Isolation, Identification and Screening of Plastic-Degrading Microorganisms: Qualitative and Structural Effects on Poly(Butylene Succinate) (PBS) Films
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Isolation of Microbial Consortia
2.2. Characterization of Isolated Microorganisms
2.3. Molecular Identification of Isolated Microorganisms
2.4. Selection of Degrading Microorganisms by the Zone of Clearing Method
2.5. PBS Film Preparation
Film Conditioning
2.6. Biodegradation of PBS Films in Liquid Medium
Plastic Film Recovery
2.7. Morphological and Structural Analysis of Films and Their Degradation
2.7.1. Macroscopic and Microscopic Characterization of Plastic Films
2.7.2. Fourier Transform Infrared Spectroscopy (FT-IR)
2.7.3. Structural Analysis Using Atmospheric Solids Analysis Probe–Mass Spectrometry (ASAP-MS)
2.8. Statistical Analysis
3. Results and Discussion
3.1. Molecular Identification of the Isolated Strains
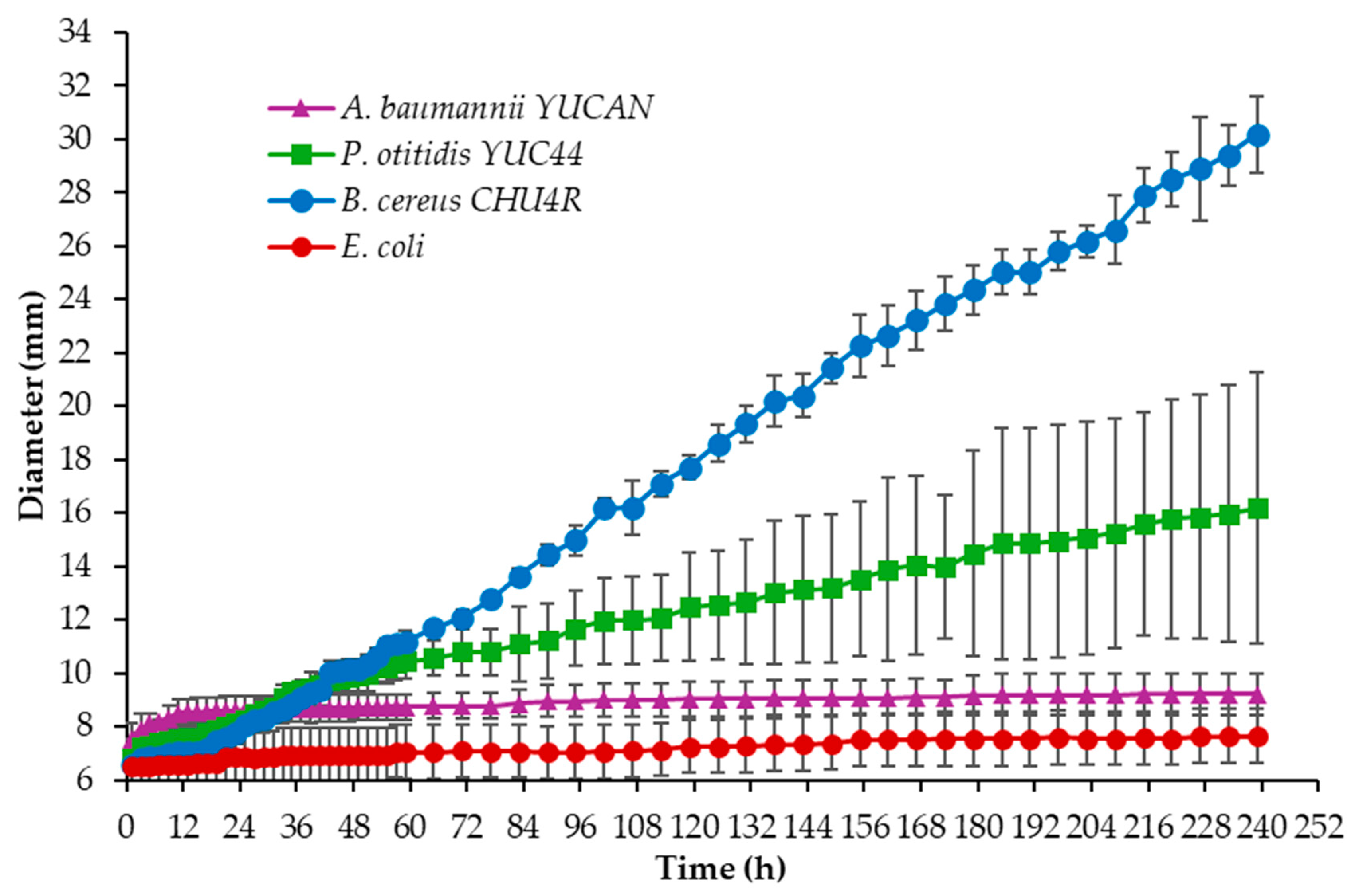
3.2. Evaluation of the Degradation Activity of Selected Strains Using the Clear Zone Method
3.3. Liquid Biodegraded Film Characterization
3.3.1. Macroscopic and Microscopic Characterization by Scanning Electron Microscopy (SEM)
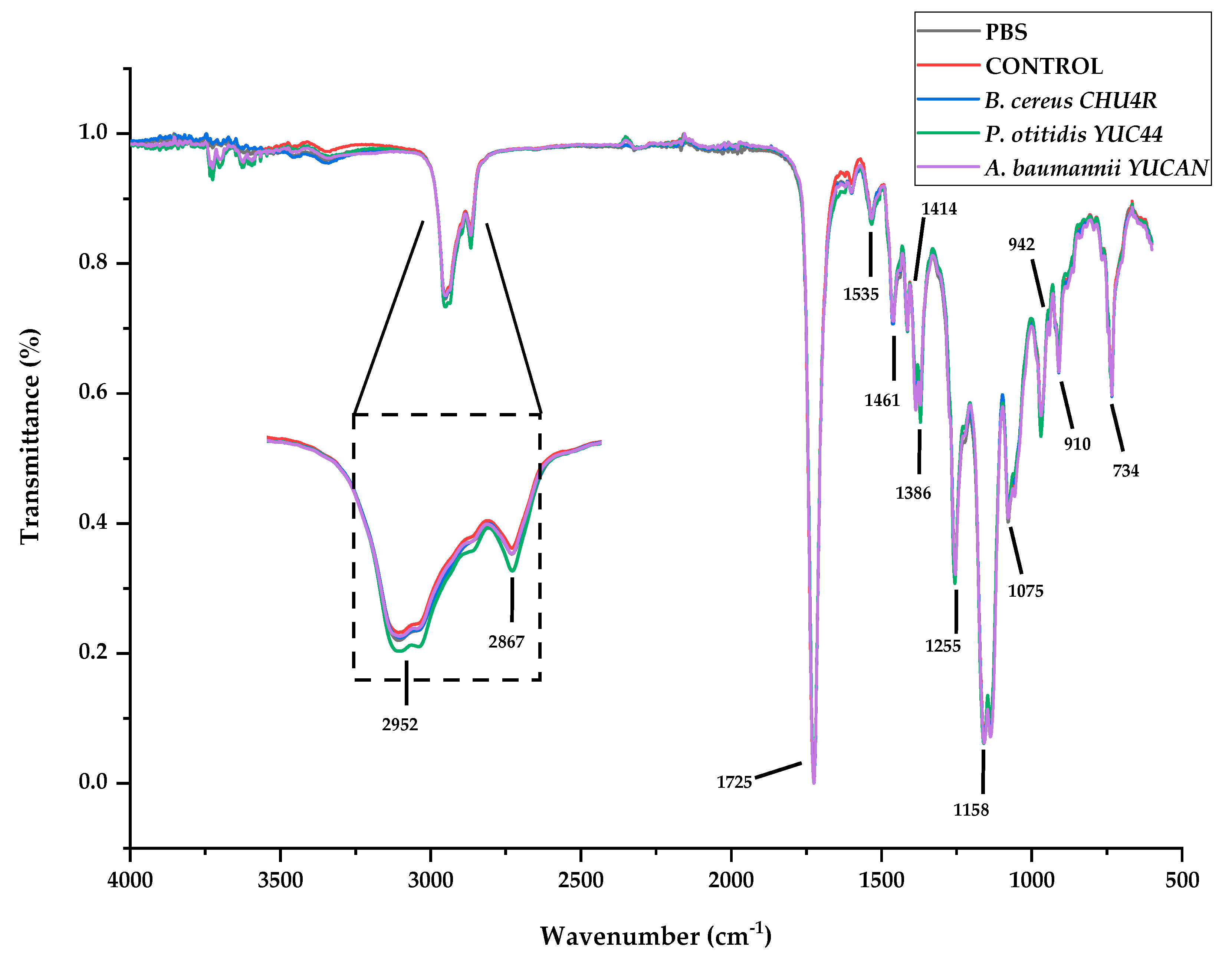
3.3.2. FTIR
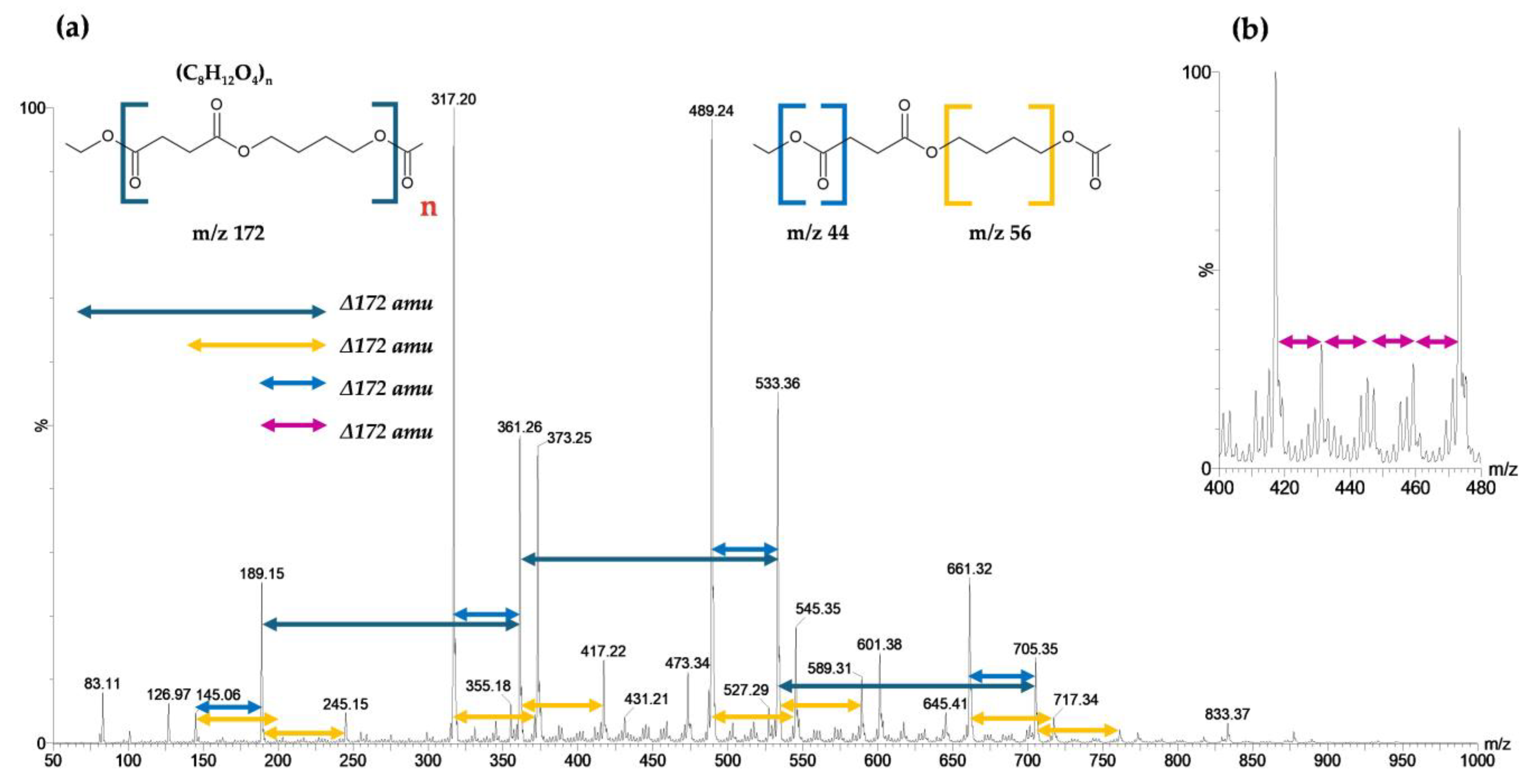
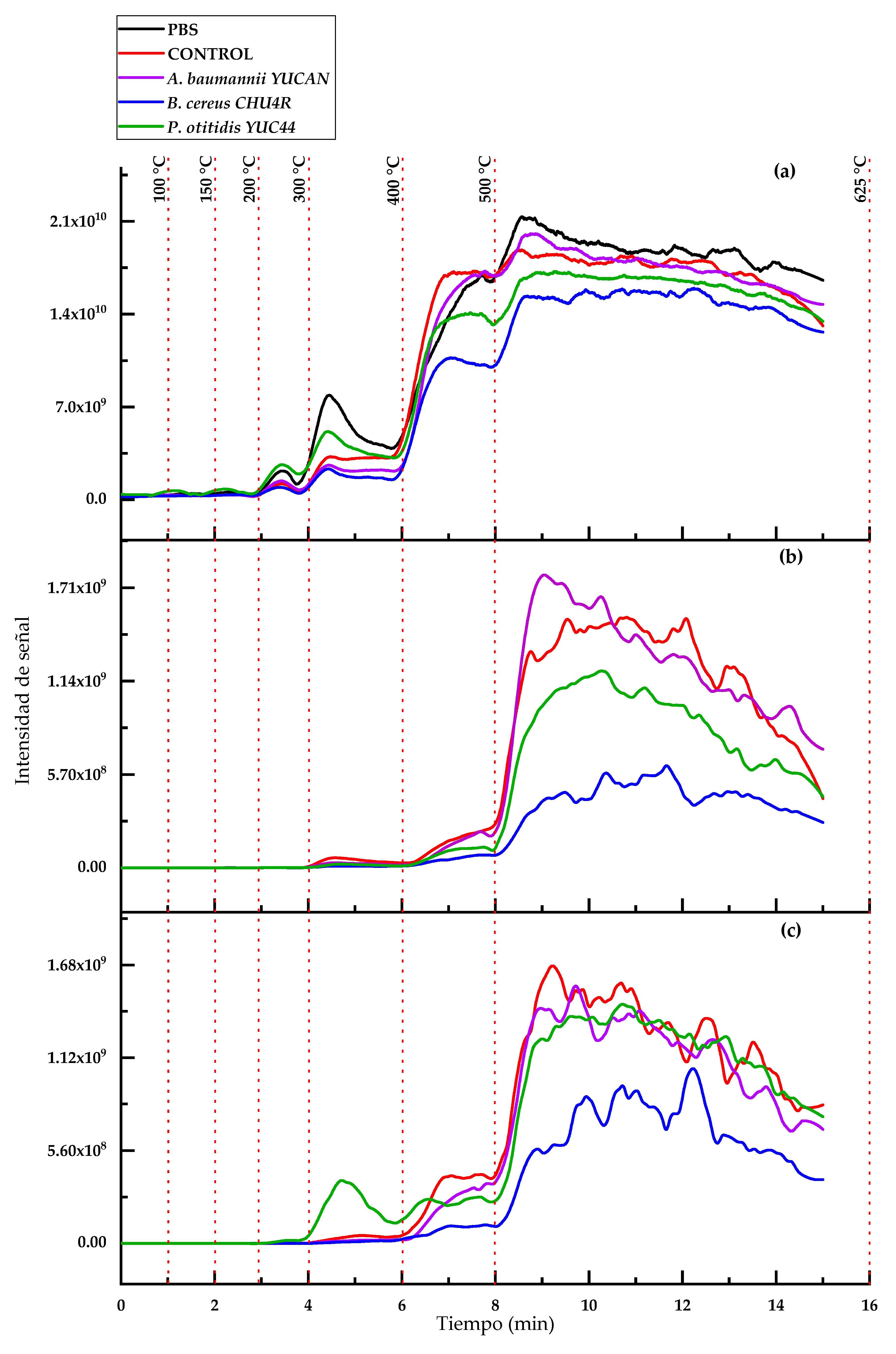
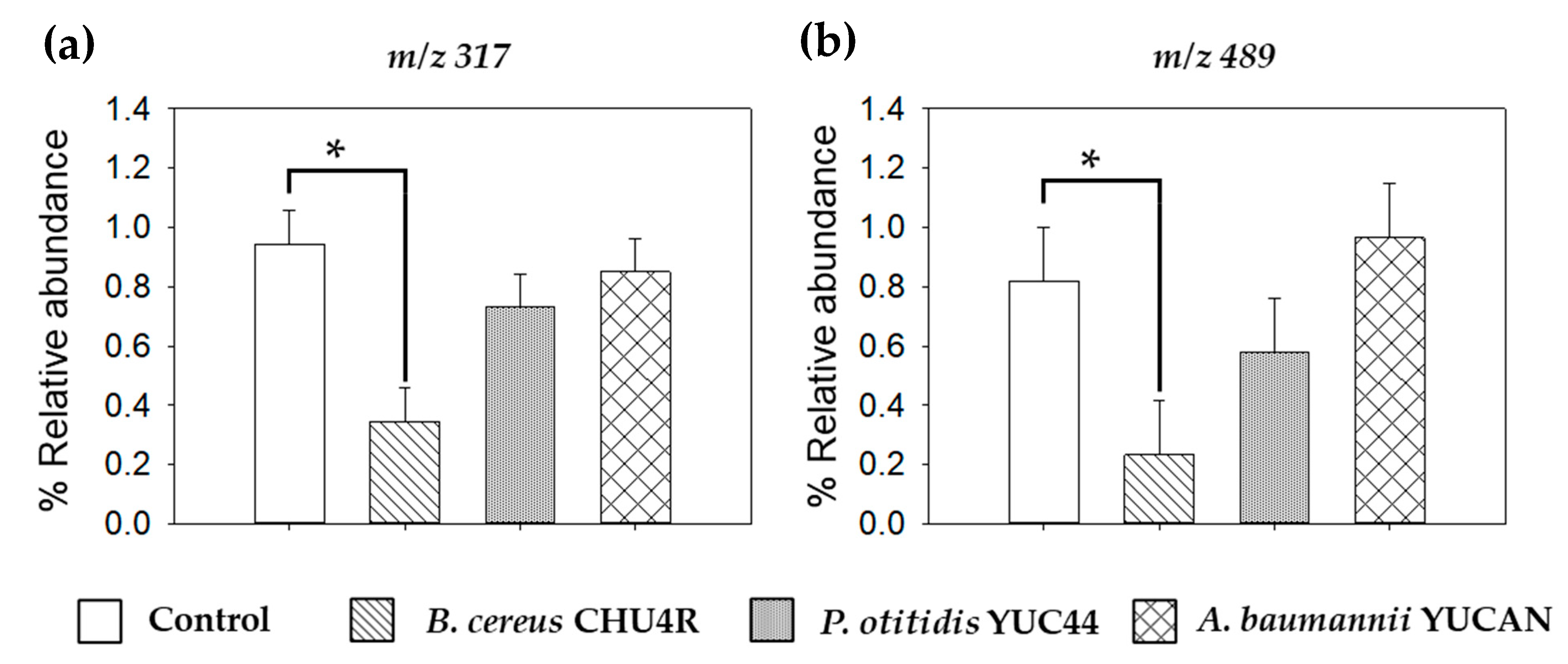
3.3.3. Analysis by ASAP-MS
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kale, S.K.; Deshmukh, A.G.; Dudhare, M.S.; Patil, V.B. Microbial degradation of plastic:a review. J. Biochem. Technol. 2015, 6, 952–961. [Google Scholar]
- Howell, S.G. A ten year review of plastics recycling. J. Hazard. Mater. 1992, 29, 143–164. [Google Scholar] [CrossRef]
- Grini, H.; Metallaoui, S.; González-Fernández, D.; Bensouilah, M. First evidence of plastic pollution in beach sediments of the Skikda coast (northeast of Algeria). Mar. Pollut. Bull. 2022, 181, 113831. [Google Scholar] [CrossRef]
- Chamas, A.; Moon, H.; Zheng, J.; Qiu, Y.; Tabassum, T.; Jang, J.H.; Abu-Omar, M.M.; Scott, S.L.; Suh, S. Degradation Rates of Plastics in the Environment. ACS Sustain. Chem. Eng. 2020, 8, 3494–3511. [Google Scholar] [CrossRef]
- Setälä, O.; Norkko, J.; Lehtiniemi, M. Feeding type affects microplastic ingestion in a coastal invertebrate community. Mar. Pollut. Bull. 2016, 102, 95–101. [Google Scholar] [CrossRef]
- Salazar-Pérez, C.; Amezcua, F.; Rosales-Valencia, A.; Green, L.; Pollorena-Melendrez, J.E.; Sarmiento-Martínez, M.A.; Tomita Ramírez, I.; Gil-Manrique, B.D.; Hernandez-Lozano, M.Y.; Muro-Torres, V.M.; et al. First insight into plastics ingestion by fish in the Gulf of California, Mexico. Mar. Pollut. Bull. 2021, 171, 112705. [Google Scholar] [CrossRef]
- Blettler, M.C.M.; Mitchell, C. Dangerous traps: Macroplastic encounters affecting freshwater and terrestrial wildlife. Sci. Total Environ. 2021, 798, 149317. [Google Scholar] [CrossRef]
- Azoulay, D.; Villa, P.; Arellano, Y.; Gordon, M.; Moon, D.; Miller, K.; Thompson, K. Plastic & Health—The Hidden Costs of a Plastic Planet; CIEL: Geneva, Switzerland, 2019. [Google Scholar]
- Mannina, G.; Presti, D.; Montiel-Jarillo, G.; Suárez-Ojeda, M.E. Bioplastic recovery from wastewater: A new protocol for polyhydroxyalkanoates (PHA) extraction from mixed microbial cultures. Bioresour. Technol. 2019, 282, 361–369. [Google Scholar] [CrossRef]
- Martínez-Tobón, D.I.; Gul, M.; Elias, A.L.; Sauvageau, D. Polyhydroxybutyrate (PHB) biodegradation using bacterial strains with demonstrated and predicted PHB depolymerase activity. Appl. Microbiol. Biotechnol. 2018, 102, 8049–8067. [Google Scholar] [CrossRef]
- Wongsirichot, P.; Gonzalez-Miquel, M.; Winterburn, J. Rapeseed meal valorization strategies via nitrogen- and oxygen-limited production of polyhydroxyalkanoates with Pseudomonas putida. Waste Manag. 2020, 105, 482–491. [Google Scholar] [CrossRef]
- Kalita, N.K.; Hakkarainen, M. Integrating biodegradable polyesters in a circular economy. Curr. Opin. Green Sustain. Chem. 2023, 40, 100751. [Google Scholar] [CrossRef]
- Mandic, M.; Spasic, J.; Ponjavic, M.; Nikolic, M.S.; Cosovic, V.R.; O’Connor, K.E.; Nikodinovic-Runic, J.; Djokic, L.; Jeremic, S. Biodegradation of poly(ε-caprolactone) (PCL) and medium chain length polyhydroxyalkanoate (mcl-PHA) using whole cells and cell free protein preparations of Pseudomonas and Streptomyces strains grown on waste cooking oil. Polym. Degrad. Stab. 2019, 162, 160–168. [Google Scholar] [CrossRef]
- Farzi, A.; Dehnad, A.; Fotouhi, A.F. Biodegradation of polyethylene terephthalate waste using Streptomyces species and kinetic modeling of the process. Biocatal. Agric. Biotechnol. 2019, 17, 25–31. [Google Scholar] [CrossRef]
- Gupta, K.K.; Devi, D. Characteristics investigation on biofilm formation and biodegradation activities of Pseudomonas aeruginosa strain ISJ14 colonizing low density polyethylene (LDPE) surface. Heliyon 2020, 6, e04398. [Google Scholar] [CrossRef]
- Scalenghe, R. Resource or waste? A perspective of plastics degradation in soil with a focus on end-of-life options. Heliyon 2018, 4, e00941. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, L.; Dombrowski, A.; Völker, C.; Wagner, M. Are bioplastics and plant-based materials safer than conventional plastics? In vitro toxicity and chemical composition. Environ. Int. 2020, 145, 106066. [Google Scholar] [CrossRef]
- Chen, X.; Yan, N. A brief overview of renewable plastics. Mater. Today Sustain. 2019, 7–8, 100031. [Google Scholar] [CrossRef]
- Mohamad, N.; Mazlan, M.M.; Tawakkal, I.S.M.A.; Talib, R.A.; Kian, L.K.; Jawaid, M. Characterization of Active Polybutylene Succinate Films Filled Essential Oils for Food Packaging Application. J. Polym. Environ. 2022, 30, 585–596. [Google Scholar] [CrossRef]
- de Costa, A.R.M.; Crocitti, A.; de Carvalho, L.H.; Carroccio, S.C.; Cerruti, P.; Santagata, G. Properties of Biodegradable Films Based on Poly(butylene Succinate) (PBS) and Poly(butylene Adipate-co-Terephthalate) (PBAT) Blends †. Polymers 2020, 12, 2317. [Google Scholar] [CrossRef]
- Bátori, V.; Åkesson, D.; Zamani, A.; Taherzadeh, M.J.; Sárvári Horváth, I. Anaerobic degradation of bioplastics: A review. Waste Manag. 2018, 80, 406–413. [Google Scholar] [CrossRef]
- Ibiene, A.A.; Stanley, H.O.; Immanuel, O.M. Biodegradation of Polyethylene by Bacillus sp. Indigenous to the Niger Delta Mangrove Swamp. Niger. J. Biotechnol. 2013, 26, 68–78. [Google Scholar]
- Auta, H.S.; Emenike, C.U.; Fauziah, S.H. Screening of Bacillus strains isolated from mangrove ecosystems in Peninsular Malaysia for microplastic degradation. Environ. Pollut. 2017, 231, 1552–1559. [Google Scholar] [CrossRef]
- Auta, H.S.; Emenike, C.U.; Jayanthi, B.; Fauziah, S.H. Growth kinetics and biodeterioration of polypropylene microplastics by Bacillus sp. and Rhodococcus sp. isolated from mangrove sediment. Mar. Pollut. Bull. 2018, 127, 15–21. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, C.G. Biodegradation of micro-polyethylene particles by bacterial colonization of a mixed microbial consortium isolated from a landfill site. Chemosphere 2019, 222, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Kunlere, I.O.; Fagade, O.E.; Nwadike, B.I. Biodegradation of low density polyethylene (LDPE) by certain indigenous bacteria and fungi. Int. J. Environ. Stud. 2019, 76, 428–440. [Google Scholar] [CrossRef]
- Hu, T.; Wang, Y.; Ma, L.; Wang, Z.; Tong, H. Biodegradation of polybutylene succinate by an extracellular esterase from Pseudomonas mendocina. Int. Biodeterior. Biodegrad. 2024, 195, 105910. [Google Scholar] [CrossRef]
- Rajgond, V.; Mohite, A.; More, N.; More, A. Biodegradable Polyester-Polybutylene Succinate (PBS): A Review; Springer: Berlin/Heidelberg, Germany, 2024; Volume 81, ISBN 0123456789. [Google Scholar]
- Šašinková, D.; Serbruyns, L.; Julinová, M.; FayyazBakhsh, A.; De Wilde, B.; Koutný, M. Evaluation of the biodegradation of polymeric materials in the freshwater environment—An attempt to prolong and accelerate the biodegradation experiment. Polym. Degrad. Stab. 2022, 203, 110085. [Google Scholar] [CrossRef]
- Baidurah, S. Methods of Analyses for Biodegradable Polymers: A Review. Polymers 2022, 14, 4928. [Google Scholar] [CrossRef]
- Barrère, C.; Selmi, W.; Hubert-Roux, M.; Coupin, T.; Assumani, B.; Afonso, C.; Giusti, P. Rapid analysis of polyester and polyethylene blends by ion mobility-mass spectrometry. Polym. Chem. 2014, 5, 3576–3582. [Google Scholar] [CrossRef]
- deb Dutta, S.; Tarafder, M.; Islam, R.; Datta, B. Characterization of cellulolytic enzymes of Fusarium soil Isolates. Biocatal. Agric. Biotechnol. 2018, 14, 279–285. [Google Scholar] [CrossRef]
- Okoye, A.U.; Chikere, C.B.; Okpokwasili, G.C. Isolation and Characterization of Hexadecane Degrading Bacteria from Oil- polluted soil in Gio Community, Niger Delta, Nigeria. Sci. Afr. 2020, 9, e00340. [Google Scholar] [CrossRef]
- Rakesh, E.; Rajakomuraiah, T. Isolation, Identification and Evaluation of Antibacterial Activity of Actinomycetes from Soil Sample of Adilabad, Telangana State (India). Ann. Rom. Soc. Cell Biol. 2021, 25, 19327–19335. [Google Scholar]
- Shirling, E.B.; Gottlieb, D. Methods for characterization of Streptomyces species. Int. J. Syst. Bacteriol. 1966, 16, 313–340. [Google Scholar] [CrossRef]
- Egamberdiyeva, D. Plant-growth-promoting rhizobacteria isolated from a Calcisol in a semi-arid region of Uzbekistan: Biochemical characterization and effectiveness. J. Plant Nutr. Soil Sci. 2005, 168, 94–99. [Google Scholar] [CrossRef]
- Smith, A.C.; Hussey, M.A. Gram Stain Protocols. 2005. Available online: https://asm.org/Protocols/Gram-Stain-Protocols (accessed on 29 March 2022).
- Reiner, K. Catalase Test Protocol Disponible en. Available online: https://scispace.com/papers/catalase-test-protocol-4d3ky0g5lq (accessed on 29 March 2022).
- Reynolds, J.; Moyes, R.; Breakwell, D.P. Differential Staining of Bacteria: Endospore Stain. Curr. Protoc. Microbiol. 2009, 15, A.3J.1–A.3J.5. [Google Scholar] [CrossRef]
- Madeira, F.; Madhusoodanan, N.; Lee, J.; Eusebi, A.; Niewielska, A.; Tivey, A.R.N.; Lopez, R.; Butcher, S. The EMBL-EBI Job Dispatcher sequence analysis tools framework in 2024. Nucleic Acids Res. 2024, 52, W521–W525. [Google Scholar] [CrossRef]
- Ramos-Díaz, A.; Morando-Grijalva, C.A.; Pacheco-López, N.; Cuevas-Bernardino, J.C.; Pech-Cohuo, S.C.; Ku-González, A.F. Microorganismos Degradadores De Materiales plásticos 2023. MX/a/2023/015172 Patent Application. Available online: https://ciatej.mx/files/patentes/patent_67bf576ed19da.pdf (accessed on 26 February 2025).
- Budkum, J.; Thammasittirong, N.S.; Thammasittirong, A. High poly ε-caprolactone biodegradation activity by a new Acinetobacter seifertii isolate. Folia Microbiol. 2022, 67, 659–669. [Google Scholar] [CrossRef]
- Martín-López, H.; Pech-Cohuo, S.C.; Ayora-Talavera, T.; Cuevas-Bernardino, J.C.; Ramos-Díaz, A.; Espinosa-Andrews, H.; Shirai, K.; Pacheco, N. Deacetylation of chitin obtained by biological method and its application in melipona honey-incorporated antimicrobial biofilms. MRS Adv. 2021, 6, 885–892. [Google Scholar] [CrossRef]
- ASTM D 882-02; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. ASTM International: West Conshohocken, PA, USA, 2002; Volume 14, pp. 1–12.
- Tribedi, P.; Sarkar, S.; Mukherjee, K.; Sil, A.K. Isolation of a novel Pseudomonas sp from soil that can efficiently degrade polyethylene succinate. Environ. Sci. Pollut. Res. 2012, 19, 2115–2124. [Google Scholar] [CrossRef]
- Clark, K.; Karsch-Mizrachi, I.; Lipman, D.J.; Ostell, J.; Sayers, E.W. GenBank. Nucleic Acids Res. 2016, 44, D67–D72. [Google Scholar] [CrossRef]
- Pramila, R.; Vijaya Ramesh, K. Potential biodegradation of low density polyethylene (LDPE) by Acinetobacter baumannii. Afr. J. Bacteriol. Res. 2015, 7, 24–28. [Google Scholar]
- El-Kurdi, N.; Elbaghdady, K.; El-Shatoury, S.; Hammad, S.; Ghazy, M. Sustainable Removal of Nanoplastics: Exploiting the Lipolytic Activity of Pseudomonas aeruginosa O6 Isolated from Mariout Wetland, Egypt. Egypt. J. Aquat. Biol. Fish. 2024, 28, 247–272. [Google Scholar] [CrossRef]
- Lee, S.J.; Park, E.H.; Han, Y.H.; Kim, Y.O.; Park, S.W. Isolation of a marine bacterium capable of biodegrading poly(butylene succinate). Fish. Aquat. Sci. 2013, 16, 41–44. [Google Scholar] [CrossRef]
- Dang, T.C.H.; Nguyen, D.T.; Thai, H.; Nguyen, T.C.; Tran, T.T.H.; Le, V.H.; Nguyen, V.H.; Tran, X.B.; Pham, T.P.T.; Nguyen, T.G.; et al. Plastic degradation by thermophilic Bacillus sp. BCBT21 isolated from composting agricultural residual in Vietnam. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 1–11. [Google Scholar] [CrossRef]
- Fibriarti, B.L.; Feliatra, F.; Amin, B.; Darwis, D. Biodegradation of ldpe plastic by local strain of Bacillus sp. Isolated from dump soil of pekanbaru, indonesia. Biodiversitas 2021, 22, 5484–5490. [Google Scholar] [CrossRef]
- Kim, S.H.; Cho, J.Y.; Cho, D.H.; Jung, H.J.; Kim, B.C.; Bhatia, S.K.; Park, S.H.; Park, K.; Yang, Y.H. Acceleration of Polybutylene Succinate Biodegradation by Terribacillus sp. JY49 Isolated from a Marine Environment. Polymers 2022, 14, 3978. [Google Scholar] [CrossRef]
- Suzuki, M.; Tachibana, Y.; Takizawa, R.; Morikawa, T.; Takeno, H.; Kasuya, K. A novel poly(3-hydroxybutyrate)-degrading actinobacterium that was isolated from plastisphere formed on marine plastic debris. Polym. Degrad. Stab. 2021, 183, 109461. [Google Scholar] [CrossRef]
- Cho, J.Y.; Park, S.L.; Kim, S.H.; Jung, H.J.; Cho, D.H.; Kim, B.C.; Bhatia, S.K.; Gurav, R.; Park, S.H.; Park, K.; et al. Novel Poly(butylene adipate-co-terephthalate)-degrading Bacillus sp. JY35 from wastewater sludge and its broad degradation of various bioplastics. Waste Manag. 2022, 144, 1–10. [Google Scholar] [CrossRef]
- Vaishnav, A.; Lal, J.; Singh, N.S.; Pati, B.K.; Mehta, N.K.; Priyadarshini, M.B. Role of Microbial Enzymes and Their Modification for Plastic Biodegradation. In Advanced Strategies for Biodegradation of Plastic Polymers; Soni, R., Debbarma, P., Chandra, D., Reeta, S., Eds.; Springer: Cham, Switzerland, 2024; pp. 373–404. ISBN 9783031556616. [Google Scholar]
- Yin, R.; Cheng, J.; Wang, J.; Li, P.; Lin, J. Treatment of Pseudomonas aeruginosa infectious biofilms: Challenges and strategies. Front. Microbiol. 2022, 13, 955286. [Google Scholar] [CrossRef]
- Sokołowska, M.; Nowak-Grzebyta, J.; Stachowska, E.; El Fray, M. Enzymatic Catalysis in Favor of Blocky Structure and Higher Crystallinity of Poly(Butylene Succinate)-Co-(Dilinoleic Succinate) (PBS-DLS) Copolymers of Variable Segmental Composition. Materials 2022, 15, 1132. [Google Scholar] [CrossRef]
- Barletta, M.; Pizzi, E. Optimizing crystallinity of engineered poly(lactic acid)/poly(butylene succinate) blends: The role of single and multiple nucleating agents. J. Appl. Polym. Sci. 2020, 138, e50236. [Google Scholar] [CrossRef]
- Abderrahim, B.; Abderrahman, E.; Mohamed, A.; Fatima, T.; Abdesselam, T.; Krim, O. Kinetic Thermal Degradation of Cellulose, Polybutylene Succinate and a Green Composite: Comparative Study. World J. Environ. Eng. 2015, 3, 95–110. [Google Scholar]
- Gupta, R.S.; Patel, S.; Saini, N.; Chen, S. Robust demarcation of 17 distinct bacillus species clades, proposed as novel bacillaceae genera, by phylogenomics and comparative genomic analyses: Description of robertmurraya kyonggiensis sp. nov. and proposal for an emended genus bacillus limiting it o. Int. J. Syst. Evol. Microbiol. 2020, 70, 5753–5798. [Google Scholar] [CrossRef]
- Hoffmann, H.; Stindl, S.; Ludwig, W.; Stumpf, A.; Mehlen, A.; Monget, D.; Pierard, D.; Ziesing, S.; Heesemann, J.; Roggenkamp, A.; et al. Enterobacter hormaechei subsp. oharae subsp. nov., E. hormaechei subsp. hormaechei comb. nov., and E. hormaechei subsp. steigerwaltii subsp. nov., three new subspecies of clinical importance. J. Clin. Microbiol. 2005, 43, 3297–3303. [Google Scholar] [CrossRef]
- Schoch, C.L.; Ciufo, S.; Domrachev, M.; Hotton, C.L.; Kannan, S.; Khovanskaya, R.; Leipe, D.; McVeigh, R.; O’Neill, K.; Robbertse, B.; et al. Nehensive update on curation, resources and tools. Database 2020, 2020, 1–21. [Google Scholar] [CrossRef]
- Bouvet, P.J.M.; Grimont, P. a D. Taxonomy of the Genus Acinetobacter with the Recognition of nov. and Emended Descriptions of Acinetobacter calcoaceticus and Acinetobacter lwofii. Int. J. Syst. Bacteriol. 1986, 36, 228–240. [Google Scholar] [CrossRef]
- Rosenblueth, M.; Martínez, L.; Silva, J.; Martínez-Romero, E. Klebsiella variicola, A Novel Species with Clinical and Plant-Associated Isolates. Syst. Appl. Microbiol. 2004, 27, 27–35. [Google Scholar] [CrossRef]
- Yang, G.; Han, L.; Wen, J.; Zhou, S. Pseudomonas guangdongensis sp. nov., isolated from an electroactive biofilm, and emended description of the genus Pseudomonas Migula 1894. Int. J. Syst. Evol. Microbiol. 2013, 63, 4599–4605. [Google Scholar] [CrossRef]
- Clark, L.L.; Dajcs, J.J.; McLean, C.H.; Bartell, J.G.; Stroman, D.W. Pseudomonas otitidis sp. nov., isolated from patients with otic infections. Int. J. Syst. Evol. Microbiol. 2006, 56, 709–714. [Google Scholar] [CrossRef]





| Isolated Strain | Similarity (%) | Tentative Identity | NCBI Accession Number Assigned |
|---|---|---|---|
| MORI88 | 100 | Bacillus sp. | NA |
| SIE11 | 100 | Bacillus sp. | NA |
| CHU22 | 100 | Bacillus sp. | NA |
| CHU4R | 99.69 | Bacillus cereus | ON721265 |
| SIE4AN | 100 | Bacillus sp. | NA |
| MORI66 | 99.31 | Enterobacter hormaechei | ON721279 |
| SIE33 | 100 | Acinetobacter sp. | NA |
| SIE22 | 99.71 | Acinetobacter sp. | NA |
| MORI77 | 100 | Acinetobacter baumannii | NA |
| YUCAN | 100 | Acinetobacter baumannii | ON721281 |
| YUC99 | 100 | Klebsiella sp. | NA |
| MORI33 | 100 | Klebsiella pneumoniae | ON721265 |
| MORI22 | 100 | Pseudomonas sp. | NA |
| YUC44 | 99.90 | Pseudomonas otitidis | ON721280 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morando-Grijalva, C.A.; Ramos-Díaz, A.; Cabrera-Ramirez, A.H.; Cuevas-Bernardino, J.C.; Pech-Cohuo, S.C.; Kú-González, A.F.; Cano-Sosa, J.; Herrera-Pool, I.E.; Valdivia-Rivera, S.; Ayora-Talavera, T.; et al. Isolation, Identification and Screening of Plastic-Degrading Microorganisms: Qualitative and Structural Effects on Poly(Butylene Succinate) (PBS) Films. Polymers 2025, 17, 1128. https://doi.org/10.3390/polym17081128
Morando-Grijalva CA, Ramos-Díaz A, Cabrera-Ramirez AH, Cuevas-Bernardino JC, Pech-Cohuo SC, Kú-González AF, Cano-Sosa J, Herrera-Pool IE, Valdivia-Rivera S, Ayora-Talavera T, et al. Isolation, Identification and Screening of Plastic-Degrading Microorganisms: Qualitative and Structural Effects on Poly(Butylene Succinate) (PBS) Films. Polymers. 2025; 17(8):1128. https://doi.org/10.3390/polym17081128
Chicago/Turabian StyleMorando-Grijalva, Cristina América, Ana Ramos-Díaz, Angel H. Cabrera-Ramirez, Juan Carlos Cuevas-Bernardino, Soledad Cecilia Pech-Cohuo, Angela Francisca Kú-González, Julia Cano-Sosa, Iván Emanuel Herrera-Pool, Sergio Valdivia-Rivera, Teresa Ayora-Talavera, and et al. 2025. "Isolation, Identification and Screening of Plastic-Degrading Microorganisms: Qualitative and Structural Effects on Poly(Butylene Succinate) (PBS) Films" Polymers 17, no. 8: 1128. https://doi.org/10.3390/polym17081128
APA StyleMorando-Grijalva, C. A., Ramos-Díaz, A., Cabrera-Ramirez, A. H., Cuevas-Bernardino, J. C., Pech-Cohuo, S. C., Kú-González, A. F., Cano-Sosa, J., Herrera-Pool, I. E., Valdivia-Rivera, S., Ayora-Talavera, T., & Pacheco, N. (2025). Isolation, Identification and Screening of Plastic-Degrading Microorganisms: Qualitative and Structural Effects on Poly(Butylene Succinate) (PBS) Films. Polymers, 17(8), 1128. https://doi.org/10.3390/polym17081128











