Polyester Dendrimers: Smart Carriers for Drug Delivery
Abstract
:1. Introduction
2. Dendrimers and Common Drug Delivery Pathways
2.1. Oral Drug Delivery
2.2. Transdermal Drug Delivery
2.3. Ocular Drug Delivery
2.4. Drug Delivery by Injection
3. Covalent and Non-Covalent Dendrimer-Drug Systems
4. Polyester Dendrimers
4.1. Attractive Features of Polyester Dendrimers for Drug Delivery Applications
4.2. Advances in Polyester Dendrimers for Drug Delivery Applications













5. Conclusions
Abbreviations:
| BACPT | 7-butyl-10-aminocamptothecin |
| bis-HMPA | 2,2-bis(hydroxymethyl)propanoic acid |
| BNCT | boron neutron capture therapy |
| COMU | 1-[(1-(cyano-2-ethoxy-2-oxoethylideneaminooxy)dimethylaminomorpho-linomethylene)]methanaminium hexafluorophosphate |
| CPEGC | citric acid–polyethylene glycol–citric acid |
| DBU | 1,8-diazabicyclo[5.4.0]undec-7-ene |
| DIC | N,N'-diisopropylcarbodiimide |
| DIEA | diisopropylethylamine |
| DLSP | drug-loaded degradable dendrimer-like star polymer |
| DMAP | 4-dimethylaminopyridine |
| DOX | doxorubicin |
| EPR | enhanced permeability and retention |
| FA-DLSP | folate-functionalized degradable amphiphilic dendrimer-like star polymer |
| FDA | United States food and drug administration |
| 10HCPT | 10-hydroxycamptothecin |
| MTX | methotrexate |
| ROP | ring-opening polymerization |
| PAMAM | polyamidoamine |
| PEG | poly(ethylene oxide) |
| PEO | poly(ethylene oxide) |
| PEPE | polyester-co-polyether |
| PLLA | poly(l-lactide) |
| TATU | 2-(1H-7-azabenzotriazole-1-yl)-1,1,3,3-tetramethyluronium tetrafluoroborate |
| TBTU | 2-(1H-benzotriazole-1-yl)-1,1,3,3-tetramethyluronium tetrafluoroborate |
| TFA | trifluoroacetic acid |
Acknowledgments
Conflict of Interest
References
- Ringsdorf, H. Structure and properties of pharmacologically active polymers. J. Polym. Sci. Pol. Sym. 1975, 51, 135–153. [Google Scholar]
- Bader, H.; Ringsdorf, H.; Schmidt, B. Water soluble polymers in medicine. Angew. Makromol. Chem. 1984, 123, 457–485. [Google Scholar] [CrossRef]
- Kopeček, J. Soluble biomedical polymers. Polym. Med. 1977, 7, 191–221. [Google Scholar]
- Kopeček, J.; Kopeckova, P.; Minko, T.; Lu, Z.R. HPMA copolymer-anticancer drug conjugates: Design, activity, and mechanism of action. Eur. J. Pharmaceut. Biopharmaceut. 2000, 50, 61–81. [Google Scholar] [CrossRef]
- Duncan, R. Drug-polymer conjugates: Potential for improved chemotherapy. Cancer Res. 1992, 46, 175–210. [Google Scholar]
- Maeda, H.; Seymour, L.W.; Miyamoto, Y. Conjugates of anticancer agents and polymers-Advantages of macromolecular therapeutics in vivo. Bioconjugate Chem. 1992, 3, 351–362. [Google Scholar] [CrossRef]
- Seymour, L.W.; Miyamoto, Y.; Maeda, H.; Brereton, M.; Strohalm, J.; Ulbrich, K.; Duncan, R. Influence of molecular weight on passive tumor accumulation of a soluble macromolecular drug carrier. Eur. J. Cancer 1995, 31A, 766–770. [Google Scholar]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control. Release 2000, 65, 271–284. [Google Scholar] [CrossRef]
- Seymour, L.W. Passive tumor targeting of soluble macromolecules and drug conjugates. Crit. Rev. Ther. Drug 1992, 9, 135–187. [Google Scholar]
- Duncan, R.; Sat, Y.N. Tumour targeting by enhanced permeability and retention (EPR) effect. Ann. Oncol. 1998, 9, 39–39. [Google Scholar] [CrossRef]
- Wang, Z.; Itoh, Y.; Hosaka, Y.; Kobayashi, I.; Nakano, Y.; Maeda, I.; Umeda, F.; Yamakawa, J.; Nishimine, M.; Suenobu, T.; Fukuzumi, S.; Kawase, M.; Yagi, K. Mechanism of enhancement effect of dendrimer on transdermal drug permeation through polyhydroxyalkanoate matrix. J. Biosci. Bioeng. 2003, 96, 537–540. [Google Scholar] [CrossRef]
- Gillies, E.R.; Fréchet, J.M.J. Designing macromolecules for therapeutic applications: Polyester dendrimer-poly(ethylene oxide) “bow-tie” hybrids with tunable molecular weight and architecture. J. Am. Chem. Soc. 2002, 124, 14137–14146. [Google Scholar] [CrossRef]
- Kolhe, P.; Misra, E.; Kannan, R.M.; Kannan, S.; Lieh-Lai, M. Drug complexation, in vitro release and cellular entry of dendrimers and hyperbranched polymers. Int. J. Pharmaceut. 2003, 259, 143–160. [Google Scholar] [CrossRef]
- Baker, J.R. Why I believe nanoparticles are crucial as a carrier for targeted drug delivery. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2013, 5, 423–429. [Google Scholar]
- Gu, L.; Wu, Z.H.; Qi, X.L.; He, H.; Ma, X.L.; Chou, X.H.; Wen, X.G.; Zhang, M.; Jiao, F. Polyamidomine dendrimers: An excellent drug carrier for improving the solubility and bioavailability of puerarin. Pharm. Dev. Technol. 2013, 18, 1051–1057. [Google Scholar] [CrossRef]
- Zhou, Z.Y.; D’Emanuele, A.; Attwood, D. Solubility enhancement of paclitaxel using a linear-dendritic block copolymer. Int. J. Pharmaceut. 2013, 452, 173–179. [Google Scholar] [CrossRef]
- Gula, A.; Ren, L.; Zhou, Z.; Lu, D.D.; Wang, S.Q. Design and evaluation of biodegradable enteric microcapsules of amifostine for oral delivery. Int. J. Pharmaceut. 2013, 453, 441–447. [Google Scholar] [CrossRef]
- Ma, X.P.; Zhou, Z.X.; Jin, E.L.; Sun, Q.H.; Zhang, B.; Tang, J.B.; Shen, Y.Q. Facile synthesis of polyester dendrimers as drug delivery carriers. Macromolecules 2013, 46, 37–42. [Google Scholar] [CrossRef]
- Thomas, T.P.; Joice, M.; Sumit, M.; Silpe, J.E.; Kotlyar, A.; Bharathi, S.; Kukowska-Latallo, J.; Baker, J.R.; Choi, S.K. Design and in vitro validation of multivalent dendrimer methotrexates as a folate-targeting anticancer therapeutic. Curr. Pharm. Design 2013, 19, 6594–6605. [Google Scholar] [CrossRef]
- Leng, Z.H.; Zhuang, Q.F.; Li, Y.C.; He, Z.; Chen, Z.; Huang, S.P.; Jia, H.Y.; Zhou, J.W.; Liu, Y.; Du, L.B. Polyamidoamine dendrimer conjugated chitosan nanoparticles for the delivery of methotrexate. Carbohyd. Polym. 2013, 98, 1173–1178. [Google Scholar] [CrossRef]
- Murugan, E.; Rani, D.P.G.; Srinivasan, K.; Muthumary, J. New surface hydroxylated and internally quaternised poly(propylene imine) dendrimers as efficient biocompatible drug carriers of norfloxacin. Expert Opin. Drug. Del. 2013, 10, 1319–1334. [Google Scholar] [CrossRef]
- Sadekar, S.; Thiagarajan, G.; Bartlett, K.; Hubbard, D.; Ray, A.; McGill, L.D.; Ghandehari, H. Poly(amido amine) dendrimers as absorption enhancers for oral delivery of camptothecin. Int. J. Pharmaceut. 2013, 456, 175–185. [Google Scholar] [CrossRef]
- Wang, L.; Xu, X.P.; Zhang, Y.; Zhang, Y.Q.; Zhu, Y.; Shi, J.Y.; Sun, Y.H.; Huang, Q. Encapsulation of curcumin within poly(amidoamine) dendrimers for delivery to cancer cells. J. Mater. Sci Mater. M. 2013, 24, 2137–2144. [Google Scholar] [CrossRef]
- Yellepeddi, V.K.; Vangara, K.K.; Palakurthi, S. Poly(amido)amine (PAMAM) dendrimer-cisplatin complexes for chemotherapy of cisplatin-resistant ovarian cancer cells. J. nanopart. Res. 2013, 15. [Google Scholar] [CrossRef]
- Koc, F.E.; Senel, M. Solubility enhancement of non-steroidal anti-inflammatory drugs (NSAIDs) using polypolypropylene oxide core PAMAM dendrimers. Int. J. Pharmaceut. 2013, 451, 18–22. [Google Scholar]
- Yabbarov, N.G.; Posypanova, G.A.; Vorontsov, E.A.; Obydenny, S.I.; Severin, E.S. A new system for targeted delivery of doxorubicin into tumor cells. J. Control. Release 2013, 168, 135–141. [Google Scholar] [CrossRef]
- Agrawal, U.; Mehra, N.K.; Gupta, U.; Jain, N.K. Hyperbranched dendritic nano-carriers for topical delivery of dithranol. J. Drug Target. 2013, 21, 497–506. [Google Scholar] [CrossRef]
- Daneshvar, N.; Abdullah, R.; Shamsabadi, F.T.; How, C.W.; Aizat, M.M.H.; Mehrbod, P. PAMAM dendrimer roles in gene delivery methods and stem cell research. Cell Biol. Int. 2013, 37, 415–419. [Google Scholar] [CrossRef]
- Richardson, R.K.; Dougherty, C.; DiMaggio, S.; Banaszak-Holl, M. Synthesis, isolation, and characterization of dendrimer conjugates as potential chemotherapy drug delivery systems. Abstr. pap. Am. Chem. S. 2013, 245, 856. [Google Scholar]
- Cai, X.P.; Hu, J.J.; Xiao, J.R.; Cheng, Y.Y. Dendrimer and cancer: A patent review (2006–2013). Expert Opin. Ther. Pat. 2013, 23, 515–529. [Google Scholar] [CrossRef]
- Garea, S.A.; Ghebaur, E.V. Hybrid drug release systems based on dendrimers and montmorillonite. Mater. Plast. 2013, 50, 8–11. [Google Scholar]
- Ly, T.U.; Tran, N.Q.; Thi, K.D.H.; Phan, K.N.; Truong, H.N.; Nguyen, C.K. Pegylated dendrimer and its effect in fluorouracil loading and release for enhancing antitumor activity. J. Biomed. Nanotechnol. 2013, 9, 213–220. [Google Scholar] [CrossRef]
- Wen, S.H.; Li, K.G.; Cai, H.D.; Chen, Q.; Shen, M.W.; Huang, Y.P.; Peng, C.; Hou, W.X.; Zhu, M.F.; Zhang, G.X.; et al. Multifunctional dendrimer-entrapped gold nanoparticles for dual mode CT/MR imaging applications. Biomaterials 2013, 34, 1570–1580. [Google Scholar] [CrossRef]
- Sharma, A.; Jain, N.; Sareen, R. Nanocarriers for diagnosis and targeting of breast cancer. Biomed. Res. Int. 2013, 2013, 960821:1–960821:10. [Google Scholar]
- Xu, L.Y.; Yeudall, W.A.; Yang, H. Tailored Polymer Architectures for Pharmaceutical and Biomedical Applications; Scholz, C., Kressler, J., Eds.; American Chemical Society: Washington, DC, USA, 2013; Volume 1135, pp. 197–213. [Google Scholar]
- Zhu, J.Y.; Shi, X.Y. Dendrimer-based nanodevices for targeted drug delivery applications. J. Mater. Chem. 2013, 1, 4199–4211. [Google Scholar]
- Huang, C.H.; Nwe, K.; Al Zaki, A.; Brechbiel, M.W.; Tsourkas, A. Biodegradable polydisulfide dendrimer nanoclusters as MRI contrast agents. ACS Nano 2012, 6, 9416–9424. [Google Scholar] [CrossRef]
- Lim, J.; Turkbey, B.; Bernardo, M.; Bryant, L.H.; Garzoni, M.; Pavan, G.M.; Nakajima, T.; Choyke, P.L.; Simanek, E.E.; Kobayashi, H. Gadolinium MRI contrast agents based on triazine dendrimers: Relaxivity and in vivo pharmacokinetics. Bioconjugate Chem. 2012, 23, 2291–2299. [Google Scholar] [CrossRef]
- Carberry, T.P.; Tarallo, R.; Falanga, A.; Finamore, E.; Galdiero, M.; Weck, M.; Galdiero, S. Dendrimer functionalization with a membrane-interacting domain of herpes simplex virus type 1: Towards intracellular delivery. Chem. Eur. J. 2012, 18, 13678–13685. [Google Scholar] [CrossRef]
- Gardikis, K.; Micha-Screttas, M.; Demetzos, C.; Steele, B.R. Dendrimers and the development of new complex nanomaterials for biomedical applications. Curr. Med. Chem. 2012, 19, 4913–4928. [Google Scholar] [CrossRef]
- Klajnert, B.; Rozanek, M.; Bryszewska, M. Dendrimers in photodynamic therapy. Curr. Med. Chem. 2012, 19, 4903–4912. [Google Scholar] [CrossRef]
- Wate, P.S.; Banerjee, S.S.; Jalota-Badhwar, A.; Mascarenhas, R.R.; Zope, K.R.; Khandare, J.; Misra, R.D.K. Cellular imaging using biocompatible dendrimer-functionalized graphene oxide-based fluorescent probe anchored with magnetic nanoparticles. Nanotechnology 2012, 23. [Google Scholar] [CrossRef]
- Andreani, T.; Macedo, A.S.; Ferreira, S.F.; Silva, A.M.; Rosmaninho, A.; Souto, E.B. Topical targeting therapies for sexually transmitted diseases. Curr. Nanosci. 2012, 8, 486–490. [Google Scholar] [CrossRef]
- Guo, R.; Shi, X.Y. Dendrimers in Cancer therapeutics and diagnosis. Curr. Drug Metab. 2012, 13, 1097–1109. [Google Scholar] [CrossRef]
- Thomas, T.P.; Huang, B.H.; Choi, S.K.; Silpe, J.E.; Kotlyar, A.; Desai, A.M.; Zong, H.; Gam, J.; Joice, M.; Baker, J.R. Polyvalent dendrimer-methotrexate as a folate receptor-targeted cancer therapeutic. Mol. Pharmaceut. 2012, 9, 2669–2676. [Google Scholar] [CrossRef]
- Holden, C.A.; Tyagi, P.; Thakur, A.; Kadam, R.; Jadhav, G.; Kompella, U.B.; Yang, H. Polyamidoamine dendrimer hydrogel for enhanced delivery of antiglaucoma drugs. Nanomed Nanotechnol. 2012, 8, 776–783. [Google Scholar]
- Lim, J.; Simanek, E.E. Triazine dendrimers as drug delivery systems: From synthesis to therapy. Adv. Drug Delivery Rev. 2012, 64, 826–835. [Google Scholar] [CrossRef]
- Haque, S.; Md, S.; Alam, M.I.; Sahni, J.K.; Ali, J.; Baboota, S. Nanostructure-based drug delivery systems for brain targeting. Drug Dev. Ind. Pharm. 2012, 38, 387–411. [Google Scholar] [CrossRef]
- Svenson, S.; Tomalia, D.A. Commentary-dendrimers in biomedical applications—Reflections on the field. Adv. Drug Delivery Rev. 2005, 57, 2106–2129. [Google Scholar] [CrossRef]
- D’Emanuele, A.; Attwood, D. Dendrimer-drug interactions. Adv. Drug Delivery Rev. 2005, 57, 2147–2162. [Google Scholar] [CrossRef]
- Wolinsky, J.B.; Grinstaff, M.W. Therapeutic and diagnostic applications of dendrimers for cancer treatment. Adv. Drug Delivery Rev. 2008, 60, 1037–1055. [Google Scholar] [CrossRef]
- Cheng, Y.Y.; Xu, T.W. The effect of dendrimers on the pharmacodynamic and pharmacokinetic behaviors of non-covalently or covalently attached drugs. Eur. J. Med. Chem. 2008, 43, 2291–2297. [Google Scholar] [CrossRef]
- Nanjwade, B.K.; Bechra, H.M.; Derkar, G.K.; Manvi, F.V.; Nanjwade, V.K. Dendrimers: Emerging polymers for drug-delivery systems. Eur. J. Pharmaceut. Sci. 2009, 38, 185–196. [Google Scholar] [CrossRef]
- Svenson, S. Dendrimers as versatile platform in drug delivery applications. Eur. J. Pharmaceut. Biopharmaceut. 2009, 71, 445–462. [Google Scholar] [CrossRef]
- Gillies, E.R.; Dy, E.; Fréchet, J.M.J.; Szoka, F.C. Biological evaluation of polyester dendrimer: Poly(ethylene oxide) “Bow-Tie” hybrids with tunable molecular weight and architecture. Mol. Pharmaceut. 2005, 2, 129–138. [Google Scholar] [CrossRef]
- Lee, C.C.; Gillies, E.R.; Fox, M.E.; Guillaudeu, S.J.; Fréchet, J.M.J.; Dy, E.E.; Szoka, F.C. A single dose of doxorubicin-functionalized bow-tie dendrimer cures mice bearing C-26 colon carcinomas. Proc. Natl. Acad. Sci. USA 2006, 103, 16649–16654. [Google Scholar]
- Carlmark, A.; Malmström, E.; Malkoch, M. Dendritic architectures based on bis-MPA: Functional polymeric scaffolds for application-driven research. Chem. Soc. Rev. 2013, 42, 5858–5879. [Google Scholar] [CrossRef]
- Feliu, N.; Walter, M.V.; Montanez, M.I.; Kunzmann, A.; Hult, A.; Nyström, A.; Malkoch, M.; Fadeel, B. Stability and biocompatibility of a library of polyester dendrimers in comparison to polyamidoamine dendrimers. Biomaterials 2012, 33, 1970–1981. [Google Scholar] [CrossRef]
- Walter, M.V.; Malkoch, M. Simplifying the synthesis of dendrimers: Accelerated approaches. Chem. Soc. Rev. 2012, 41, 4593–4609. [Google Scholar] [CrossRef]
- Medina, S.H.; El-Sayed, M.E.H. Dendrimers as carriers for delivery of chemotherapeutic agents. Chem. Rev. 2009, 109, 3141–3157. [Google Scholar] [CrossRef]
- Ihre, H.R.; Padilla de Jesús, O.L.; Szoka, F.C.; Fréchet, J.M.J. Polyester dendritic systems for drug delivery applications: Design, synthesis, and characterization. Bioconjugate Chem. 2002, 13, 443–452. [Google Scholar] [CrossRef]
- Padilla de Jesús, O.L.; Ihre, H.R.; Gagne, L.; Fréchet, J.M.J.; Szoka, F.C. Polyester dendritic systems for drug delivery applications: In vitro and in vivo evaluation. Bioconjugate Chem. 2002, 13, 453–461. [Google Scholar] [CrossRef]
- Lazniewska, J.; Milowska, K.; Gabryelak, T. Dendrimers—Revolutionary drugs for infectious diseases. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2012, 4, 469–491. [Google Scholar] [CrossRef]
- El Kazzouli, S.; El Brahmi, N.; Mignani, S.; Bousmina, M.; Zablocka, M.; Majoral, J.P. From metallodrugs to metallodendrimers for nanotherapy in oncology: A concise overview. Curr. Med. Chem. 2012, 19, 4995–5010. [Google Scholar] [CrossRef]
- Malik, N.; Evagorou, E.G.; Duncan, R. Dendrimer-platinate: A novel approach to cancer chemotherapy. Anticancer Drugs 1999, 10, 767–776. [Google Scholar] [CrossRef]
- Ahn, S.; Lee, I.H.; Lee, E.; Kim, H.; Kim, Y.C.; Jon, S. Oral delivery of an anti-diabetic peptide drug via conjugation and complexation with low molecular weight chitosan. J. Control. Release 2013, 170, 226–232. [Google Scholar] [CrossRef]
- Sangwai, M.; Vavia, P. Amorphous ternary cyclodextrin nanocomposites of telmisartan for oral drug delivery: Improved solubility and reduced pharmacokinetic variability. Int. J. Pharmaceut. 2013, 453, 423–432. [Google Scholar] [CrossRef]
- Higuchi, W.I.; Ho, N.F.H.; Merkle, H.P. Design of oral-drug delivery systems—Past, present and future. Drug Dev. Ind. Pharm. 1983, 9, 1227–1239. [Google Scholar] [CrossRef]
- Mitra, S.B. Oral sustained-release drug delivery system using polymer film composites. Abstr. Pap. Am. Chem. S. 1983, 185, 119. [Google Scholar]
- Kulhari, H.; Kulhari, D.P.; Prajapati, S.K.; Chauhan, A.S. Pharmacokinetic and pharmacodynamic studies of poly(amidoamine) dendrimer based simvastatin oral formulations for the treatment of hypercholesterolemia. Mol. Pharmaceut. 2013, 10, 2528–2533. [Google Scholar] [CrossRef]
- Gajbhiye, V.; Kumar, P.V.; Sharma, A.; Agarwal, A.; Asthana, A.; Jain, N.K. Dendrimeric nanoarchitectures mediated transdermal and oral delivery of bioactives. Indian J. Pharm. Sci. 2008, 70, 431–439. [Google Scholar] [CrossRef]
- Ke, W.L.; Zhao, Y.S.; Huang, R.Q.; Jiang, C.; Pei, Y.Y. Enhanced oral bioavailability of doxorubicin in a dendrimer drug delivery system. J. Pharm. Sci. 2008, 97, 2208–2216. [Google Scholar] [CrossRef]
- Kolhatkar, R.B.; Swaan, P.; Ghandehari, H. Potential oral delivery of 7-Ethyl-10-hydroxy-camptothecin (SN-38) using poly(amidoamine) dendrimers. Pharm. Res. 2008, 25, 1723–1729. [Google Scholar] [CrossRef]
- Najlah, M.; Freeman, S.; Attwood, D.; D’Emanuele, A. Design and assessment of drug-dendrimer conjugates for oral drug delivery. J. Pharm. Pharmacol. 2004, 56, S67–S67. [Google Scholar]
- Kalhapure, R.S.; Akamanchi, K.G. Oleodendrimers: A novel class of multicephalous heterolipids as chemical penetration enhancers for transdermal drug delivery. Int. J. Pharmaceut. 2013, 454, 158–166. [Google Scholar] [CrossRef]
- Filipowicz, A.; Wolowiec, S. Solubility and in vitro transdermal diffusion of riboflavin assisted by PAMAM dendrimers. Int. J. Pharmaceut. 2011, 408, 152–156. [Google Scholar] [CrossRef]
- Borowska, K.; Laskowska, B.; Magon, A.; Mysliwiec, B.; Pyda, M.; Wolowiec, S. PAMAM dendrimers as solubilizers and hosts for 8-methoxypsoralene enabling transdermal diffusion of the guest. Int. J. Pharmaceut. 2010, 398, 185–189. [Google Scholar] [CrossRef]
- Venuganti, V.V.K.; Perumal, O.P. Poly(amidoamine) dendrimers as skin penetration enhancers: Influence of charge, generation, and concentration. J. Pharm. Sci. 2009, 98, 2345–2356. [Google Scholar] [CrossRef]
- Cheng, Y.Y.; Man, N.; Xu, T.W.; Fu, R.Q.; Wang, X.Y.; Wang, X.M.; Wen, L.P. Transdermal delivery of nonsteroidal anti-inflammatory drugs mediated by polyamidoamine (PAMAM) dendrimers. J. Pharm. Sci. 2007, 96, 595–602. [Google Scholar] [CrossRef]
- Chauhan, A.S.; Sridevi, S.; Chalasani, K.B.; Jain, A.K.; Jain, S.K.; Jain, N.K.; Diwan, P.V. Dendrimer-mediated transdermal delivery: Enhanced bioavailability of indomethacin. J. Control. Release 2003, 90, 335–343. [Google Scholar] [CrossRef]
- Gaudana, R.; Ananthula, H.; Parenky, A.; Mitra, A. Ocular drug delivery. AAPS J. 2010, 12, 348–360. [Google Scholar] [CrossRef]
- Yang, H.; Kao, W.J. Dendrimers for pharmaceutical and biomedical applications. J. Biomat. Sci Polym. E. 2006, 17, 3–19. [Google Scholar] [CrossRef]
- Kambhampati, S.P.; Kannan, R.M. Dendrimer nanoparticles for ocular drug delivery. J. Ocul. Pharmacol. Ther. 2013, 29, 151–165. [Google Scholar] [CrossRef]
- Fernandez, L.; Calderon, M.; Martinelli, M.; Strumia, M.; Cerecetto, H.; Gonzalez, M.; Silber, J.J.; Santo, M. Evaluation of a new dendrimeric structure as prospective drugs carrier for intravenous administration of antichagasic active compounds. J. Phys. Org. Chem. 2008, 21, 1079–1085. [Google Scholar] [CrossRef]
- Kaminskas, L.M.; Kota, J.; McLeod, V.M.; Kelly, B.D.; Karellas, P.; Porter, C.J.H. PEGylation of polylysine dendrimers improves absorption and lymphatic targeting following SC administration in rats. J. Control. Release 2009, 140, 108–116. [Google Scholar] [CrossRef]
- Merkel, O.M.; Mintzer, M.A.; Librizzi, D.; Samsonova, O.; Dicke, T.; Sproat, B.; Garn, H.; Barth, P.J.; Simanek, E.E.; Kissel, T. Triazine dendrimers as nonviral vectors for in vitro and in vivo RNAi: The effects of peripheral groups and core structure on biological activity. Mol. Pharmaceut. 2010, 7, 969–983. [Google Scholar] [CrossRef]
- Navath, R.S.; Kurtoglu, Y.E.; Wang, B.; Kannan, S.; Romero, R.; Kannan, R.M. Dendrimer-drug conjugates for tailored intracellular drug release based on glutathione levels. Bioconjugate Chem. 2008, 19, 2446–2455. [Google Scholar] [CrossRef]
- Ward, B.B.; Huang, B.H.; Desai, A.; Cheng, X.M.; Vartanian, M.; Zong, H.; Shi, X.Y.; Thomas, T.P.; Kotlyar, A.E.; van der Spek, A.; et al. Sustained analgesia achieved through esterase-activated morphine prodrugs complexed with PAMAM dendrimer. Pharm. Res. 2013, 30, 247–256. [Google Scholar] [CrossRef]
- Duncan, R. Polymer conjugates as anticancer nanomedicines. Nat. Rev. Cancer 2006, 6, 688–701. [Google Scholar] [CrossRef]
- Harris, J.M.; Chess, R.B. Effect of pegylation on pharmaceuticals. Nat. Rev. Drug Discov. 2003, 2, 214–221. [Google Scholar] [CrossRef]
- Bae, Y.; Fukushima, S.; Harada, A.; Kataoka, K. Design of environment-sensitive supramolecular assemblies for intracellular drug delivery: Polymeric micelles that are responsive to intracellular pH change. Angew. Chem. Int. Ed. 2003, 42, 4640–4643. [Google Scholar] [CrossRef]
- Kataoka, K.; Harada, A.; Nagasaki, Y. Block copolymer micelles for drug delivery: Design, characterization and biological significance. Adv. Drug Delivery Rev. 2001, 47, 113–131. [Google Scholar] [CrossRef]
- Kataoka, K.; Harashima, H. Gene delivery systems: Viral vs. non-viral vectors. Adv. Drug Delivery Rev. 2001, 52, 151–151. [Google Scholar] [CrossRef]
- Vasey, P.A.; Kaye, S.B.; Morrison, R.; Twelves, C.; Wilson, P.; Duncan, R.; Thomson, A.H.; Murray, L.S.; Hilditch, T.E.; Murray, T.; et al. Phase I clinical and pharmacokinetic study of PK1 N-(2-hydroxypropyl)methacrylamide copolymer doxorubicin: First member of a new class of chemotherapeutic agents—Drug-polymer conjugates. Clinic. Cancer Res. 1999, 5, 83–94. [Google Scholar]
- Gillies, E.R.; Fréchet, J.M.J. Dendrimers and dendritic polymers in drug delivery. Drug Discov. Today 2005, 10, 35–43. [Google Scholar] [CrossRef]
- Gillies, E.R.; Goodwin, A.P.; Fréchet, J.M.J. Acetals as pH-sensitive linkages for drug delivery. Bioconjugate Chem. 2004, 15, 1254–1263. [Google Scholar] [CrossRef]
- Van der Poll, D.G.; Kieler-Ferguson, H.M.; Floyd, W.C.; Guillaudeu, S.J.; Jerger, K.; Szoka, F.C.; Fréchet, J.M. Design, synthesis, and biological evaluation of a robust, biodegradable dendrimer. Bioconjugate Chem. 2010, 21, 764–773. [Google Scholar] [CrossRef]
- Nakanishi, T.; Fukushima, S.; Okamoto, K.; Suzuki, M.; Matsumura, Y.; Yokoyama, M.; Okano, T.; Sakurai, Y.; Kataoka, K. Development of the polymer micelle carrier system for doxorubicin. J. Control. Release 2001, 74, 295–302. [Google Scholar] [CrossRef]
- Haag, R. Supramolecular drug-delivery systems based on polymeric core-shell architectures. Angew. Chem. Int. Ed. 2004, 43, 278–282. [Google Scholar] [CrossRef]
- Morgan, M.T.; Nakanishi, Y.; Kroll, D.J.; Griset, A.P.; Carnahan, M.A.; Wathier, M.; Oberlies, N.H.; Manikumar, G.; Wani, M.C.; Grinstaff, M.W. Dendrimer-encapsulated camptothecins: Increased solubility, cellular uptake, and cellular retention affords enhanced anticancer activity in vitro. Cancer Res. 2006, 66, 11913–11921. [Google Scholar] [CrossRef]
- Dhanikula, R.S.; Hildgen, P. Synthesis and evaluation of novel dendrimers with a hydrophilic interior as nanocarriers for drug delivery. Bioconjugate Chem. 2006, 17, 29–41. [Google Scholar] [CrossRef]
- Nazemi, A.; Haeryfar, S.M.M.; Gillies, E.R. Multifunctional dendritic sialopolymersomes as potential antiviral agents: Their lectin binding and drug release properties. Langmuir 2013, 29, 6420–6428. [Google Scholar] [CrossRef]
- Kim, S.H.; Tan, J.P.K.; Nederberg, F.; Fukushima, K.; Yang, Y.Y.; Waymouth, R.M.; Hedrick, J.L. Mixed micelle formation through stereocomplexation between enantiomeric poly(lactide) block copolymers. Macromolecules 2009, 42, 25–29. [Google Scholar] [CrossRef]
- Skey, J.; Hansell, C.F.; O’Reilly, R.K. Stabilization of amino acid derived diblock copolymer micelles through favorable D:L side chain interactions. Macromolecules 2010, 43, 1309–1318. [Google Scholar] [CrossRef]
- Moughton, A.O.; O’Reilly, R.K. Noncovalently connected micelles, nanoparticles, and metal-functionalized nanocages using supramolecular self-assembly. J. Am. Chem. Soc. 2008, 130, 8714–8725. [Google Scholar] [CrossRef]
- Fukushima, K.; Pratt, R.C.; Nederberg, F.; Tan, J.P.K.; Yang, Y.Y.; Waymouth, R.M.; Hedrick, J.L. Organocatalytic approach to amphiphilic comb-block copolymers capable of stereocomplexation and self-assembly. Biomacromolecules 2008, 9, 3051–3056. [Google Scholar] [CrossRef]
- Nederberg, F.; Appel, E.; Tan, J.P.K.; Kim, S.H.; Fukushima, K.; Sly, J.; Miller, R.D.; Waymouth, R.M.; Yang, Y.Y.; Hedrick, J.L. Simple approach to stabilized micelles employing miktoarm terpolymers and stereocomplexes with application in paclitaxel delivery. Biomacromolecules 2009, 10, 1460–1468. [Google Scholar] [CrossRef]
- Weaver, J.V.M.; Tang, Y.Q.; Liu, S.Y.; Iddon, P.D.; Grigg, R.; Billingham, N.C.; Armes, S.P.; Hunter, R.; Rannard, S.P. Preparation of shell cross-linked micelles by polyelectrolyte complexation. Angew. Chem. Int. Ed. 2004, 43, 1389–1392. [Google Scholar] [CrossRef]
- Giacomelli, C.; Schmidt, V.; Borsali, R. Specific interactions improve the loading capacity of block copolymer micelles in aqueous media. Langmuir 2007, 23, 6947–6955. [Google Scholar] [CrossRef]
- Chiang, Y.T.; Cheng, Y.T.; Lu, C.Y.; Yen, Y.W.; Yu, L.Y.; Yu, K.S.; Lyu, S.Y.; Yang, C.Y.; Lo, C.L. Polymer-liposome complexes with a functional hydrogen-bond cross-linker for preventing protein adsorption and improving tumor accumulation. Chem. Mater. 2013, 25, 4364–4372. [Google Scholar] [CrossRef]
- Hutin, M.; Burakowska-Meise, E.; Appel, W.P.J.; Dankers, P.Y.W.; Meijer, E.W. From molecular structure to macromolecular organization: Keys to design supramolecular biomaterials. Macromolecules 2013, 46, 8528–8537. [Google Scholar] [CrossRef]
- Zhu, Z.C.; Gao, N.; Wang, H.J.; Sukhishvili, S.A. Temperature-triggered on-demand drug release enabled by hydrogen-bonded multilayers of block copolymer micelles. J. Control. Release 2013, 171, 73–80. [Google Scholar] [CrossRef]
- Tadi, K.K.; Motghare, R.V. Rational synthesis of pindolol imprinted polymer by non-covalent protocol based on computational approach. J. Mol. Model. 2013, 19, 3385–3396. [Google Scholar] [CrossRef]
- Sanyakamdhorn, S.; Agudelo, D.; Tajmir-Riahi, H.A. Encapsulation of antitumor drug doxorubicin and its analogue by chitosan nanoparticles. Biomacromolecules 2013, 14, 557–563. [Google Scholar] [CrossRef]
- Kim, S.H.; Tan, J.P.K.; Nederberg, F.; Fukushima, K.; Colson, J.; Yang, C.A.; Nelson, A.; Yang, Y.Y.; Hedrick, J.L. Hydrogen bonding-enhanced micelle assemblies for drug delivery. Biomaterials 2010, 31, 8063–8071. [Google Scholar] [CrossRef]
- Zhou, J.; Soontornworajit, B.; Martin, J.; Sullenger, B.A.; Gilboa, E.; Wang, Y. A hybrid DNA aptamer-dendrimer nanomaterial for targeted cell labeling. Macromol. Biosci. 2009, 9, 831–835. [Google Scholar] [CrossRef]
- Battig, M.R.; Soontornworajit, B.; Wang, Y. Programmable release of multiple protein drugs from aptamer-functionalized hydrogels via nucleic acid hybridization. J. Am. Chem. Soc. 2012, 134, 12410–12413. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Chen, N.C.; Li, S.H.; Battig, M.R.; Wang, Y. Programmable hydrogels for controlled cell catch and release using hybridized aptamers and complementary sequences. J. Am. Chem. Soc. 2012, 134, 15716–15719. [Google Scholar] [CrossRef]
- Soontornworajit, B.; Zhou, J.; Zhang, Z.Y.; Wang, Y. Aptamer-functionalized in situ injectable hydrogel for controlled protein release. Biomacromolecules 2010, 11, 2724–2730. [Google Scholar] [CrossRef]
- Zhou, J.; Soontornworajit, B.; Wang, Y. A temperature-responsive antibody-like nanostructure. Biomacromolecules 2010, 11, 2087–2093. [Google Scholar] [CrossRef]
- Morgan, M.T.; Carnahan, M.A.; Immoos, C.E.; Ribeiro, A.A.; Finkelstein, S.; Lee, S.J.; Grinstaff, M.W. Dendritic molecular capsules for hydrophobic compounds. J. Am. Chem. Soc. 2003, 125, 15485–15489. [Google Scholar] [CrossRef]
- Antoni, P.; Hed, Y.; Nordberg, A.; Nyström, D.; von Holst, H.; Hult, A.; Malkoch, M. Bifunctional dendrimers: From robust synthesis and accelerated one-pot postfunctionalization strategy to potential applications. Angew. Chem. Int. Ed. 2009, 48, 2126–2130. [Google Scholar] [CrossRef]
- Jain, K.; Kesharwani, P.; Gupta, U.; Jain, N.K. Dendrimer toxicity: Let’s meet the challenge. Int. J. Pharmaceut. 2010, 394, 122–142. [Google Scholar] [CrossRef]
- Bernkop-Schnurch, A.; Scholler, S.; Biebel, R.G. Development of controlled drug release systems based on thiolated polymers. J. Control. Release 2000, 66, 39–48. [Google Scholar] [CrossRef]
- Hawker, C.J.; Fréchet, J.M.J. Unusual macromolecular architectures—The convergent growth approach to dendritic polyesters and novel block copolymers. J. Am. Chem. Soc. 1992, 114, 8405–8413. [Google Scholar] [CrossRef]
- Haddleton, D.M.; Sahota, H.S.; Taylor, P.C.; Yeates, S.G. Synthesis of polyester dendrimers. J. Chem. Soc. Perkin Trans. 1 1996, 649–656. [Google Scholar]
- Antoni, P.; Nyström, D.; Hawker, C.J.; Hult, A.; Malkoch, M. A chemoselective approach for the accelerated synthesis of well-defined dendritic architectures. Chem. Commun. 2007, 2249–2251. [Google Scholar]
- Ihre, H.; Hult, A.; Söderlind, E. Synthesis, characterization, and H-1 NMR self-diffusion studies of dendritic aliphatic polyesters based on 2,2-bis(hydroxymethyl)propionic acid and 1,1,1-tris(hydroxyphenyl)ethane. J. Am. Chem. Soc. 1996, 118, 6388–6395. [Google Scholar] [CrossRef]
- Ihre, H.; Padilla de Jesús, O.L.; Fréchet, J.M.J. Fast and convenient divergent synthesis of aliphatic ester dendrimers by anhydride coupling. J. Am. Chem. Soc. 2001, 123, 5908–5917. [Google Scholar] [CrossRef]
- Parrott, M.C.; Benhabbour, S.R.; Saab, C.; Lemon, J.A.; Parker, S.; Valliant, J.F.; Adronov, A. Synthesis, radiolabeling, and bio-imaging of high-generation polyester dendrimers. J. Am. Chem. Soc. 2009, 131, 2906–2916. [Google Scholar]
- Bouillon, C.; Tintaru, A.; Monnier, V.; Charles, L.; Quelever, G.; Peng, L. Synthesis of poly(amino)ester dendrimers via active cyanomethyl ester intermediates. J. Org. Chem. 2010, 75, 8685–8688. [Google Scholar] [CrossRef]
- Twibanire, J.K.; Al-Mughaid, H.; Grindley, T.B. Synthesis of new cores and their use in the preparation of polyester dendrimers. Tetrahedron 2010, 66, 9602–9609. [Google Scholar] [CrossRef]
- Keefe, G.E.; Twibanire, J.K.; Grindley, T.B.; Shaver, M.P. Poly(lactic acid) polymer stars built from early generation dendritic polyols. Can. J. Chem. 2013, 91, 392–397. [Google Scholar] [CrossRef]
- Twibanire, J.K.; Grindley, T.B. Polyester dendrimers. Polymers 2012, 4, 794–879. [Google Scholar] [CrossRef]
- Knorr, R.; Trzeciak, A.; Bannwarth, W.; Gillessen, D. New coupling reagents in peptide chemistry. Tetrahedron Lett. 1989, 30, 1927–1930. [Google Scholar] [CrossRef]
- Carpino, L.A. 1-Hydroxy-7-azabenzotriazole-An efficient peptide coupling additive. J. Am. Chem. Soc. 1993, 115, 4397–4398. [Google Scholar] [CrossRef]
- El-Faham, A.; Subirós Funosas, R.; Prohens, R.; Albericio, F. COMU: A safer and more effective replacement for benzotriazole-based uronium coupling reagents. Chem. Eur. J. 2009, 15, 9404–9416. [Google Scholar] [CrossRef]
- Twibanire, J.K.; Grindley, T.B. Efficient and controllably selective preparation of esters using uronium-based coupling agents. Org. Lett. 2011, 13, 2988–2991. [Google Scholar] [CrossRef]
- Twibanire, J.K.; Omran, R.P.; Grindley, T.B. Facile synthesis of a library of lyme disease glycolipid antigens. Org. Lett. 2012, 14, 3909–3911. [Google Scholar] [CrossRef]
- Paul, N.K.; Twibanire, J.K.; Grindley, T.B. Direct synthesis of maradolipids and other trehalose 6-monoesters and 6,6'-diesters. J. Org. Chem. 2013, 78, 363–369. [Google Scholar] [CrossRef]
- Hawker, C.J.; Fréchet, J.M.J. Preparation of polymers with controlled molecular architecture—A new convergent approach to dendritic macromolecules. J. Am. Chem. Soc. 1990, 112, 7638–7647. [Google Scholar] [CrossRef]
- Hawker, C.J.; Fréchet, J.M.J. A new convergent approach to monodisperse dendritic macromolecules. J. Chem. Soc. Chem. Commun. 1990, 1990, 1010–1013. [Google Scholar] [CrossRef]
- Hawker, C.J.; Lee, R.; Fréchet, J.M.J. One-step synthesis of hyperbranched dendritic polyesters. J. Am. Chem. Soc. 1991, 113, 4583–4588. [Google Scholar] [CrossRef]
- Hawker, C.J.; Fréchet, J.M.J. Monodispersed dendritic polyesters with removable chain ends—A versatile approach to globular macromolecules with chemically reversible polarities. J. Chem. Soc. Perkin Trans. 1 1992, 2459–2469. [Google Scholar] [CrossRef]
- Guillaudeu, S.J.; Fox, M.E.; Haidar, Y.M.; Dy, E.E.; Szoka, F.C.; Fréchet, J.M.J. PEGylated dendrimers with core functionality for biological applications. Bioconjugate Chem. 2008, 19, 461–469. [Google Scholar] [CrossRef]
- Gillies, E.R.; Fréchet, J.M.J. Synthesis and self-assembly of supramolecular dendritic “Bow-Ties”: Effect of peripheral functionality on association constants. J. Org. Chem. 2004, 69, 46–53. [Google Scholar] [CrossRef]
- Krishna, R.; Mayer, L.D. Liposomal doxorubicin circumvents PSC 833-free drug interactions, resulting in effective therapy of multidrug-resistant solid tumors. Cancer Res. 1997, 57, 5246–5253. [Google Scholar]
- Kaneko, T.; Willner, D.; Monkovic, I.; Knipe, J.O.; Braslawsky, G.R.; Greenfield, R.S.; Vyas, D.M. New hydrazone derivatives of Adriamycin and their immunoconjugates: A correlation between acid stability and cytotoxicity. Bioconjugate Chem. 1991, 2, 133–141. [Google Scholar] [CrossRef]
- Lee, C.C.; Cramer, A.T.; Szoka, F.C.; Frechet, J.M.J. An intramolecular cyclization reaction is responsible for the in vivo inefficacy and apparent pH insensitive hydrolysis kinetics of hydrazone carboxylate derivatives of doxorubicin. Bioconjugate Chem. 2006, 17, 1364–1368. [Google Scholar] [CrossRef]
- Duncan, R. The dawning era of polymer therapeutics. Nat. Rev. Drug Discov. 2003, 2, 347–360. [Google Scholar] [CrossRef]
- Seymour, L.W.; Ulbrich, K.; Steyger, P.S.; Brereton, M.; Subr, V.; Strohalm, J.; Duncan, R. Tumor tropism and anticancer efficacy of polymer-based doxorubicin prodrugs in the treatment of subcutaneous murine b16f10 melanoma. Brit. J. Cancer 1994, 70, 636–641. [Google Scholar] [CrossRef]
- Huang, S.K.; Mayhew, E.; Gilani, S.; Lasic, D.D.; Martin, F.J.; Papahadjopoulos, D. Pharmacokinetics and therapeutics of sterically stabilized liposomes in mice bearing C-26 colon-carcinoma. Cancer Res. 1992, 52, 6774–6781. [Google Scholar]
- Mayhew, E.G.; Goldrosen, M.H.; Vaage, J.; Rustum, Y.M. Effects of liposome-entrapped doxorubicin on liver metastases of mouse colon carcinoma-26 and carcinoma-38. J. Natl. Cancer Inst. 1987, 78, 707–713. [Google Scholar]
- Namazi, H.; Bahrami, S.; Entezami, A.A. Synthesis and controlled release of biocompatible prodrugs of beta-cyclodextrin linked with PEG containing ibuprofen or indomethacin. Iran. Polym. J. 2005, 14, 921–927. [Google Scholar]
- Namazi, H.; Adeli, M. Novel linear-globular thermoreversible hydrogel ABA type copolymers from dendritic citric acid as the A blocks and poly(ethyleneglycol) as the B block. Eur. Polym. J. 2003, 39, 1491–1500. [Google Scholar] [CrossRef]
- Namazi, H.; Adell, M. Dendrimers of citric acid and poly (ethylene glycol) as the new drug-delivery agents. Biomaterials 2005, 26, 1175–1183. [Google Scholar] [CrossRef]
- Hawthorne, M.F. The role of chemistry in the development of boron neutron-capture therapy of cancer. Angew. Chem. Int. Ed. Engl. 1993, 32, 950–984. [Google Scholar] [CrossRef]
- Soloway, A.H.; Tjarks, W.; Barnum, B.A.; Rong, F.G.; Barth, R.F.; Codogni, I.M.; Wilson, J.G. The chemistry of neutron capture therapy. Chem. Rev. 1998, 98, 1515–1562. [Google Scholar] [CrossRef]
- Kawabata, S.; Hiramatsu, R.; Kuroiwa, T.; Ono, K.; Miyatake, S.-I. Boron neutron capture therapy for recurrent high-grade meningiomas Clinical article. J. Neurosurg. 2013, 119, 837–844. [Google Scholar] [CrossRef]
- Sun, T.; Zhang, Z.; Li, B.; Chen, G.; Xie, X.; Wei, Y.; Wu, J.; Zhou, Y.; Du, Z. Boron neutron capture therapy induces cell cycle arrest and cell apoptosis of glioma stem/progenitor cells in vitro. Radiat. Oncol. 2013, 8. [Google Scholar] [CrossRef]
- Hawthorne, M.F.; Lee, M.W. A critical assessment of boron target compounds for boron neutron capture therapy. J. NeuroOncol. 2003, 62, 33–45. [Google Scholar]
- Parrott, M.C.; Marchington, E.B.; Valliant, J.F.; Adronov, A. Synthesis and properties of carborane-functionalized aliphatic polyester dendrimers. J. Am. Chem. Soc. 2005, 127, 12081–12089. [Google Scholar] [CrossRef]
- Newkome, G.R.; Moorefield, C.N.; Keith, J.M.; Baker, G.R.; Escamilla, G.H. Chemistry within a unimolecular micelle precursor: Boron superclusters by site-specific and depth-specific transformations of dendrimers. 37. Chemistry of micelles. Angew. Chem. Int. Ed. Engl. 1994, 33, 666–668. [Google Scholar] [CrossRef]
- Cragg, G.M.; Newman, D.J.; Snader, K.M. Natural products in drug discovery and development. J. Nat. Prod. 1997, 60, 52–60. [Google Scholar] [CrossRef]
- Cragg, G.M.; Newman, D.J.; Weiss, R.B. Coral reefs, forests, and thermal vents: The worldwide exploration of nature for novel antitumor agents. Semin. Oncol. 1997, 24, 156–163. [Google Scholar]
- Oberlies, N.H.; Kroll, D.J. Camptothecin and taxol: Historic achievements in natural products research. J. Nat. Prod. 2004, 67, 129–135. [Google Scholar] [CrossRef]
- Garcia-Carbonero, R.; Supko, J.G. Current perspectives on the clinical experience, pharmacology, and continued development of the camptothecins. Clinic. Cancer Res. 2002, 8, 641–661. [Google Scholar]
- Thomas, C.J.; Rahier, N.J.; Hecht, S.M. Camptothecin: Current perspectives. Bioorg. Med. Chem. 2004, 12, 1585–1604. [Google Scholar] [CrossRef]
- Sriram, D.; Yogeeswari, P.; Thirumurugan, R.; Bal, T.R. Camptothecin and its analogues: A review on their chemotherapeutic potential. Nat. Prod. Res. 2005, 19, 393–412. [Google Scholar] [CrossRef]
- Hecht, J.R. Gastrointestinal toxicity of irinotecan. Oncology NY 1998, 12, 72–78. [Google Scholar]
- Armstrong, D.K. Topotecan dosing guidelines in ovarian cancer: Reduction and management of hematologic toxicity. Oncologist 2004, 9, 33–42. [Google Scholar] [CrossRef]
- Feng, X.S.; Pinaud, J.; Chaikof, E.L.; Taton, D.; Gnanou, Y. Sequential functionalization of janus-type dendrimer-like poly(ethylene oxide)s with camptothecin and folic acid. J. Polym. Sci. Part A Polym. Sci. 2011, 49, 2839–2849. [Google Scholar] [CrossRef]
- Fox, M.E.; Guillaudeu, S.; Fréchet, J.M.J.; Jerger, K.; Macaraeg, N.; Szoka, F.C. Synthesis and in vivo antitumor efficacy of pegylated poly(l-lysine) dendrimer-camptothecin conjugates. Mol. Pharmaceut. 2009, 6, 1562–1572. [Google Scholar] [CrossRef]
- Thiagarajan, G.; Ray, A.; Malugin, A.; Ghandehari, H. PAMAM-camptothecin conjugate inhibits proliferation and induces nuclear fragmentation in colorectal carcinoma cells. Pharm. Res. 2010, 27, 2307–2316. [Google Scholar] [CrossRef]
- Bolten, B.M.; DeGregorio, T. Trends in development cycles. Nat. Rev. Drug Discov. 2002, 1, 335–336. [Google Scholar] [CrossRef]
- Langer, R. Drug delivery and targeting. Nature 1998, 392, 5–10. [Google Scholar]
- Dhanikula, R.S.; Hildgen, P. Influence of molecular architecture of polyether-co-polyester dendrimers on the encapsulation and release of methotrexate. Biomaterials 2007, 28, 3140–3152. [Google Scholar] [CrossRef]
- Lee, C.C.; Yoshida, M.; Fréchet, J.M.J.; Dy, E.E.; Szoka, F.C. In vitro and in vivo evaluation of hydrophilic dendronized linear polymers. Bioconjugate Chem. 2005, 16, 535–541. [Google Scholar] [CrossRef]
- Lee, C.C.; Grayson, S.M.; Fréchet, J.M.J. Synthesis of narrow-polydispersity degradable dendronized aliphatic polyesters. J. Polym. Sci. Part A Polym. Sci. 2004, 42, 3563–3578. [Google Scholar] [CrossRef]
- Goldspiel, B.R. Clinical overview of the taxanes. Pharmacotherapy 1997, 17, S110–S125. [Google Scholar]
- Wang, T.H.; Wang, H.S.; Soong, Y.K. Paclitaxel-induced cell death—Where the cell cycle and apoptosis come together. Cancer 2000, 88, 2619–2628. [Google Scholar] [CrossRef]
- Szebeni, J.; Muggia, F.M.; Alving, C.R. Complement activation by cremophor EL as a possible contributor to hypersensitivity to paclitaxel: An in vitro study. J. Natl. Cancer Inst. 1998, 90, 300–306. [Google Scholar] [CrossRef]
- Hennenfent, K.L.; Govindan, R. Novel formulations of taxanes: A review. Old wine in a new bottle? Ann. Oncol. 2006, 17, 735–749. [Google Scholar] [CrossRef]
- Wu, J.; Liu, Q.; Lee, R.J. A folate receptor-targeted liposomal formulation for paclitaxel. Int. J. Pharmaceut. 2006, 316, 148–153. [Google Scholar] [CrossRef]
- Ooya, T.; Lee, J.; Park, K. Hydrotropic dendrimers of generations 4 and 5: Synthesis, characterization, and hydrotropic solubilization of paclitaxel. Bioconjugate Chem. 2004, 15, 1221–1229. [Google Scholar] [CrossRef]
- Le Garrec, D.; Gori, S.; Luo, L.; Lessard, D.; Smith, D.C.; Yessine, M.A.; Ranger, M.; Leroux, J.C. Poly(N-vinylpyrrolidone)-block-poly(d,l-lactide) as a new polymeric solubilizer for hydrophobic anticancer drugs: In vitro and in vivo evaluation. J. Control. Release 2004, 99, 83–101. [Google Scholar] [CrossRef]
- Lee, S.C.; Huh, K.M.; Lee, J.; Cho, Y.W.; Galinsky, R.E.; Park, K. Hydrotropic polymeric micelles for enhanced paclitaxel solubility: In vitro and in vivo characterization. Biomacromolecules 2007, 8, 202–208. [Google Scholar] [CrossRef]
- Khandare, J.J.; Jayant, S.; Singh, A.; Chandna, P.; Wang, Y.; Vorsa, N.; Minko, T. Dendrimer versus linear conjugate: Influence of polymeric architecture on the delivery and anticancer effect of paclitaxel. Bioconjugate Chem. 2006, 17, 1464–1472. [Google Scholar] [CrossRef]
- Majoros, I.J.; Myc, A.; Thomas, T.; Mehta, C.B.; Baker, J.R. PAMAM dendrimer-based multifunctional conjugate for cancer therapy: Synthesis, characterization, and functionality. Biomacromolecules 2006, 7, 572–579. [Google Scholar] [CrossRef]
- Zhang, Z.P.; Feng, S.S. The drug encapsulation efficiency, in vitro drug release, cellular uptake and cytotoxicity of paclitaxel-loaded poly(lactide)-tocopheryl polyethylene glycol succinate nanoparticles. Biomaterials 2006, 27, 4025–4033. [Google Scholar] [CrossRef]
- Ceruti, M.; Crosasso, P.; Brusa, P.; Arpicco, S.; Dosio, F.; Cattel, L. Preparation, characterization, cytotoxicity and pharmacokinetics of liposomes containing water-soluble prodrugs of paclitaxel. J. Control. Release 2000, 63, 141–153. [Google Scholar] [CrossRef]
- Crosasso, P.; Ceruti, M.; Brusa, P.; Arpicco, S.; Dosio, F.; Cattel, L. Preparation, characterization and properties of sterically stabilized paclitaxel-containing liposomes. J. Control. Release 2000, 63, 19–30. [Google Scholar] [CrossRef]
- Kontoyianni, C.; Sideratou, Z.; Theodossiou, T.; Tziveleka, L.A.; Tsiourvas, D.; Paleos, C.M. A novel micellar PEGylated hyperbranched polyester as a prospective drug delivery system for paclitaxel. Macromol. Biosci. 2008, 8, 871–881. [Google Scholar] [CrossRef]
- Malmström, E.; Johansson, M.; Hult, A. Hyperbranched aliphatic polyesters. Macromolecules 1995, 28, 1698–1703. [Google Scholar] [CrossRef]
- Gupta, U.; Agashe, H.B.; Asthana, A.; Jain, N.K. Dendrimers: Novel polymeric nanoarchitectures for solubility enhancement. Biomacromolecules 2006, 7, 649–658. [Google Scholar] [CrossRef]
- Reul, R.; Renette, T.; Bege, N.; Kissel, T. Nanoparticles for paclitaxel delivery: A comparative study of different types of dendritic polyesters and their degradation behavior. Int. J. Pharmaceut. 2011, 407, 190–196. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, X.Z.; Cheng, S.X.; Zhuo, R.X.; Gu, Z.W. Functionalized amphiphilic hyperbranched polymers for targeted drug delivery. Biomacromolecules 2008, 9, 2578–2585. [Google Scholar] [CrossRef]
- Wang, J.; Xu, T. Facile construction of multivalent targeted drug delivery system from Boltorn-® series hyperbranched aliphatic polyester and folic acid. Polym. Adv. Technol. 2011, 22, 763–767. [Google Scholar] [CrossRef]
- Zeng, X.H.; Zhang, Y.N.; Wu, Z.H.; Lundberg, P.; Malkoch, M.; Nyström, A.M. Hyperbranched copolymer micelles as delivery vehicles of doxorubicin in breast cancer cells. J. Polym. Sci. Part A Polym. Sci. 2012, 50, 280–288. [Google Scholar] [CrossRef]
- Fox, M.E.; Szoka, F.C.; Fréchet, J.M.J. Soluble polymer carriers for the treatment of cancer: The importance of molecular architecture. Acc. Chem. Res. 2009, 42, 1141–1151. [Google Scholar] [CrossRef]
- Akiyoshi, K.; Deguchi, S.; Moriguchi, N.; Yamaguchi, S.; Sunamoto, J. Self-aggregates of hydrophobized polysaccharides in water-Formation and characteristics of nanoparticles. Macromolecules 1993, 26, 3062–3068. [Google Scholar] [CrossRef]
- Kwon, G.S.; Kataoka, K. Block-copolymer micelles as long-circulating drug vehicles. Adv. Drug Delivery Rev. 1995, 16, 295–309. [Google Scholar] [CrossRef]
- Torchilin, V.P. Structure and design of polymeric surfactant-based drug delivery systems. J. Control. Release 2001, 73, 137–172. [Google Scholar] [CrossRef]
- Jones, M.C.; Leroux, J.C. Polymeric micelles-A new generation of colloidal drug carriers. Eur. J. Pharmaceut. Biopharmaceut. 1999, 48, 101–111. [Google Scholar] [CrossRef]
- Lawrence, M.J. Surfactant systems-Their use in drug-delivery. Chem. Soc. Rev. 1994, 23, 417–424. [Google Scholar] [CrossRef]
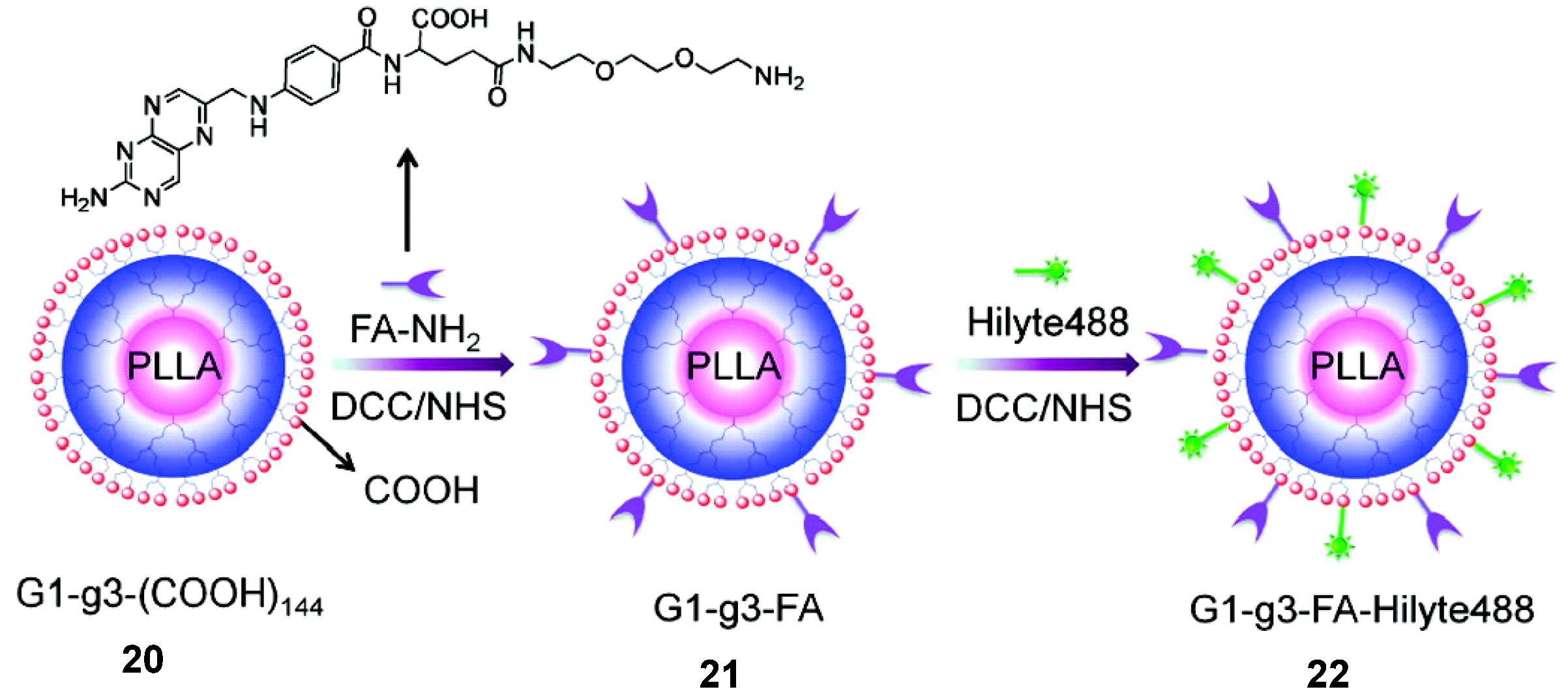
- Cao, W.Q.; Zhou, J.; Mann, A.; Wang, Y.; Zhu, L. Folate-functionalized unimolecular micelles based on a degradable amphiphilic dendrimer-like star polymer for cancer cell-targeted drug delivery. Biomacromolecules 2011, 12, 2697–2707. [Google Scholar] [CrossRef]
- Cao, W.Q.; Zhu, L. Synthesis and unimolecular micelles of amphiphilic dendrimer-like star polymer with various functional surface groups. Macromolecules 2011, 44, 1500–1512. [Google Scholar] [CrossRef]
- Cao, W.Q.; Zhou, J.; Wang, Y.; Zhu, L. Synthesis and in vitro cancer cell targeting of folate-functionalized biodegradable amphiphilic dendrimer-like star polymers. Biomacromolecules 2010, 11, 3680–3687. [Google Scholar] [CrossRef]
- Pan, X.Q.; Wang, H.Q.; Lee, R.J. Antitumor activity of folate receptor-targeted liposomal doxorubicin in a KB oral carcinoma murine xenograft model. Pharm. Res. 2003, 20, 417–422. [Google Scholar] [CrossRef]
- Lu, Y.J.; Low, P.S. Folate-mediated delivery of macromolecular anticancer therapeutic agents. Adv. Drug Delivery Rev. 2002, 54, 675–693. [Google Scholar] [CrossRef]
- Wang, S.; Low, P.S. Folate-mediated targeting of antineoplastic drags, imaging agents, and nucleic acids to cancer cells. J. Control. Release 1998, 53, 39–48. [Google Scholar] [CrossRef]
- Brinkhuis, R.P.; Rutjes, F.; van Hest, J.C.M. Polymeric vesicles in biomedical applications. Polym. Chem. 2011, 2, 1449–1462. [Google Scholar] [CrossRef]
- Pourtau, L.; Oliveira, H.; Thevenot, J.; Wan, Y.L.; Brisson, A.R.; Sandre, O.; Miraux, S.; Thiaudiere, E.; Lecommandoux, S. Antibody-functionalized magnetic polymersomes: In vivo targeting and imaging of bone metastases using high resolution MRI. Adv. Heathc. Mater. 2013, 2, 1420–1424. [Google Scholar] [CrossRef]
- Huang, Z.H.; Teng, W.; Liu, L.S.; Wang, L.C.; Wang, Q.M.; Dong, Y.G. Efficient cytosolic delivery mediated by polymersomes facilely prepared from a degradable, amphiphilic, and amphoteric copolymer. Nanotechnology 2013, 24. [Google Scholar] [CrossRef]
- Oliveira, H.; Perez-Andres, E.; Thevenot, J.; Sandre, O.; Berra, E.; Lecommandoux, S. Magnetic field triggered drug release from polymersomes for cancer therapeutics. J. Control. Release 2013, 169, 165–170. [Google Scholar] [CrossRef]
- Debets, M.F.; Leenders, W.P.J.; Verrijp, K.; Zonjee, M.; Meeuwissen, S.A.; Otte-Holler, I.; van Hest, J.C.M. Nanobody-functionalized polymersomes for tumor-vessel targeting. Macromol. Biosci. 2013, 13, 938–945. [Google Scholar] [CrossRef]
- Spulber, M.; Najer, A.; Winkelbach, K.; Glaied, O.; Waser, M.; Pieles, U.; Meier, W.; Bruns, N. Photoreaction of a hydroxyalkyphenone with the membrane of polymersomes: A versatile method to generate semipermeable nanoreactors. J. Am. Chem. Soc. 2013, 135, 9204–9212. [Google Scholar] [CrossRef]
- Petersen, M.A.; Hillmyer, M.A.; Kokkoli, E. Bioresorbable polymersomes for targeted delivery of cisplatin. Bioconjugate Chem. 2013, 24, 533–543. [Google Scholar] [CrossRef]
- Qiao, Z.Y.; Ji, R.; Huang, X.N.; Du, F.S.; Zhang, R.; Liang, D.H.; Li, Z.C. Polymersomes from dual responsive block copolymers: Drug encapsulation by heating and acid-triggered release. Biomacromolecules 2013, 14, 1555–1563. [Google Scholar] [CrossRef]
- Stano, A.; Scott, E.A.; Dane, K.Y.; Swartz, M.A.; Hubbell, J.A. Tunable T cell immunity towards a protein antigen using polymersomes vs. solid-core nanoparticles. Biomaterials 2013, 34, 4339–4346. [Google Scholar] [CrossRef]
- Cui, H.G.; Chen, Z.Y.; Zhong, S.; Wooley, K.L.; Pochan, D.J. Block copolymer assembly via kinetic controlled. Science 2007, 317, 647–650. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Zhao, Y. Rapid release of entrapped contents from multi-functionalizable, surface cross-linked micelles upon different stimulation. J. Am. Chem. Soc. 2010, 132, 10642–10644. [Google Scholar] [CrossRef]
- Tong, R.; Cheng, J.J. Anticancer polymeric nanomedicines. Polym. Rev. 2007, 47, 345–381. [Google Scholar] [CrossRef]
- Danhier, F.; Lecouturier, N.; Vroman, B.; Jerome, C.; Marchand-Brynaert, J.; Feron, O.; Preat, V. Paclitaxel-loaded PEGylated PLGA-based nanoparticles: In vitro and in vivo evaluation. J. Control. Release 2009, 133, 11–17. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Twibanire, J.K.; Grindley, T.B. Polyester Dendrimers: Smart Carriers for Drug Delivery. Polymers 2014, 6, 179-213. https://doi.org/10.3390/polym6010179
Twibanire JK, Grindley TB. Polyester Dendrimers: Smart Carriers for Drug Delivery. Polymers. 2014; 6(1):179-213. https://doi.org/10.3390/polym6010179
Chicago/Turabian StyleTwibanire, Jean–d’Amour K., and T. Bruce Grindley. 2014. "Polyester Dendrimers: Smart Carriers for Drug Delivery" Polymers 6, no. 1: 179-213. https://doi.org/10.3390/polym6010179
APA StyleTwibanire, J. K., & Grindley, T. B. (2014). Polyester Dendrimers: Smart Carriers for Drug Delivery. Polymers, 6(1), 179-213. https://doi.org/10.3390/polym6010179




