Metal-Free Polymethyl Methacrylate (PMMA) Nanoparticles by Enamine “Click” Chemistry at Room Temperature
Abstract
: “Click” chemistry has become an efficient avenue to unimolecular polymeric nanoparticles through the self-crosslinking of individual polymer chains containing appropriate functional groups. Herein we report the synthesis of ultra-small (7 nm in size) polymethyl methacrylate (PMMA) nanoparticles (NPs) by the “metal-free” cross-linking of PMMA-precursor chains prepared by reversible addition-fragmentation chain transfer (RAFT) polymerization containing β-ketoester functional groups. Intramolecular collapse was performed by the one-pot reaction of β-ketoester moieties with alkyl diamines in tetrahydrofurane at r.t. (i.e., by enamine formation). The collapsing process was followed by size exclusion chromatography and by nuclear magnetic resonance spectroscopy. The size of the resulting PMMA-NPs was determined by dynamic light scattering. Enamine “click” chemistry increases the synthetic toolbox for the efficient synthesis of metal-free, ultra-small polymeric NPs.1. Introduction
Unimolecular polymeric nanoparticles (NPs) are emerging soft nano-objects showing dimensions below 10–15 nm displaying promising properties for different end-use applications such as processing additives [1], blend compatibilizers [2], artificial enzymes [3], photostable bio-imaging agents [4] and drug/siRNA-delivery systems [5], among others. Significant efforts have been devoted in recent years to open new routes to unimolecular nanoparticles relying on efficient synthetic methods for the controlled, irreversible intramolecular collapse of individual polymer chains. In this sense, different synthetic approaches have been reported: free-radical cross-linking of vinyl units [6-8], thermal cross-linking of benzocyclobutene and o-quinodimethane functional groups by Diels-Alder reactions [9,10], copper-catalyzed azide-alkyne “click” chemistry [11-13] and fast cross-linking of isocyanate groups from appropriate precursor chains by diamines [14].
Pioneering results were obtained by Mecerreyes et al. [6] for polycaprolactone (PCL)-, polystyrene (PS)- and polymethyl methacrylate (PMMA)-NPs synthesized by free-radical cross-linking of vinyl units by using ultra-diluted conditions. Following a similar route, Park et al. [7] reported the preparation of nanoparticles with sizes ranging from 10 to 30 nm by self-crosslinking of polymetacrylate terpolymers in ultra-dilute solutions. Additionally, amine-functionalized PS-nanoparticles were prepared by Jiang et al. [8] from PS precursors with a very high amount of vinyl cross-linking units (33 and 50 mol%). One of the main shortcomings of the ultra-dilute technique was the relatively high dispersity in size of the resulting polymeric NPs.
Harth et al. [9] developed a continuous addition technique in which intramolecular cross-linking was performed in benzyl ether (BE) at 250 °C from PS-, PMMA- and poly(n-butyl acrylate) (PnBA)-precursors containing benzocyclobutene (BCB) cross-linking units. PS-nanoparticles were also synthesized by Croce et al. [10] following this route from PS-precursors containing o-quinodimethane cross-linking units instead of BCB moieties. A clear limitation of the technique is that reaction conditions are harsh, precluding the use of thermally labile polymers and/or functional groups.
In an attempt to solve the limitations of the previous techniques, we introduced the macromolecular “click” cycloaddition route [11-13] which relies on the use of alkyne and azide functional groups allowing the synthesis of unimolecular NPs by copper-catalyzed azide-alkyne “click” chemistry. Currently, a great interest exists for developing “click” chemistry procedures beyond the copper-catalyzed azide-alkyne cycloaddition [15], allowing the synthesis of “metal-free” unimolecular polymeric nanoparticles at r.t. In addition to cross-linking of isocyanate by diamines leading to PMMA-NPs of relatively high polydispersity [14], several alternative (metal-free) “click” chemistry reactions are promising candidates, including: (i) enamine formation [16]; (ii) thiol-ene [17]/thiol-yne coupling [18]; (iii) thiol-isocyanate addition [19]; (iv) nitrile oxide-alkyne cycloaddition [20]; and (v) hydroxylamine-carbonyl reaction [21].
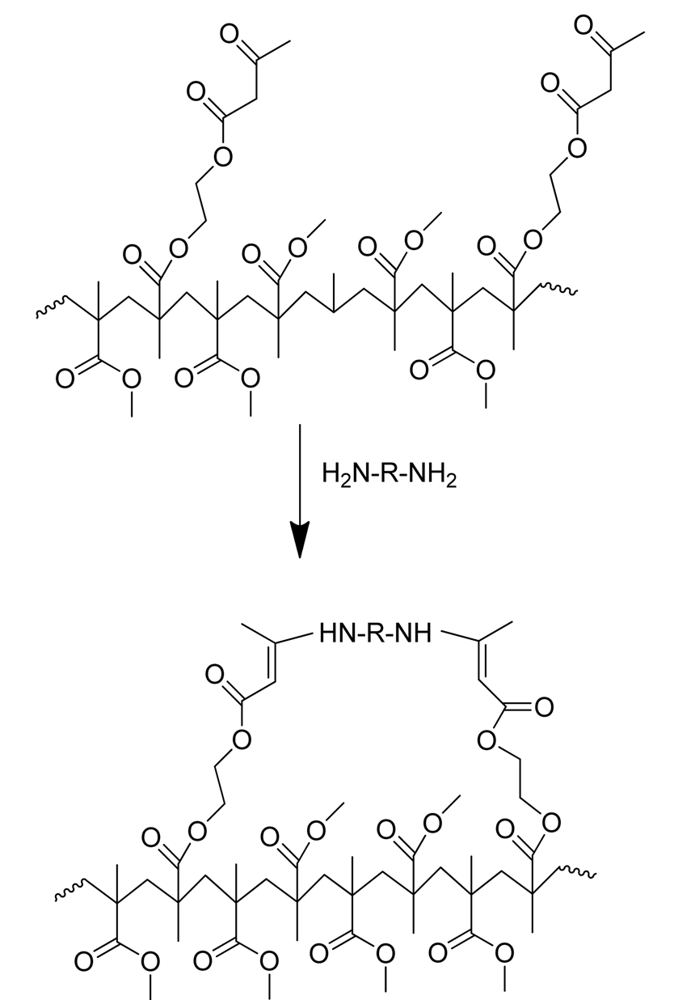
Here we disclose intramolecular enamine cross-linking as an alternative “click” technique for the metal-free, room temperature synthesis of unimolecular polymeric nanoparticles. The direct reaction of β-ketoester groups with ammonia to give enamine groups is well-known in polymer chemistry [22] as a useful reaction to protect latexes containing β-ketoester moieties from hydrolysis (see Figure 1). Enamine formation follows the “click” chemistry concept [23,24] since: (i) it can be performed under equimolar conditions and proceeds in appropriate time-scales even at room temperature, with high yields; (ii) the reaction is insensitive to oxygen and can be performed in solvents that can be easily removed; (iii) products can be easily isolated by simple methods; (iv) tedious fine-tuning of reaction conditions is not required; and (v) there are no side-reactions involved.
In this communication, we report the synthesis of ultra-small PMMA-NPs synthesized by metal-free enamine cross-linking from PMMA precursors containing β-ketoester units by using alkyl diamines. The collapsing process was followed by size exclusion chromatography and nuclear magnetic resonance spectroscopy. The size of the resulting PMMA-NPs was determined by dynamic light scattering. Similar attempts via reductive amination did not lead to defined nanoparticles and the enamine formation might be the only way to form ultra-small nanoparticles from ketones and amines. Subtle hydrogen bonding effects, affecting the solubility of the resulting NPs upon isolation and drying, are also indicated.
2. Results and Discussion
The “metal-free” synthetic pathway to PMMA-NPs is summarized in Figure 2. As PMMA-nanoparticle precursors we targeted random copolymers of methyl methacrylate (MMA) and (2-acetoacetoxy)ethyl methacrylate (AEMA) containing different molar fractions of β-ketoester units and/or different molecular weight (denoted as 1a, 1b and 1c in Table 1). The precursors were synthesized by reversible addition-fragmentation chain transfer (RAFT) polymerization using 2-cyanoprop-2-yl-dithiobenzoate (CDB) as chain transfer agent and 2,2′-azobis(2-methylpropionitrile) (AIBN) as free-radical initiator. RAFT is a very convenient polymerization technique for obtaining nanoparticle precursors with low molecular-weight dispersity [11-13] and hence nanoparticles with uniform size. The main characteristics of the random P(MMA-co-AEMA) copolymers investigated in this work are summarized in Table 1, as determined by size exclusion chromatography with absolute molar mass characterization and proton nuclear magnetic resonance spectroscopy. Well-defined copolymers showing low molecular-weight dispersity (Ð = Mw/Mn) values were obtained. The copolymer composition was found to be very close to the feed monomer composition, as expected for a statistical copolymerization process (reactivity ratios: rMMA = 0.90 and rAEMA = 0.95) allowing the random placement of the β-ketoester moieties along the copolymer chains.
The collapsing process upon enamine formation was followed by size-exclusion chromatography (SEC) which is a technique very valuable to discriminate macromolecules according to their hydrodynamic radius (RH). In this sense, a significant reduction in RH is expected by SEC/refractive index (RI) upon collapse of individual polymer chains to ultra-small nanoparticles. Conversely, for totally collapsed single-chain NPs only a slight increase of the actual molecular weight due to enamine cross-linking (i.e., less than 10%) is expected by SEC/multi-angle laser light scattering (MALLS) when compared to the molecular weight of the starting linear precursor chains. A significant increase in retention time (corresponding to a clear reduction in RH) is observed in Figure 3 accompanying NP formation by enamine cross-linking.
It is noteworthy that since intermolecular reactions give rise inevitably to a large increase in the molecular weight (i.e., ≥100%), an “apparent” molecular weight reduction, as deduced from Figure 3 due to the increase in retention time upon enamine cross-linking, is a clear signature of chain collapse. A slight increase in “absolute” mass-average molecular weight, Mw, upon reaction was found by SEC/MALLS (Mw = 89,000 g/mol) when compared to the expected value (Mw = 61,900 g/mol) suggesting that some degree of inter-particle coupling could be also present in addition to the principal single-chain intramolecular cross-linking process. On average, the number of polymer chains per nanoparticle is estimated to be around 89,000/61,900 = 1.4.
As expected, nanoparticle collapse by enamine formation introduces significant changes in the 1H-NMR spectrum of the material. Figure 4(a) illustrates the 1H-NMR spectrum of the starting precursor 1a, whereas Figure 4(b) gives the spectrum of the resulting nanoparticles. The corresponding peak assignation is also indicated.
The peak broadening observed in Figure 4(b) is a clear signature of NP formation and is well-documented in the literature [9]. This band broadening cannot be attributed to an increase of the “absolute” molecular weight upon nanoparticle formation but to its own nanoparticle structure upon changing from a solvated random coil to a compact, globular object. As an example, insets in Figure 4 show band broadening in signals from –CH= protons of enamine groups (4.5 ppm, broad) and –CH3 protons of main-chain methyl groups (0.5–1.1 ppm, very broad).
The signal from the =N–H protons of enamine groups is visible in the 1H-NMR spectrum at 8.7 ppm. It is worth noting that the weak signal from the proton enol-form which can be seen at around 12 ppm in the precursor (Figure 4(a)) disappears upon nanoparticle formation.
Upon NP formation, a shift of a significant part of the original infra-red C=O band of AEMA from 1,732 cm−1 to 1,656 cm−1 is observed as well as the presence of a new band at 1,605 cm−1 which can be assigned to stretching vibrations of enamine double bonds (Figure 5). Based on previous works on model compounds [25,26], the large shifts observed can be attributed to the presence of significant NH/C=O hydrogen bonding interactions upon enamine formation.
As depicted in Figure 6, nanoparticle size measurements by DLS supported the SEC and 1H-NMR results. Ultra-small PMMA-NPs with 7 nm in size were routinely obtained at r.t. upon reaction completion. Interestingly, the size of the PMMA-NPs remained unaltered while stored in dilute THF solutions (c < 1 g/L).
However, when isolated by precipitation and further drying the resulting PMMA-NPs became insoluble in THF probably as a consequence of strong inter-nanoparticle hydrogen bonding (HB) interactions [14]. Attempts to solubilize the nanoparticles by adding lithium chloride, methanol, or trifluoroacetic acid failed. Similar attempts to change from THF solvent to dimethyl formamide before precipitation do not avoid nanoparticle aggregation presumably by cooperative HB interactions upon isolation.
Work is in progress to investigate the influence of the diamine structure on this striking behavior by using diamines containing bulky tert-butyl groups in the structure, as well as to determine the precise NP morphology by transmission electron microscopy and atomic force microscopy. Such research, which is outside the scope of the present paper, will be published in the near future.
3. Experimental Section
3.1. Materials
2,2′-Azobis(2-methylpropionitrile) (AIBN), methyl methacrylate (MMA), (2-acetoacetoxy)ethyl methacrylate (AEMA), diethylenetriamine (DETA), deuterated tetrahydrofurane (THF-d8) and deuterated chloroform (99.96 atom% D) containing 0.03% (v/v) tetramethylsilane (TMS) were supplied by Aldrich. AIBN was purified by re-crystallization. MMA was distilled under reduced pressure (Buchi Glass Oven B-585) immediately before use. AEMA was purified by passing through a neutral alumina column. 2-Cyanoprop-2-yl-dithiobenzoate (CDB) and HPLC grade tetrahydrofurane (THF) were obtained from Strem Chemicals and Scharlau, respectively, and were used as received.
3.2. Methods
Size-exclusion chromatography (SEC) was performed with an Agilent G-1310A liquid chromatograph equipped with a high performance PLgel Mixed column and on-line differential refractometer (RI) and multi-angle laser light scattering (MALLS) Wyatt detectors (Optilab-Rex and Mini-Dawn Treos, respectively) and. THF (flow rate of 1.0 mL/min) was used as eluent at 30 °C. 1H-NMR spectra were recorded on a Bruker AVANCE spectrometer (300 MHz). Nanoparticle size was measured by dynamic light scattering (DLS) in a Malvern Zetasizer apparatus at r.t. Infrared (IR) spectra were recorded in a Jasco 3600 FT-IR Spectrometer.
3.3. Precursor Synthesis
In a typical procedure, a solution containing AIBN (1 mg, 6 μmol), CDB (9 mg, 40 μmol), MMA (1 mL, 9.3 mmol) and AEMA (1.2 mL, 6.2 mmol) was degassed and heated to 65 °C under magnetic stirring. After reaction, the resulting copolymer, 1a, was purified by precipitation in methanol and drying under dynamic vacuum for 24 h. Yield (gravimetric): 1.52 g (67%). Mass-average molecular weight and dispersity (SEC/RI/MALLS): 57,100 g/mol and 1.1, respectively. Molar fraction of MMA (1H-NMR): 0.65.
3.4. Nanoparticle Synthesis
On a round-bottom flask, the precursor 1a (150 mg, 0.3 mmol of AEMA) was dissolved in 25 mL of THF. On a separate flask, DETA (equimolar amount of amine groups) was dissolved in 25 mL of THF. Both solutions were mixed at once and allowed to stir at r.t. Samples were removed periodically from the reaction medium for SEC/RI/MALLS and DLS analysis. In separate experiments, nanoparticle formation was monitored by 1H-NMR by using THF-d8 as solvent. After reaction completion, the transparent solution was concentrated and precipitated onto methanol to give 2a as a powder which was dried under dynamic vacuum for 24 h. Reaction yield: 100 mg, (67%). Average NP size (by DLS in THF): 7 nm.
4. Conclusions
A new, “metal-free” route to PMMA-NPs has been developed based on enamine “click” chemistry. As appropriate precursors, well-defined random copolymers of MMA and AEMA have been synthesized by RAFT polymerization, showing low molecular-weight dispersity and a random placement of β-ketoester moieties along the copolymer chains. Intramolecular collapse of the PMMA-NP precursors was performed by the one-pot reaction of the β-ketoester moieties with alkyl diamines in THF at r.t. (i.e., by enamine formation). Significant changes were observed both in the 1H-NMR and FTIR spectra upon enamine cross-linking.
Nanoparticle formation was followed by SEC/RI/MALLS and, in separate experiments, by 1H-NMR spectroscopy in THF-d8. The size of the resulting PMMA-NPs was <10 nm as determined by DLS and remained unaltered while the nanoparticles were stored in dilute THF solutions (c < 1 g/L). Strong inter-nanoparticle hydrogen bonding upon nanoparticle drying rendered the isolated PMMA-NPs insoluble in THF.





| Precursor | Mw(g/mol) | Dispersity | AEMA (mol%) |
|---|---|---|---|
| 1a | 57,100 | 1.2 | 35 |
| 1b | 35,000 | 1.1 | 34 |
| 1c | 43,000 | 1.1 | 25 |
Acknowledgments
We thank Maria Isabel Asenjo-Sanz for excellent experimental technical support. L.B. acknowledges financial support by Basque Excellence Research Center-Materials Physics Center (BERC-MPC). Authors gratefully acknowledge financial support from the Spanish Ministry of Science and Innovation, project MAT-22007-63681, and Diputación de Gipuzkoa, project 2011-CIEN-000085-01.
References
- Tuteja, A.; Duxbury, P.M.; Mackay, M.E. Multifunctional Nanocomposites with Reduced Viscosity. Macromolecules 2007, 40, 9427–9434. [Google Scholar]
- Pomposo, J.A.; Ruiz de Luzuriaga, A.; García, I.; Etxeberria, A.; Colmenero, J.A. Nanotechnology Pathway to Arresting Phase Separation in Soft Nanocomposites. Macromol. Rapid Commun. 2011, 32, 573–578. [Google Scholar]
- Wulff, G.; Chong, B.-O.; Kolb, U. Soluble Single-Molecule Nanogels of Controlled Structure as a Matrix for Efficient Artificial Enzymes. Angew. Chem. Int. Ed. 2006, 45, 2955–2958. [Google Scholar]
- Adkins, C.T.; Muchalski, H.; Harth, E. Nanoparticles with Individual Site-Isolated Semiconducting Polymers from Intramolecular Chain Collapse Processes. Macromolecules 2009, 42, 5786–5792. [Google Scholar]
- Tamura, A.; Nagasaki, Y. Smart siRNA Delivery Systems Based on Polymeric Nanoassemblies and Nanoparticles. Nanomedicine 2010, 5, 1089–1102. [Google Scholar]
- Mecerreyes, D.; Lee, V.; Hawker, C.J.; Hedrick, J.L.; Wursch, A.; Volksen, W.; Magbitang, T.; Huang, E.; Miller, R.D. A Novel Approach to Functionalized Nanoparticles: Self-Crosslinking of Macromolecules in Ultradilute Solution. Adv. Mater. 2001, 13, 204–208. [Google Scholar]
- Park, K.S.; Kim, D.Y.; Choi, S.K.; Suh, D.H. Novel Approach to Chemically Amplified Resist Materials for Next Generation of Lithography. Jpn. J. Appl. Phys. 2003, 42, 3877–3880. [Google Scholar]
- Jiang, J.; Thayumanavan, S. Synthesis and Characterization of Amine-Functionalized Polystyrene Nanoparticles. Macromolecules 2005, 38, 5886–5891. [Google Scholar]
- Harth, E.; Horn, B.V.; Lee, V.Y.; Germack, D.S.; Gonzales, C.P.; Miller, R.D.; Hawker, C.J. A Facile Approach to Architecturally Defined Nanoparticles via Intramolecular Chain Collapse. J. Am. Chem. Soc. 2002, 124, 8653–8660. [Google Scholar]
- Croce, T.A.; Hamilton, S.K.; Chen, M.L.; Muchalski, H.; Harth, E. Alternative o-Quinodimethane Cross-Linking Precursors for Intramolecular Chain Collapse Nanoparticles. Macromolecules 2007, 40, 6028–6031. [Google Scholar]
- Oria, L.; Aguado, R.; Pomposo, J.A.; Colmenero, J.A. Versatile “Click” Chemistry Precursor of Functional Polystyrene Nanoparticles. Adv. Mater. 2010, 22, 3038–3041. [Google Scholar]
- Ruiz de Luzuriaga, A.; Perez-Baena, I.; Montes, S.; Loinaz, I.; Odriozola, I.; Garcia, I.; Pomposo, J.A. New Route to Polymeric Nanoparticles by Click Chemistry Using Bifunctional Cross-Linkers. Macromol. Symp. 2010, 296, 303–310. [Google Scholar]
- Ruiz de Luzuriaga, A.; Ormategui, N.; Grande, H.J.; Odriozola, I.; Pomposo, J.A.; Loinaz, I. Intramolecular Click Cycloaddition: An Efficient Room-Temperature Route towards Bioconjugable Polymeric Nanoparticles. Macromol. Rapid Commun. 2008, 29, 1156–1160. [Google Scholar]
- Beck, J.B.; Killops, K.L.; Kang, T.; Sivanandan, K.; Bayles, A.; Mackay, M.E.; Wooley, K.; Hawker, C.J. Facile Preparation of Nanoparticles by Intramolecular Cross-Linking of Isocyanate Functionalized Copolymers. Macromolecules 2009, 42, 5629–5635. [Google Scholar]
- Canalle, L.A.; van Berkel, S.S.; de Haan, L.T.; van Hest, J.C.M. Copper-Free Clickable Coatings. Adv. Funct. Mat. 2009, 19, 3464–3470. [Google Scholar]
- Cook, A.G. Enamines: Synthesis, Structure and Reactions; Dekker, M., Ed.; CRC Press: New York, NY, USA, 1988. [Google Scholar]
- Lowe, A.B. Thiol-Ene “Click” Reactions and Recent Applications in Polymer and Materials Synthesis. Polym. Chem. 2010, 1, 17–36. [Google Scholar]
- Lowe, A.B.; Hoyle, C.E.; Bowman, C.N. Thiol-Yne Click Chemistry: A Powerful and Versatile Methodology for Materials Synthesis. J. Mater. Chem. 2010, 20, 4745–4750. [Google Scholar]
- Li, H.; Yu, B.; Matsushima, H.; Hoyle, C.E.; Lowe, A.B. The Thiol-Isocyanate Click Reaction: Facile and Quantitative Access to ω-End-Functional Poly(N,N -diethylacrylamide) Synthesized by RAFT Radical Polymerization. Macromolecules 2009, 42, 6537–6542. [Google Scholar]
- Singh, I.; Zarafshani, Z.; Lutz, J.-F.; Heaney, F. Metal-Free “Click” Chemistry: Efficient Polymer Modification via 1,3-Dipolar Cycloaddition of Nitrile Oxides and Alkynes. Macromolecules 2009, 42, 5411–5413. [Google Scholar]
- Heredia, K.L.; Maynard, H.D. Synthesis of Protein-Polymer Conjugates. Org. Biomol. Chem. 2007, 5, 45–53. [Google Scholar]
- Gonzalez, I.; Arzamendi, G.; Asua, J.M.; Leiza, J.R. Unexpected Crosslinking during Acetoacetoxy Group Protection on Waterborne Crosslinkable Latexes. Macromol. Mater. Eng. 2006, 291, 1185–1193. [Google Scholar]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar]
- Barner-Kowollik, C.; Du Prez, F.E.; Espeel, P.; Hawker, C.J.; Junkers, T.; Schlaad, H.; van Camp, W. “Clicking” Polymers or Just Efficient Linking: What Is the Difference? Angew. Chem. Int. Ed. 2011, 50, 60–62. [Google Scholar]
- Labelle, M.; Gravel, D. Tautomeric Equilibrium of Cyclic β-Ketoester Enamines. Can. J. Chem. 1985, 63, 1884–1890. [Google Scholar]
- Witkop, B. Imine-Enamine Systems and the Mechanism of Their Oxidation. J. Am. Chem. Soc. 1956, 78, 2873–2882. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Buruaga, L.; Pomposo, J.A. Metal-Free Polymethyl Methacrylate (PMMA) Nanoparticles by Enamine “Click” Chemistry at Room Temperature. Polymers 2011, 3, 1673-1683. https://doi.org/10.3390/polym3041673
Buruaga L, Pomposo JA. Metal-Free Polymethyl Methacrylate (PMMA) Nanoparticles by Enamine “Click” Chemistry at Room Temperature. Polymers. 2011; 3(4):1673-1683. https://doi.org/10.3390/polym3041673
Chicago/Turabian StyleBuruaga, Lorea, and José A. Pomposo. 2011. "Metal-Free Polymethyl Methacrylate (PMMA) Nanoparticles by Enamine “Click” Chemistry at Room Temperature" Polymers 3, no. 4: 1673-1683. https://doi.org/10.3390/polym3041673
APA StyleBuruaga, L., & Pomposo, J. A. (2011). Metal-Free Polymethyl Methacrylate (PMMA) Nanoparticles by Enamine “Click” Chemistry at Room Temperature. Polymers, 3(4), 1673-1683. https://doi.org/10.3390/polym3041673





