Bioreducible Micelles Self-Assembled from Poly(ethylene glycol)-Cholesteryl Conjugate As a Drug Delivery Platform
Abstract
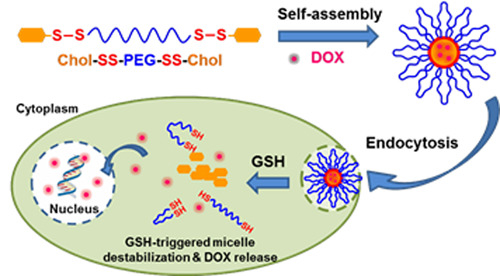
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Measurements
2.3. Synthesis
2.4. Micellization and CMC Measurement of Chol-ss-PEG-ss-Chol
2.5. Reductive Cleavage of Chol-ss-PEG-ss-Chol in Response to DTT
2.6. Determination of Loading Level of DOX
2.7. Thiol-Mediated Release of DOX from DOX-Loaded Micelles
2.8. Cytotoxicity of Micelles
2.9. Intracellular DOX Release
2.10. Cellular Uptake Determination Using CLSM
2.11. Statistical Analysis
3. Results and Discussion
3.1. Synthesis and Characterization of Chol-ss-PEG-ss-Chol


3.2. Characterization of Chol-ss-PEG-ss-Chol Micelles


3.3. Thiol-Responsive Degradation of Chol-ss-PEG-ss-Chol



3.4. Drug Loading and DOX Release upon Thiol-Responsive Destabilization from DOX-Loaded Micelles

3.5. Cytotoxicity of Micelles and Intracellular DOX Release

3.6. Cellular Uptake

4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Glen, S.K.; Teruo, O. Polymeric micelles as new drug carriers. Adv. Drug Del. Rev. 1996, 21, 107–116. [Google Scholar] [CrossRef]
- Kazunori, K.; Atsushi, H.; Yukio, N. Block copolymer micelles for drug delivery: Design characterization and biological significance. Adv. Drug Del. Rev. 2001, 47, 113–131. [Google Scholar] [CrossRef]
- Rodríguez-Hernández, J.; Chécot, F.; Gnanou, Y.; Lecommandoux, S. Toward “smart” nano-objects by self-assembly of block copolymers in solution. Prog. Polym. Sci. 2005, 30, 691–724. [Google Scholar] [CrossRef]
- Vladimir, T.P. Structure and design of polymeric surfactant-based drug delivery systems. J. Control. Release 2001, 73, 137–172. [Google Scholar] [CrossRef] [PubMed]
- Moghimi, S.M.; Hunter, A.C.; Murray, J.C. Long-circulating and target-specific nanoparticles: Theory to practice. Pharmacol. Rev. 2001, 53, 283–318. [Google Scholar] [PubMed]
- Bae, Y.H.; Yin, H. Stability issues of polymeric micelles. J. Control. Release 2008, 131, 2–4. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shabat, S.; Kumar, N.; Domb, A.J. PEG-PLA block copolymer as potential drug carrier: Preparation and characterization. Macromol. Biosci. 2006, 6, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.Z.; Zeng, Z.W.; Zhou, G.L.; Wang, J.J.; Li, F.Z.; Wang, A.M. Recent advances in PEG–PLA block copolymer nanoparticles. Int. J. Nanomed. 2010, 5, 1057–1065. [Google Scholar]
- Jain, A.K.; Goyal, A.K.; Mishra, N.; Vaidya, B.; Mangal, S.; Vyas, S.P. PEG–PLA–PEG block copolymeric nanoparticles for oral immunization against hepatitis B. Int. J. Pharm. 2010, 387, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhao, J.; Zhang, B.; Qian, Y.; Gao, H.; Yu, Y.; Wei, Y.; Yang, Z.; Jiang, X.; Pang, Z. Polyethylene glycol–polylactic acid nanoparticles modified with cysteine–arginine–glutamic acid–lysine–alanine fibrin-homing peptide for glioblastoma therapy by enhanced retention effect. Int. J. Nanomed. 2014, 9, 5261–5271. [Google Scholar]
- Hwang, M.J.; Suh, J.M.; Bae, Y.H.; Kim, S.W.; Jeong, B. Caprolactonic poloxamer analog: PEG-PCL-PEG. Biomacromolecules 2005, 6, 885–890. [Google Scholar] [CrossRef] [PubMed]
- Diao, Y.-Y.; Li, H.-Y.; Fu, Y.-H.; Han, M.; Hu, Y.-L.; Jiang, H.-L.; Tsutsumi, Y.; Wei, Q.-C.; Chen, D.-W.; Gao, J.-Q. Doxorubicin-loaded PEG-PCL copolymer micelles enhance cytotoxicity and intracellular accumulation of doxorubicin in adriamycin-resistant tumor cells. Int. J. Nanomed. 2011, 6, 1955–1962. [Google Scholar]
- Cuong, N.-V.; Jiang, J.-L.; Li, Y.-L.; Chen, J.-R.; Jwo, S.-C.; Hsieh, M.-F. Doxorubicin-loaded PEG-PCL-PEG micelle using xenograft model of nude mice: Effect of multiple administration of micelle on the suppression of human breast cancer. Cancers 2011, 3, 61–78. [Google Scholar] [CrossRef] [PubMed]
- Machida, Y.; Onishi, H.; Kurita, A.; Hata, H.; Morikaw, A.; Machid, Y. Pharmacokinetics of prolonged-release CPT-11-loaded microspheres in rats. J. Control. Release 2000, 66, 159–175. [Google Scholar] [CrossRef]
- Musumeci, T.; Ventura, C.A.; Giannone, I.; Ruozi, B.; Montenegro, L.; Pignatello, R.; Puglisi, G. PLA/PLGA nanoparticles for sustained release of docetaxel. Int. J. Pharm. 2006, 325, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Ishiwata, H.; Sato, S.B.; Vertut-Doï, A.; Hamashima, Y.; Miyajima, K. Cholesterol derivative of poly(ethylene glycol) inhibits clathrin-independent, but not clathrin-dependent endocytosis. Biochim. Biophys. Acta 1997, 1359, 123–135. [Google Scholar] [CrossRef]
- Dufort, S.; Sancey, L.; Coll, J.-L. Physico-chemical parameters that govern nanoparticles fate also dictate rules for their molecular evolution. Adv. Drug Deliv. Rev. 2012, 64, 179–189. [Google Scholar] [CrossRef] [PubMed]
- He, Z.-Y.; Chu, B.-Y.; Wei, X.-W.; Li, J.; Edwards, C.K.; Song, X.-R.; He, G.; Xie, Y.-M.; Wei, Y.-Q.; Qian, Z.-Y. Recent development of poly(ethylene glycol)-cholesterol conjugates as drug delivery systems. Int. J. Pharm. 2014, 469, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Saito, G.; Swanson, J.A.; Lee, K.-D. Drug delivery strategy utilizing conjugation via reversible disulfide linkages: Role and site of cellular reducing activities. Adv. Drug Deliv. Rev. 2003, 55, 199–215. [Google Scholar] [CrossRef]
- Jonesa, D.P.; Carlson, J.L.; Samiec, P.S.; Paul, S.J.; Vino, C.M.J.; Reed, R.L.; Brown, L.A.S. Glutathione measurement in human plasma evaluation of sample collection, storage and derivatization conditions for analysis of dansyl derivatives by HPLC. Clin. Chim. Acta 1998, 275, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Yan, X.; Yuan, T.; Liang, J.; Fan, Y.; Gu, Z.; Zhang, X. Disassemblable micelles based on reduction-degradable amphiphilic graft copolymers for intracellular delivery of doxorubicin. Biomaterials 2010, 31, 7124–7131. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Huo, M.; Wang, J.; Zhou, J.; Mohammad, J.M.; Zhang, Y.; Zhu, Q.; Waddad, A.Y.; Zhang, Q. Redox-sensitive micelles self-assembled from amphiphilic hyaluronic acid-deoxycholic acid conjugates for targeted intracellular delivery of paclitaxel. Biomaterials 2012, 33, 2310–2320. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-C.; Wang, F.; Sun, T.-M.; Wang, J. Redox-responsive nanoparticles from the single disulfide bond-bridged block copolymer as drug carriers for overcoming multidrug resistance in cancer cells. Bioconjugate Chem. 2011, 22, 1939–1945. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-C.; Kim, E.; Ha, T.-L.; Jeong, S.W.; Lee, S.G.; Lee, S.J.; Lee, B. Thiol-responsive gemini poly(ethylene glycol)-poly(lactide) with a cystine disulfide spacer as an intracellular drug delivery nanocarrier. Colloid Surf. B Biointerfaces 2015, 127, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Boomer, A.J.; Qualls, M.M.; Inerowicz, H.D.; Haynes, R.H.; Patri, V.S.; Kim, J.-M.; Thompson, D.H. Cytoplasmic delivery of liposomal contents mediated by an acid-labile cholesterol-vinyl ether-PEG conjugate. Bioconjugate Chem. 2009, 20, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Jiang, X.; Huang, Y.; Zhang, C.; Ping, Q. pH-Sensitive mPEG-Hz-cholesterol conjugates as a liposome delivery system. J. Bioact. Compat. Polym. 2010, 25, 527–542. [Google Scholar] [CrossRef]
- Shirazi, R.S.; Ewert, K.K.; Leal, C.; Majzoub, R.N.; Bouxsein, N.F.; Safinya, C.R. Synthesis and characterization of degradable multivalent cationic lipids with disulfide-bond spacers for gene delivery. Biochim. Biophys. Acta 2011, 1808, 2156–2166. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M.; Cullis, P.R. Drug delivery systems: Entering the mainstream. Science 2004, 303, 1818–1822. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.; Maysinger, D.; Eisenberg, A. Nano-engineering block copolymer aggregates for drug delivery. Colloid Surf. B Biointerfaces 1999, 16, 3–27. [Google Scholar] [CrossRef]
- Mary, E.A.; Alton, M. Glutathione monoesters. Anal. Biochem. 1989, 183, 16–20. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baek, C.; Ha, T.-L.; Kim, E.; Jeong, S.W.; Lee, S.G.; Lee, S.J.; Kim, H.-C. Bioreducible Micelles Self-Assembled from Poly(ethylene glycol)-Cholesteryl Conjugate As a Drug Delivery Platform. Polymers 2015, 7, 2245-2258. https://doi.org/10.3390/polym7111511
Baek C, Ha T-L, Kim E, Jeong SW, Lee SG, Lee SJ, Kim H-C. Bioreducible Micelles Self-Assembled from Poly(ethylene glycol)-Cholesteryl Conjugate As a Drug Delivery Platform. Polymers. 2015; 7(11):2245-2258. https://doi.org/10.3390/polym7111511
Chicago/Turabian StyleBaek, Chulsu, Tae-Lin Ha, Eunjoo Kim, Sang Won Jeong, Se Guen Lee, Sung Jun Lee, and Hyun-Chul Kim. 2015. "Bioreducible Micelles Self-Assembled from Poly(ethylene glycol)-Cholesteryl Conjugate As a Drug Delivery Platform" Polymers 7, no. 11: 2245-2258. https://doi.org/10.3390/polym7111511
APA StyleBaek, C., Ha, T.-L., Kim, E., Jeong, S. W., Lee, S. G., Lee, S. J., & Kim, H.-C. (2015). Bioreducible Micelles Self-Assembled from Poly(ethylene glycol)-Cholesteryl Conjugate As a Drug Delivery Platform. Polymers, 7(11), 2245-2258. https://doi.org/10.3390/polym7111511