Starch Hydrogels for Slow and Controlled-Release Fertilizers: A Review
Abstract
1. Introduction
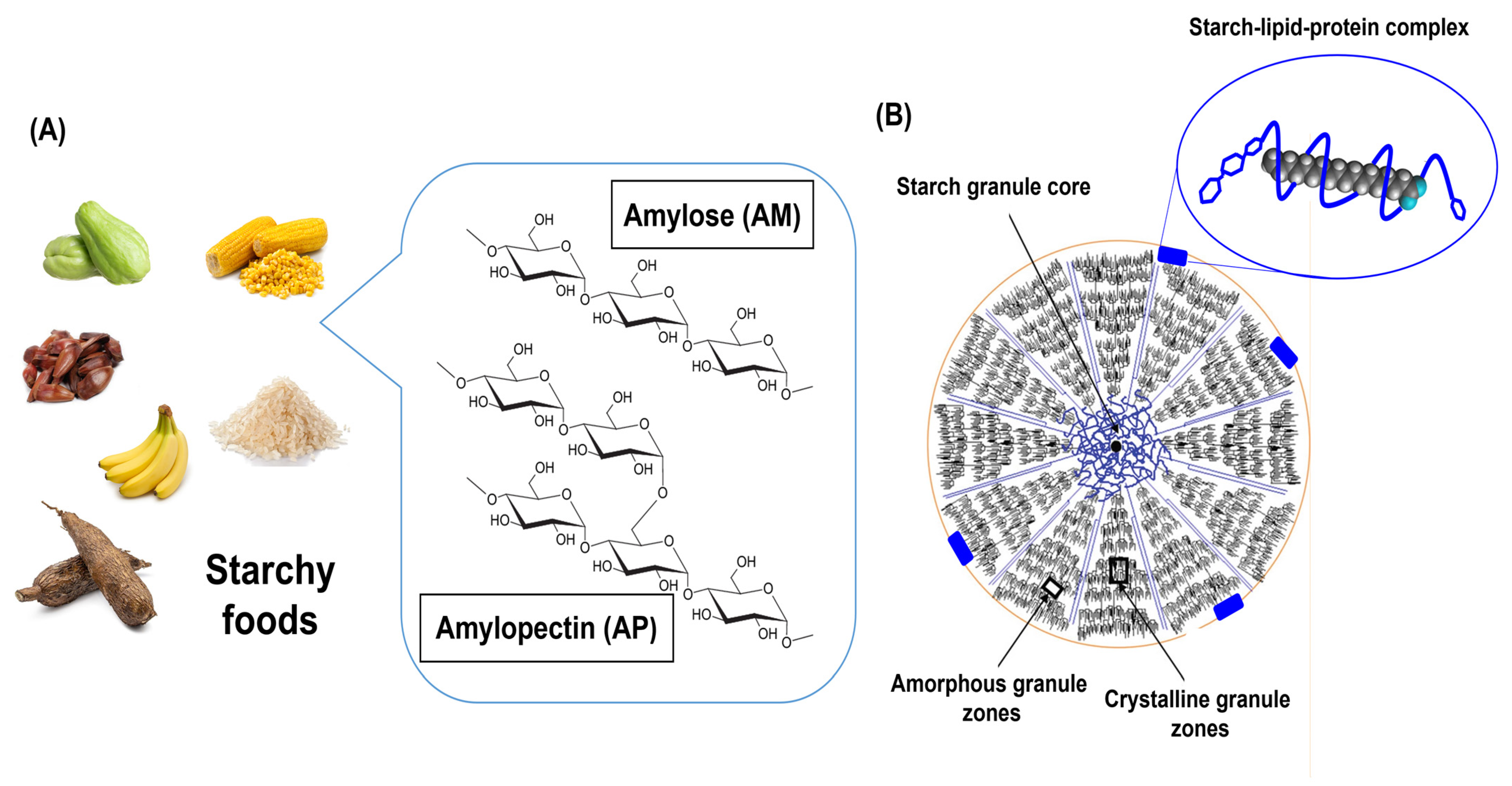
2. Starch Sources and Chemical Composition
| Source | % Starch | % AM | Size Granule (μm) | % Protein | % Ash | % Lipids | % Fiber | Ref. |
|---|---|---|---|---|---|---|---|---|
| Cassava | 93.17 ± 0.30 | 16.71 ± 1.00 | 17.24 ± 1.0 | 0.31 ± 0.01 | 0.23 ± 0.06 | 0.20 ± 0.00 | 0.23 ± 0.06 | [35] |
| Potato | 85.90–88.10 | 26.20–29.10 | 20.60–30.90 | 0.31–0.34 | - | - | - | [36] |
| Rice | 90.42 | - | - | 7.00 ± 0.06 | 1.81 ± 0.05 | 0.77 ± 0.15 | - | [37] |
| Corn | 9.00–23.00 | - | - | 10.00–13.00 | 2.00–5.00 | 2.00–3.00 | 12.30 | [38] |
| Maize | - | 29.30 ± 0.17 | 7.00–28.00 | 0.50 ± 0.00 | 0.38 ± 0.00 | 0.68 ± 0.02 | - | [39] |
| Wheat | 69.50 | - | 2.80–42.80 | 11.90 | - | - | - | [40] |
| Chayote | - | 12.90 ± 0.64 | 7.00–50.00 | 0.29 ± 0.00 | 0.46 ± 0.04 | 0.16 ± 0.00 | - | [39] |
| Barley | 72.20 ± 0.60 | 25.80 ± 0.70 | 16.30 | 13.50 | - | 3.04 ± 0.40 | 11.80 ± 1.00 | [41] |
| Yam bean | - | - | - | 1.23 ± 0.02 | 1.24 ± 0.03 | 1.17 ± 0.04 | 10.94 ± 0.02 | [42] |
| Yam | 99.70 | 22.20 | 28.50–30.60 | 0.06 | 0.13 | 0.03 | 0.11 | [43] |
| Taro | 96.75 | 19.37 ± 0.93 | 1.46 ± 0.10 | 0.98 ± 0.05 | 0.43 ± 0.01 | 0.39 ± 0.01 | [44] | |
| Taro | 99.70 ± 0.40 | 8.40 ± 0.20 | 1.30–2.20 | 0.35 ± 0.00 | 0.28 ± 0.00 | - | - | [45] |
| Canna | - | 20.60 | - | 0.07 | 0.25 | 0.01 | - | [46] |
| Mung bean | - | 19.60 | - | 0.56 | 0.16 | 0.14 | - | [46] |
| Sorghum | - | 27.18 ± 9.98 | - | 0.31 ± 0.00 | 0.04 ± 0.00 | 0.05 ± 0.02 | 0.14 ± 0.03 | [47] |
| Pejibaye fruit | 79.00 ± 0.12 | 12.40 ± 0.18 | - | 0.54 ± 0.07 | 0.18 ± 0.07 | 0.93 ± 0.01 | - | [48] |
| Banana | 97.20 ± 2.40 | - | 2.03 ± 0.15 | 1.30 ± 0.30 | - | - | [49] | |
| Mango seeds | 21.00 | - | - | 0.68 | 1.15 | 0.36 | - | [50] |
| Chestnut | 93.20 ± 1.10 | 24.70 ± 0.90 | 4.00–21.00 | 0.48 ± 0.02 | - | - | - | [51] |
| Pinhão | - | 22.25 | - | 0.07 | 0.08 | 1.00 | - | [52] |
| Yambean (Sphenostylis stenocarpa) | - | 34.40 ± 0.40 | - | 0.42 ± 0.01 | 0.25 ± 0.10 | 0.96 ± 0.10 | [53] | |
| Anchote | - | 32.14 ± 0.19 | - | - | - | - | - | [54] |
| Peach palm | 71.00 ± 2.15 | 1.52 ± 0.04 | - | 0.47 ± 0.13 | 0.18 ± 0.02 | 0.55 ± 0.06 | - | [55] |
| Breadfruit | 98.86 | 27.68 ± 0.75 | 4.24–7.88 | 0.61 ± 0.01 | 0.47 ± 0.04 | 0.06 ± 0.01 | - | [56] |
| Seed of loquat | 93.78–54.31 | 45.69–6.22 | 29.05–43.66 | 0.61–1.86 | 0.39–0.28 | 0.80–0.41 | - | [57] |
| Tomato | - | 17.40–19.10 | 13.50–14.30 | - | - | - | - | [58] |
| Ramon | 92.57 ± 2.89 | 25.36 ± 2.37 | 3.00–26.00 | - | - | - | 1.15 ± 0.01 | [59] |
| Unripe | 99.31 ± 0.01 | 28.79 ± 0.10 | 27.30–42.00 | 0.36 ± 0.01 | 0.22 ± 0.01 | 0.08 ± 0.01 | 0.03 ± 0.01 | [60] |
| Longan | 84.40 ± 1.00 | 25.10 ± 0.30 | 1.57–7.66 | 0.08 ± 0.01 | - | - | - | [61] |
| Loquat | 85.70 ± 0.40 | 26.40 ± 1.10 | 5.21–9.36 | 0.12 ± 0.01 | - | - | - | [61] |
| Myrosma cannifolia | 438.60 (g kg−1) | 225 (g kg−1) | 8.00–17.50 | 12.64 (g kg−1) | 5.48 (g kg−1) | 2.51 (g kg−1) | 0.07 (g kg−1) | [62] |
3. SHs Formation Methods
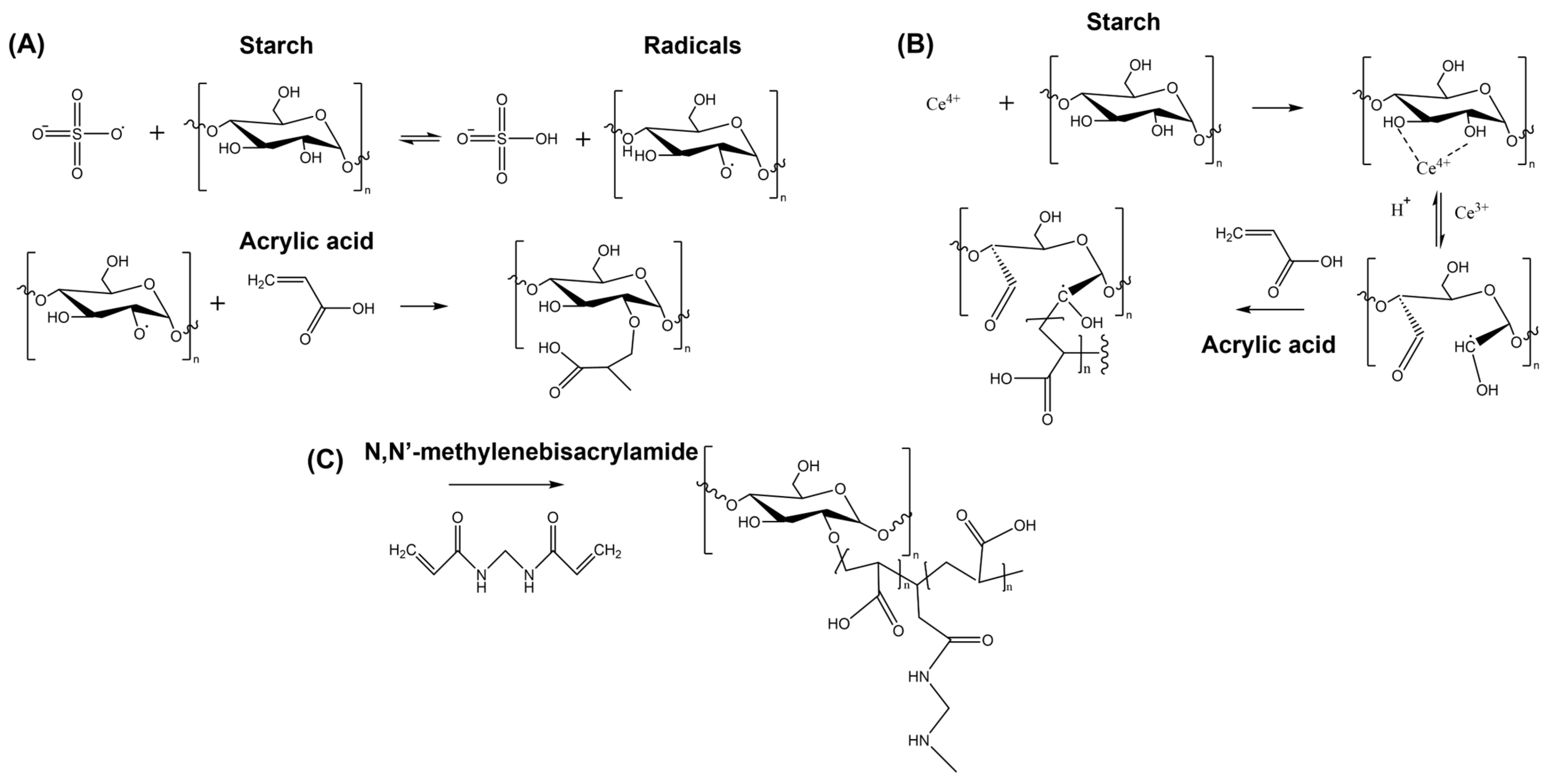
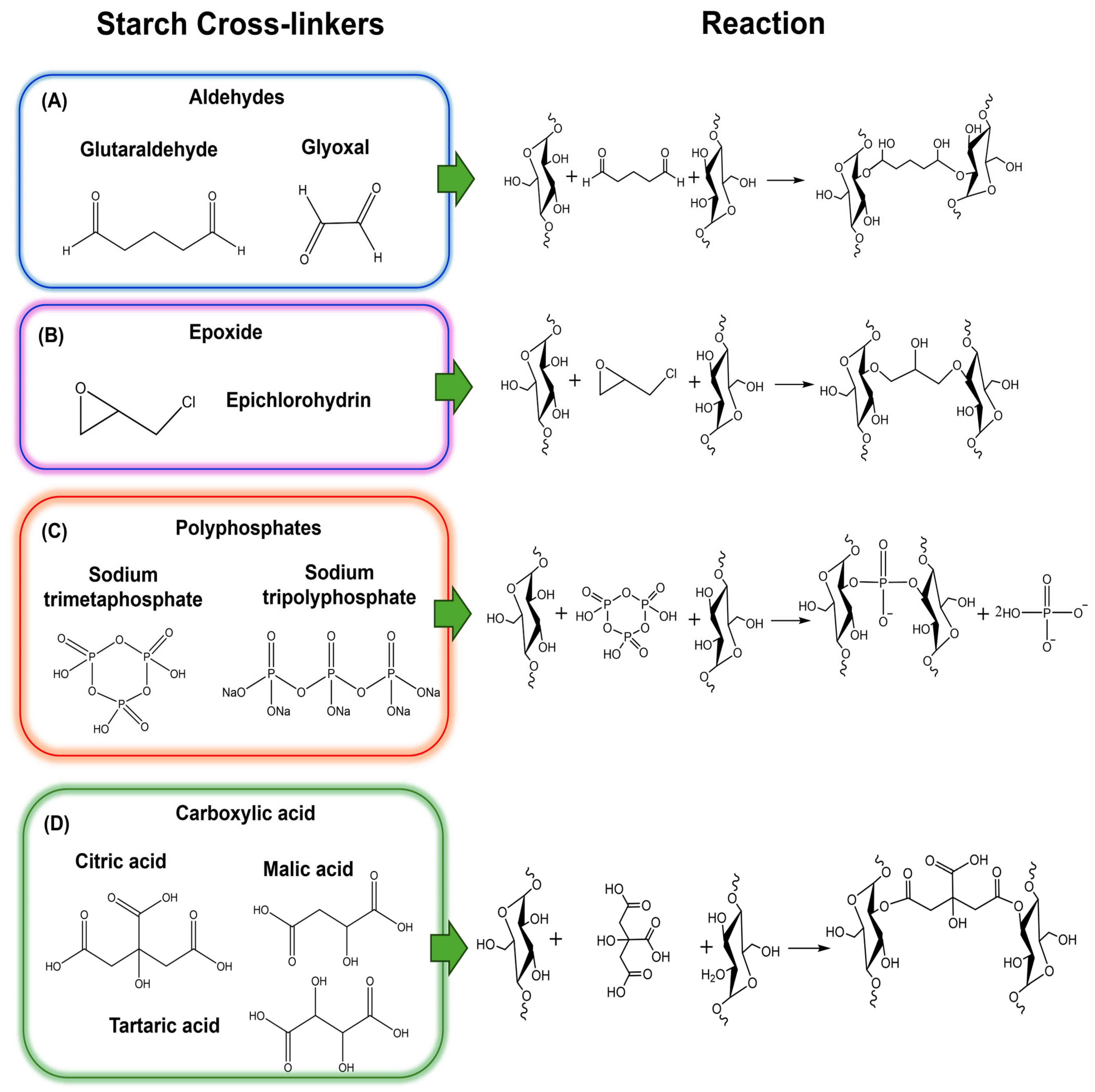
3.1. Crosslinking Chemical Methods
3.2. Crosslinking Physical Methods
4. Application of SHs to Transport Fertilizers
4.1. Fertilizers Encapsulation and In Vitro Assays

| Material | Nutrient | Observation | Reference |
|---|---|---|---|
| Hydrogel: Sulfonated corn starch/poly(acrylic acid) synthesized by polymerization | P | P exhibited a sustained release over 72 h; however, 40% of the entrapped P was released within the first 24 days. | [116] |
| Hydrogel: PVA-modified starch biopolymer crosslinked with CA | N | The transport of nutrients through the swollen network of macromolecular polymer chains follows a non-Fickian or anomalous diffusion mechanism. | [117] |
| CM starch-graft-polyacrylamide (PCMS-g-PAM) | P | The phosphate release mechanism from P-CMS-g-PAM was analyzed using the Korsmeyer–Peppas model. | [118] |
| Starch acrylamide, N,N′-methylene-bisacrylamide (crosslinker) by hot compression vulcanizer | N | The results showed that a higher gel strength was achieved with lower urea loading, increased grafting content, and greater crosslinking density in starch-based hydrogels. Additionally, the hydrogel network exhibited zero-order, or at least time-independent, urea release kinetics during the intermediate stable stage. | [113] |
| Starch acetate (SA)/PVA/glycerol (GLY) | N, P | The time required to reach the maximum concentration of N release was 2.4 and 3.2 times longer than that of uncoated DAP when DAP was coated with CF-3 (70% SA) in a single layer and double layer, respectively, indicating improved slow-release properties for N. | [109] |
| Cassava SHs crosslinked with CA | N, K, P | The hydrogel showed a slow release of the nutrient to P, releasing up to 30% in 150 h; however, N and K showed a burst release effect in the first 15 h, releasing until 80% of the nutrient was entrapped in the polymeric matrix. Additionally, the nutrient release followed a Fickian diffusion from the hydrogel to the bulk of the diffusion medium. | [119,120] |
| Maize starch (AS) was modified by graft copolymerization with sodium acid maleate and disodium maleate and covalently crosslinked with N,N′-bismethylene acrylamide | K, P | The fertilizer loading percentages for KNO3 ranged from 95% to 57%, while for KH2PO4, they ranged from 90% to 55%. The release percentages were between 92% and 67% for KNO3 and between 89% and 61% for KH2PO4, respectively. | [4] |
| Starch-g-poly (acrylic acid-acrylamide)/\zeolite hydrogel composite | N, P, K | Measuring the concentrations of N, P, and K released in buffer solutions at pH 5, pH 7, and in water over a period of 0 to 100 h revealed a gradual release pattern. | [121] |
| Hydrogel formed by starch oxidation with KMO4 and NaHSO4 | N, K | The loading and release of the fertilizer depended on the initial fertilizer concentration in the medium, as well as the nature, structure, and morphology of the starch used. | [111] |
| Hydrogel formed by the starch (corn, sweet cassava and bitter cassava) oxidation with KMO4 and NaHSO4 | N, K | The SC of the oxidized samples follows the trend of Na2SO4 > NaCl > KNO3 > CaCl2. All starch samples exhibit a similar “S”-shaped release kinetic profile, consisting of three main stages. A noticeable “burst effect” was observed up to 0.5 h, particularly for KNO3. | [122] |
| CS/starch hydrogel crosslinking with sodium TPP | P, N | The fertilizer release ratio exceeded 70% of the total loading. The hydrogel composition and crosslinking time determined the mechanism governing K release, which was controlled by matrix relaxation. | [110] |
| Hydrogel formed by potato starch, acrylic acid, acrylamide, and β-cyclodextrin modified by maleic anhydride, accompanying halloysite nanotubes | N | The results suggested that the prepared fertilizer exhibited excellent water retention and urea release control. Moreover, the addition of halloysite enhanced the fertilizer’s release properties. The study on urea release kinetics indicated that urea release in water was controlled by its concentration, whereas its release in soil followed the Fickian diffusion mechanism. | [123] |
| Starch-based superabsorbent hydrogels reinforced with natural char nano/microparticles | N | Starch-based superabsorbent nanocomposites exhibited a Fickian water diffusion mechanism and followed a pseudo-second-order swelling kinetic model. Moreover, after 14 days, the NCNP/hydrogel nanocomposite demonstrated a threefold increase in water retention capacity compared to the neat hydrogel (23.1% vs. 7.1%, respectively). | [124] |
| Starch-based superabsorbent polymers (SBSAPs) using as initiator (ceric ammonium nitrate, or CAN) and crosslinker (N,N0-methylene-bisacrylamide) | N | More than 50% of the urea was released at a significantly higher rate. Subsequently, between 20 and 40 days, over 80% of the urea was released. | [114] |
4.2. In Vitro Evaluation of SHs in Soil and Plant System
| Material | Nutrient | Plant and Seeds | Observation | Reference |
|---|---|---|---|---|
| Natural rubber and cassava starch crosslinking with sulfur and GA | Urea | Corn and basil plants | The performance of encapsulated urea beads in corn and basil plantations was significantly higher compared to non-encapsulated urea. The material releases urea through the porous membrane via non-Fickian diffusion. | [135] |
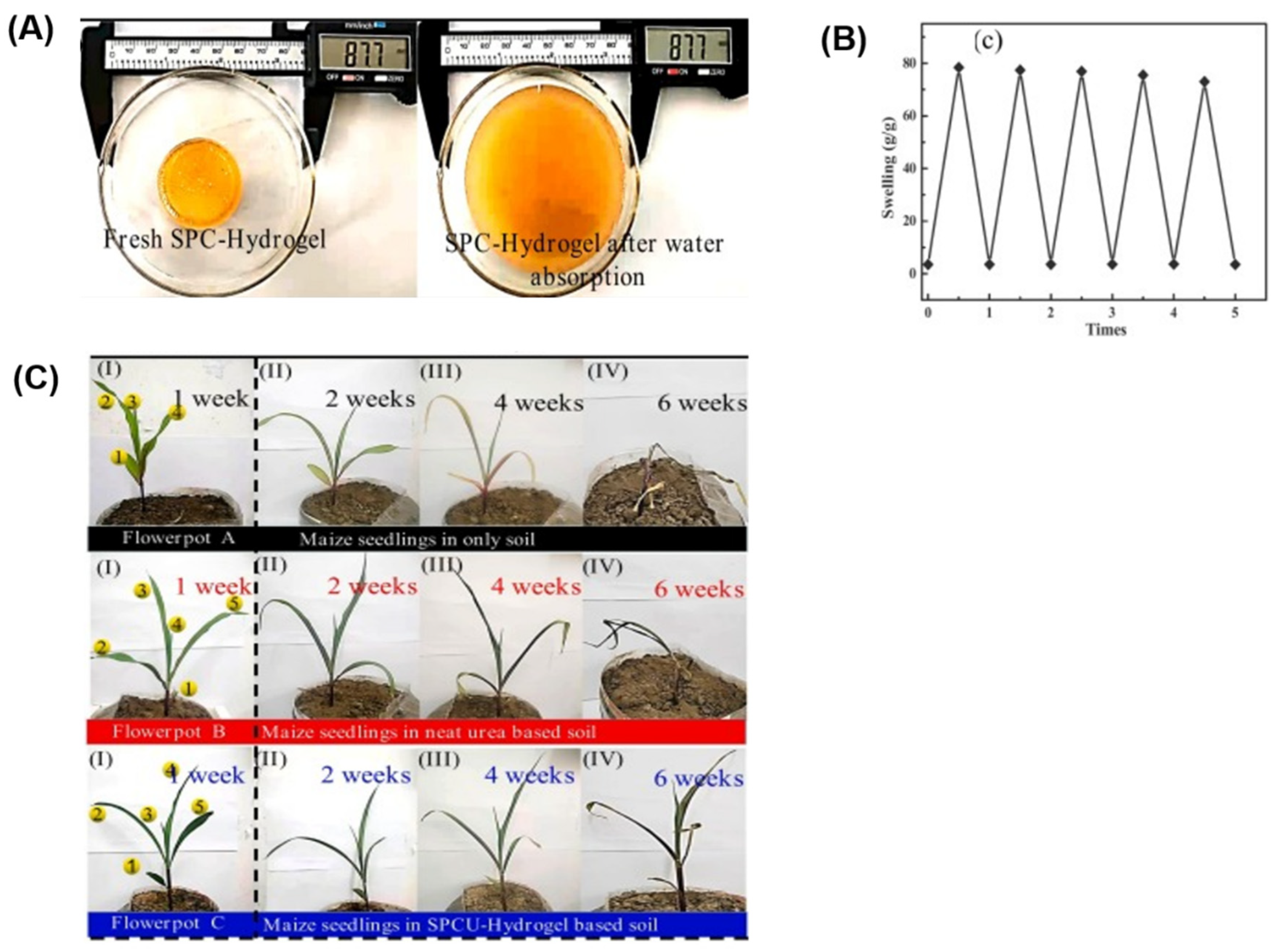
| Starch phosphate carbamate hydrogel (SPC-hydrogel) | Urea | Maize seeds | The hydrogel exhibits a continuous slow-release performance, with 50.3% of urea released within 15 h and nearly complete release in just over 25 h in water. | [136] |
| Cassava starch-g-polyacrylic acid/natural rubber/PVA | Urea | Chili plant | The formulation demonstrated excellent slow-release N delivery in both water (47.5% at 168 h) and soil (38.5% at 30 days). The chili plant growth was effectively enhanced, with a production cost 4–5 times lower than that of commercial slow-release fertilizers. | [112] |
| Succinate-modified potato starch | Water | Corn seed | At a water supply of 77% field capacity, the coated seeds exhibited a significantly higher emergence rate compared to uncoated seeds. | [131] |
| Starch-g-poly(styrene-co-butylacrylate) nanocomposite | Urea | Tomato | An increase in the total N percentage and a decrease in nitrate content in the aerial parts of plants were observed compared to traditional urea. | [137] |
| Starch-modified poly(acrylic acid with N, MBA as crosslinker) | Urea | Chickpea plant | Seeds placed in soil treated with urea-encapsulated hydrogel exhibited a shoot length of 2.8 cm, whereas those in untreated soil had a shoot length of only 0.5 cm. | [134] |
| Starch-poly (sodium acrylate-co-acrylamide) | Water | Maize (Zea may) seed | The soil-hydrogel analysis conducted at monthly intervals revealed a significant improvement in soil moisture retention and enhanced growth performance of maize seedlings compared to the control. | [132] |
| Nanozeolite– CS/sago starch-based biopolymer composite | Urea | Philodendron sp. plant | The hydrogel significantly promoted the growth of Philodendron sp., leading to improved growth indices, including survival rate, number of leaves, leaf length, and plant height, compared to the control and neat urea. | [138] |
| Poly(starch/acrylic acid) superabsorbent hydrogel (SAH) | Water | Sunflower under drought stress | The shoot and root length increased by 49.84% and 5.35%, respectively, compared to the absence of SAH. Growth parameters and photosynthetic pigment levels in sunflower plants grown under drought conditions were reduced without SAH. However, hydrogel application enhanced photosynthesis. | [139] |
| Cassava starch-graft-poly(acrylamide) copolymer | Water | Chili plants | The addition of hydrogel to the soil, combined with watering every three days, increased soil porosity and water retention, maintained higher nutrient levels, and preserved the soil’s biological properties. This treatment resulted in better plant growth compared to the control, which received daily watering without the polymer. | [140] |
| Starch-grafted-poly(sodium acrylate) | Water | Melon (Cucumis melo L.) seeds | Field experiments were conducted to evaluate the effects of hydrogel quantity, substrate type (sandy soil and coconut fibers), and soil type (sandy soil and clay soil) on various plant growth parameters. Overall, plants grown in coconut fibers exhibited the highest growth (5.60 cm) compared to those cultivated in sandy soil (4.12 cm). | [141] |
| Corn starch—urea extrusion material composite | Urea—melamine | Sweet corn | Greenhouse trials revealed that melamine plays a crucial role as a structural modifier, enhancing the effective utilization of N from urea in maize pot experiments. Additionally, it was observed that N-melamine remained unavailable during the first 60 days of the trial, indicating that the lower amount of N released (solely from urea) was more efficiently utilized by plants treated with composite material. | [142] |
| Starch-g-poly(acrylic acid-co-acrylic amide) (SBS-g-P(AA/AM)) as the skeleton and urea-formaldehyde oligomers | Urea | Maize | N release experiments confirmed that SBS-g-P(AA/AM)-UF provided a gradual N supply in the soil. Compared to conventional urea and UF fertilizers, maize yield increased by 20.3% and 9.7%, respectively, with the application of SBS-g-P(AA/AM)-UF. | [143] |
5. Conclusions, Challenges, and Outlook
- Most studies on SHs have focused on the release of N sources, with limited research on P, K, and micronutrients. In particular, researchers need to focus on developing versatile SHs capable of promoting the simultaneous release of NPK and water to plants.
- Many researchers have concentrated on SH production at the laboratory scale, with few studies exploring large-scale applications in crop production. As a result, there is limited information on application costs and feasibility, making it difficult to compare SH-based fertilization with conventional methods.
- The limited information on the application costs of SHs in soils also creates a knowledge gap in areas such as storage, mass production, and standardized protocols. Furthermore, the high biodegradability of SHs presents a challenge; research is needed to develop slow-release nutrient systems with high nutrient loading to reduce application frequency in soil. Additionally, new formulations should aim to improve the resistance of SHs to microbial degradation, which can otherwise lead to a rapid release of nutrients.
- Although SHs enhance the biodegradability of hydrogels formed with synthetic polymers, they exhibit low water retention under salt stress conditions, which negatively impacts continuous nutrient release in soil applications. Therefore, further research is needed to develop new SHs with improved performance under saline conditions. These materials must also be cost-effective to ensure adoption by farmers. For instance, the use of agricultural residues is emerging as a promising strategy to produce affordable SHs without requiring complex production processes that could increase the final product cost.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AP | amylopectin |
| AM | amylose |
| KPS | ammonium persulfate |
| CM | carboxymethyl |
| CS | chitosan |
| CA | citric acid |
| Dg | degree of grafting |
| DS | degree of substitution |
| DMSO | dimethyl sulfoxide |
| EH | epichlorohydrin |
| GA | glutaraldehyde |
| GYX | glyoxal |
| MA | malic acid |
| MBA | methylene bisacrylamide |
| Mw | molecular weight |
| N | nitrogen |
| NCNPs | natural charcoal nanoparticles |
| Nps | nanoparticles |
| NR | natural rubber latex |
| NZs | nanozeolites |
| P | phosphorus |
| PEC | polyelectrolyte complex |
| PVA | polyvinyl acetate |
| K | potassium |
| REX | reactive extrusion |
| SAH | starch-modified poly(acrylic acid) |
| SCM | starch–carboxymethyl |
| STMP | sodium trimetaphosphate |
| SPC | starch phosphate carbamate |
| SHs | starch-based hydrogels |
| SC | swelling capacity |
| SW | swelling ratio |
| TPP | tripolyphosphate |
References
- Fukase, E.; Martin, W. Economic growth, convergence, and world food demand and supply. World Dev. 2020, 132, 104954. [Google Scholar] [CrossRef]
- Guo, H.; White, J.C.; Wang, Z.; Xing, B. Nano-enabled fertilizers to control the release and use efficiency of nutrients. Curr. Opin. Env. Sci. Health 2018, 6, 77–83. [Google Scholar] [CrossRef]
- Vejan, P.; Khadiran, T.; Abdullah, R.; Ahmad, N. Controlled release fertilizer: A review on developments, applications, and potential in agriculture. J. Control. Release 2021, 339, 321–334. [Google Scholar] [CrossRef]
- León, O.; Soto, D.; Muñoz, B.A.; Fernández, G.M. Amylose modified starches as superabsorbent systems for release of potassium fertilizers. J. Polym. Environ. 2022, 30, 2314–2328. [Google Scholar] [CrossRef]
- Michalik, R.; Ilona, W. A mini-review on chitosan-based hydrogels with potential for sustainable agricultural applications. Polymers 2020, 12, 2425. [Google Scholar] [CrossRef]
- Chen, J.; Lü, S.; Zhang, Z.; Zhao, X.; Li, X.; Ning, P.; Liu, M. Environmentally friendly fertilizers: A review of materials used and their effects on the environment. Sci. Total Environ. 2018, 613, 829–839. [Google Scholar] [CrossRef]
- Tharanathan, R.N. Starch—Value addition by modification. Crit. Rev. Food Sci. Nutr. 2005, 45, 371–384. [Google Scholar] [CrossRef]
- Mottiar, Y.; Altosaar, I. Iodine sequestration by amylose to combat iodine deficiency disorders. Trends Food Sci. Technol. 2011, 22, 335–340. [Google Scholar] [CrossRef]
- Wang, S.; Blazek, J.; Gilbert, E.; Copeland, L. New insights on the mechanism of acid degradation of pea starch. Carbohydr. Polymers 2012, 87, 1941–1949. [Google Scholar] [CrossRef]
- Wang, S.; Li, C.; Copeland, L.; Niu, Q.; Wang, S. Starch retrogradation: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 568–585. [Google Scholar] [CrossRef]
- Vamadevan, V.; Bertoft, E. Structure-function relationships of starch components. Starch-Stärke 2015, 67, 55–68. [Google Scholar] [CrossRef]
- Callaghan, P.T.; Lelievre, J. The size and shape of amylopectin: A study using pulsed-field gradient nuclear magnetic resonance. Biopolymers 1985, 24, 441–460. [Google Scholar] [CrossRef]
- Kringel, D.H.; El Halal, S.L.M.; Zavareze, E.D.R.; Dias, A.R.G. Methods for the extraction of roots, tubers, pulses, pseudocereals, and other unconventional starches sources: A review. Starch-Stärke 2020, 72, 1900234. [Google Scholar] [CrossRef]
- Akhter, J.; Mahmood, K.; Malik, K.A.; Mardan, A.; Ahmad, M.; Iqbal, M.M. Effects of hydrogel amendment on water storage of sandy loam and loam soils and seedling growth of barley, wheat and chickpea. Plant Soil Environ. 2004, 50, 463–469. [Google Scholar] [CrossRef]
- Han, Y.G.; Yang, P.L.; Luo, Y.P.; Ren, S.M.; Zhang, L.X.; Xu, L. Porosity change model for watered super absorbent polymer-treated soil. Environ. Earth Sci. 2010, 61, 1197–1205. [Google Scholar] [CrossRef]
- Koupai, J.A.; Eslamian, S.S.; Kazemi, J.A. Enhancing the available water content in unsaturated soil zone using hydrogel, to improve plant growth indices. Ecohydrol. Hydrobiol. 2008, 8, 67–75. [Google Scholar] [CrossRef]
- Sojka, R.E.; Entry, J.A. Influence of polyacrylamide application to soil on movement of microorganisms in runoff water. Environ. Pollut. 2000, 108, 405–412. [Google Scholar] [CrossRef]
- Ni, B.; Liu, M.; Lü, S. Multifunctional slow-release urea fertilizer from ethylcellulose and superabsorbent coated formulations. Chem. Eng. J. 2009, 155, 892–898. [Google Scholar] [CrossRef]
- Rupollo, G.; Vanier, N.L.; da Rosa, Z.E.; de Oliveira, M.; Pereira, J.M.; Paraginski, R.T.; Guerra, A.R.; Cardoso, M.E. Pasting, morphological, thermal and crystallinity properties of starch isolated from beans stored under different atmospheric conditions. Carbohydr. Polym. 2011, 86, 1403–1409. [Google Scholar] [CrossRef]
- Jan, K.N.; Panesar, P.S.; Rana, J.C.; Singh, S. Structural, thermal and rheological properties of starches isolated from Indian quinoa varieties. Int. J. Biol. Macromol. 2017, 102, 315–322. [Google Scholar] [CrossRef]
- Leal, C.E.J.; García, T.Y.; Hernández, S.H.; Alamilla, B.L.; Téllez, M.D.I.; Calderón, D.G.; García, H.S.; Gutiérrez, L.G.F. Pickering emulsions stabilized with native and lauroylated amaranth starch. Food Hydrocoll. 2018, 80, 177–185. [Google Scholar] [CrossRef]
- Ratnayake, W.S.; Hoover, R.; Warkentin, T. Pea starch: Composition, structure and properties review. Starch-Stärke 2002, 54, 217–234. [Google Scholar] [CrossRef]
- Joshi, M.; Aldred, P.; McKnight, S.; Panozzo, J.F.; Kasapis, S.; Adhikari, R.; Adhikari, B. Physicochemical and functional characteristics of lentil starch. Carbohydr. Polym. 2013, 92, 1484–1496. [Google Scholar] [CrossRef] [PubMed]
- Oates, C.G. Towards an understanding of starch granule structure and hydrolysis. Trends Food Sci. Technol. 1997, 8, 375–382. [Google Scholar] [CrossRef]
- Singh, S.; Singh, N.; Isono, N.; Noda, T. Relationship of granule size distribution and amylopectin structure with pasting, thermal, and retrogradation properties in wheat starch. J. Agric. Food Chem. 2010, 58, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
- Ao, Z.; Jane, J.L. Characterization and modeling of the A and B-granule starches of wheat, triticale, and barley. Carbohydr. Polym. 2007, 67, 46–55. [Google Scholar] [CrossRef]
- Cornejo, R.Y.I.; Martínez, C.O.; del Toro, S.C.L.; Wong, C.F.J.; Borboa, F.J.; Cinco, M.F.J. The structural characteristics of starches and their functional properties. CyTA-J. Food 2018, 16, 1003–1017. [Google Scholar] [CrossRef]
- Jane, J.L. Current understanding on starch granule structures. J. Appl. Glycosci. 2006, 53, 205–213. [Google Scholar] [CrossRef]
- Konopka, I.; Rotkiewicz, D.; Tańska, M. Wheat endosperm hardness. Part II. Relationships to content and composition of flour lipids. Eur. Food Res. Technol. 2005, 220, 20–24. [Google Scholar] [CrossRef]
- Gaines, C.S.; Raeker, M.Ö.; Tilley, M.; Finney, P.L.; Wilson, J.D.; Bechtel, D.B.; Donelson, T. Associations of starch gel hardness, granule size, waxy allelic expression, thermal pasting, milling quality, and kernel texture of 12 soft wheat cultivars. Cereal Chem. 2000, 77, 163–168. [Google Scholar] [CrossRef]
- Fu, Z.; Chen, J.; Luo, S.J.; Liu, C.M.; Liu, W. Effect of food additives on starch retrogradation: A review. Starch-Stärke 2015, 67, 69–78. [Google Scholar] [CrossRef]
- Wang, S.; Chao, C.; Cai, J.; Niu, B.; Copeland, L.; Wang, S. Starch–lipid and starch–lipid–protein complexes: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1056–1079. [Google Scholar] [CrossRef]
- Shah, N.; Mewada, R.K.; Mehta, T. Crosslinking of starch and its effect on viscosity behaviour. Rev. Chem. Eng. 2016, 32, 265–270. [Google Scholar] [CrossRef]
- Kim, H.S.; Huber, K.C. Channels within soft wheat starch A and B-type granules. J. Cereal Sci. 2008, 48, 159–172. [Google Scholar] [CrossRef]
- Numfor, F.A.; Walter, W.M., Jr.; Schwartz, S.J. Physicochemical changes in cassava starch and flour associated with fermentation: Effect on textural properties. Starch-Stärke 1995, 47, 86–91. [Google Scholar] [CrossRef]
- Alvani, K.; Qi, X.; Tester, R.F.; Snape, C.E. Physico-chemical properties of potato starches. Food Chem. 2011, 125, 958–965. [Google Scholar] [CrossRef]
- de Souza, D.; Sbardelotto, A.F.; Ziegler, D.R.; Marczak, L.D.F.; Tessaro, I.C. Characterization of rice starch and protein obtained by a fast alkaline extraction method. Food Chem. 2016, 191, 36–44. [Google Scholar] [CrossRef]
- Zhang, R.; Ma, S.; Li, L.; Zhang, M.; Tian, S.; Wang, D.; Liu, K.; Liu, H.; Zhu, W.; Wang, X. Comprehensive utilization of corn starch processing by-products: A review. Grain Oil Sci. Technol. 2021, 4, 89–107. [Google Scholar] [CrossRef]
- Jiménez, H.J.; Salazar, J.A.; Ramos, E.G. Physical, chemical and microscopic characterization of a new starch from chayote (Sechium edule) tuber and its comparison with potato and maize starches. Carbohydr. Polym. 2007, 68, 679–686. [Google Scholar] [CrossRef]
- Raeker, M.Ö.; Gaines, C.S.; Finney, P.L.; Donelson, T. Granule size distribution and chemical composition of starches from 12 soft wheat cultivars. Cereal Chem. 1998, 75, 721–728. [Google Scholar] [CrossRef]
- Asare, E.K.; Jaiswal, S.; Maley, J.; Baga, M.; Sammynaiken, R.; Rossnagel, B.G.; Chibbar, R.N. Barley grain constituents, starch composition, and structure affect starch in vitro enzymatic hydrolysis. J. Agric. Food Chem. 2011, 59, 4743–4754. [Google Scholar] [CrossRef] [PubMed]
- Moongngarm, A. Chemical compositions and resistant starch content in starchy foods. Am. J. Agric. Biol. Sci. 2013, 8, 107. [Google Scholar] [CrossRef]
- Emiola, L.O.; Delarosa, L.C. Physicochemical characteristics of yam starches. J. Food Biochem. 1981, 2, 115–130. [Google Scholar] [CrossRef]
- Andrade, L.A.; Barbosa, N.A.; Pereira, J. Extraction and properties of starches from the non-traditional vegetables Yam and Taro. Polímeros 2017, 27, 151–157. [Google Scholar] [CrossRef]
- Zeng, F.K.; Liu, H.; Liu, G. Physicochemical properties of starch extracted from Colocasia esculenta (L.) Schott (Bun-long taro) grown in Hunan, China. Starch-Stärke 2014, 66, 142–148. [Google Scholar] [CrossRef]
- Thitipraphunkul, K.; Uttapap, D.; Piyachomkwan, K.; Takeda, Y. A comparative study of edible canna (Canna edulis) starch from different cultivars. Part I. Chemical composition and physicochemical properties. Carbohydr. Polym. 2003, 53, 317–324. [Google Scholar] [CrossRef]
- Chanapamokkhot, H.; Thongngam, M. The chemical and physico-chemical properties of sorghum starch and flour. Int. J. Agric. Nat. Resour. 2007, 41, 343–349. [Google Scholar]
- de Melo Neto, B.A.; Barbosa, A.A.; dos Santos Leite, C.X.; de Almeida, P.F.; Bonomo, R.C.F.; Pontes, K.V. Chemical composition and functional properties of starch extracted from the pejibaye fruit (Bactris gasepaes Kunth.). Acta Sci. Technol. 2015, 37, 105–110. [Google Scholar] [CrossRef]
- Bello, L.A.; Agama, E.; Sánchez, L.; Paredes, L.O. Isolation and partial characterization of banana starches. J. Agr Food Chem 1999, 47, 854–857. [Google Scholar] [CrossRef]
- Saadany, R.E.; Foda, Y.H.; Saadany, F.E. Studies on starch extracted from mango seeds (Mangifira indica) as a new source of starch. Starch-Stärke 1980, 32, 113–116. [Google Scholar] [CrossRef]
- Cruz, B.R.; Abraão, A.S.; Lemos, A.M.; Nunes, F.M. Chemical composition and functional properties of native chestnut starch (Castanea sativa Mill). Carbohydr. Polym. 2013, 94, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Wosiacki, G.; Cereda, M.P. Characterization of pinhão starch. Part I. Extraction and properties of the starch granules. Starch-Stärke 1985, 37, 224–227. [Google Scholar] [CrossRef]
- Agunbiade, S.O. The chemical composition and in vitro digestibility of yam bean starch. Food Chem. 1998, 61, 173–176. [Google Scholar] [CrossRef]
- Tessema, A.; Admassu, H. Extraction and characterization of starch from anchote (Coccinia abyssinica): Physico-chemical, functional, morphological and crystalline properties. J. Food Meas. Charact. 2021, 15, 3096–3110. [Google Scholar] [CrossRef]
- Pires, M.B.; Amante, E.R.; de Oliveira Petkowicz, C.L.; Esmerino, E.A.; da Cruz, R.A.M.; da Silva, L.H.M. Impact of extraction methods and genotypes on the properties of starch from peach palm (Bactris gasipaes Kunth) fruits. LWT 2021, 150, 111983. [Google Scholar] [CrossRef]
- Rincón, A.M.; Padilla, F.C. Physicochemical properties of breadfruit (Artocarpus altilis) starch from Margarita island, Venezuela. Arch. Latinoam. Nutr. 2004, 54, 449–456. [Google Scholar]
- Barbi, R.C.T.; Teixeira, G.L.; Hornung, P.S.; Ávila, S.; Hoffmann-Ribani, R. Eriobotrya japonica seed as a new source of starch: Assessment of phenolic compounds, antioxidant activity, thermal, rheological, and morphological properties. Food Hydrocoll. 2018, 77, 646–658. [Google Scholar] [CrossRef]
- Luengwilai, K.; Beckles, D.M. Structural investigations and morphology of tomato fruit starch. J. Agric. Food Chem. 2009, 57, 282–291. [Google Scholar] [CrossRef]
- Pérez, P.E.; Moo, H.V.M.; Estrada, L.R.J.; Ortiz, F.A.; May, H.L.H.; Ríos, C.R.; Betancur, D.A. Isolation and characterization of starch obtained from Brosimum alicastrum Swarts Seeds. Carbohydr. Polym. 2014, 101, 920–927. [Google Scholar] [CrossRef]
- Ascheri, D.P.R.; Pereira, L.D.; Bastos, S.M.C. Chemical, morphological, rheological and thermal properties of Solanum lycocarpum phosphorylated starches. Ceres 2014, 61, 458–466. [Google Scholar] [CrossRef]
- Guo, K.; Lin, L.; Fan, X.; Zhang, L.; Wei, C. Comparison of structural and functional properties of starches from five fruit kernels. Food Chem. 2018, 257, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Rincón, A.M.; Padilla, F.C.; Araujo, C.; Tillett, S. Myrosma cannifolia, chemical composition and physicochemical properties of the extracted starch. J. Sci. Food Agric. 1999, 79, 532–536. [Google Scholar] [CrossRef]
- Ismail, H.; Irani, M.; Ahmad, Z. Starch-based hydrogels: Present status and applications. Int. J. Polym. Mater. Polym. Biomater. 2013, 62, 411–420. [Google Scholar] [CrossRef]
- Czarnecka, E.; Nowaczyk, J. Semi-Natural superabsorbents based on Starch-g-poly (acrylic acid): Modification, synthesis and application. Polymers 2020, 12, 1794. [Google Scholar] [CrossRef] [PubMed]
- Lanthong, P.; Nuisin, R.; Kiatkamjornwong, S. Graft copolymerization, characterization, and degradation of cassava starch-g-acrylamide/itaconic acid superabsorbents. Carbohydr. Polym. 2006, 66, 229–245. [Google Scholar] [CrossRef]
- Cho, C.G.; Lee, K. Preparation of starch-g-polystyrene copolymer by emulsion polymerization. Carbohydr. Polym. 2002, 48, 125–130. [Google Scholar] [CrossRef]
- Fakhru’L-Razi, A.; Qudsieh, I.Y.; Yunus, W.M.Z.W.; Ahmad, M.B.; Rahman, M.Z.A. Graft copolymerization of methyl methacrylate onto sago starch using ceric ammonium nitrate and potassium persulfate as redox initiator systems. J. Appl. Polym. Sci. 2001, 82, 1375–1381. [Google Scholar] [CrossRef]
- Sandle, N.K.; Verma, O.P.S.; Varma, I.K. Thermal characterization of starch-g-acrylonitrile copolymers. Thermochim. Acta 1987, 115, 189–198. [Google Scholar] [CrossRef]
- Meimoun, J.; Wiatz, V.; Saint-Loup, R.; Parcq, J.; Favrelle, A.; Bonnet, F.; Zinck, P. Modification of starch by graft copolymerization. Starch-Stärke 2018, 70, 1600351. [Google Scholar] [CrossRef]
- Kiatkamjornwong, S.; Chomsaksakul, W.; Sonsuk, M. Radiation modification of water absorption of cassava starch by acrylic acid/acrylamide. Radiat. Phys. Chem. 2000, 59, 413–427. [Google Scholar] [CrossRef]
- Lertsarawut, P.; Rattanawongwiboon, T.; Tangthong, T.; Laksee, S.; Kwamman, T.; Phuttharak, B.; Hemvichian, K. Starch-Based Super Water Absorbent: A Promising and Sustainable Way to Increase Survival Rate of Trees Planted in Arid Areas. Polymers 2021, 13, 1314. [Google Scholar] [CrossRef]
- Mu, X.; Zhou, J.; Wang, P.; Chen, H.; Yang, T.; Chen, S.; Mori, T. A robust starch–polyacrylamide hydrogel with scavenging energy harvesting capacity for efficient solar thermoelectricity–freshwater cogeneration. Energy Environ. Sci. 2022, 15, 3388–3399. [Google Scholar] [CrossRef]
- Pratiwi, S.N.; Afifah, D.N.; Widyastiti, N.S.; Karlowee, V.; Anjani, G.; Istiadi, H. Banana resistant starch inhibitory inflammation and cyclooxygenase-2 in BALB/c mice induced by azoxymethane and dextran sodium sulfate. Food Res. 2022, 6, 337–344. [Google Scholar] [CrossRef]
- Zhai, M.; Yoshii, F.; Kume, T.; Hashim, K. Syntheses of PVA/starch grafted hydrogels by irradiation. Carbohydr. Polym. 2002, 50, 295–303. [Google Scholar] [CrossRef]
- Xiao, C. Current advances of chemical and physical starch-based hydrogels. Starch-Stärke 2013, 65, 82–88. [Google Scholar] [CrossRef]
- Gonenc, I.; Us, F. Effect of glutaraldehyde crosslinking on degree of substitution, thermal, structural, and physicochemical properties of corn starch. Starch-Stärke 2019, 71, 1800046. [Google Scholar] [CrossRef]
- Bursali, E.A.; Coskun, S.; Kizil, M.; Yurdakoc, M. Synthesis, characterization and in vitro antimicrobial activities of boron/starch/polyvinyl alcohol hydrogels. Carbohydr. Polym. 2011, 83, 1377–1383. [Google Scholar] [CrossRef]
- Thakur, K.; Rajhans, A.; Kandasubramanian, B. Starch/PVA hydrogels for oil/water separation. Environ. Sci. Pollut. R. 2019, 26, 32013–32028. [Google Scholar] [CrossRef]
- Kulicke, W.M.; Aggour, Y.A.; Nottelmann, H.; Elsabee, M.Z. Swelling and rheological studies of some starch hydrogels. Starch-Stärke 1989, 41, 140–146. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, Y.; Shen, L.; Wang, X.; Chen, J.; Ma, A.; Jiang, W. Lead (II) and methylene blue removal using a fully biodegradable hydrogel based on starch immobilized humic acid. Chem. Eng. J. 2015, 268, 348–355. [Google Scholar] [CrossRef]
- Kulicke, W.M.; Aggour, Y.A.; Elsabee, M.Z. Preparation, Characterisation, and Rheological Behaviour of Starch-Sodium Trimetaphosphate Hydrogels. Starch-Stärke 1990, 42, 134–141. [Google Scholar] [CrossRef]
- Seker, M.; Hanna, M.A. Sodium hydroxide and trimetaphosphate levels affect properties of starch extrudates. Ind. Crop. Prod. 2006, 23, 249–255. [Google Scholar] [CrossRef]
- Cagnin, C.; Simões, B.M.; Yamashita, F.; de Carvalho, G.M.; Grossmann, M.V.E. pH sensitive phosphate crosslinked films of starch-carboxymethyl cellulose. Polym. Eng. Sci. 2021, 61, 388–396. [Google Scholar] [CrossRef]
- Lemos, P.V.F.; Opretzka, L.C.F.; Almeida, L.S.; Cardoso, L.G.; da Silva, J.B.A.; de Souza, C.O.; Druzian, J.I. Preparation and characterization of C-phycocyanin coated with STMP/STPP cross-linked starches from different botanical sources. Int. J. Biol. Macromol. 2020, 159, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Lawal, O.S. Succinyl and acetyl starch derivatives of a hybrid maize: Physicochemical characteristics and retrogradation properties monitored by differential scanning calorimetry. Carbohydr. Res. 2004, 339, 2673–2682. [Google Scholar] [CrossRef]
- Olivato, J.B.; Grossmann, M.V.E.; Bilck, A.P.; Yamashita, F. Effect of organic acids as additives on the performance of thermoplastic starch/polyester blown films. Carbohydr. Polym. 2012, 90, 159–164. [Google Scholar] [CrossRef]
- Simões, B.M.; Cagnin, C.; Yamashita, F.; Olivato, J.B.; Garcia, P.S.; de Oliveira, S.M.; Grossmann, M.V.E. Citric acid as crosslinking agent in starch/xanthan gum hydrogels produced by extrusion and thermopressing. LWT 2020, 125, 108950. [Google Scholar] [CrossRef]
- Reddy, N.; Yang, Y. Citric acid cross-linking of starch films. Food Chem. 2010, 118, 702–711. [Google Scholar] [CrossRef]
- Seligra, P.G.; Jaramillo, C.M.; Famá, L.; Goyanes, S. Biodegradable and non-retrogradable eco-films based on starch–glycerol with citric acid as crosslinking agent. Carbohydr. Polym. 2016, 138, 66–74. [Google Scholar] [CrossRef]
- Vudjung, C.; Chaisuwan, U.; Pangan, U.; Chaipugdee, N.; Boonyod, S.; Santawitee, O.; Saengsuwan, S. Effect of natural rubber contents on biodegradation and water absorption of interpenetrating polymer network (IPN) hydrogel from natural rubber and cassava starch. Energy Procedia 2014, 56, 255–263. [Google Scholar] [CrossRef]
- Pang, S.C.; Chin, S.F.; Tay, S.H.; Tchong, F.M. Starch–maleate–polyvinyl alcohol hydrogels with controllable swelling behaviors. Carbohydr. Polym. 2011, 84, 424–429. [Google Scholar] [CrossRef]
- Passauer, L.; Liebner, F.; Fischer, K. Starch phosphate hydrogels. Part I: Synthesis by mono-phosphorylation and cross-linking of starch. Starch-Stärke 2009, 61, 621–627. [Google Scholar] [CrossRef]
- Sonawane, R.O.; Patil, S.D. Fabrication and statistical optimization of starch-κ-carrageenan cross-linked hydrogel composite for extended-release pellets of zaltoprofen. Int. J. Biol. Macromol. 2018, 120, 2324–2334. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tan, Y.; Xu, K.; Lu, C.; Wang, P. A biodegradable starch hydrogel synthesized via thiol-ene click chemistry. Polym. Degrad. Stab. 2017, 137, 75–82. [Google Scholar] [CrossRef]
- da Silva Fernandes, R.; Tanaka, F.N.; de Moura, M.R.; Aouada, F.A. Development of alginate/starch-based hydrogels crosslinked with different ions: Hydrophilic, kinetic and spectroscopic properties. Mater. Today Commun. 2019, 21, 100636. [Google Scholar] [CrossRef]
- Perez, J.J.; Francois, N.J. Chitosan-starch beads prepared by ionotropic gelation as potential matrices for controlled release of fertilizers. Carbohydr. Polym. 2016, 148, 134–142. [Google Scholar] [CrossRef]
- Perez, J.J.; Francois, N.J.; Maroniche, G.A.; Borrajo, M.P.; Pereyra, M.A.; Creus, C.M. A novel, green, low-cost chitosan-starch hydrogel as potential delivery system for plant growth-promoting bacteria. Carbohydr. Polym. 2018, 202, 409–417. [Google Scholar] [CrossRef]
- Rosiak, J.M.; Yoshii, F. Hydrogels and their medical applications. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 1999, 151, 56–64. [Google Scholar] [CrossRef]
- Hennink, W.E.; van Nostrum, C.F. Novel crosslinking methods to design hydrogels. Adv. Drug Deliv. Rev. 2012, 64, 223–236. [Google Scholar] [CrossRef]
- Qamruzzaman, M.; Ahmed, F.; Mondal, M.; Ibrahim, H. An overview on starch-based sustainable hydrogels: Potential applications and aspects. J. Polym. Environ. 2022, 30, 19–50. [Google Scholar] [CrossRef]
- Zhu, F. Starch based aerogels: Production, properties and applications. Trends Food Sci. Technol. 2019, 89, 1–10. [Google Scholar] [CrossRef]
- Druel, L.; Bardl, R.; Vorwerg, W.; Budtova, T. Starch aerogels: A member of the family of thermal superinsulating materials. Biomacromolecules 2017, 18, 4232–4239. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Yang, M. Controlled preparation of physical cross-linked starch-g-PVA hydrogel. Carbohydr. Polym. 2006, 64, 37–40. [Google Scholar] [CrossRef]
- Kenawy, E.R.; Kamoun, E.A.; Eldin, M.S.M.; El-Meligy, M.A. Physically crosslinked poly (vinyl alcohol)-hydroxyethyl starch blend hydrogel membranes: Synthesis and characterization for biomedical applications. Arab. J. Chem. 2014, 7, 372–380. [Google Scholar] [CrossRef]
- Nanaki, S.G.; Koutsidis, I.A.; Koutri, I.; Karavas, E.; Bikiaris, D. Miscibility study of chitosan/2-hydroxyethyl starch blends and evaluation of their effectiveness as drug sustained release hydrogels. Carbohydr. polym. 2012, 87, 1286–1294. [Google Scholar] [CrossRef]
- Prado, H.J.; Matulewicz, M.C.; Bonelli, P.R.; Cukierman, A.L. Preparation and characterization of a novel starch-based interpolyelectrolyte complex as matrix for controlled drug release. Carbohydr. Res. 2009, 344, 1325–1331. [Google Scholar] [CrossRef]
- Assaad, E.; Blemur, L.; Lessard, M.; Mateescu, M.A. Polyelectrolyte complex of carboxymethyl starch and chitosan as protein carrier: Oral administration of ovalbumin. J. Biomat. Sci. Polym. Ed. 2012, 23, 1713–1728. [Google Scholar] [CrossRef]
- Wang, Y.J.; Assaad, E.; Ispas-Szabo, P.; Mateescu, M.A.; Zhu, X.X. NMR imaging of chitosan and carboxymethyl starch tablets: Swelling and hydration of the polyelectrolyte complex. Int. J. Pharmaceut. 2011, 419, 215–221. [Google Scholar] [CrossRef]
- Sofyane, A.; Ablouh, E.; Lahcini, M.; Elmeziane, A.; Khouloud, M.; Kaddami, H.; Raihane, M. Slow-release fertilizers based on starch acetate/glycerol/polyvinyl alcohol biocomposites for sustained nutrient release. Mater. Today Proc. 2021, 36, 74–81. [Google Scholar] [CrossRef]
- Perez, B.J.J.; François, N.J. Chitosan/starch matrices prepared by ionotropic gelation: Rheological characterization, swelling behavior and potassium nitrate release kinetics. J. Polym. Environ. 2020, 28, 2681–2690. [Google Scholar] [CrossRef]
- León, O.; Soto, D.; González, J.; Piña, C.; Muñoz, B.A.; Fernández, G.M. Environmentally friendly fertilizers based on starch superabsorbents. Materials 2019, 12, 3493. [Google Scholar] [CrossRef] [PubMed]
- Tanan, W.; Panichpakdee, J.; Suwanakood, P.; Saengsuwan, S. Biodegradable hydrogels of cassava starch-g-polyacrylic acid/natural rubber/polyvinyl alcohol as environmentally friendly and highly efficient coating material for slow-release urea fertilizers. J. Ind. Eng. Chem. 2021, 101, 237–252. [Google Scholar] [CrossRef]
- Wei, X.; Bao, X.; Yu, L.; Liu, H.; Lu, K.; Chen, L. Correlation between gel strength of starch-based hydrogel and slow-release behavior of its embedded urea. J. Polym. Environ. 2020, 28, 863–870. [Google Scholar] [CrossRef]
- Xiao, X.; Yu, L.; Xie, F.; Bao, X.; Liu, H. One-step method to prepare starch-based superabsorbent polymer for slow release of fertilizer. Chem. Eng. J. 2017, 309, 607–616. [Google Scholar] [CrossRef]
- Jiang, T.; Chen, F.; Duan, Q.; Bao, X.; Jiang, S.; Liu, H.; Liu, C.; Yu, L. Designing and application of reactive extrusion with twice initiations for graft copolymerization of acrylamide on starch. Eur. Polym. J. 2022, 165, 111008. [Google Scholar] [CrossRef]
- Zhong, K.; Lin, Z.T.; Zheng, X.L.; Jiang, G.B.; Fang, Y.S.; Mao, X.Y.; Liao, Z.W. Starch derivative-based superabsorbent with integration of water-retaining and controlled-release fertilizers. Carbohydr. Polym. 2013, 92, 1367–1376. [Google Scholar] [CrossRef]
- Azeem, B.; KuShaari, K.; Man, Z. Effect of coating thickness on release characteristics of controlled release urea produced in fluidized bed using waterborne starch biopolymer as coating material. Procedia Eng. 2016, 148, 282–289. [Google Scholar] [CrossRef]
- Alharbi, K.; Ghoneim, A.; Ebid, A.; El-Hamshary, H.; El-Newehy, M.H. Controlled release of phosphorous fertilizer bound to carboxymethyl starch-g-polyacrylamide and maintaining a hydration level for the plant. Int. J. Biol. Macromol. 2018, 116, 224–231. [Google Scholar] [CrossRef]
- Chamorro, A.F.; Palencia, M.; Arrieta, Á.A. Development of High-Efficiency Fertilizer by Hydrogels Obtained from Cassava Starch and Citric Acid for Slow Release of Ammonium and Potassium. Gels 2024, 10, 434. [Google Scholar] [CrossRef]
- Chamorro, A.F.; Palencia, M.; Combatt, E.M. Biodegradable Cassava Starch/Phosphorite/Citric Acid Based Hydrogel for Slow Release of Phosphorus: In Vitro Study. Gels 2024, 10, 431. [Google Scholar] [CrossRef]
- Sjaifullah, A.; Faidza, L.Z.; Mitoma, Y. Arrowroot starch-g-poly (acrylic acid-acrylamide)/zeolite hydrogel composite as matrix for CRF of nitrogen, phosphorous and kalium. AIP Conf. Proc. 2020, 2278, 020025. [Google Scholar] [CrossRef]
- León, O.; Soto, D.; Antúnez, A.; Fernández, R.; González, J.; Fernández, G.M. Hydrogels based on oxidized starches from different botanical sources for release of fertilizers. Int. J. Biol. Macromol. 2019, 136, 813–822. [Google Scholar] [CrossRef]
- Wei, H.; Wang, H.; Chu, H.; Li, J. Preparation and characterization of slow-release and water-retention fertilizer based on starch and halloysite. Int. J. Biol. Macromol. 2019, 133, 1210–1218. [Google Scholar] [CrossRef] [PubMed]
- Motamedi, E.; Motesharezedeh, B.; Shirinfekr, A.; Samar, S.M. Synthesis and swelling behavior of environmentally friendly starch-based superabsorbent hydrogels reinforced with natural char nano/micro particles. J. Environ. Chem. Eng. 2020, 8, 103583. [Google Scholar] [CrossRef]
- Moad, G. Chemical modification of starch by reactive extrusion. Prog. Polym. Sci. 2011, 36, 218–237. [Google Scholar] [CrossRef]
- Salimi, M.; Motamedi, E.; Motesharezedeh, B.; Hosseini, H.M.; Alikhani, H.A. Starch-g-poly (acrylic acid-co-acrylamide) composites reinforced with natural char nanoparticles toward environmentally benign slow-release urea fertilizers. J. Environ. Chem. Eng. 2020, 8, 103765. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, P.; Zhao, L.; Chen, Y. Preparation and swelling properties of a starch-g-poly (acrylic acid)/organo-mordenite hydrogel composite. Front. Chem. Sci. Eng. 2016, 10, 147–161. [Google Scholar] [CrossRef]
- Qiao, D.; Liu, H.; Yu, L.; Bao, X.; Simon, G.P.; Petinakis, E.; Chen, L. Preparation and characterization of slow-release fertilizer encapsulated by starch-based superabsorbent polymer. Carbohydr. Polym. 2016, 147, 146–154. [Google Scholar] [CrossRef]
- Gungula, D.T.; Andrew, F.P.; Joseph, J.; Kareem, S.A.; Barminas, J.T.; Adebayo, E.F.; Saddiq, A.M.; Tame, V.T.; Dere, I.; Ahinda, W.J.; et al. Formulation and characterization of water retention and slow-release urea fertilizer based on Borassus aethiopum starch and Maesopsis eminii hydrogels. Res. Mat. 2021, 12, 100223. [Google Scholar] [CrossRef]
- Jyothi, A.N.; Pillai, S.S.; Aravind, M.; Salim, S.A.; Kuzhivilayil, S.J. Cassava starch-graft-poly (acrylonitrile)-coated urea fertilizer with sustained release and water retention properties. Adv. Polym. Technol. 2018, 37, 2687–2694. [Google Scholar] [CrossRef]
- Pathak, V.; Ambrose, R.K. Starch-based biodegradable hydrogel as seed coating for corn to improve early growth under water shortage. J. Appl. Polym. Sci. 2020, 137, 48523. [Google Scholar] [CrossRef]
- Ekebafe, L.O.; Ogbeifun, D.E.; Okieimen, F.E. Effect of native cassava starch-poly (sodium acrylate-co-acrylamide) hydrogel on the growth performance of maize (Zea may) seedlings. Am. J. Polym. Sci. 2011, 1, 6–11. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, W.A.; Luo, W.; Fang, Y.E. Synthesis of superabsorbent polymers by irradiation and their applications in agriculture. J. Appl. Polym. Sci. 2004, 93, 1748–1755. [Google Scholar] [CrossRef]
- Sarmah, D.; Karak, N. Biodegradable superabsorbent hydrogel for water holding in soil and controlled-release fertilizer. J. Appl. Polym. Sci. 2020, 137, 48495. [Google Scholar] [CrossRef]
- Vudjung, C.; Saengsuwan, S. Biodegradable IPN hydrogels based on pre-vulcanized natural rubber and cassava starch as coating membrane for environment-friendly slow-release urea fertilizer. J. Polym. Environ. 2018, 26, 3967–3980. [Google Scholar] [CrossRef]
- Dong, G.; Mu, Z.; Liu, D.; Shang, L.; Zhang, W.; Gao, Y.; Zhao, M.; Zhang, X.; Chen, S.; Wei, M. Starch phosphate carbamate hydrogel based slow-release urea formulation with good water retentivity. Int. J. Biol. Macromol. 2021, 190, 189–197. [Google Scholar] [CrossRef]
- Salimi, M.; Motamedi, E.; Safari, M.; Motesharezadeh, B. Synthesis of urea slow-release fertilizer using a novel starch-g-poly (styrene-co-butylacrylate) nanocomposite latex and its impact on a model crop production in greenhouse. J. Clean. Prod. 2021, 322, 129082. [Google Scholar] [CrossRef]
- Pimsen, R.; Porrawatkul, P.; Nuengmatcha, P.; Ramasoot, S.; Chanthai, S. Efficiency enhancement of slow release of fertilizer using nanozeolite–chitosan/sago starch-based biopolymer composite. J. Coat. Technol. Res. 2021, 18, 1321–1332. [Google Scholar] [CrossRef]
- Ghobashy, M.M.; Amin, M.A.; Nady, N.; Meganid, A.S.; Alkhursani, S.A.; Alshangiti, D.M.; Madani, M.; Al-Gahtany, S.A.; Zaher, A.A. Improving impact of poly (starch/acrylic acid) superabsorbent hydrogel on growth and biochemical traits of sunflower under drought stress. J. Polym. Environ. 2022, 30, 1973–1983. [Google Scholar] [CrossRef]
- Parvathy, P.C.; Jyothi, A.N.; John, K.S.; Sreekumar, J. Cassava Starch Based Superabsorbent Polymer as Soil Conditioner: Impact on Soil Physico-Chemical and Biological Properties and Plant Growth. CLEAN–Soil Air Water 2014, 42, 1610–1617. [Google Scholar] [CrossRef]
- Vasconcelos, M.C.; Gomes, R.F.; Sousa, A.A.; Moreira, F.J.; Rodrigues, F.H.; Fajardo, A.R.; Neto, L.G.P. Superabsorbent hydrogel composite based on starch/rice husk ash as a soil conditioner in melon (Cucumis melo L.) seedling culture. J. Polym. Environ. 2020, 28, 131–140. [Google Scholar] [CrossRef]
- Giroto, A.S.; Guimarães, G.G.; Colnago, L.A.; Klamczynski, A.; Glenn, G.; Ribeiro, C. Controlled release of nitrogen using urea-melamine-starch composites. J. Clean. Prod. 2019, 217, 448–455. [Google Scholar] [CrossRef]
- Zhao, Y.; Fan, Z.; Chen, Y.; Huang, X.; Zhai, S.; Sun, S.; Tian, X. A Bio-Based Hydrogel Derived from Moldy Steamed Bread as Urea-Formaldehyde Loading for Slow-Release and Water-Retention Fertilizers. ACS Omega 2021, 6, 33462–33469. [Google Scholar] [CrossRef]
- Mikhak, A.; Sohrabi, A.; Kassaee, M.Z.; Feizian, M. Synthetic nanozeolite/nanohydroxyapatite as a phosphorus fertilizer for German chamomile (Matricariachamomilla L.). Ind. Crop. Prod. 2017, 95, 444–452. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chamorro, A.F.; Palencia, M.; Combatt, E.M. Starch Hydrogels for Slow and Controlled-Release Fertilizers: A Review. Polymers 2025, 17, 1117. https://doi.org/10.3390/polym17081117
Chamorro AF, Palencia M, Combatt EM. Starch Hydrogels for Slow and Controlled-Release Fertilizers: A Review. Polymers. 2025; 17(8):1117. https://doi.org/10.3390/polym17081117
Chicago/Turabian StyleChamorro, Andrés Felipe, Manuel Palencia, and Enrique Miguel Combatt. 2025. "Starch Hydrogels for Slow and Controlled-Release Fertilizers: A Review" Polymers 17, no. 8: 1117. https://doi.org/10.3390/polym17081117
APA StyleChamorro, A. F., Palencia, M., & Combatt, E. M. (2025). Starch Hydrogels for Slow and Controlled-Release Fertilizers: A Review. Polymers, 17(8), 1117. https://doi.org/10.3390/polym17081117








