3.1. Structure Analysis
The infrared spectrum of acetic acid vinyl ester and maleic anhydride copolymer is tested by Fourier transformation infrared spectrometric analyzer. It can be seen from the
Figure 2 that the absorption peak of wave number is 1780 cm
−1 for carboxylic acid C=O stretching vibration and 1231 cm
−1 for C–O stretching vibration; they are the characteristic structures of acid anhydride. The wave number is 1780 cm
−1 for carboxyl ester C=O stretching vibration and 1374 cm
−1 for methyl groups C–H flexural vibration. The intensity of the absorption band in wave number 1374 cm
−1 is greater than in 1433 cm
−1, which is an obvious characteristic of vinyl acetate structure [
11,
12]. Thereby, it proves the reaction of copolymerization between acetic acid vinyl ester and maleic anhydride. See the reaction equation of VM in
Figure 2.
Figure 2.
FT-IR spectrum of copolymer (a) VM; and (b) VM-g-PA66.
Figure 2.
FT-IR spectrum of copolymer (a) VM; and (b) VM-g-PA66.
See the infrared spectroscopic analysis of grafted copolymer in
Figure 2b. The peaks of 1650 and 1540 cm
−1 are corresponding to the absorption peak of C=O and the absorption peak of N–H and C–N of amide. There was a large and wide peak envelope in the range of 2700–3600 cm
−1. This is the characteristic peak of the carboxyl group. However, 1433 and 1374 cm
−1 are the characteristic absorption peaks of vinyl acetate, which proves that PA66 and VM can react to grafted copolymer by melt blending.
Figure 3a is the FT-IR spectrum of PA66/PVDF blends. It can be seen from the figure that PA66 has a significant absorption peak at 3301 cm
−1, which is corresponding to the stretching vibration of N–H. The strong amide I band at 1640 cm
−1 is produced by the C=O stretching vibration of amide group. The characteristic absorption peaks of amide at 1555 and 1405 cm
−1 were produced by C–N stretching vibration and N–H flexural vibration. For PA66 and PVDF, FT-IR spectrum of PA66/PVDF blend film has no new peak or significant deviation. It means there is no bulking agent, PA and PVDF cannot have chemical reaction by melt blending.
Figure 3b is the infrared spectrum of PA66/PVDF and VM-
g-PA66/PVDF. When the infrared spectral curve of VM-
g-PA66/PVDF was compared with that of VM, it can be found that the two acid anhydride peaks disappear at 1780 and 1740 cm
−1. When the infrared spectral curve of VM-
g-PA66/PVDF is compared with it of VM, it can be found that VM-
g-PA66/PVDF characteristic peak of cyclic amide disappears at 1414 cm
−1, which shows the anhydride of VM molecule has chemical reaction with –NH of PA66 molecule.
Figure 3.
FT-IR spectrum of PA66/PVDF blends: (a) without VM; (b) with VM.
Figure 3.
FT-IR spectrum of PA66/PVDF blends: (a) without VM; (b) with VM.
Figure 4 is DSC curve of PA66/PVDF blends. It can be seen from the figure that the melting peak position of PA66 is at 223.2 °C. The melting peak of PA66/PVDF blends were coexisting after blending PA66 and PVDF, it shows PA66 and PVDF of PA66/PVDF blends can also be crystallized.
Figure 4.
DSC thermograms of PA66/PVDF blends at 10 °C·min−1: (a) without VM; (b) with VM.
Figure 4.
DSC thermograms of PA66/PVDF blends at 10 °C·min−1: (a) without VM; (b) with VM.
Figure 5 is XRD pattern of PA66 and PVDF blends. It can be seen from the figure that there are the α crystal-form diffraction peak of pure PA66 monomer at 2θ = 20.3° and β crystal-form diffraction peak at 2θ = 22.8°. It can be seen by blending PVDF and PA66 that the α crystal-form diffraction peak of PA66 monomer at 2θ = 20.3° was weakened with the accession of PVDF, but the β crystal-form diffraction peak at 2θ = 22.8° was enhanced, which shows that PVDF can inhibit the formation of α crystal form of PA66 and promote the β crystal of PA66. Besides, there are α crystals formed of PVDF in PVDF/PA66 blends, which shows that the accession of PA66 cannot promote to form β crystal or inhibit the formation of α crystal form in PVDF.
Figure 6 is the SEM image of PA66/PVDF blends.
Figure 6a is the SEM image of PA66/PVDF. It can be seen from
Figure 6a that PA66/PVDF blends present a unique structure of ordered arrangement which is evenly distributed. It can be seen clearly that the particles of PA66 are evenly distributed in PVDF. VM-
g-PA66 is as the dispersion phase of the main ingredient which is dispersed in flakes, where the continuous phase gives priority to PVDF. With regard to the amplification of the continuous phase, it can also be found that there are small VM-
g-PA66 particles. All of these are demonstrated that PA/PVDF blends are a two-phase system.
Figure 6b is the SEM image of VM-
g-PA66/PVDF blends after adding the bulking agent of VM. It can be seen from the figure that PA66/PVDF blend film presents a two-phase separation structure in PA66/PVDF blends. PA66 as the dispersion phase of the main ingredient is dispersed in flakes in the continuous phase where it gives priority to PVDF, and the interface adhesion is not good.
Figure 6b displays that the accession of 3 wt % bulking agent VM makes the interface of dispersion phase and continuous phase blurred with the melt blending of PA66 and PVDF. This shows that the accession of bulking agent VA-MA can improve interface adhesion between the PVDF phase and PA66 phase.
Figure 5.
XRD pattern of PA66/PVDF blends.
Figure 5.
XRD pattern of PA66/PVDF blends.
Figure 6.
SEM micrographs for the PA66/PVDF and VMg-PA66/PVDF blends. (a) PA66/PVDF(40/60); (b) 3 wt %VM-g-PA66/PVDF(40/60).
Figure 6.
SEM micrographs for the PA66/PVDF and VMg-PA66/PVDF blends. (a) PA66/PVDF(40/60); (b) 3 wt %VM-g-PA66/PVDF(40/60).
3.2. Dielectric Properties
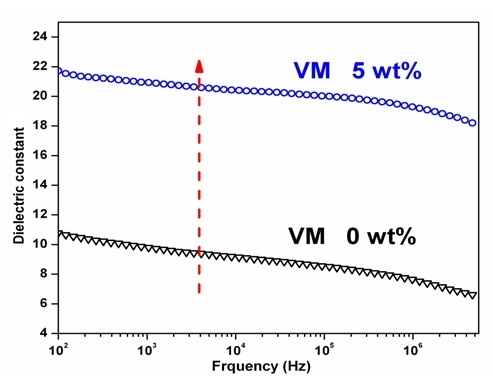
Figure 7 is the relationship of dielectric constant ε of PA66/PVDF blend system to frequency. In PA66/PVDF blend system. It can be seen from the figure that the dielectric constant ε of PA/PVDF blend system has no significant change with the increase of electric field frequency, which presents excellent frequency stability. After PA and PVDF are melted and blended according to different volume ratios, the dielectric constants of all the volume ratios of PA66/PVDF blends decrease with the increase of frequency. It can also be seen from the figure that the dielectric loss of PA66/PVDF blends in different volume ratios increases with the increase of frequency after PA66 and PVDF are melted and blended in different volume ratios, but it is slower than the speed of pure PVDF. In the low frequency region (
f < 10
5 Hz), the dielectric loss tanδ of PA66/PVDF is less than 0.10, and there is no significant difference among the volume ratios. In the high frequency region (such as 10
6 Hz), the dielectric loss of PA66/PVDF blends decreases continuously with the increase of PA66 volume content in the blend system. This is because the dielectric loss of PA66 is lower than PVDF in high frequency region, and polar amide groups can promote the orientation of CF
2 in high frequency, decreasing thermal loss of the blends. This shows that the interaction of PA66 and PVDF requires a better compatibility with the melt blending of PA66 and PVDF.
Figure 7.
Frequency dependence of (a) ε and (b) tanδ for PA66/PVDF blends.
Figure 7.
Frequency dependence of (a) ε and (b) tanδ for PA66/PVDF blends.
Figure 8 is the melt blending of PA66 and PVDF after VM is added, by which it can not only improve the dielectric constant of PA66/PVDF blends to some extent. Meanwhile, it can reduce the dielectric loss of blends. VA-MA makes polar dipoles easier to be oriented in the PA66/PVDF blend system, thus lowering the dielectric loss of blends.
Figure 8.
Variation of (a) ε and tanδ (b) versus VM content of VM-g-PA66/PVDF blends.
Figure 8.
Variation of (a) ε and tanδ (b) versus VM content of VM-g-PA66/PVDF blends.
PA66/PVDF blends present excellent dielectric properties. However, combining the result of thermal property analysis on three PA/PVDF blends in the last section, it can be found that the melting point (Tm = 223.2 °C) of PA66 is high. During the melt blending with PVDF (Tm = 163.4 °C), it requires a very high processing temperature. In the temperature rising process, it may destroy the crystal form of the polymer and cause inconvenience for preparation. Therefore, VM is selected to provide graft modification on PA66 to study the influence it has on PVDF blends’ other properties.
At room temperature, when the frequency is 10
3 Hz, the dielectric properties of VM-
g-PA66/PVDF blends are obtained for analysis after adding different mass fractions of VM. The quantities of VM added are 1 wt %, 3 wt %, and 5 wt %. The relation curve of the dielectric constants of VM-
g-PA66/PVDF blends to the content of VM is shown in
Figure 8. Besides, the imaginary line means VM-
g-PA66/PVDF blends of VM which are not added. Obviously, the influence of bulking agent VM-
g-PA66/PVDF on the dielectric constant of blends was 3 wt % > 1 wt % > 5 wt %. When PA66 and PVDF are melted and blended, it can improve the flexibility of molecules with the accession of VM, and the dielectric constant of VM-
g-PA66/PVDF can be improved. However, excessive VM can improve the grafting ratio of PA66, and the crystallinity of PA66 is declined, which is not good for the collection and delivery of system charge.