Thin-Layer Drying Model and Antifungal Properties of Rubber Sheets Produced with Wood Vinegar as a Substitute for Formic and Acetic Acids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Commercial Acids and Wood Vinegar
2.3. Characterization by Fourier-Transform Infrared Spectroscopy (FTIR)
2.4. Drying Chamber and Procedure
2.5. Thin-Layer Drying Models and Data Analysis
| Model | ||
|---|---|---|
| Logarithmic | (1) | |
| Midilli et al. [25] | (2) | |
| Page | (3) | |
| Two-term exponential | (4) | |
| Wang and Singh [26] | (5) |
2.6. Evaluation of Wood Vinegar for Inhibiting Fungal Growth on Rubber Sheets
2.7. Fungal Growth Inhibition Analysis
2.8. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition of Wood Vinegar
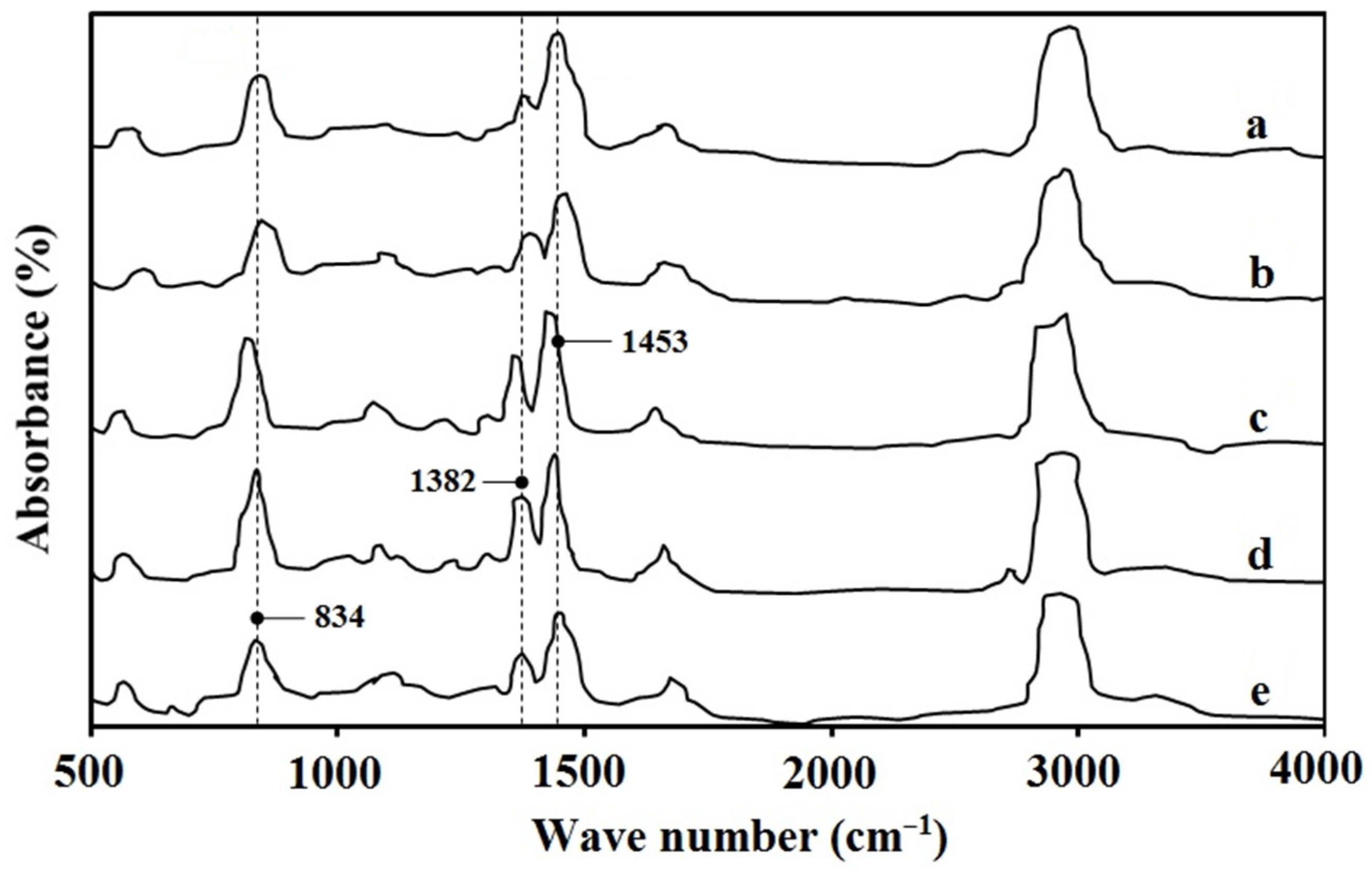
3.2. The FTIR Spectra of NR Films
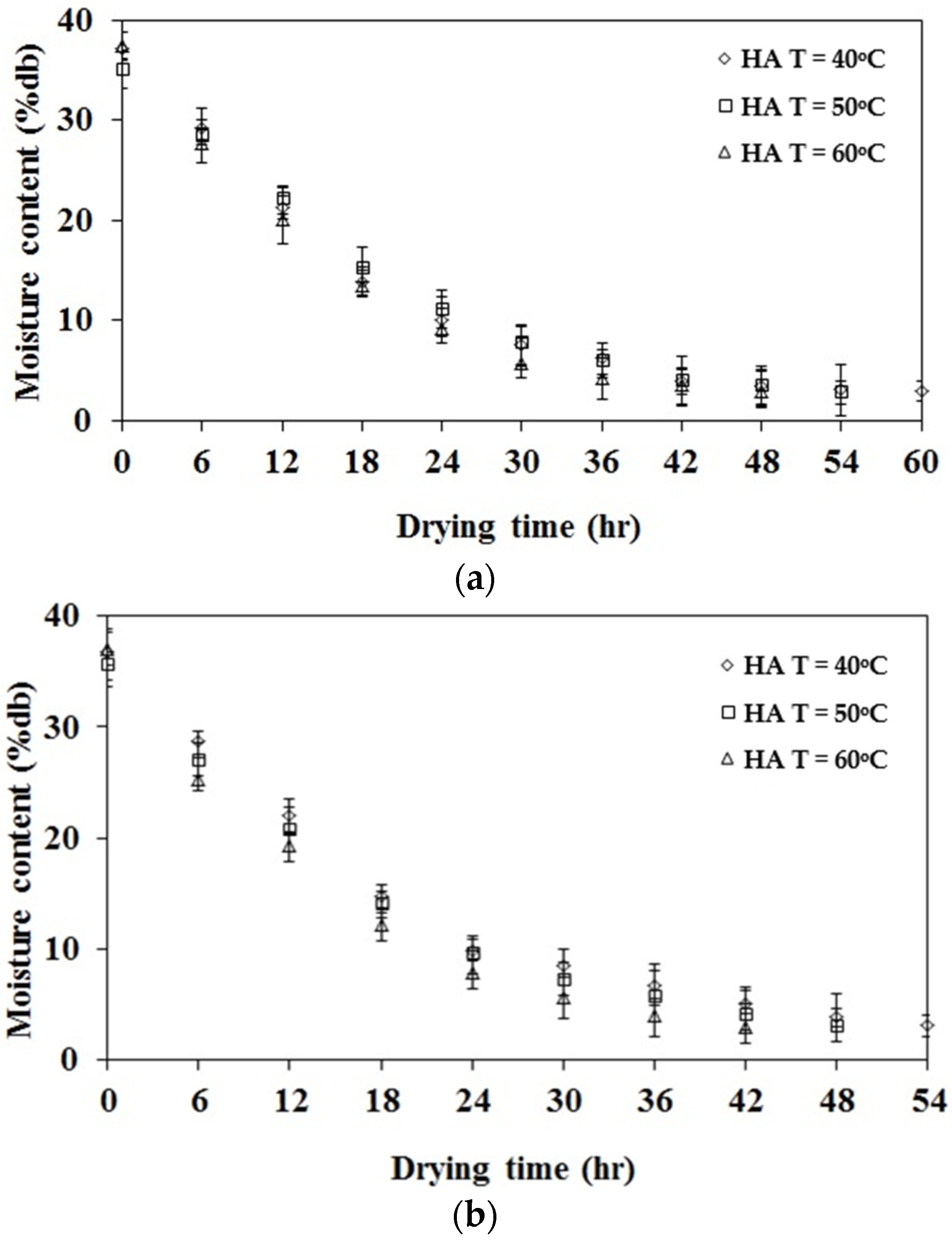
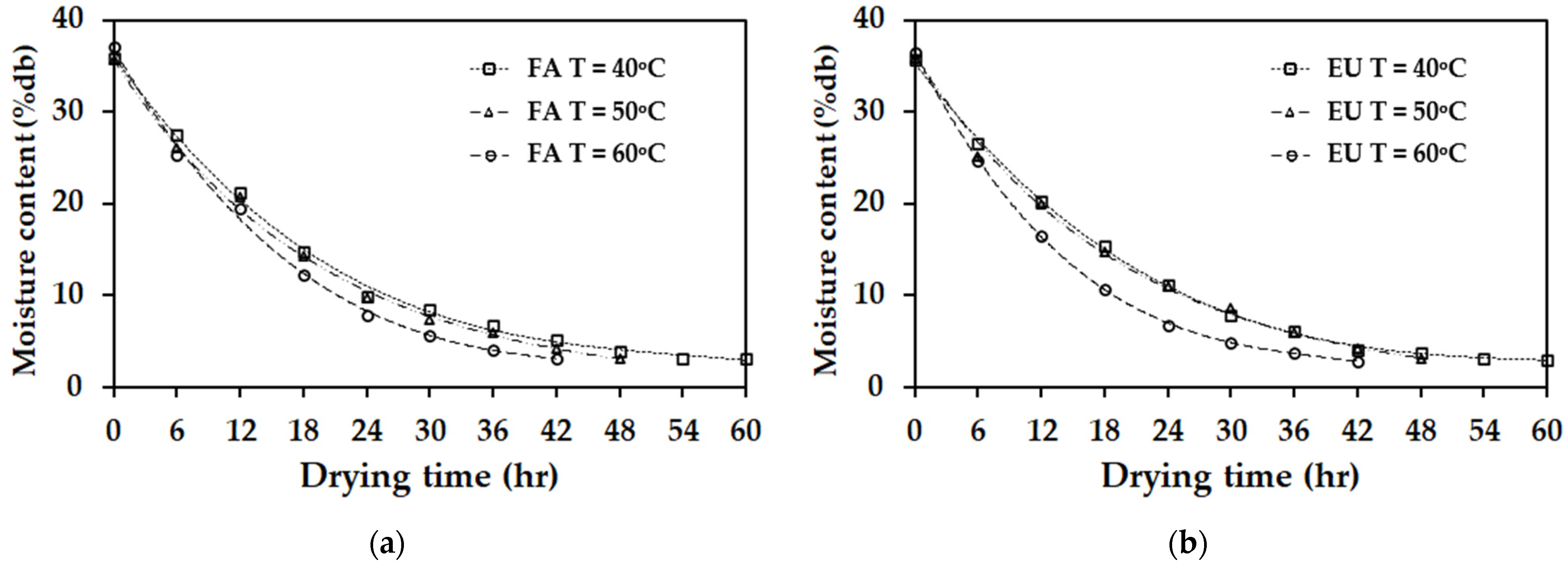
3.3. The Experimental Results for Drying Kinetics of Rubber Sheets
3.4. The Results for Thin-Layer Drying Models
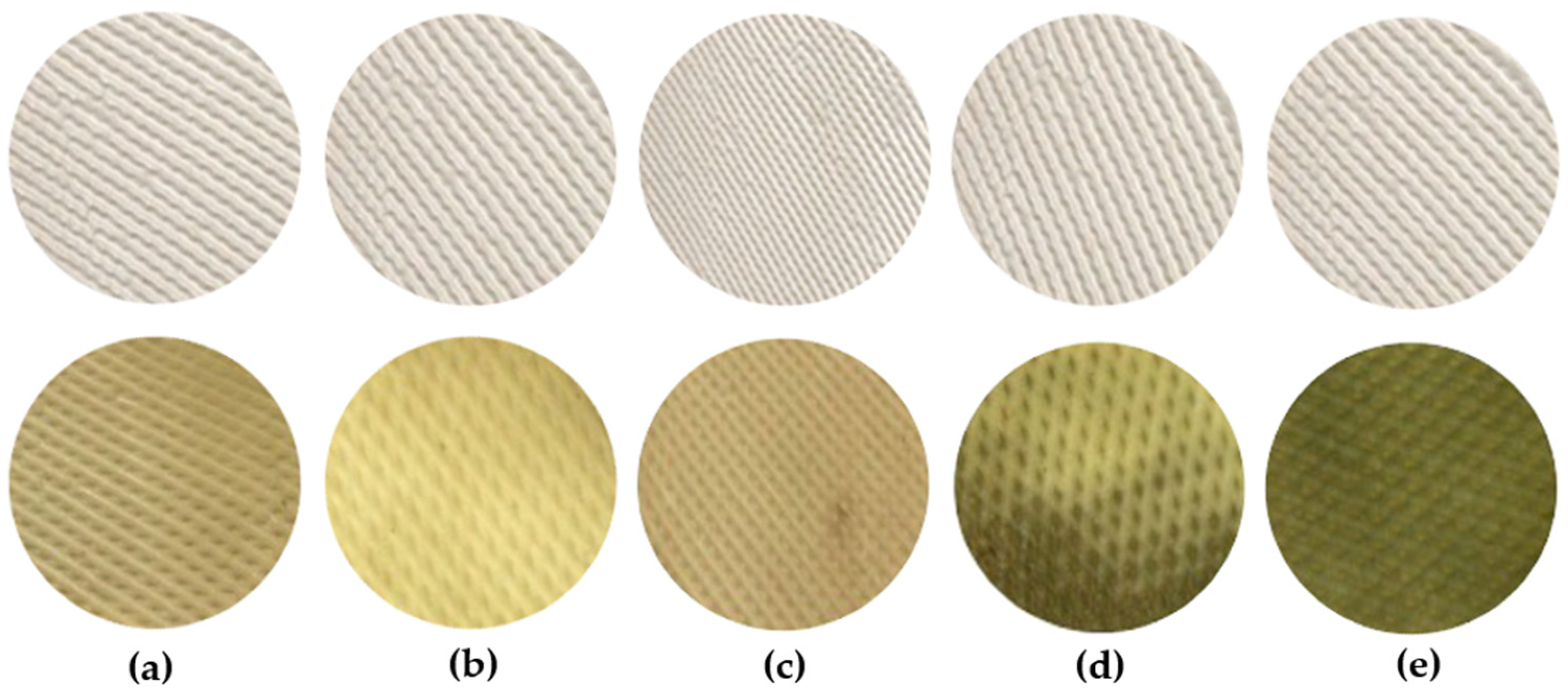
3.5. Effectiveness of Wood Vinegar in Inhibiting Fungal Growth on Rubber Sheets
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rattanamechaiskul, C.; Junka, N.; Potichalung, J.; Wingwon, T.; Boontum, W.; Srisang, N. Whiteness Index Prediction of Para Rubber Sheet During Hot Air Drying. Eng. Appl. Sci. Res. 2016, 43, 331–333. [Google Scholar]
- Junka, N.; Rattanamechaiskul, C.; Prachayawarakorn, S.; Soponronnarit, S. Drying Guideline to Control Colour Quality of Para Rubber Sheet by Computation Method. Biosyst. Eng. 2018, 176, 151–161. [Google Scholar] [CrossRef]
- Office of Industrial Economics. 2023. Available online: http://rubber.oie.go.th (accessed on 19 January 2024).
- Agricultural Commodities Price Report of Thailand. 2023. Available online: https://www.bot.or.th (accessed on 25 January 2024).
- Kalasee, W.; Teekapakvisit, C. A Review of Air Pollution and Solutions Way Management Related to Ribbed Smoked Sheets (RSS) Production of Community-Level Rubber Cooperatives in Thailand: Smoke, Soot and PAHs Particles. Pollution 2020, 6, 267–284. [Google Scholar] [CrossRef]
- Kalasee, W.; Dangwilailux, P. Effect of Wood Vinegar Substitutes on Acetic Acid for Coagulating Natural Para Rubber Sheets during the Drying Process. Appl. Sci. 2021, 11, 7891. [Google Scholar] [CrossRef]
- Dejchanchaiwong, R.; Arkasuwan, A.; Kumar, A.; Tekasakul, P. Mathematical Modeling and Performance Investigation of Mixed-Mode and Indirect Solar Dryers for Natural Rubber Sheet Drying. Energy Sustain. Dev. 2016, 34, 44–53. [Google Scholar] [CrossRef]
- Eakvanich, V.; Kalasee, W.; Lakachaiworakun, P.; Dangwilailux, P.; Wattana, W. Mathematical Models of Natural Rubber Sheets Drying: Difference Acid Coagulation Cases. J. Adv. Res. Fluid Mech. Therm. Sci. 2024, 117, 37–45. [Google Scholar] [CrossRef]
- Nun-Anan, P.; Suchat, S.; Mahathaninwong, N.; Chueangchayaphan, N.; Karrila, S.; Limhengha, S. Study of Aquilaria crassna Wood as an Antifungal Additive to Improve the Properties of Natural Rubber as Air-Dried Sheets. Polymers 2021, 13, 4178. [Google Scholar] [CrossRef]
- Ratanapisit, J.; Apiraksakul, S.; Rerngnarong, A.; Chungsiriporn, J.; Bunyakarn, C. Preliminary Evaluation of Production and Characterization of Wood Vinegar from Rubberwood. Songklanakarin J. Sci. Technol. 2009, 31, 343–349. [Google Scholar]
- Yang, J.; Yang, C.; Liang, M.; Gao, Z.; Wu, Y.; Chuang, L. Chemical Composition, Antioxidant, and Antibacterial Activity of Wood Vinegar from Litchi chinensis. Molecules 2016, 21, 1150. [Google Scholar] [CrossRef]
- Jeentada, W.; Kongboon, P.; Boonyanuwat, S.; Sirirak, C. Thin-Layer Drying Models for Para Rubber Sheet. Eng. Appl. Sci. Res. 2014, 41, 99–108. [Google Scholar]
- Kalasee, W.; Dangwilailux, P. Prediction of Size Distribution and Mass Concentration of Smoke Particles on Moisture Content and Combustion Period from Para Rubber Wood Burning. Appl. Sci. 2021, 11, 5649. [Google Scholar] [CrossRef]
- Tekasakul, P.; Furuuchi, M.; Tekasakul, S.; Chomanee, J.; Otani, Y. Characteristics of PAHs in Particulates in the Atmospheric Environment of Hat Yai City, Thailand, and Relationship with Rubber-wood Burning in Rubber Sheet Production. Aerosol Air Qual. Res. 2008, 8, 265–278. [Google Scholar] [CrossRef]
- Choosong, T.; Furuuchi, M.; Tekasakul, P.; Tekasakul, S.; Chomanee, J.; Jinno, T.; Hata, M.; Otani, Y. Working Environment in a Rubber Sheet Smoking Factory Polluted by Smoke from Biomass Fuel Burning and Health Influences to Workers. J. Ecotechnology Res. 2007, 13, 91–96. [Google Scholar] [CrossRef]
- Hata, M.; Chomanee, J.; Thongyen, T.; Bao, L.; Tekasakul, S.; Tekasakul, P.; Otani, Y.; Furuuchi, M. Characteristics of Nanoparticles Emitted from Burning of Biomass Fuels. J. Environ. Sci. 2014, 26, 1913–1920. [Google Scholar] [CrossRef]
- Ekburanawat, J. Biomass Pellets Electrical Power Generation System by Using Gasification Technology. Res. Mod. Sci. Util. Technol. Innov. J. 2018, 11, 32–42. [Google Scholar]
- Namtaku, K. Efficiency of Wood Vinegar from Coconut Shell to Anti-Fungal on Para Rubber Sheet. Prawarun Agric. J. 2017, 14, 41–49. [Google Scholar]
- ASTM D1076:1988; Standard Specification for Rubber—Concentrated, Ammonia Stabilized, Creamed, and Centrifuged Natural Latex. ASTM: West Conshohocken, PA, USA, 2023.
- Kalasee, W. A Wood Vinegar Machine for Sawdust and Wood Dust. SDU Res. J. Sci. Technol. 2008, 1, 45–54. [Google Scholar]
- Dejchanchaiwong, R.; Tirawanichakul, Y.; Tirawanichakul, S.; Tekasakul, P. Single-Phase and Multiphase Models for Temperature and Relative Humidity Calculations During Forced Convection in a Rubber-Sheet Drying Chamber. Maejo Int. J. Sci. Technol. 2014, 8, 207–220. [Google Scholar] [CrossRef]
- Tekasakul, P.; Dejchanchaiwong, R.; Tirawanichakul, Y.; Tirawanichakul, S. Three-Dimension Numerical Modeling of Heat and Moisture Transfer in Natural Rubber Sheet Drying Process. Dry. Technol. 2015, 33, 1124–1137. [Google Scholar] [CrossRef]
- Ninchuewong, T.; Ekphon, A.; Tirawanichakul, S.; Tirawanichakul, Y. Drying of Air Dried Sheet Rubber Using Hot Air Dryer and Solar Dryer for Small Entrepreneurs and Small Rubber Cooperatives. Burapha Sci. J. 2012, 17, 50–59. [Google Scholar]
- Pupakapanpong, C.; Tirawanichakul, S.; Tirawanichakul, Y. Drying Modeling and Energy Consumption of Air Dried Sheet (ADS) Rubber by Solar and Biomass Energy. Appl. Mech. Mater. 2014, 541–542, 1017–1021. [Google Scholar] [CrossRef]
- Midilli, A.; Kucuk, H.; Yapar, Z. A New Model for Single-Layer Drying. Dry. Technol. 2002, 20, 1503–1513. [Google Scholar] [CrossRef]
- Wang, C.Y.; Singh, R.P. A Single Layer Drying Equation for Rough Rice; ASAE Paper No: 78-3001; ASAE: St. Joseph, MI, USA, 1978. [Google Scholar]
- FDA. Food Code 2001, Recommendations of the United States Public Health Service Food and Drug Administration; FDA: Silver Spring, MD, USA, 2001.



| No. | Compound | Percentage of Total Area | ||
|---|---|---|---|---|
| Para-Rubber | Bamboo | Eucalyptus | ||
| 1 | Acetic acid | 41.34 | 38.19 | 31.25 |
| 2 | Phenol | 8.29 | 7.56 | 7.12 |
| 3 | 2,6-dimethoxyphenol (Syringol) | 7.38 | 5.53 | 11.07 |
| 4 | 2-Methoxyphenol (Guaiacol) | 2.81 | 3.27 | 3.89 |
| 5 | p-Cresol | 1.57 | 1.72 | 1.85 |
| 6 | Benzenemathanol | 0.82 | 1.89 | 0.97 |
| 7 | Cyclotene | 2.79 | 2.15 | 2.38 |
| 8 | Butanoic acid | 0.95 | 1.18 | 0.83 |
| 9 | Propanoic acid | 2.13 | 3.14 | 1.75 |
| 10 | 2-propanone, 1-hydroxy (hydroxyacetone) | 4.21 | 4.13 | 3.12 |
| Total | 72.29 | 68.76 | 64.23 | |
| Model | Parameters and Goodness of Fit | Para-Rubber Wood-Derived Vinegar | Bamboo Wood-Derived Vinegar | Eucalyptus Wood-Derived Vinegar | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Drying Temperature | Drying Temperature | Drying Temperature | ||||||||
| 40 °C | 50 °C | 60 °C | 40 °C | 50 °C | 60 °C | 40 °C | 50 °C | 60 °C | ||
| Logarithmic | a | 1.024 | 1.263 | 1.108 | 1.027 | 1.585 | 1.131 | 1.347 | 1.091 | 1.052 |
| b | −0.051 | −0.032 | −0.077 | −0.089 | −0.072 | −0.084 | −0.104 | −0.079 | −0.089 | |
| k | 0.024 | 0.031 | 0.038 | 0.028 | 0.045 | 0.052 | 0.036 | 0.058 | 0.071 | |
| r2 | 0.865 | 0.882 | 0.892 | 0.871 | 0.878 | 0.909 | 0.842 | 0.911 | 0.893 | |
| MRD | 0.069 | 0.050 | 0.039 | 0.065 | 0.062 | 0.038 | 0.081 | 0.037 | 0.052 | |
| Midilli et al. [25] | a | 0.056 | 0.075 | 0.062 | 0.047 | 0.083 | 0.041 | 0.036 | 0.058 | 0.069 |
| b | 0.034 | 0.043 | 0.038 | 0.022 | 0.053 | 0.019 | 0.017 | 0.035 | 0.041 | |
| k | 0.297 | 0.315 | 0.283 | 0.228 | 0.352 | 0.213 | 0.205 | 0.281 | 0.304 | |
| n | 1.265 | 1.421 | 1.154 | 0.927 | 1.625 | 0.752 | 0.748 | 1.152 | 1.395 | |
| r2 | 0.904 | 0.871 | 0.907 | 0.902 | 0.893 | 0.878 | 0.973 | 0.979 | 0.971 | |
| MRD | 0.039 | 0.065 | 0.038 | 0.039 | 0.052 | 0.062 | 0.018 | 0.016 | 0.019 | |
| Page | k | 0.082 | 0.112 | 0.067 | 0.045 | 0.083 | 0.098 | 0.057 | 0.118 | 0.091 |
| n | 0.543 | 0.718 | 0.412 | 0.382 | 0.543 | 0.583 | 0.399 | 0.729 | 0.582 | |
| r2 | 0.887 | 0.853 | 0.865 | 0.945 | 0.907 | 0.853 | 0.902 | 0.918 | 0.905 | |
| MRD | 0.048 | 0.074 | 0.069 | 0.027 | 0.038 | 0.074 | 0.039 | 0.031 | 0.040 | |
| Two-term exponential | a | 0.287 | 0.415 | 0.532 | 0.891 | 0.306 | 0.254 | 0.286 | 0.308 | 0.218 |
| k | 4.021 | 5.327 | 6.189 | 9.852 | 4.115 | 3.926 | 4.019 | 4.117 | 3.728 | |
| r2 | 0.967 | 0.975 | 0.982 | 0.968 | 0.987 | 0.971 | 0.902 | 0.893 | 0.885 | |
| MRD | 0.022 | 0.018 | 0.015 | 0.021 | 0.011 | 0.019 | 0.039 | 0.052 | 0.049 | |
| Wang and Singh [26] | a | 0.072 | 0.056 | 0.028 | 0.039 | 0.085 | 0.095 | 0.062 | 0.074 | 0.853 |
| b | 0.629 | 0.497 | 0.379 | 0.422 | 0.781 | 0.832 | 0.593 | 0.631 | 0.983 | |
| r2 | 0.902 | 0.897 | 0.873 | 0.893 | 0.887 | 0.902 | 0.865 | 0.941 | 0.897 | |
| MRD | 0.039 | 0.051 | 0.065 | 0.052 | 0.048 | 0.039 | 0.069 | 0.029 | 0.051 | |
| Model | Parameters and Goodness of Fit | Commercial Formic Acid | Commercial Acetic Acid | ||||
|---|---|---|---|---|---|---|---|
| Drying Temperature | Drying Temperature | ||||||
| 40 °C | 50 °C | 60 °C | 40 °C | 50 °C | 60 °C | ||
| Logarithmic | a | 1.185 | 1.095 | 1.029 | 1.127 | 1.052 | 1.136 |
| b | −0.086 | −0.088 | −0.025 | −0.069 | −0.082 | −0.065 | |
| k | 0.043 | 0.052 | 0.059 | 0.035 | 0.049 | 0.057 | |
| r2 | 0.887 | 0.853 | 0.902 | 0.885 | 0.871 | 0.905 | |
| MRD | 0.048 | 0.074 | 0.039 | 0.049 | 0.065 | 0.040 | |
| Midilli et al. [25] | a | 0.035 | 0.071 | 0.078 | 0.032 | 0.045 | 0.038 |
| b | 0.015 | 0.041 | 0.047 | 0.013 | 0.021 | 0.018 | |
| k | 0.201 | 0.304 | 0.329 | 0.195 | 0.228 | 0.215 | |
| n | 0.742 | 1.395 | 1.512 | 0.739 | 0.925 | 0.758 | |
| r2 | 0.967 | 0.938 | 0.956 | 0.893 | 0.886 | 0.842 | |
| MRD | 0.022 | 0.029 | 0.024 | 0.052 | 0.049 | 0.081 | |
| Page | k | 0.105 | 0.091 | 0.068 | 0.034 | 0.056 | 0.108 |
| n | 0.687 | 0.582 | 0.413 | 0.337 | 0.398 | 0.689 | |
| r2 | 0.902 | 0.893 | 0.878 | 0.975 | 0.871 | 0.902 | |
| MRD | 0.039 | 0.052 | 0.062 | 0.031 | 0.065 | 0.039 | |
| Two-term exponential | a | 0.262 | 0.251 | 0.492 | 0.177 | 0.218 | 0.395 |
| k | 3.958 | 3.922 | 5.993 | 3.855 | 3.728 | 4.937 | |
| r2 | 0.907 | 0.871 | 0.901 | 0.942 | 0.983 | 0.953 | |
| MRD | 0.038 | 0.065 | 0.038 | 0.028 | 0.014 | 0.026 | |
| Wang and Singh [26] | a | 0.049 | 0.028 | 0.041 | 0.074 | 0.025 | 0.095 |
| b | 0.461 | 0.379 | 0.425 | 0.631 | 0.367 | 0.832 | |
| r2 | 0.842 | 0.842 | 0.909 | 0.882 | 0.892 | 0.935 | |
| MRD | 0.081 | 0.081 | 0.038 | 0.050 | 0.039 | 0.041 | |
| Model | Parameters and Goodness of Fit | Para-Rubber Wood-Derived Vinegar | Bamboo Wood-Derived Vinegar | Eucalyptus Wood-Derived Vinegar | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Drying Temperature | Drying Temperature | Drying Temperature | ||||||||
| 40 °C | 50 °C | 60 °C | 40 °C | 50 °C | 60 °C | 40 °C | 50 °C | 60 °C | ||
| Logarithmic | a | 1.042 | 1.363 | 1.267 | 1.165 | 1.028 | 1.375 | 1.025 | 1.024 | 1.048 |
| b | −0.083 | −0.031 | −0.043 | −0.081 | −0.029 | −0.039 | −0.024 | −0.051 | −0.081 | |
| k | 0.067 | 0.067 | 0.065 | 0.049 | 0.053 | 0.068 | 0.058 | 0.044 | 0.065 | |
| r2 | 0.903 | 0.885 | 0.897 | 0.842 | 0.918 | 0.905 | 0.975 | 0.865 | 0.853 | |
| MRD | 0.037 | 0.049 | 0.051 | 0.081 | 0.031 | 0.040 | 0.031 | 0.069 | 0.074 | |
| Midilli et al. [25] | a | 0.041 | 0.038 | 0.081 | 0.038 | 0.072 | 0.089 | 0.043 | 0.059 | 0.065 |
| b | 0.019 | 0.019 | 0.045 | 0.015 | 0.042 | 0.041 | 0.022 | 0.027 | 0.033 | |
| k | 0.213 | 0.211 | 0.332 | 0.197 | 0.305 | 0.338 | 0.217 | 0.281 | 0.327 | |
| n | 0.752 | 0.749 | 1.527 | 0.741 | 1.397 | 1.582 | 0.758 | 0.916 | 1.338 | |
| r2 | 0.902 | 0.971 | 0.906 | 0.907 | 0.975 | 0.906 | 0.978 | 0.952 | 0.945 | |
| MRD | 0.039 | 0.019 | 0.039 | 0.038 | 0.031 | 0.039 | 0.016 | 0.026 | 0.027 | |
| Page | k | 0.062 | 0.085 | 0.048 | 0.067 | 0.068 | 0.105 | 0.059 | 0.102 | 0.105 |
| n | 0.385 | 0.489 | 0.312 | 0.388 | 0.413 | 0.687 | 0.395 | 0.681 | 0.688 | |
| r2 | 0.902 | 0.842 | 0.873 | 0.893 | 0.905 | 0.889 | 0.885 | 0.897 | 0.878 | |
| MRD | 0.039 | 0.082 | 0.065 | 0.052 | 0.040 | 0.048 | 0.049 | 0.051 | 0.062 | |
| Two-term exponential | a | 0.258 | 0.498 | 0.311 | 0.256 | 0.398 | 0.825 | 0.365 | 0.295 | 0.157 |
| k | 3.925 | 6.009 | 4.128 | 3.912 | 5.007 | 9.657 | 4.369 | 4.035 | 2.956 | |
| r2 | 0.952 | 0.942 | 0.972 | 0.965 | 0.975 | 0.948 | 0.897 | 0.918 | 0.902 | |
| MRD | 0.026 | 0.028 | 0.019 | 0.023 | 0.018 | 0.027 | 0.051 | 0.031 | 0.039 | |
| Wang and Singh [26] | a | 0.031 | 0.065 | 0.077 | 0.052 | 0.087 | 0.092 | 0.075 | 0.085 | 0.078 |
| b | 0.382 | 0.598 | 0.635 | 0.486 | 0.789 | 0.834 | 0.634 | 0.781 | 0.635 | |
| r2 | 0.885 | 0.853 | 0.865 | 0.897 | 0.887 | 0.873 | 0.842 | 0.908 | 0.906 | |
| MRD | 0.049 | 0.074 | 0.069 | 0.051 | 0.048 | 0.065 | 0.081 | 0.038 | 0.039 | |
| Model | Parameters and Goodness of Fit | Commercial Formic Acid | Commercial Acetic Acid | ||||
|---|---|---|---|---|---|---|---|
| Drying Temperature | Drying Temperature | ||||||
| 40 °C | 50 °C | 60 °C | 40 °C | 50 °C | 60 °C | ||
| Logarithmic | a | 1.185 | 1.099 | 1.372 | 1.135 | 1.029 | 1.091 |
| b | −0.086 | −0.081 | −0.038 | −0.086 | −0.025 | −0.079 | |
| k | 0.043 | 0.049 | 0.071 | 0.053 | 0.059 | 0.058 | |
| r2 | 0.887 | 0.862 | 0.842 | 0.908 | 0.902 | 0.911 | |
| MRD | 0.048 | 0.070 | 0.081 | 0.038 | 0.039 | 0.037 | |
| Midilli et al. [25] | a | 0.037 | 0.051 | 0.063 | 0.082 | 0.096 | 0.078 |
| b | 0.020 | 0.031 | 0.035 | 0.053 | 0.065 | 0.051 | |
| k | 0.215 | 0.329 | 0.324 | 0.351 | 0.422 | 0.343 | |
| n | 0.756 | 1.356 | 1.325 | 1.628 | 1.825 | 1.605 | |
| r2 | 0.972 | 0.965 | 0.971 | 0.842 | 0.907 | 0.905 | |
| MRD | 0.019 | 0.023 | 0.019 | 0.081 | 0.038 | 0.040 | |
| Page | k | 0.089 | 0.065 | 0.115 | 0.099 | 0.093 | 0.075 |
| n | 0.452 | 0.412 | 0.726 | 0.585 | 0.592 | 0.421 | |
| r2 | 0.887 | 0.842 | 0.934 | 0.853 | 0.893 | 0.907 | |
| MRD | 0.048 | 0.081 | 0.041 | 0.074 | 0.052 | 0.038 | |
| Two-term exponential | a | 0.867 | 0.854 | 0.895 | 0.225 | 0.535 | 0.425 |
| k | 9.726 | 9.712 | 9.863 | 3.738 | 6.187 | 5.371 | |
| r2 | 0.909 | 0.897 | 0.935 | 0.983 | 0.971 | 0.987 | |
| MRD | 0.038 | 0.051 | 0.041 | 0.015 | 0.019 | 0.011 | |
| Wang and Singh [26] | a | 0.089 | 0.105 | 0.097 | 0.041 | 0.028 | 0.057 |
| b | 0.901 | 0.995 | 0.846 | 0.427 | 0.375 | 0.499 | |
| r2 | 0.921 | 0.865 | 0.872 | 0.893 | 0.941 | 0.902 | |
| MRD | 0.025 | 0.069 | 0.067 | 0.052 | 0.029 | 0.039 | |
| No. | Samples | Fungal Count (CFU/cm2) |
|---|---|---|
| 1 | Control I: Acetic acids | 4.53 ± 0.35 × 103 b |
| 2 | Control II: Formic acids | 4.55 ± 0.27 × 103 b |
| 3 | 10% v/v eucalyptus wood vinegar | 135 ± 0.56 e |
| 4 | 10% v/v para-rubber wood vinegar | 411 ± 1.24 c |
| 5 | 10% v/v bamboo wood vinegar | 514 ± 1.72 a |
| 6 | 20% v/v eucalyptus wood vinegar | 71 ± 1.82 c |
| 7 | 20% v/v para-rubber wood vinegar | 82 ± 2.19 f |
| 8 | 20% v/v bamboo wood vinegar | 93 ± 1.45 d |
| 9 | 30% v/v eucalyptus wood vinegar | 25 ± 0.79 h |
| 10 | 30% v/v para-rubber wood vinegar | 38 ± 1.85 g |
| 11 | 30% v/v bamboo wood vinegar | 47 ± 1.67 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wattana, W.; Lakachaiworakun, P.; Rachsiriwatcharabul, N.; Eakvanich, V.; Dangwilailux, P.; Kalasee, W. Thin-Layer Drying Model and Antifungal Properties of Rubber Sheets Produced with Wood Vinegar as a Substitute for Formic and Acetic Acids. Polymers 2025, 17, 1201. https://doi.org/10.3390/polym17091201
Wattana W, Lakachaiworakun P, Rachsiriwatcharabul N, Eakvanich V, Dangwilailux P, Kalasee W. Thin-Layer Drying Model and Antifungal Properties of Rubber Sheets Produced with Wood Vinegar as a Substitute for Formic and Acetic Acids. Polymers. 2025; 17(9):1201. https://doi.org/10.3390/polym17091201
Chicago/Turabian StyleWattana, Wassachol, Putipong Lakachaiworakun, Natworapol Rachsiriwatcharabul, Visit Eakvanich, Panya Dangwilailux, and Wachara Kalasee. 2025. "Thin-Layer Drying Model and Antifungal Properties of Rubber Sheets Produced with Wood Vinegar as a Substitute for Formic and Acetic Acids" Polymers 17, no. 9: 1201. https://doi.org/10.3390/polym17091201
APA StyleWattana, W., Lakachaiworakun, P., Rachsiriwatcharabul, N., Eakvanich, V., Dangwilailux, P., & Kalasee, W. (2025). Thin-Layer Drying Model and Antifungal Properties of Rubber Sheets Produced with Wood Vinegar as a Substitute for Formic and Acetic Acids. Polymers, 17(9), 1201. https://doi.org/10.3390/polym17091201







