Polymethylmethacrylate/Polyacrylonitrile Membranes via Centrifugal Spinning as Separator in Li-Ion Batteries
Abstract
:1. Introduction

2. Experimental Section
2.1. Materials
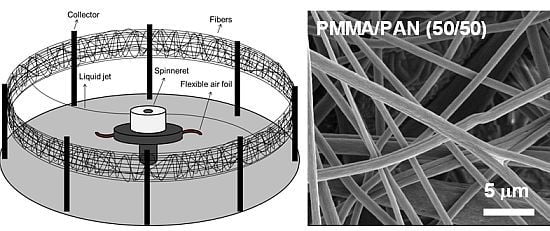
2.2. Separator Preparation
2.3. Structure Characterization
2.4. Performance Evaluation
3. Results and Discussion
3.1. Separator Morphology


| Samples | Porosity (%) | Electrolyte Uptake (%) | Ionic Conductivity (mS/cm) |
|---|---|---|---|
| PMMA membrane | 57 | 300 | 2.8 |
| PMMA/PAN (75/25) membrane | 64 | 330 | 3.0 |
| PMMA/PAN (50/50) membrane | 73 | 370 | 3.2 |
| Microporous PP membrane | 41 | 158 | 0.8 |
3.2. Liquid Electrolyte Uptake
3.3. Ionic Conductivity
3.4. Electrochemical Oxidation Limit

3.5. Interfacial Resistance


3.6. Charge-Discharge and Cycling Performance


3.7. C-Rate Performance

4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yanilmaz, M.; Lu, Y.; Dirican, M.; Fu, K.; Zhang, X. Nanoparticle-on-nanofiber hybrid membrane separators for lithium-ion batteries via combining electrospraying and electrospinning techniques. J. Membr. Sci. 2014, 456, 57–65. [Google Scholar] [CrossRef]
- Dirican, M.; Yanilmaz, M.; Fu, K.; Lu, Y.; Kizil, H.; Zhang, X. Carbon-enhanced electrodeposited SnO2/carbon nanofiber composites as anode for lithium-ion batteries. J. Power Sources 2014, 264, 240–247. [Google Scholar] [CrossRef]
- Li, Y.; Xu, G.; Yao, Y.; Xue, L.; Yanilmaz, M.; Lee, H.; Zhang, X. Coaxial electrospun Si/C–C core–shell composite nanofibers as binder-free anodes for lithium-ion batteries. Solid State Ion. 2014, 258, 67–73. [Google Scholar] [CrossRef]
- Fu, K.; Lu, Y.; Dirican, M.; Chen, C.; Yanilmaz, M.; Shi, Q.; Bradford, P.D.; Zhang, X. Chamber-confined silicon–carbon nanofiber composites for prolonged cycling life of Li-ion battery. Nanoscale 2014, 6, 7489–7495. [Google Scholar] [CrossRef] [PubMed]
- Dirican, M.; Yanilmaz, M.; Fu, K.; Yildiz, O.; Kizil, H.; Hu, Y.; Zhang, X. Carbon-confined PVA-derived silicon/silica/carbon nanofiber composites as anode for lithium-ion batteries. J. Electrochem. Soc. 2014, 161, A2197–A2203. [Google Scholar] [CrossRef]
- Lee, H.; Yanilmaz, M.; Toprakci, O.; Fu, K.; Zhang, X. A review of recent developments in membrane separators for rechargeable lithium-ion batteries. Energy Environ. Sci. 2014, 7, 3857–3886. [Google Scholar] [CrossRef]
- Yanilmaz, M.; Chen, C.; Zhang, X. Fabrication and characterization of SiO2/PVDF composite nanofiber-coated PP nonwoven separators for lithium-ion batteries. J. Polym. Sci. B Polym. Phys. 2013, 51, 1719–1726. [Google Scholar] [CrossRef]
- Yanilmaz, M.; Lu, Y.; Li, Y.; Zhang, X. SiO2/polyacrylonitrile membranes via centrifugal spinning as a separator for Li-ion batteries. J. Power Sources 2015, 273, 1114–1119. [Google Scholar] [CrossRef]
- Yanilmaz, M.; Kalaoglu, F.; Karakas, H.; Sarac, A.S. Preparation and characterization of electrospun polyurethane–polypyrrole nanofibers and films. J. Appl. Polym. Sci. 2012, 125, 4100–4108. [Google Scholar] [CrossRef]
- Yanilmaz, M.; Kalaoglu, F.; Karakas, H. Investigation on the effect of process variables on polyurethane nanofibre diameter using a factorial design. Fibres Text. East Eur. 2013, 21, 19–21. [Google Scholar]
- Yanilmaz, M.; Kalaoğlu, F.; Karakaş, H. Study on optimising the morphology of electrospun polyurethane nanofibers. J. Text. Appar. (Tekstil ve Konfeksiyon) 2012, 22, 212–217. [Google Scholar]
- Yanilmaz, M.; Dirican, M.; Zhang, X. Evaluation of electrospun SiO2/nylon 6,6 nanofiber membranes as a thermally-stable separator for lithium-ion batteries. Electrochim. Acta 2014, 133, 501–508. [Google Scholar] [CrossRef]
- Yanilmaz, M.; Sarac, A.S. A review: Effect of conductive polymers on the conductivities of electrospun mats. Text. Res. J. 2014, 84, 1325–1342. [Google Scholar] [CrossRef]
- Lu, Y.; Fu, K.; Zhang, S.; Li, Y.; Chen, C.; Zhu, J.; Yanilmaz, M.; Dirican, M.; Zhang, X. Centrifugal spinning: A novel approach to fabricate porous carbon fibers as binder-free electrodes for electric double-layer capacitors. J. Power Sources 2015, 273, 502–510. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, Y. Centrifugal spinning: An alternative approach to fabricate nanofibers at high speed and low cost. Polym. Rev. 2014, 54, 677–701. [Google Scholar] [CrossRef]
- Lu, Y.; Li, Y.; Zhang, S.; Xu, G.; Fu, K.; Lee, H.; Zhang, X. Parameter study and characterization for polyacrylonitrile nanofibers fabricated via centrifugal spinning process. Eur. Polym. J. 2013, 49, 3834–3845. [Google Scholar] [CrossRef]
- Zhang, S.S.; Xu, K.; Jow, T.R. An inorganic composite membrane as the separator of Li-ion batteries. J. Power Sources 2005, 140, 361–364. [Google Scholar] [CrossRef]
- Gopalan, A.I.; Santhosh, P.; Manesh, K.M.; Nho, J.H.; Kim, S.H.; Hwang, C.-G.; Lee, K.-P. Development of electrospun PVDF–PAN membrane-based polymer electrolytes for lithium batteries. J. Membr. Sci. 2008, 325, 683–690. [Google Scholar] [CrossRef]
- Flora, X.H.; Ulaganathan, M.; Babu, R.S.; Rajendran, S. Evaluation of lithium ion conduction in PAN/PMMA-based polymer blend electrolytes for Li-ion battery applications. Ionics 2012, 18, 731–736. [Google Scholar] [CrossRef]
- Sohn, J.-Y.; Im, J.-S.; Shin, J.; Nho, Y.-C. PVDF-HFP/PMMA-coated PE separator for lithium ion battery. J. Solid State Electrochem. 2012, 16, 551–556. [Google Scholar] [CrossRef]
- Li, H.; Lin, C.-E.; Shi, J.-L.; Ma, X.-T.; Zhu, B.-K.; Zhu, L.-P. Preparation and characterization of safety PVDF/P (MMA-co-PEGMA) active separators by studying the liquid electrolyte distribution in this kind of membrane. Electrochim. Acta 2014, 115, 317–325. [Google Scholar] [CrossRef]
- Subramania, A.; Sundaram, N.; Kumar, G.V. Structural and electrochemical properties of micro-porous polymer blend electrolytes based on PVDF-co-HFP-PAN for Li-ion battery applications. J. Power Sources 2006, 153, 177–182. [Google Scholar] [CrossRef]
- Ma, T.; Cui, Z.; Wu, Y.; Qin, S.; Wang, H.; Yan, F.; Han, N.; Li, J. Preparation of PVDF based blend microporous membranes for lithium ion batteries by thermally induced phase separation: I. Effect of PMMA on the membrane formation process and the properties. J. Membr. Sci. 2013, 444, 213–222. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, P.; Long, Z.; Jiang, Y.; Xu, F.; Di, W. The ionic conductivity and mechanical property of electrospun P(VdF-HFP)/PMMA membranes for lithium ion batteries. J. Membr. Sci. 2009, 329, 56–59. [Google Scholar] [CrossRef]
- Hong, C.K.; Yang, K.S.; Oh, S.H.; Ahn, J.H.; Cho, B.H.; Nah, C. Effect of blend composition on the morphology development of electrospun fibres based on PAN/PMMA blends. Polym. Int. 2008, 57, 1357–1362. [Google Scholar] [CrossRef]
- Jung, H.-R.; Ju, D.-H.; Lee, W.-J.; Zhang, X.; Kotek, R. Electrospun hydrophilic fumed silica/polyacrylonitrile nanofiber-based composite electrolyte membranes. Electrochim. Acta 2009, 54, 3630–3637. [Google Scholar] [CrossRef]
- Raghavan, P.; Zhao, X.; Shin, C.; Baek, D.-H.; Choi, J.-W.; Manuel, J.; Heo, M.-Y.; Ahn, J.-H.; Nah, C. Preparation and electrochemical characterization of polymer electrolytes based on electrospun poly(vinylidene fluoride-co-hexafluoropropylene)/polyacrylonitrile blend/composite membranes for lithium batteries. J. Power Sources 2010, 195, 6088–6094. [Google Scholar] [CrossRef]
- Choi, S.; Kim, J.; Jo, S.; Lee, W.; Kim, Y.-R. Electrochemical and spectroscopic properties of electrospun pan-based fibrous polymer electrolytes. J. Electrochem. Soc. 2005, 152, A989–A995. [Google Scholar] [CrossRef]
- Raja, M.; Angulakshmi, N.; Thomas, S.; Kumar, T.P.; Stephan, A.M. Thin, flexible and thermally stable ceramic membranes as separator for lithium-ion batteries. J. Membr. Sci. 2014, 471, 103–109. [Google Scholar] [CrossRef]
- Shubha, N.; Prasanth, R.; Hng, H.H.; Srinivasan, M. Study on effect of poly (ethylene oxide) addition and in-situ porosity generation on poly (vinylidene fluoride)–glass ceramic composite membranes for lithium polymer batteries. J. Power Sources 2014, 267, 48–57. [Google Scholar] [CrossRef]
- Cao, J.; Wang, L.; Fang, M.; Shang, Y.; Deng, L.; Yang, J.; Li, J.; Chen, H.; He, X. Interfacial compatibility of gel polymer electrolyte and electrode on performance of Li-ion battery. Electrochim. Acta 2013, 114, 527–532. [Google Scholar] [CrossRef]
- Cao, C.; Tan, L.; Liu, W.; Ma, J.; Li, L. Polydopamine coated electrospun poly (vinyldiene fluoride) nanofibrous membrane as separator for lithium-ion batteries. J. Power Sources 2014, 248, 224–229. [Google Scholar] [CrossRef]
- Yang, P.; Zhang, P.; Shi, C.; Chen, L.; Dai, J.; Zhao, J. The functional separator coated with core–shell structured silica–poly (methyl methacrylate) sub-microspheres for lithium-ion batteries. J. Membr. Sci. 2015, 474, 148–155. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yanilmaz, M.; Zhang, X. Polymethylmethacrylate/Polyacrylonitrile Membranes via Centrifugal Spinning as Separator in Li-Ion Batteries. Polymers 2015, 7, 629-643. https://doi.org/10.3390/polym7040629
Yanilmaz M, Zhang X. Polymethylmethacrylate/Polyacrylonitrile Membranes via Centrifugal Spinning as Separator in Li-Ion Batteries. Polymers. 2015; 7(4):629-643. https://doi.org/10.3390/polym7040629
Chicago/Turabian StyleYanilmaz, Meltem, and Xiangwu Zhang. 2015. "Polymethylmethacrylate/Polyacrylonitrile Membranes via Centrifugal Spinning as Separator in Li-Ion Batteries" Polymers 7, no. 4: 629-643. https://doi.org/10.3390/polym7040629
APA StyleYanilmaz, M., & Zhang, X. (2015). Polymethylmethacrylate/Polyacrylonitrile Membranes via Centrifugal Spinning as Separator in Li-Ion Batteries. Polymers, 7(4), 629-643. https://doi.org/10.3390/polym7040629