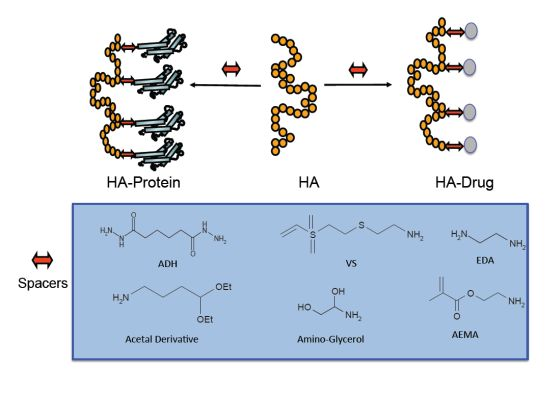
Hyaluronic Acid Bioconjugates for the Delivery of Bioactive Molecules
Abstract
:1. Introduction

2. HA Drug Conjugates
3. HA Anticancer Drug



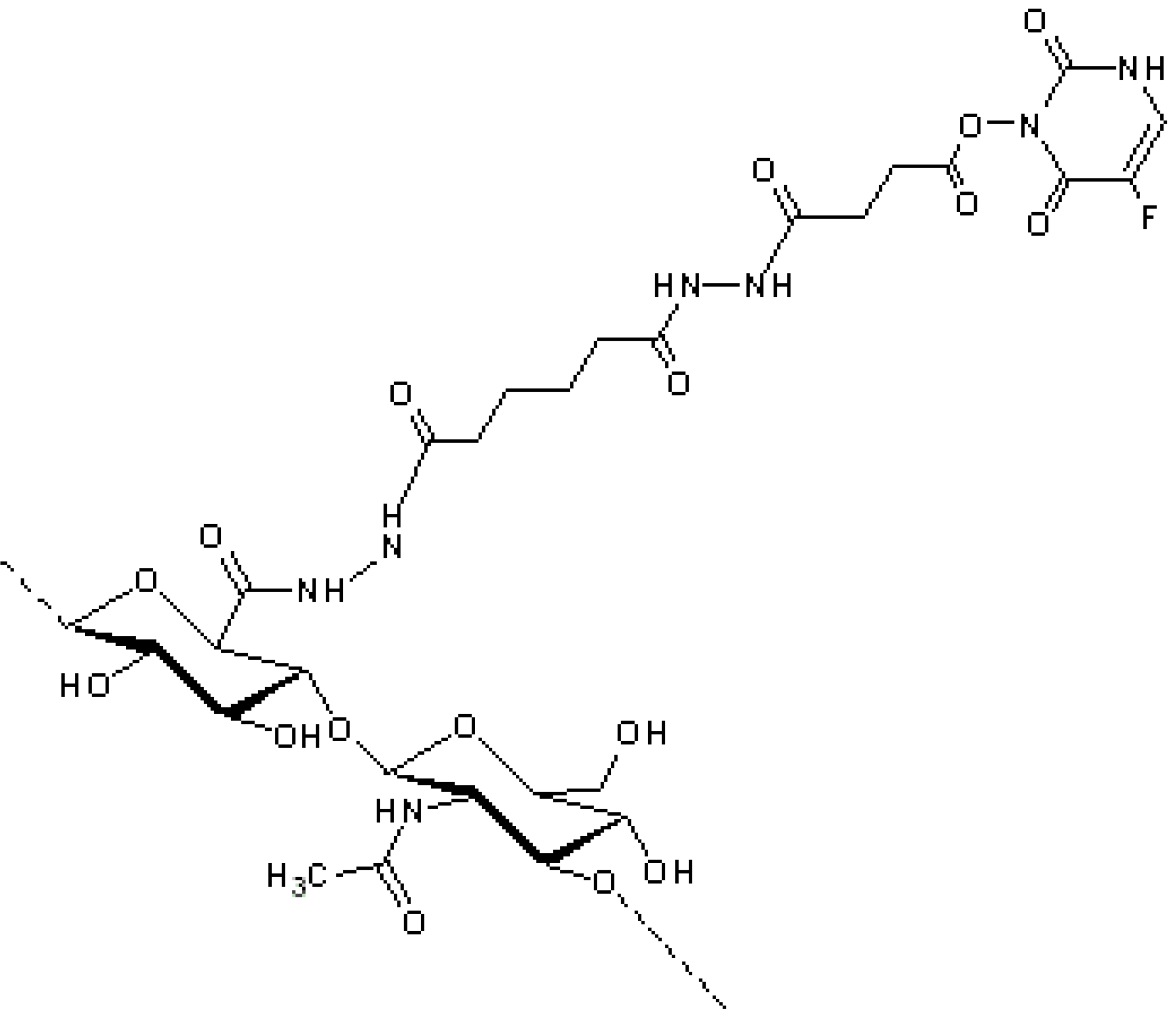
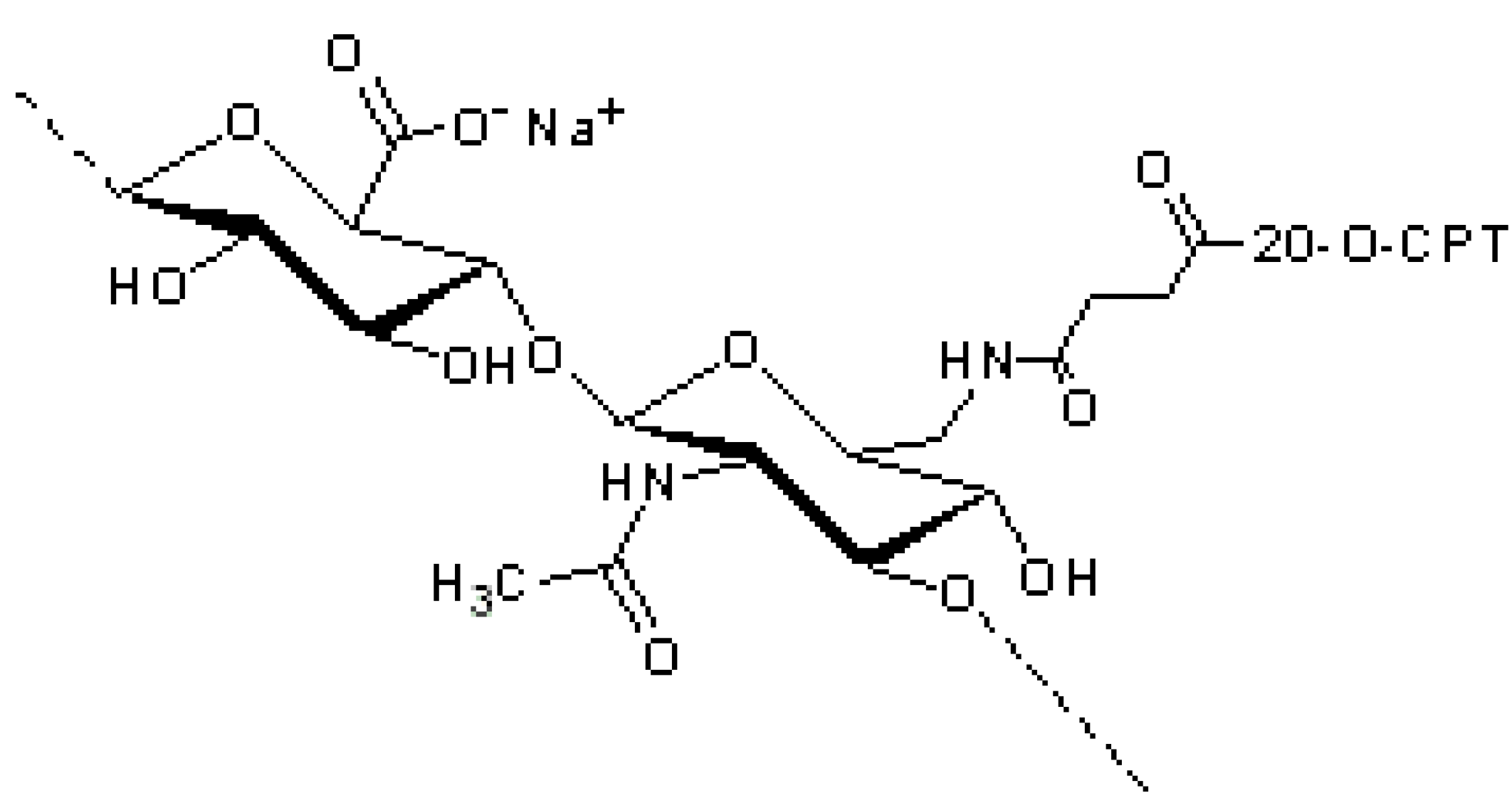
4. HA Anti-Inflammatory Drug


5. HA Conjugates in the Form of Hydrogels

6. HA–Peptide Conjugates
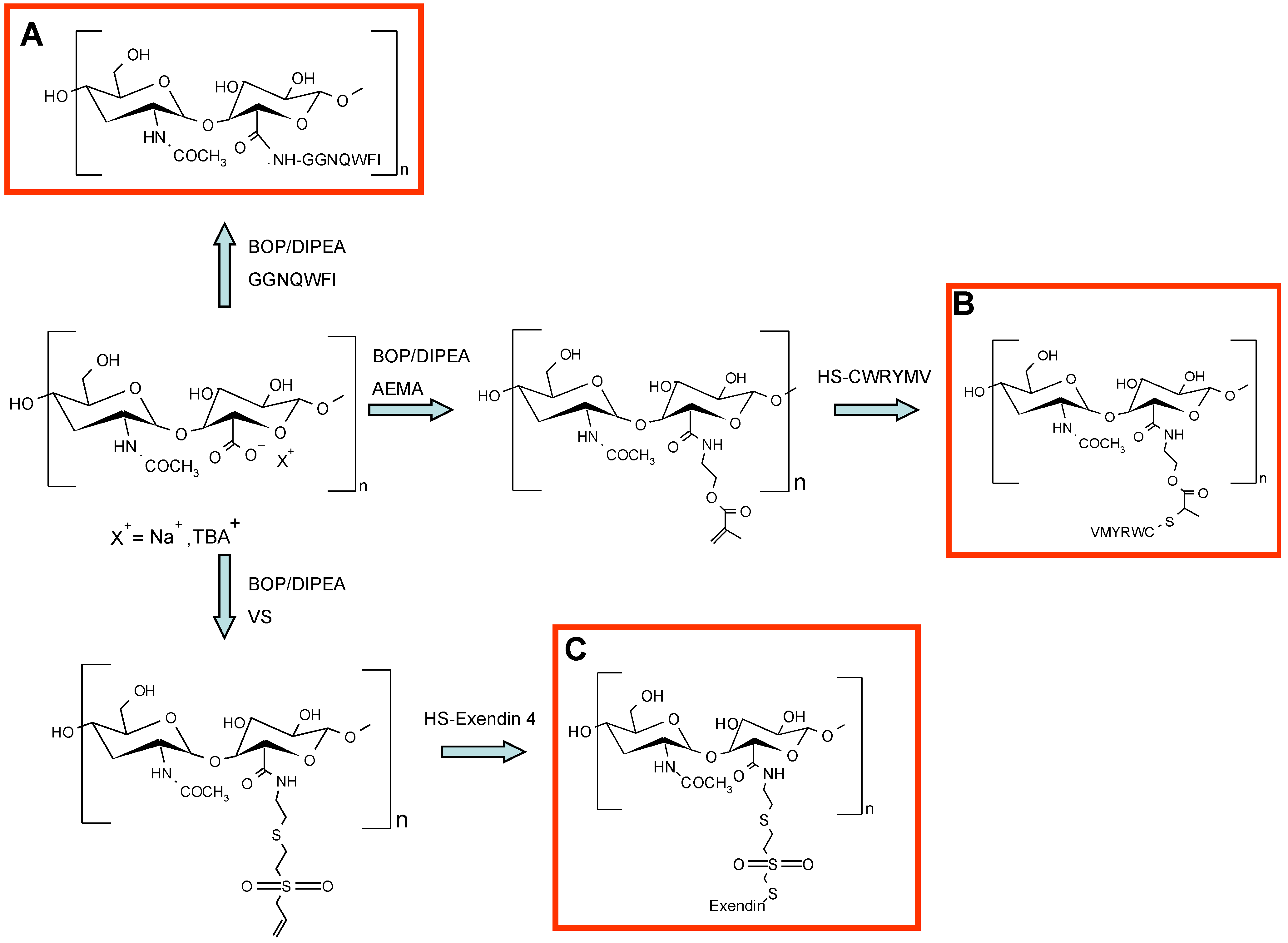
7. HA–Protein Conjugates

8. Delivery of Protein Mediated by Non-Covalent Conjugation with HA
9. Conclusions
Conflicts of Interest
References
- Meyer, K.; Palmer, J.W. The Polysaccharide of the vitreous humor. J. Biol. Chem. 1934, 107, 629–634. [Google Scholar]
- Laurent, T.C.; Fraser, J. Hyaluronan. FASEB J. 1992, 6, 2397–2404. [Google Scholar]
- Cowman, M.K.; Matsuoka, S. Experimental approaches to hyaluronan structure. Carbohydr. Res. 2005, 340, 791–809. [Google Scholar] [CrossRef]
- Ågren, U.M.; Tammi, M.; Ryynänen, M.; Tammi, R. Developmentally programmed expression of hyaluronan in human skin and its appendages. J. Invest. Dermatol. 1997, 109, 219–224. [Google Scholar]
- Sherman, L.; Sleeman, J.; Herrlich, P.; Ponta, H. Hyaluronate receptors: Key players in growth, differentiation, migration and tumor progression. Curr. Opin. Cell Biol. 1994, 6, 726–733. [Google Scholar] [CrossRef]
- Marhaba, R.; Zöller, M. CD44 in cancer progression: Adhesion, migration and growth regulation. J. Mol. Histol. 2004, 35, 211–231. [Google Scholar] [CrossRef]
- Arshinoff, S.A.; Jafari, M. New classification of ophthalmic viscosurgical devices—2005. J. Cataract Refract. Surg. 2005, 31, 2167–2171. [Google Scholar] [CrossRef]
- Lapčík, L.; Lapcik, L.; de Smedt, S.; Demeester, J.; Chabrecek, P. Hyaluronan: Preparation, structure, properties, and applications. Chem. Rev. 1998, 98, 2663–2684. [Google Scholar] [CrossRef]
- Laurent, T.C. The Chemistry, Biology and Medical Applications of Hyaluronan and Its Derivatives; Cambridge University Press: Cambridge, UK; volume 72, 1998. [Google Scholar]
- Davidson, J.M.; Nanney, L.B.; Broadley, K.N.; Whitsett, J.S.; Aquino, A.M.; Beccaro, M.; Rastrelli, A. Hyaluronate derivatives and their application to wound healing: Preliminary observations. Clin. Mater. 1991, 8, 171–177. [Google Scholar] [CrossRef]
- Chen, W.J.; Abatangelo, G. Functions of hyaluronan in wound repair. Wound Repair Regen. 1999, 7, 79–89. [Google Scholar] [CrossRef]
- Lipponen, P.; Aaltoma, S.; Kosma, V.; Ala‐Opas, M.; Eskelinen, M. Expression of CD44 standard and variant-v6 proteins in transitional cell bladder tumours and their relation to prognosis during a long-term follow-up. J. Pathol. 1998, 186, 157–164. [Google Scholar] [CrossRef]
- Kong, Q.; Liu, J.; Chen, X.; Wang, X.; Sun, Y.; Li, H. Differential expression patterns of hyaluronan receptors CD44 and RHAMM in transitional cell carcinomas of urinary bladder. Oncol. Rep. 2003, 10, 51–55. [Google Scholar]
- Misra, S.; Heldin, P.; Hascall, V.C.; Karamanos, N.K.; Skandalis, S.S.; Markwald, R.R.; Ghatak, S. Hyaluronan–CD44 interactions as potential targets for cancer therapy. FEBS J. 2011, 278, 1429–1443. [Google Scholar] [CrossRef]
- Banzato, A.; Bobisse, S.; Rondina, M.; Renier, D.; Bettella, F.; Esposito, G.; Quintieri, L.; Meléndez-Alafort, L.; Mazzi, U.; Zanovello, P. A paclitaxel–hyaluronan bioconjugate targeting ovarian cancer affords a potent in vivo therapeutic activity. Clin. Cancer Res. 2008, 14, 3598–3606. [Google Scholar] [CrossRef]
- Pitarresi, G.; Palumbo, F.S.; Albanese, A.; Fiorica, C.; Picone, P.; Giammona, G. Self-assembled amphiphilic hyaluronic acid graft copolymers for targeted release of antitumoral drug. J. Drug Target. 2010, 18, 264–276. [Google Scholar] [CrossRef]
- Saravanakumar, G.; Choi, K.Y.; Yoon, H.Y.; Kim, K.; Park, J.H.; Kwon, I.C.; Park, K. Hydrotropic hyaluronic acid conjugates: Synthesis, characterization, and implications as a carrier of paclitaxel. Int. J. Pharm. 2010, 394, 154–161. [Google Scholar] [CrossRef]
- Homma, A.; Sato, H.; Okamachi, A.; Emura, T.; Ishizawa, T.; Kato, T.; Matsuura, T.; Sato, S.; Tamura, T.; Higuchi, Y. Novel hyaluronic acid–methotrexate conjugates for osteoarthritis treatment. Bioorg. Med. Chem. 2009, 17, 4647–4656. [Google Scholar] [CrossRef]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control. Release 2000, 65, 271–284. [Google Scholar]
- Walsh, G. Biopharmaceutical benchmarks 2010. Nat. Biotechnol. 2010, 28, 917–924. [Google Scholar] [CrossRef]
- Pasut, G.; Veronese, F.M. State of the art in PEGylation: The great versatility achieved after forty years of research. J. Control. Release 2012, 161, 461–472. [Google Scholar] [CrossRef]
- Shi, Y.; Li, L. Current advances in sustained-release systems for parenteral drug delivery. Inf. Healthc. 2005, 2, 1039–1058. [Google Scholar]
- Tuncer Degim, I.; Celebi, N. Controlled delivery of peptides and proteins. Curr. Pharm. Des. 2007, 13, 99–117. [Google Scholar] [CrossRef]
- Hardwicke, J.; Ferguson, E.L.; Moseley, R.; Stephens, P.; Thomas, D.W.; Duncan, R. Dextrin–rhEGF conjugates as bioresponsive nanomedicines for wound repair. J. Control. Release 2008, 130, 275–283. [Google Scholar] [CrossRef]
- Schanté, C.E.; Zuber, G.; Herlin, C.; Vandamme, T.F. Chemical modifications of hyaluronic acid for the synthesis of derivatives for a broad range of biomedical applications. Carbohydr. Polym. 2011, 85, 469–489. [Google Scholar] [CrossRef]
- Drobnik, J. Hyaluronan in drug delivery. Adv. Drug Deliv. Rev. 1991, 7, 295–308. [Google Scholar] [CrossRef]
- Platt, V.M.; Szoka, F.C., Jr. Anticancer therapeutics: Targeting macromolecules and nanocarriers to hyaluronan or CD44, a hyaluronan receptor. Mol. Pharm. 2008, 5, 474–486. [Google Scholar] [CrossRef]
- Luo, Y.; Bernshaw, N.J.; Lu, Z.; Kopecek, J.; Prestwich, G.D. Targeted delivery of doxorubicin by HPMA copolymer–hyaluronan bioconjugates. Pharm. Res. 2002, 19, 396–402. [Google Scholar] [CrossRef]
- Luo, Y.; Prestwich, G.D. Synthesis and selective cytotoxicity of a hyaluronic acid–antitumor bioconjugate. Bioconjug. Chem. 1999, 10, 755–763. [Google Scholar]
- Luo, Y.; Ziebell, M.R.; Prestwich, G.D. A hyaluronic acid–taxol antitumor bioconjugate targeted to cancer cells. Biomacromolecules 2000, 1, 208–218. [Google Scholar] [CrossRef]
- Leonelli, F.; La Bella, A.; Francescangeli, A.; Joudioux, R.; Capodilupo, A.; Quagliariello, M.; Migneco, L.M.; Bettolo, R.M.; Crescenzi, V.; de Luca, G. A new and simply available class of hydrosoluble bioconjugates by coupling paclitaxel to hyaluronic acid through a 4-hydroxybutanoic acid derived linker. Helv. Chim. Acta 2005, 88, 154–159. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.; Shu, X.Z.; Gray, S.D.; Prestwich, G.D. Synthesis and biological evaluation of a cross-linked hyaluronan-mitomycin C hydrogel. Biomacromolecules 2004, 5, 895–902. [Google Scholar] [CrossRef]
- Jordan, M.A.; Wilson, L. Microtubules as a target for anticancer drugs. Nat. Rev. Cancer 2004, 4, 253–265. [Google Scholar] [CrossRef]
- Cormio, G.; Vagno, G.D.; Melilli, G.; Cazzolla, A.; Gesù, G.D.; Carriero, C.; Cramarossa, D.; Loverro, G.; Selvaggi, L. Hypersensitivity reactions in ovarian cancer patients receiving paclitaxel. J. chemother. 1999, 11, 407–409. [Google Scholar] [CrossRef]
- Weiss, R.B.; Donehower, R.; Wiernik, P.; Ohnuma, T.; Gralla, R.; Trump, D.; Baker, J.; van Echo, D.; von Hoff, D.; Leyland-Jones, B. Hypersensitivity reactions from taxol. J. Clin. Oncol. 1990, 8, 1263–1268. [Google Scholar]
- Thierry, B.; Kujawa, P.; Tkaczyk, C.; Winnik, F.M.; Bilodeau, L.; Tabrizian, M. Delivery platform for hydrophobic drugs: Prodrug approach combined with self-assembled multilayers. J. Am. Chem. Soc. 2005, 127, 1626–1627. [Google Scholar]
- Capodilupo, A.; Crescenzi, V.; Francescangeli, A.; Joudioux, R.; Leonelli, F.; Marini, R.; Renier, D.; Banzato, A.; Rosato, A. Hydrosoluble, metabolically fragile bioconjugates by coupling tetrabutylammonium hyaluronan with 2′-paclitaxel-4-bromobutyrate: Synthesis and antitumor properties. Hyaluronan 2003, 1, 391–395. [Google Scholar]
- Montagner, I.M.; Banzato, A.; Zuccolotto, G.; Renier, D.; Campisi, M.; Bassi, P.; Zanovello, P.; Rosato, A. Paclitaxel–hyaluronan hydrosoluble bioconjugate: Mechanism of action in human bladder cancer cell lines. Urol. Oncol. Semin. Orig. Investig. 2003, 31, 1261–1269. [Google Scholar]
- De Stefano, I.; Battaglia, A.; Zannoni, G.F.; Prisco, M.G.; Fattorossi, A.; Travaglia, D.; Baroni, S.; Renier, D.; Scambia, G.; Ferlini, C. Hyaluronic acid–paclitaxel: Effects of intraperitoneal administration against CD44 (+) human ovarian cancer xenografts. Cancer Chemother. Pharmacol. 2011, 68, 107–116. [Google Scholar] [CrossRef]
- Campisi, M.; Renier, D.; Pierimarchi, P.; Serafino, A. Therapeutic use of new pharmaceutical preparations containing antitumoral drugs bound to hyaluronic acid in the treatment of neoplasias. EP2279006, 30 October 2009. [Google Scholar]
- Renier, D.; Bettella, F. Antitumoral bioconjugates of hyaluronic acid or its derivatives obtained by indirect chemical conjugation, and their use in the pharmaceutical field. WIPO Patent No. 2007014784, 9 February 2007. [Google Scholar]
- Serafino, A.; Zonfrillo, M.; Andreola, F.; Psaila, R.; Mercuri, L.; Moroni, N.; Renier, D.; Campisi, M.; Secchieri, C.; Pierimarchi, P. CD44-targeting for antitumor drug delivery: A new SN–38-hyaluronan bioconjugate for locoregional treatment of peritoneal carcinomatosis. Curr. Cancer Drug Targets 2011, 11, 572–585. [Google Scholar] [CrossRef]
- Tringali, G.; Bettella, F.; Greco, M.C.; Campisi, M.; Renier, D.; Navarra, P. Pharmacokinetic profile of oncofid-S after intraperitoneal and intravenous administration in the rat. J. Pharm. Pharmacol. 2012, 64, 360–365. [Google Scholar] [CrossRef]
- Xin, D.; Wang, Y.; Xiang, J. The use of amino acid linkers in the conjugation of paclitaxel with hyaluronic acid as drug delivery system: Synthesis, self-assembled property, drug release, and in vitro efficiency. Pharm. Res. 2010, 27, 380–389. [Google Scholar] [CrossRef]
- Lee, H.; Lee, K.; Park, T.G. Hyaluronic acid−paclitaxel conjugate micelles: Synthesis, characterization, and antitumor activity. Bioconjug. Chem. 2008, 19, 1319–1325. [Google Scholar] [CrossRef]
- Zhang, L.; Petroll, W.M.; Greyner, H.J.; Mummert, M.E. Development of a hyaluronan bioconjugate for the topical treatment of melanoma. J. Dermatol. Sci. 2009, 55, 56–59. [Google Scholar] [CrossRef]
- Manju, S.; Sreenivasan, K. Conjugation of curcumin onto hyaluronic acid enhances its aqueous solubility and stability. J. Colloid Interface Sci. 2011, 359, 318–325. [Google Scholar] [CrossRef]
- Ossipov, D.A. Nanostructured hyaluronic acid-based materials for active delivery to cancer. Expert Opin. Drug Deliv. 2010, 7, 681–703. [Google Scholar] [CrossRef]
- Dong, Z.; Zheng, W.; Xu, Z.; Yin, Z. Improved stability and tumor targeting of 5-fluorouracil by conjugation with hyaluronan. J. Appl. Polym. Sci. 2013, 130, 927–932. [Google Scholar] [CrossRef]
- Norbedo, S.; Dinon, F.; Bergamin, M.; Bosi, S.; Aroulmoji, V.; Khan, R.; Murano, E. Synthesis of 6-amino-6-deoxyhyaluronan as an intermediate for conjugation with carboxylate-containing compounds: Application to hyaluronan–camptothecin conjugates. Carbohydr. Res. 2009, 344, 98–104. [Google Scholar] [CrossRef]
- Banerji, S.; Wright, A.J.; Noble, M.; Mahoney, D.J.; Campbell, I.D.; Day, A.J.; Jackson, D.G. Structures of the Cd44–hyaluronan complex provide insight into a fundamental carbohydrate-protein interaction. Nat. Struct. Mol. Biol. 2007, 14, 234–239. [Google Scholar] [CrossRef]
- Sorbi, C.; Bergamin, M.; Bosi, S.; Dinon, F.; Aroulmoji, V.; Khan, R.; Murano, E.; Norbedo, S. Synthesis of 6-O-methotrexylhyaluronan as a drug delivery system. Carbohydr. Res. 2009, 344, 91–97. [Google Scholar] [CrossRef]
- Sugahara, S.; Okuno, S.; Yano, T.; Hamana, H.; Inoue, K. Characteristics of tissue distribution of various polysaccharides as drug carriers: Influences of molecular weight and anionic charge on tumor targeting. Biol. Pharm. Bull. 2001, 24, 535–543. [Google Scholar] [CrossRef]
- Della Valle, F.; Romeo, A. Esters of hyaluronic acid. US 4,851,521, 25 July 1989. [Google Scholar]
- Pouyani, T.; Prestwich, G.D. Functionalized derivatives of hyaluronic acid oligosaccharides: Drug carriers and novel biomaterials. Bioconjug. Chem. 1994, 5, 339–347. [Google Scholar] [CrossRef]
- Alarcon, G.S. Methotrexate use in rheumatoid arthritis: A clinician’s perspective. Immunopharmacology 2000, 47, 259–271. [Google Scholar] [CrossRef]
- Homma, A.; Sato, H.; Tamura, T.; Okamachi, A.; Emura, T.; Ishizawa, T.; Kato, T.; Matsuura, T.; Sato, S.; Higuchi, Y. Synthesis and optimization of hyaluronic acid–methotrexate conjugates to maximize benefit in the treatment of osteoarthritis. Bioorg. Med. Chem. 2010, 18, 1062–1075. [Google Scholar] [CrossRef]
- Miyamoto, K; Yasuda, Y.; Yoshioka, K. Hyaluronic acid derivative and drug containing the same. CA 2555759, 21 July 2005. [Google Scholar]
- Kogan, G.; Šoltés, L.; Stern, R.; Gemeiner, P. Hyaluronic acid: A natural biopolymer with a broad range of biomedical and industrial applications. Biotechnol. Lett. 2007, 29, 17–25. [Google Scholar]
- Price, R.D.; Berry, M.; Navsaria, H.A. Hyaluronic acid: The scientific and clinical evidence. J. Plast. Reconstr. Aesthet. Surg. 2007, 60, 1110–1119. [Google Scholar] [CrossRef]
- Ito, T.; Fraser, I.P.; Yeo, Y.; Highley, C.B.; Bellas, E.; Kohane, D.S. Anti-inflammatory function of an in situ cross-linkable conjugate hydrogel of hyaluronic acid and dexamethasone. Biomaterials 2007, 28, 1778–1786. [Google Scholar] [CrossRef]
- Gianolio, D.A.; Philbrook, M.; Avila, L.Z.; MacGregor, H.; Duan, S.X.; Bernasconi, R.; Slavsky, M.; Dethlefsen, S.; Jarrett, P.K.; Miller, R.J. Synthesis and evaluation of hydrolyzable hyaluronan-tethered bupivacaine delivery systems. Bioconjug. Chem. 2005, 16, 1512–1518. [Google Scholar] [CrossRef]
- Vercruysse, K.P.; Prestwich, G.D. Hyaluronate derivatives in drug delivery. Crit. Rev. Ther. Drug Carr. Syst. 1998, 15, 513–555. [Google Scholar]
- Dhal, P.K.; Gianolio, D.A.; Miller, R.J. Polymer based therapies for the treatment of chronic pain. Pain Manag. Curr. Issues Opin. 2012, 28, 27–42. [Google Scholar]
- Mero, A.; Pasqualin, M.; Campisi, M.; Renier, D.; Pasut, G. Conjugation of hyaluronan to proteins. Carbohydr. Polym. 2013, 92, 2163–2170. [Google Scholar] [CrossRef]
- Oh, E.J.; Choi, J.; Kim, H.; Joo, C.; Hahn, S.K. Anti-Flt1 peptide–hyaluronate conjugate for the treatment of retinal neovascularization and diabetic retinopathy. Biomaterials 2011, 32, 3115–3123. [Google Scholar] [CrossRef]
- Hammes, H.; Lin, J.; Bretzel, R.G.; Brownlee, M.; Breier, G. Upregulation of the vascular endothelial growth factor/vascular endothelial growth factor receptor system in experimental background diabetic retinopathy of the rat. Diabetes 1998, 47, 401–406. [Google Scholar] [CrossRef]
- Bae, D.; Kim, T.; Li, G.; Yoon, W.; Chae, C. Anti-Flt1 peptide, a vascular endothelial growth factor receptor 1—Specific hexapeptide, inhibits Tumor growth and metastasis. Clin. Cancer Res. 2005, 11, 2651–2661. [Google Scholar] [CrossRef]
- Kong, J.; Oh, E.J.; Chae, S.Y.; Lee, K.C.; Hahn, S.K. Long acting hyaluronate–exendin 4 conjugate for the treatment of type 2 diabetes. Biomaterials 2010, 31, 4121–4128. [Google Scholar] [CrossRef]
- Gentilella, R.; Bianchi, C.; Rossi, A.; Rotella, C. Exenatide: A review from pharmacology to clinical practice. Diabetes Obes. Metabol. 2009, 11, 544–556. [Google Scholar] [CrossRef]
- Gedulin, B.R.; Nikoulina, S.E.; Smith, P.A.; Gedulin, G.; Nielsen, L.L.; Baron, A.D.; Parkes, D.G.; Young, A.A. Exenatide (Exendin-4) improves insulin sensitivity and β-cell mass in insulin-resistant obese fa/fa zucker rats independent of glycemia and body weight. Endocrinology 2005, 146, 2069–2076. [Google Scholar] [CrossRef]
- Oh, E.J.; Kim, J.; Kong, J.; Ryu, S.H.; Hahn, S.K. Signal transduction of hyaluronic acid−peptide conjugate for formyl peptide receptor like 1 receptor. Bioconjug. Chem. 2008, 19, 2401–2408. [Google Scholar] [CrossRef]
- Ferguson, E.L.; Alshame, A.M.; Thomas, D.W. Evaluation of hyaluronic acid–protein conjugates for polymer masked—Unmasked protein therapy. Int. J. Pharm. 2010, 402, 95–102. [Google Scholar] [CrossRef]
- Lu, H.; Chai, J.; Li, M.; Huang, B.; He, C.; Bi, R. Crystal structure of human epidermal growth factor and its dimerization. J. Biol. Chem. 2001, 276, 34913–34917. [Google Scholar]
- Wong, S.S. Reactive groups of proteins and their modifying agents. Chem. Protein Conjug. Crosslink. 1991, 2, 27–29. [Google Scholar]
- Kinstler, O.B.; Brems, D.N.; Lauren, S.L.; Paige, A.G.; Hamburger, J.B.; Treuheit, M.J. Characterization and stability of N-terminally PEGylated rhG-CSF. Pharm. Res. 1996, 13, 996–1002. [Google Scholar] [CrossRef]
- Bobbitt, J. Periodate oxidation of carbohydrates. Adv. Carbohydr. Chem. 1956, 11, 1–41. [Google Scholar]
- Yang, J.; Kim, E.; Kwon, J.H.; Kim, H.; Shin, J.H.; Yun, S.H.; Choi, K.Y.; Hahn, S.K. Transdermal delivery of hyaluronic acid–human growth hormone conjugate. Biomaterials 2012, 33, 5947–5954. [Google Scholar] [CrossRef]
- Yang, J.; Park, K.; Jung, H.; Kim, H.; Hong, S.W.; Yoon, S.K.; Hahn, S.K. Target specific hyaluronic acid–interferon alpha conjugate for the treatment of hepatitis C virus infection. Biomaterials 2011, 32, 8722–8729. [Google Scholar] [CrossRef]
- Bergman, K.; Engstrand, T.; Hilborn, J.; Ossipov, D.; Piskounova, S.; Bowden, T. Injectable cell-free template for bone-tissue formation. J. Biomed. Mater. Res. A 2009, 91, 1111–1118. [Google Scholar]
- Wang, S.; Oommen, O.P.; Yan, H.; Varghese, O.P. Mild and efficient strategy for site-selective aldehyde modification of glycosaminoglycans: Tailoring hydrogels with tunable release of growth factor. Biomacromolecules 2013, 14, 2427–2432. [Google Scholar] [CrossRef]
- Martínez-Sanz, E.; Ossipov, D.A.; Hilborn, J.; Larsson, S.; Jonsson, K.B.; Varghese, O.P. Bone reservoir: Injectable hyaluronic acid hydrogel for minimal invasive bone augmentation. J. Control. Release 2011, 152, 232–240. [Google Scholar] [CrossRef]
- Hu, J.; Sebald, W. N-Terminal specificity of PEGylation of human bone morphogenetic protein-2 at acidic pH. Int. J. Pharm. 2011, 413, 140–146. [Google Scholar] [CrossRef]
- Mero, A.; Pasqualin, M.; Schiavon, O.; Montagner, I.; Boldrin, D.; Rosato, A.; Pasut, G.; University of Padua, Padua, Italy. Personal data. 2010.
- Van Tomme, S.R.; Storm, G.; Hennink, W.E. In situ gelling hydrogels for pharmaceutical and biomedical applications. Int. J. Pharm. 2008, 355, 1–18. [Google Scholar] [CrossRef]
- Oh, E.J.; Park, K.; Kim, K.S.; Kim, J.; Yang, J.; Kong, J.; Lee, M.Y.; Hoffman, A.S.; Hahn, S.K. Target specific and long-acting delivery of protein, peptide, and nucleotide therapeutics using hyaluronic acid derivatives. J. Control. Release 2010, 141, 2–12. [Google Scholar] [CrossRef]
- Fiorica, C.; Pitarresi, G.; Palumbo, F.S.; di Stefano, M.; Calascibetta, F.; Giammona, G. A new hyaluronic acid pH sensitive derivative obtained by ATRP for potential oral administration of proteins. Int. J. Pharm. 2013, 457, 150–157. [Google Scholar] [CrossRef] [Green Version]
- Han, L.; Zhao, Y.; Yin, L.; Li, R.; Liang, Y.; Huang, H.; Pan, S.; Wu, C.; Feng, M. Insulin-loaded pH-sensitive hyaluronic acid nanoparticles enhance transcellular delivery. AAPS PharmSciTech 2012, 13, 836–845. [Google Scholar] [CrossRef]
- Xu, K.; Lee, F.; Gao, S.J.; Chung, J.E.; Yano, H.; Kurisawa, M. Injectable hyaluronic acid-tyramine hydrogels incorporating interferon-α2a for liver cancer therapy. J. Control. Release 2013, 166, 203–210. [Google Scholar] [CrossRef]
- Lee, M.; Yang, J.; Jung, H.S.; Beack, S.; Choi, J.E.; Hur, W.; Koo, H.; Kim, K.; Yoon, S.K.; Hahn, S.K. Hyaluronic acid–gold nanoparticle/interferon α complex for targeted treatment of hepatitis C virus infection. ACS nano 2012, 6, 9522–9531. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mero, A.; Campisi, M. Hyaluronic Acid Bioconjugates for the Delivery of Bioactive Molecules. Polymers 2014, 6, 346-369. https://doi.org/10.3390/polym6020346
Mero A, Campisi M. Hyaluronic Acid Bioconjugates for the Delivery of Bioactive Molecules. Polymers. 2014; 6(2):346-369. https://doi.org/10.3390/polym6020346
Chicago/Turabian StyleMero, Anna, and Monica Campisi. 2014. "Hyaluronic Acid Bioconjugates for the Delivery of Bioactive Molecules" Polymers 6, no. 2: 346-369. https://doi.org/10.3390/polym6020346
APA StyleMero, A., & Campisi, M. (2014). Hyaluronic Acid Bioconjugates for the Delivery of Bioactive Molecules. Polymers, 6(2), 346-369. https://doi.org/10.3390/polym6020346