review. J. Eng. Fibers Fabr. 2022, 17, 1–11. [Google Scholar] [CrossRef]
- Chowdhury, F.I.; Khalil, I.; Khandaker, M.U.; Rabbani, M.M.; Uddin, J.; Arof, A.K. Electrochemical and structural characterization of polyacrylonitrile (PAN)–based gel polymer electrolytes blended with tetrabutylammonium iodide for possible application in dye-sensitized solar cells. Ionics 2020, 20, 1209–1223. [Google Scholar] [CrossRef]
- Mahdavi, H.; Mousavi Davijani, S.M.; Ahmadian-Alam, L. Performance investigation of a novel interfacially polymerized poly(urea-co-urethane) thin film nanocomposite on PAN membranes for solvent-resistant nanofiltration membrane application. J. Iran. Chem. Soc. 2023, 20, 1209–1223. [Google Scholar] [CrossRef]
- Yuhang, J.; Huijie, Y.; Qingxin, M.; Shiling, J.; Shuang, L.; Huixuan, Z.; Zhongyu, F. Polymerization of acrylonitrile in dimethyl carbonate: A kinetic and mechanistic study. Polymer 2024, 316, 127878. [Google Scholar] [CrossRef]
- Isaac, B.; Taylor, R.M.; Reifsnider, K. Anisotropic Characterizations of Electrospun PAN Nanofiber Mats Using Design of Experiments. Nanomaterials 2020, 10, 2273. [Google Scholar] [CrossRef]
- Yasin, A.; Chen, Y.; Liu, Y.; Zhang, L.; Zan, X.; Zhang, Y. Hyperbranched multiple polythioamides made from elemental sulfur for mercury adsorption. Polym. Chem. 2020, 11, 810–819. [Google Scholar] [CrossRef]
- Bos, T.S.; Philipsen, H.J.; Staal, B.B.; Purmova, J.; Beerends, R.J.; Buijtenhuijs, A.; Karlson, L.; Schoenmakers, P.J.; Somsen, G.W. Quantitative assessment of polymer molecular shape based on changes in the slope of the Mark-Houwink plot derived from size-exclusion chromatography with triple detection. J. Appl. Polym. Sci. 2024, 141, e55013. [Google Scholar] [CrossRef]
- Al-Dujaili, A.H.; Mustafa, I.F. Viscometric behavior of solution of polyisobutylene in hexane and cyclohexane solvents: Viscosity-molecular weight relationships. Polym. -Plast. Technol. Eng. 1994, 33, 111–118. [Google Scholar] [CrossRef]
- Chuah, H.; Lin-Vien, D.; Soni, U. Poly (trimethylene terephthalate) molecular weight and Mark–Houwink equation. Polymer 2001, 42, 7137–7139. [Google Scholar] [CrossRef]
- Holdcroft, S. Determination of molecular weights and Mark–Houwink constants for soluble electronically conducting polymers. J. Polym. Sci. Part B Polym. Phys. 1991, 29, 1585–1588. [Google Scholar] [CrossRef]
- Schott, H. Dependence of the Constant of the Mark-Houwink Equation under Theta Conditions on the Bulk of Substituents for Hydrocarbon Polymers. J. Macromol. Sci. Part B Phys. 2006, 45, 1183–1187. [Google Scholar] [CrossRef]






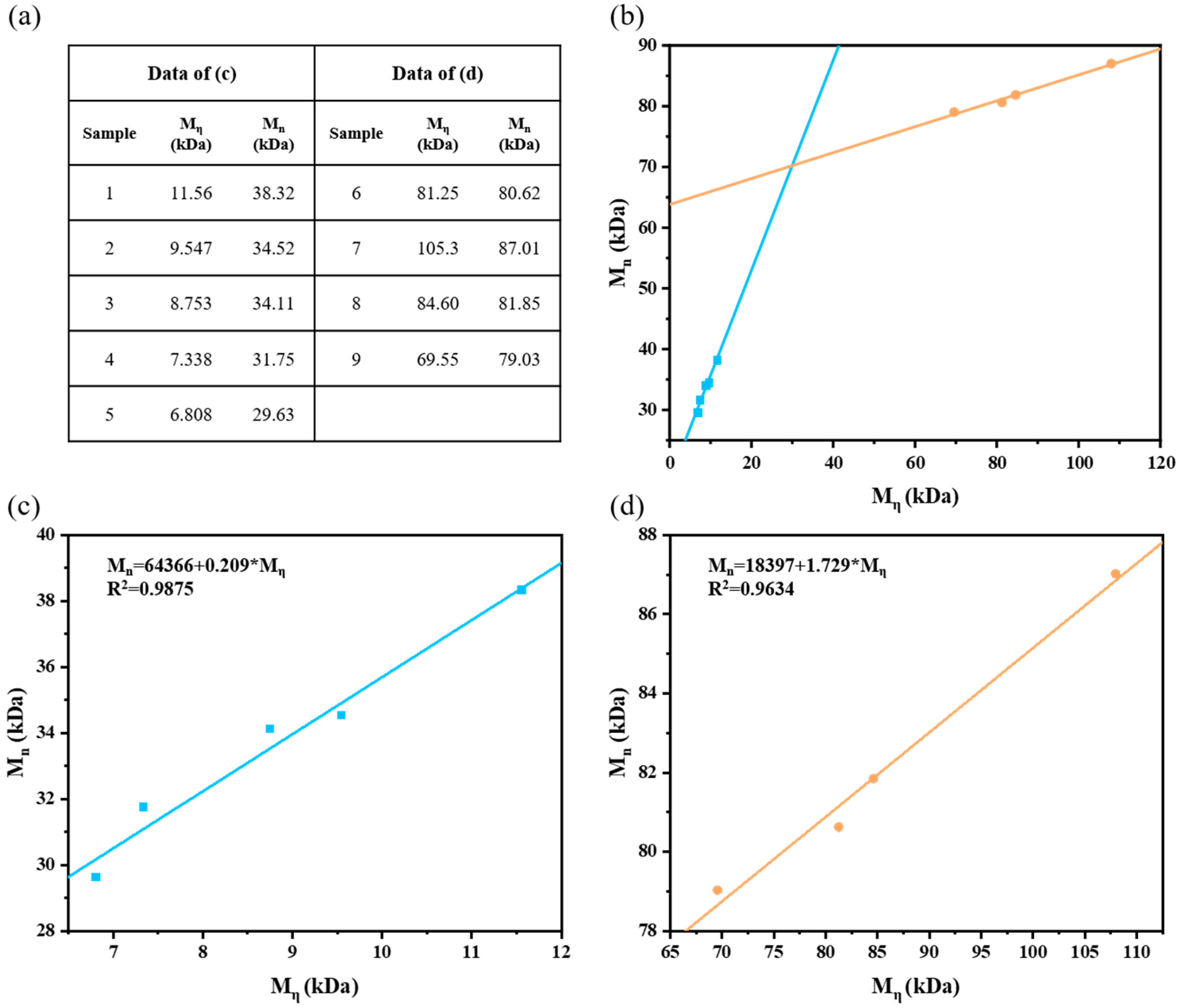
| Sample | CTA | AN–CTA–APS | Time (h) | Mη (kDa) | Mn (kDa) | Mw (kDa) | PDI | Yield (wt%) |
|---|---|---|---|---|---|---|---|---|
| 1 | Acetone | 100:20:3 | 3 | 81.25 | 80.62 | 223.9 | 2.78 | 46.4 |
| 2 | Glycol | 100:20:3 | 3 | 84.60 | 81.85 | 229.45 | 2.81 | 50.7 |
| PAN-3 | Isopropanol | 100:20:3 | 3 | 69.55 | 79.03 | 207.79 | 2.63 | 43.9 |
| 4 | 1,4-butanediol | 100:20:3 | 3 | 64.42 | / | / | / | 36.4 |
| 5 | Isopropanol | 100:100:3 | 3 | 25.90 | / | / | / | 39.5 |
| 6 | Isopropanol | 100:200:3 | 3 | 18.26 | / | / | / | 30.7 |
| PAN-2 | Isopropanol | 100:300:3 | 3 | 11.56 | 38.32 | 62.40 | 1.63 | 30.3 |
| 8 | Isopropanol | 100:400:3 | 3 | 8.753 | 34.11 | 64.84 | 1.91 | 28.3 |
| 9 | Isopropanol | 100:100:3 | 1 | 18.34 | / | / | / | 27.5 |
| 10 | Isopropanol | 100:100:3 | 2 | 21.33 | / | / | / | 37.1 |
| 11 | Isopropanol | 100:100:3 | 4 | 27.35 | / | / | / | 45.5 |
| 12 | Isopropanol | 100:100:3 | 5 | 28.71 | / | / | / | 49.8 |
| 13 | Isopropanol | 100:300:1 | 2 | 12.82 | 25.2 | |||
| 14 | Isopropanol | 100:300:3 | 2 | 9.547 | 34.52 | 59.53 | 1.72 | 29.7 |
| PAN-1 | Isopropanol | 100:300:6 | 2 | 6.808 | 29.63 | 58.50 | 1.97 | 32.6 |
| 16 | Isopropanol | 100:300:9 | 2 | 7.338 | 31.75 | 51.47 | 1.62 | 37.5 |
| Sample | Mη (kDa) | Mn (kDa) | Mw (kDa) | PDI | Yield (wt%) |
|---|---|---|---|---|---|
| PAN-1 | 6.808 | 29.63 | 58.50 | 1.97 | 32.6 |
| PAN-2 | 11.56 | 38.32 | 62.40 | 1.63 | 30.3 |
| PAN-3 | 69.55 | 79.03 | 207.8 | 2.63 | 43.9 |
| PAN-4 | 105.3 | 87.01 | 238.1 | 2.74 | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Jiang, X.; Jiang, J.; Liu, Y.; Zhao, L.; Zhu, H.; Wang, J.; Yan, Z.; Zhang, Y. Preparation and Characterization of Low-Molecular-Weight Polyacrylonitrile. Polymers 2025, 17, 1112. https://doi.org/10.3390/polym17081112
Yang Y, Jiang X, Jiang J, Liu Y, Zhao L, Zhu H, Wang J, Yan Z, Zhang Y. Preparation and Characterization of Low-Molecular-Weight Polyacrylonitrile. Polymers. 2025; 17(8):1112. https://doi.org/10.3390/polym17081112
Chicago/Turabian StyleYang, Yuanteng, Xiaoli Jiang, Jing Jiang, Yang Liu, Lin Zhao, Hongyu Zhu, Junjie Wang, Zongkai Yan, and Yagang Zhang. 2025. "Preparation and Characterization of Low-Molecular-Weight Polyacrylonitrile" Polymers 17, no. 8: 1112. https://doi.org/10.3390/polym17081112
APA StyleYang, Y., Jiang, X., Jiang, J., Liu, Y., Zhao, L., Zhu, H., Wang, J., Yan, Z., & Zhang, Y. (2025). Preparation and Characterization of Low-Molecular-Weight Polyacrylonitrile. Polymers, 17(8), 1112. https://doi.org/10.3390/polym17081112








