Heteroatom-Doped Carbon Nanostructures Derived from Conjugated Polymers for Energy Applications
Abstract
:1. Introduction
2. Carbon Nanomaterials Derived from PANI
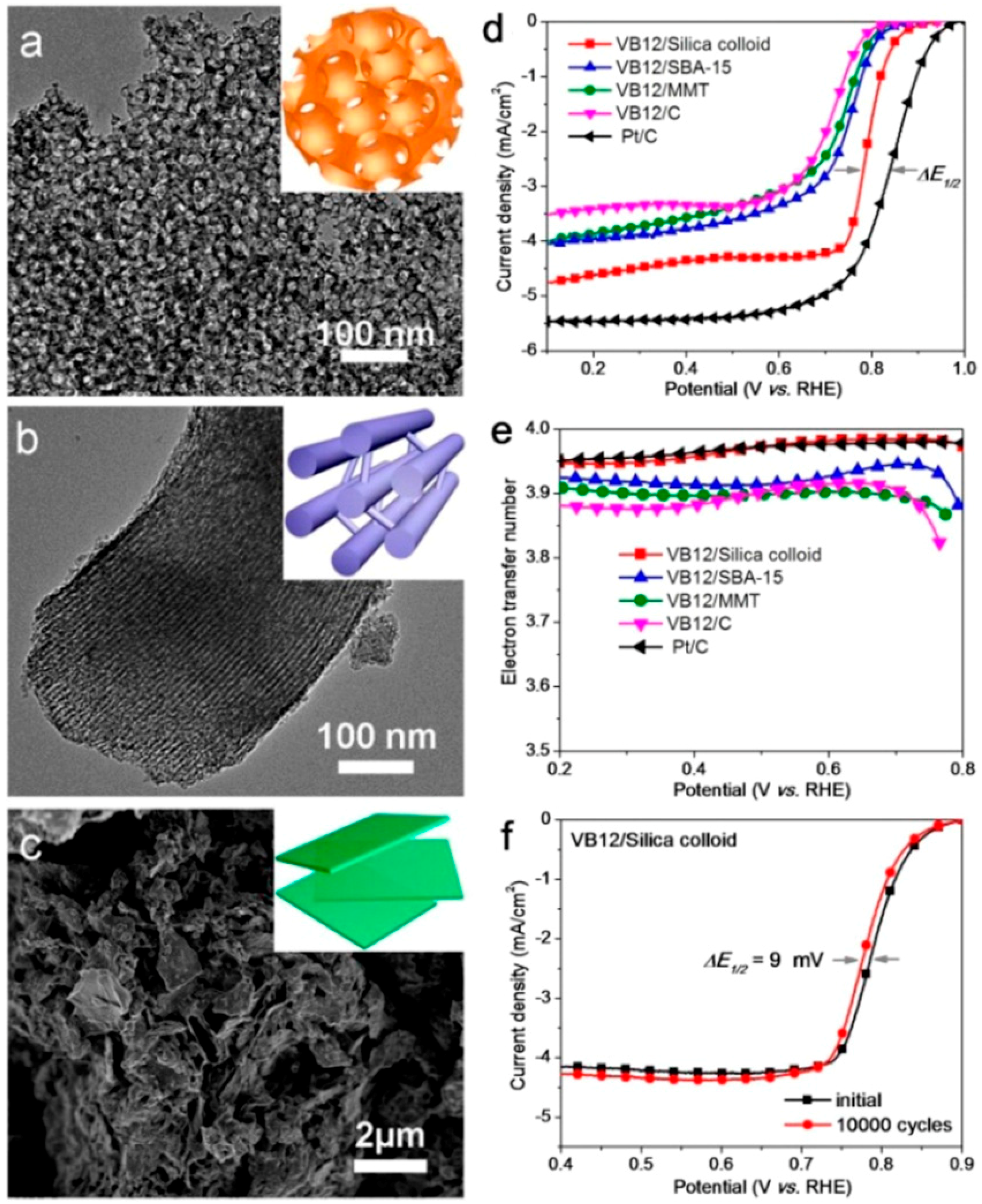
2.1. Catalytic ORR Activity of PANI-Derived Nitrogen-Doped Carbon Nanomaterials
2.2. Electrode Material of PANI-Derived Nitrogen-Doped Carbon Nanomaterials
3. Carbon Nanomaterials Derived from PPy
3.1. Catalytic ORR Activity of PPy-Derived Nitrogen-Doped Carbon Nanomaterials
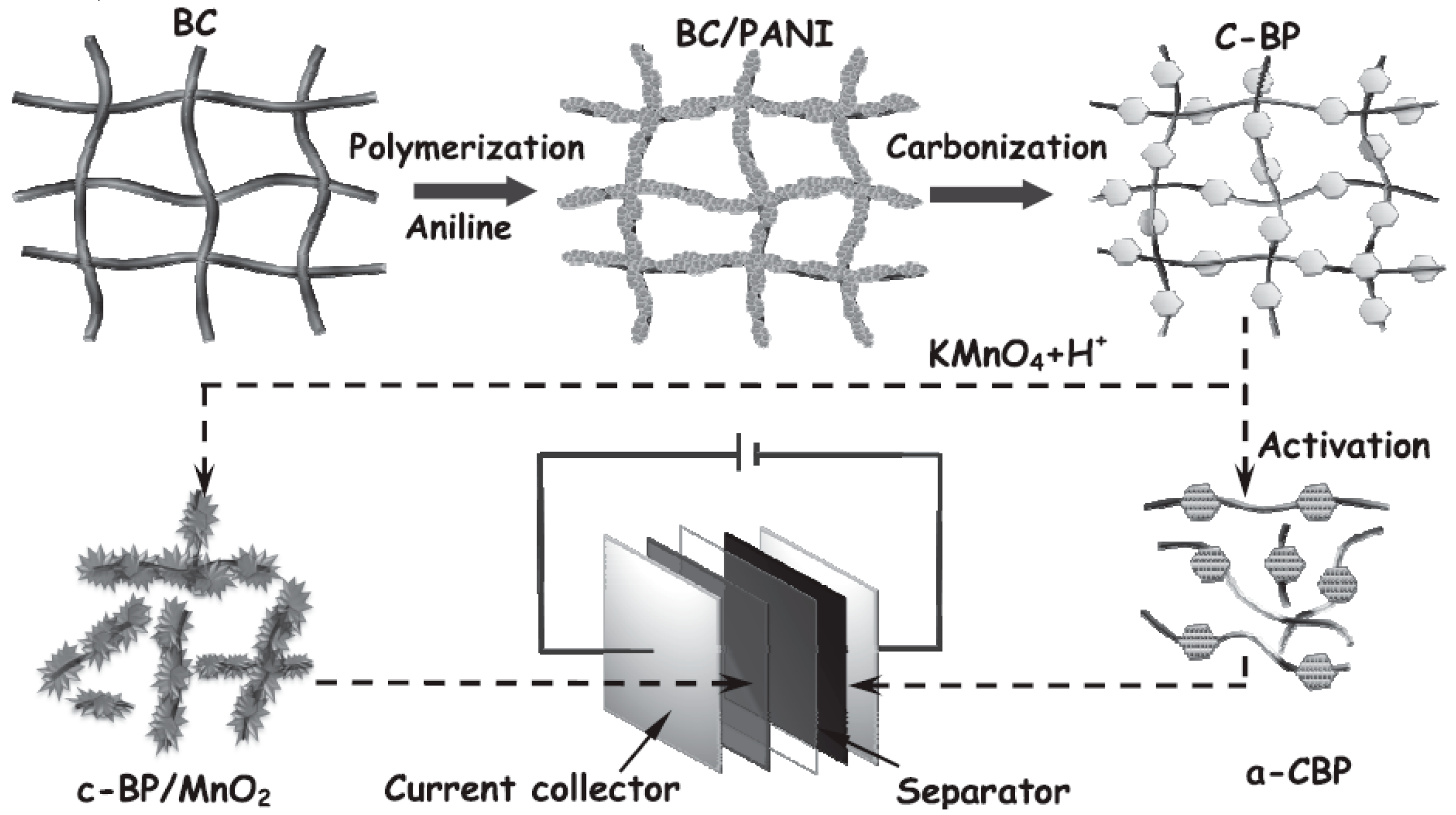
3.2. Electrode Material of PPy-Derived Nitrogen-Doped Carbon Nanomaterials
4. Carbon Nanomaterials Derived from Derivatives of CPs
5. Conclusions and Outlook
Acknowledgments
Conflicts of Interest
References
- Duan, J.; Chen, S.; Jaroniec, M.; Qiao, S.Z. Heteroatom-doped graphene-based materials for energy-relevant electrocatalytic processes. ACS Catal. 2015, 5, 5207–5234. [Google Scholar] [CrossRef]
- Chen, G.F.; Li, X.X.; Zhang, L.Y.; Li, N.; Ma, T.Y.; Liu, Z.Q. A porous perchlorate-doped polypyrrole nanocoating on nickel nanotube arrays for stable wide-potential-window supercapacitors. Adv. Mater. 2016. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Z.; Cheng, T.; Wang, Y.; Lai, W.Y.; Pang, H.; Huang, W. A simple approach to boost capacitance: Flexible supercapacitors based on manganese Oxides@MOFs via chemically induced in situ self-transformation. Adv. Mater. 2016, 28, 5242–5248. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Xu, L.; Yan, Y.; Zakhidov, A.A.; Baughman, R.H.; Chen, J. Ordered mesoporous nickel sphere arrays for highly efficient electrocatalytic water oxidation. ACS Catal. 2016, 6, 1446–1450. [Google Scholar] [CrossRef]
- Dai, L.; Xue, Y.; Qu, L.; Choi, H.J.; Baek, J.B. Metal-free catalysts for oxygen reduction reaction. Chem. Rev. 2015, 115, 4823–4892. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Shi, G. Conducting polymer-based catalysts. J. Am. Chem. Soc. 2016, 138, 2868–2876. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Wang, H.L.; Guo, S. Towards high-efficiency nanoelectrocatalysts for oxygen reduction through engineering advanced carbon nanomaterials. Chem. Soc. Rev. 2016, 45, 1273–1307. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Nabae, Y.; Hayakawa, T.; Kakimoto, M.-A. Highly selective two-electron oxygen reduction catalyzed by mesoporous nitrogen-doped carbon. ACS Catal. 2014, 4, 3749–3754. [Google Scholar] [CrossRef]
- Shao, M.; Chang, Q.; Dodelet, J.P.; Chenitz, R. Recent advances in electrocatalysts for oxygen reduction reaction. Chem. Rev. 2016, 116, 3594–3657. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhuang, X.; Su, Y.; Zhang, F.; Feng, X. Polyaniline nanosheet derived B/N co-doped carbon nanosheets as efficient metal-free catalysts for oxygen reduction reaction. J. Mater. Chem. A 2014, 2, 7742–7746. [Google Scholar] [CrossRef]
- Song, J.; Gordin, M.L.; Xu, T.; Chen, S.; Yu, Z.; Sohn, H.; Lu, J.; Ren, Y.; Duan, Y.; Wang, D. Strong lithium polysulfide chemisorption on electroactive sites of nitrogen-doped carbon composites for high-performance lithium-sulfur battery cathodes. Angew. Chem. Int. Ed. 2015, 54, 4325–4329. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Dai, L. Heteroatom-doped graphitic carbon catalysts for efficient electrocatalysis of oxygen reduction reaction. ACS Catal. 2015, 5, 7244–7253. [Google Scholar] [CrossRef]
- Yang, J.; Sun, H.; Liang, H.; Ji, H.; Song, L.; Gao, C.; Xu, H. A highly efficient metal-free oxygen reduction electrocatalyst assembled from carbon nanotubes and graphene. Adv. Mater. 2016, 28, 4606–4613. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Zhu, Z.; Dong, S. Carbon-based nanostructures for advanced catalysis. ChemCatChem 2015, 7, 2806–2815. [Google Scholar] [CrossRef]
- Wan, K.; Yu, Z.-P.; Li, X.-H.; Liu, M.-Y.; Yang, G.; Piao, J.-H.; Liang, Z.-X. pH effect on electrochemistry of nitrogen-doped carbon catalyst for oxygen reduction reaction. ACS Catal. 2015, 5, 4325–4332. [Google Scholar] [CrossRef]
- Wang, Q.; Yan, J.; Fan, Z. Carbon materials for high volumetric performance supercapacitors: Design, progress, challenges and opportunities. Energy Environ. Sci. 2016, 9, 729–762. [Google Scholar] [CrossRef]
- Yang, X.; Liu, A.; Zhao, Y.; Lu, H.; Zhang, Y.; Wei, W.; Li, Y.; Liu, S. Three-dimensional macroporous polypyrrole-derived graphene electrode prepared by the hydrogen bubble dynamic template for supercapacitors and metal-free catalysts. ACS Appl. Mater. Interfaces 2015, 7, 23731–23740. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Xie, Y.; Zou, K.; Ji, X. Review on recent advances in nitrogen-doped carbons: Preparations and applications in supercapacitors. J. Mater. Chem. A 2016, 4, 1144–1173. [Google Scholar] [CrossRef]
- Paraknowitsch, J.P.; Thomas, A. Doping carbons beyond nitrogen: An overview of advanced heteroatom doped carbons with boron, sulphur and phosphorus for energy applications. Energy Environ. Sci. 2013, 6, 2839–2855. [Google Scholar] [CrossRef]
- Li, Q.; Cao, R.; Cho, J.; Wu, G. Nanocarbon electrocatalysts for oxygen reduction in alkaline media for advanced energy conversion and storage. Adv. Energy Mater. 2014, 4, 1301415. [Google Scholar] [CrossRef]
- Xu, P.; Han, X.; Zhang, B.; Du, Y.; Wang, H.L. Multifunctional polymer-metal nanocomposites via direct chemical reduction by conjugated polymers. Chem. Soc. Rev. 2014, 43, 1349–1360. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, A.; Kumar, R.; Arya, S.K.; Nair, M.; Malhotra, B.D.; Bhansali, S. Organic-inorganic hybrid nanocomposite-based gas sensors for environmental monitoring. Chem. Rev. 2015, 115, 4571–4606. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Peng, L.; Yu, G. Nanostructured conducting polymer hydrogels for energy storage applications. Nanoscale 2015, 7, 12796–12806. [Google Scholar] [CrossRef] [PubMed]
- Khomenko, V.G.; Barsukov, V.Z.; Katashinskii, A.S. The catalytic activity of conducting polymers toward oxygen reduction. Electrochim. Acta 2005, 50, 1675–1683. [Google Scholar] [CrossRef]
- Balch, A.L.; Winkler, K. Two-component polymeric materials of fullerenes and the transition metal complexes: A bridge between metal-organic frameworks and conducting polymers. Chem. Rev. 2016, 116, 3812–3882. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Zhao, C.E.; Lin, Z.Q.; Gu, P.Y.; Zhang, Q. Nanostructured conjugated polymers for energy-related applications beyond solar cells. Chem. Asian J. 2016, 11, 1489–1511. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Kampstra, K.L.; Abidian, M.R. High performance conducting polymer nanofiber biosensors for detection of biomolecules. Adv. Mater. 2014, 26, 4954–4960. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kang, Y.J.; Majd, S. A Review of patterned organic bioelectronic materials and their biomedical applications. Adv. Mater. 2015, 27, 7583–7619. [Google Scholar] [CrossRef] [PubMed]
- Zhao, A.; Masa, J.; Muhler, M.; Schuhmann, W.; Xia, W. N-doped carbon synthesized from N-containing polymers as metal-free catalysts for the oxygen reduction under alkaline conditions. Electrochim. Acta 2013, 98, 139–145. [Google Scholar] [CrossRef]
- Jin, C.; Nagaiah, T.C.; Xia, W.; Spliethoff, B.; Wang, S.; Bron, M.; Schuhmann, W.; Muhler, M. Metal-free and electrocatalytically active nitrogen-doped carbon nanotubes synthesized by coating with polyaniline. Nanoscale 2010, 2, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.W.; Higgins, D.; Yu, A.P.; Zhang, L.; Zhang, J.J. A review on non-precious metal electrocatalysts for PEM fuel cells. Energy Environ. Sci. 2011, 4, 3167–3192. [Google Scholar] [CrossRef]
- Shrestha, S.; Mustain, W.E. Properties of nitrogen-functionalized ordered mesoporous carbon prepared using polypyrrole precursor. J. Electrochem. Soc. 2010, 157, B1665. [Google Scholar] [CrossRef]
- Wu, G.; More, K.L.; Johnston, C.M.; Zelenay, P. High-performance electrocatalysts for oxygen reduction derived from polyaniline, iron, and cobalt. Science 2011, 332, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Wei, Z.; Chen, S.; Qi, X.; Yang, T.; Hu, J.; Wang, D.; Wan, L.J.; Alvi, S.F.; Li, L. Space-confinement-induced synthesis of pyridinic- and pyrrolic-nitrogen-doped graphene for the catalysis of oxygen reduction. Angew. Chem. Int. Ed. 2013, 52, 11755–11759. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.; Voiry, D.; Chhowalla, M.; Asefa, T. Efficient metal-free electrocatalysts for oxygen reduction: Polyaniline-derived N- and O-doped mesoporous carbons. J. Am. Chem. Soc. 2013, 135, 7823–7826. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Li, L.; Xiong, K.; Wang, Y.; Li, W.; Nie, Y.; Chen, S.; Qi, X.; Wei, Z. Shape fixing via salt recrystallization: A morphology-controlled approach to convert nanostructured polymer to carbon nanomaterial as a highly active catalyst for oxygen reduction reaction. J. Am. Chem. Soc. 2015, 137, 5414–5420. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Sun, Y.; Li, D.; Liang, H.W.; Dong, R.; Feng, X.; Mullen, K. Controlled synthesis of N-doped carbon nanospheres with tailored mesopores through self-assembly of colloidal silica. Angew. Chem. Int. Ed. 2015, 54, 15191–15196. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Miao, Y.-E.; Huang, Y.; Tjiu, W.W.; Liu, T. Flexible free-standing 3D porous N-doped graphene–carbon nanotube hybrid paper for high-performance supercapacitors. RSC Adv. 2015, 5, 9228–9236. [Google Scholar] [CrossRef]
- Singh, K.P.; Song, M.Y.; Yu, J.-S. Iodine-treated heteroatom-doped carbon: Conductivity driven electrocatalytic activity. J. Mater. Chem. A 2014, 2, 18115–18124. [Google Scholar] [CrossRef]
- Xu, L.; Pan, G.; Liang, X. Nitrogen/sulfur co-doped non-noble metal material as an efficient electrocatalyst for the oxygen reduction reaction in alkaline media. RSC Adv. 2014, 4, 19756–19765. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, Z.; Xia, Z.; Dai, L. A metal-free bifunctional electrocatalyst for oxygen reduction and oxygen evolution reactions. Nat. Nanotechnol. 2015, 10, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Liang, Y.; Hu, Y.; Kong, B.; Simon, G.P.; Zhang, J.; Jiang, S.P.; Wang, H. A versatile iron-tannin-framework ink coating strategy to fabricate biomass-derived iron carbide/Fe–N–carbon catalysts for efficient oxygen reduction. Angew. Chem. Int. Ed. 2016, 55, 1355–1359. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J.; Zhang, H.G.; Lin, H.H.; Gupta, S.; Wang, C.; Tao, Z.X.; Fu, H.; Wang, T.; Zheng, J.; Wu, G.; et al. Directly converting Fe-doped metal organic frameworks into highly active and stable Fe–N–C catalysts for oxygen reduction in acid. Nano Energy 2016, 25, 110–119. [Google Scholar] [CrossRef]
- Li, J.-S.; Tang, Y.-J.; Liu, C.-H.; Li, S.-L.; Li, R.-H.; Dong, L.-Z.; Dai, Z.-H.; Bao, J.-C.; Lan, Y.-Q. Polyoxometalate-based metal–organic framework-derived hybrid electrocatalysts for highly efficient hydrogen evolution reaction. J. Mater. Chem. A 2016, 4, 1202–1207. [Google Scholar] [CrossRef]
- Liang, H.W.; Wei, W.; Wu, Z.S.; Feng, X.; Mullen, K. Mesoporous metal-nitrogen-doped carbon electrocatalysts for highly efficient oxygen reduction reaction. J. Am. Chem. Soc. 2013, 135, 16002–16005. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Deng, C.; Qiu, Y.; Yao, L.; Zhang, H. Nitrogen-doped hierarchically porous carbon as efficient oxygen reduction electrocatalysts in acid electrolyte. J. Mater. Chem. A 2014, 2, 17047–17057. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, X.; Xia, H.; Liu, J.; Wang, H.; Gao, J.; Zhang, Y.; Liu, J.; Zhou, H.; Li, X.; et al. Design, preparation and performance of novel three-dimensional hierarchically porous carbon for supercapacitors. Electrochim. Acta 2015, 173, 566–574. [Google Scholar] [CrossRef]
- Fan, H.S.; Wang, H.; Zhao, N.; Xu, J.; Pan, F. Nano-porous architecture of N-doped carbon nanorods grown on graphene to enable synergetic effects of supercapacitance. Sci. Rep. 2014, 4, 7426. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Cheng, B.; Song, H.; Chen, X. Preparation and electrochemical performance of polyaniline-based carbon nanotubes as electrode material for supercapacitor. Electrochim. Acta 2010, 55, 7021–7027. [Google Scholar] [CrossRef]
- Yuan, D.-S.; Zhou, T.-X.; Zhou, S.-L.; Zou, W.-J.; Mo, S.-S.; Xia, N.-N. Nitrogen-enriched carbon nanowires from the direct carbonization of polyaniline nanowires and its electrochemical properties. Electrochem. Commun. 2011, 13, 242–246. [Google Scholar] [CrossRef]
- Peng, H.; Ma, G.; Sun, K.; Mu, J.; Zhou, X.; Lei, Z. A novel fabrication of nitrogen-containing carbon nanospheres with high rate capability as electrode materials for supercapacitors. RSC Adv. 2015, 5, 12034–12042. [Google Scholar] [CrossRef]
- Tan, Y.; Xu, C.; Chen, G.; Liu, Z.; Ma, M.; Xie, Q.; Zheng, N.; Yao, S. Synthesis of ultrathin nitrogen-doped graphitic carbon nanocages as advanced electrode materials for supercapacitor. ACS Appl. Mater. Interfaces 2013, 5, 2241–2248. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Xu, G.; Ding, B.; Pan, J.; Dou, H.; MacFarlane, D.R. Porous nitrogen-doped hollow carbon spheres derived from polyaniline for high performance supercapacitors. J. Mater. Chem. A 2014, 2, 5352–5357. [Google Scholar] [CrossRef]
- Long, C.; Qi, D.; Wei, T.; Yan, J.; Jiang, L.; Fan, Z. Nitrogen-doped carbon networks for high energy density supercapacitors derived from polyaniline coated bacterial cellulose. Adv. Funct. Mater. 2014, 24, 3953–3961. [Google Scholar] [CrossRef]
- Sevilla, M.; Yu, L.; Fellinger, T.P.; Fuertes, A.B.; Titirici, M.-M. Polypyrrole-derived mesoporous nitrogen-doped carbons with intrinsic catalytic activity in the oxygen reduction reaction. RSC Adv. 2013, 3, 9904–9910. [Google Scholar] [CrossRef]
- Wei, H.; Xu, M.-W.; Bao, S.-J.; Yang, F.; Chai, H. Design and synthesis of carbonized polypyrrole-coated graphene aerogel acting as an efficient metal-free catalyst for oxygen reduction. RSC Adv. 2014, 4, 16979–16984. [Google Scholar] [CrossRef]
- Morozan, A.; Jegou, P.; Campidelli, S.; Palacin, S.; Jousselme, B. Relationship between polypyrrole morphology and electrochemical activity towards oxygen reduction reaction. Chem. Commun. 2012, 48, 4627–4629. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Voiry, D.; Goswami, A.; Zou, X.; Huang, X.; Chhowalla, M.; Liu, Z.; Asefa, T. N-, O-, and S-tridoped nanoporous carbons as selective catalysts for oxygen reduction and alcohol oxidation reactions. J. Am. Chem. Soc. 2014, 136, 13554–13557. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Li, H.; Fu, S.; Du, D.; Lin, Y. Highly efficient nonprecious metal catalysts towards oxygen reduction reaction based on three-dimensional porous carbon nanostructures. Chem. Soc. Rev. 2016, 45, 517–531. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Song, Y.; He, S.; Tjiu, W.W.; Pan, J.; Xia, Y.Y.; Liu, T. Nitrogen-doped graphene nanoribbons as efficient metal-free electrocatalysts for oxygen reduction. ACS Appl. Mater. Interfaces 2014, 6, 4214–4222. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Cao, T.; Chen, C.; Pedramrazi, Z.; Haberer, D.; de Oteyza, D.G.; Fischer, F.R.; Louie, S.G.; Crommie, M.F. Molecular bandgap engineering of bottom-up synthesized graphene nanoribbon heterojunctions. Nat. Nanotechnol. 2015, 10, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Du, R.; Zhu, J.; Mao, Y.; Xue, C.; Zhang, N.; Hou, Y.; Zhang, J.; Yi, T. Three-dimensional nitrogen-doped graphene nanoribbons aerogel as a highly efficient catalyst for the oxygen reduction reaction. Small 2015, 11, 1423–1429. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xu, C.; Du, R.; Mao, Y.; Xue, C.; Chen, L.; Qu, L.; Zhang, J.; Yi, T. Rational design of three-dimensional nitrogen-doped carbon nanoleaf networks for high-performance oxygen reduction. J. Mater. Chem. A 2015, 3, 5617–5627. [Google Scholar] [CrossRef]
- Ferrero, G.A.; Preuss, K.; Marinovic, A.; Jorge, A.B.; Mansor, N.; Brett, D.J.; Fuertes, A.B.; Sevilla, M.; Titirici, M.M. Fe-N-doped carbon capsules with outstanding electrochemical performance and stability for the oxygen reduction reaction in both acid and alkaline conditions. ACS Nano 2016, 10, 5922–5932. [Google Scholar] [CrossRef] [PubMed]
- Masa, J.; Xia, W.; Muhler, M.; Schuhmann, W. On the role of metals in nitrogen-doped carbon electrocatalysts for oxygen reduction. Angew. Chem. Int. Ed. 2015, 54, 10102–10120. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Li, H.; Cheng, X.; Jao, T.-C.; Weng, F.-B.; Su, A.; Chiang, Y.-C. A comparative study of pyrolyzed and doped cobalt-polypyrrole eletrocatalysts for oxygen reduction reaction. Appl. Surf. Sci. 2012, 258, 4048–4053. [Google Scholar] [CrossRef]
- Yuan, X.; Sha, H.-D.; Ding, X.-L.; Kong, H.-C.; Lin, H.; Wen, W.; Huang, T.; Guo, Z.; Ma, Z.-F.; Yang, Y. Comparative investigation on the properties of carbon-supported cobalt-polypyrrole pyrolyzed at various conditions as electrocatalyst towards oxygen reduction reaction. Int. J. Hydrog. Energy 2014, 39, 15937–15947. [Google Scholar] [CrossRef]
- Kuroki, S.; Nabae, Y.; Chokai, M.; Kakimoto, M.-A.; Miyata, S. Oxygen reduction activity of pyrolyzed polypyrroles studied by 15N solid-state NMR and XPS with principal component analysis. Carbon 2012, 50, 153–162. [Google Scholar] [CrossRef]
- Masa, J.; Zhao, A.; Xia, W.; Sun, Z.; Mei, B.; Muhler, M.; Schuhmann, W. Trace metal residues promote the activity of supposedly metal-free nitrogen-modified carbon catalysts for the oxygen reduction reaction. Electrochem. Commun. 2013, 34, 113–116. [Google Scholar] [CrossRef]
- Su, Y.; Jiang, H.; Zhu, Y.; Yang, X.; Shen, J.; Zou, W.; Chen, J.; Li, C. Enriched graphitic N-doped carbon-supported Fe3O4 nanoparticles as efficient electrocatalysts for oxygen reduction reaction. J. Mater. Chem. A 2014, 2, 7281–7287. [Google Scholar] [CrossRef]
- Wan, K.; Long, G.-F.; Liu, M.-Y.; Du, L.; Liang, Z.-X.; Tsiakaras, P. Nitrogen-doped ordered mesoporous carbon: Synthesis and active sites for electrocatalysis of oxygen reduction reaction. Appl. Catal. B Environ. 2015, 165, 566–571. [Google Scholar] [CrossRef]
- Meng, F.L.; Wang, Z.L.; Zhong, H.X.; Wang, J.; Yan, J.M.; Zhang, X.B. Reactive multifunctional template-induced preparation of Fe-N-doped mesoporous carbon microspheres towards highly efficient electrocatalysts for oxygen reduction. Adv. Mater. 2016. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Ding, B.; Nie, P.; Shen, L.; Wang, J.; Zhang, X. Porous nitrogen-doped carbon nanotubes derived from tubular polypyrrole for energy-storage applications. Chem. Eur. J. 2013, 19, 12306–12312. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xu, J.; Zhou, S.; Zhao, N.; Wong, C.-P. Nitrogen-doped hierarchically porous carbon foam: A free-standing electrode and mechanical support for high-performance supercapacitors. Nano Energy 2016, 25, 193–202. [Google Scholar] [CrossRef]
- Chen, L.F.; Zhang, X.D.; Liang, H.W.; Kong, M.; Guan, Q.F.; Chen, P.; Wu, Z.Y.; Yu, S.H. Synthesis of nitrogen-doped porous carbon nanofibers as an efficient electrode material for supercapacitors. ACS Nano 2012, 6, 7092–7102. [Google Scholar] [CrossRef] [PubMed]
- An, B.; Xu, S.; Li, L.; Tao, J.; Huang, F.; Geng, X. Carbon nanotubes coated with a nitrogen-doped carbon layer and its enhanced electrochemical capacitance. J. Mater. Chem. A 2013, 1, 7222–7228. [Google Scholar] [CrossRef]
- Wu, R.; Wang, D.P.; Rui, X.; Liu, B.; Zhou, K.; Law, A.W.; Yan, Q.; Wei, J.; Chen, Z. In-situ formation of hollow hybrids composed of cobalt sulfides embedded within porous carbon polyhedra/carbon nanotubes for high-performance lithium-ion batteries. Adv. Mater. 2015, 27, 3038–3044. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Wen, Z.; Cui, S.; Ci, S.; Mao, S.; Chen, J. An advanced nitrogen-doped graphene/cobalt-embedded porous carbon polyhedron hybrid for efficient catalysis of oxygen reduction and water splitting. Adv. Funct. Mater. 2015, 25, 872–882. [Google Scholar] [CrossRef]
- Qie, L.; Chen, W.; Xu, H.; Xiong, X.; Jiang, Y.; Zou, F.; Hu, X.; Xin, Y.; Zhang, Z.; Huang, Y. Synthesis of functionalized 3D hierarchical porous carbon for high-performance supercapacitors. Energy Environ. Sci. 2013, 6, 2497–2504. [Google Scholar] [CrossRef]
- Ćirić-Marjanović, G.; Mentus, S.; Pašti, I.; Gavrilov, N.; Krstić, J.; Travas-Sejdic, J.; Strover, L.T.; Kopecká, J.; Moravková, Z.; Trchová, M.; et al. Synthesis, characterization, and electrochemistry of nanotubular polypyrrole and polypyrrole-derived carbon nanotubes. J. Phys. Chem. C 2014, 118, 14770–14784. [Google Scholar] [CrossRef]
- Ning, X.; Zhong, W.; Li, S.; Wang, Y.; Yang, W. High performance nitrogen-doped porous graphene/carbon frameworks for supercapacitors. J. Mater. Chem. A 2014, 2, 8859–8867. [Google Scholar] [CrossRef]
- Xiao, M.; Zhu, J.; Feng, L.; Liu, C.; Xing, W. Meso/macroporous nitrogen-doped carbon architectures with iron carbide encapsulated in graphitic layers as an efficient and robust catalyst for the oxygen reduction reaction in both acidic and alkaline solutions. Adv. Mater. 2015, 27, 2521–2527. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Wang, X.; Liu, X.; Yang, X. Integrated synthesis of poly(o-phenylenediamine)-derived carbon materials for high performance supercapacitors. Adv. Mater. 2012, 24, 6524–6529. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Ma, G.; Sun, K.; Mu, J.; Zhang, Z.; Lei, Z. Facile synthesis of poly(p-phenylenediamine)-derived three-dimensional porous nitrogen-doped carbon networks for high performance supercapacitors. J. Phys. Chem. C 2014, 118, 29507–29516. [Google Scholar] [CrossRef]
- Yuan, C.; Liu, X.; Jia, M.; Luo, Z.; Yao, J. Facile preparation of N- and O-doped hollow carbon spheres derived from poly(o-phenylenediamine) for supercapacitors. J. Mater. Chem. A 2015, 3, 3409–3415. [Google Scholar] [CrossRef]
- Li, Y.; Li, T.; Yao, M.; Liu, S. Metal-free nitrogen-doped hollow carbon spheres synthesized by thermal treatment of poly(o-phenylenediamine) for oxygen reduction reaction in direct methanol fuel cell applications. J. Mater. Chem. 2012, 22, 10911–10917. [Google Scholar] [CrossRef]
- Liang, H.W.; Zhuang, X.; Bruller, S.; Feng, X.; Mullen, K. Hierarchically porous carbons with optimized nitrogen doping as highly active electrocatalysts for oxygen reduction. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, B.; Liu, X.; Wang, D.W.; Su, D.S. Unravelling the structure of electrocatalytically active Fe-N complexes in carbon for the oxygen reduction reaction. Angew. Chem. Int. Ed. 2014, 53, 10673–10677. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Han, X.; Du, Y.; Song, B.; Xu, P.; Zhang, B. Bifunctional nitrogen-doped microporous carbon microspheres derived from poly(o-methylaniline) for oxygen reduction and supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 3601–3608. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.; Bo, X.; Wang, H.; Zhang, Y.; Luhana, C.; Guo, L. Poly-o-toluidine cobalt supported on ordered mesoporous carbon as an efficient electrocatalyst for oxygen reduction. Electrochem. Commun. 2012, 25, 35–38. [Google Scholar] [CrossRef]
- Wang, Z.; Xiong, X.; Qie, L.; Huang, Y. High-performance lithium storage in nitrogen-enriched carbon nanofiber webs derived from polypyrrole. Electrochim. Acta 2013, 106, 320–326. [Google Scholar] [CrossRef]
- Zhou, X.; Tang, J.; Yang, J.; Xie, J.; Huang, B. Seaweed-like porous carbon from the decomposition of polypyrrole nanowires for application in lithium ion batteries. J. Mater. Chem. A 2013, 1, 5037–5044. [Google Scholar] [CrossRef]
- Qie, L.; Chen, W.M.; Wang, Z.H.; Shao, Q.G.; Li, X.; Yuan, L.X.; Hu, X.L.; Zhang, W.X.; Huang, Y.H. Nitrogen-doped porous carbon nanofiber webs as anodes for lithium ion batteries with a superhigh capacity and rate capability. Adv. Mater. 2012, 24, 2047–2050. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Duan, Y.; Feng, X.; Yang, J.; Che, S. Synthesis of enantiopure carbonaceous nanotubes with optical activity. Angew. Chem. Int. Ed. 2013, 52, 6858–6862. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Zhong, Y.; Xuan, Z.; Mao, C.; Du, F.; Li, G. Polypyrrole-assisted synthesis of roselike MoS2/nitrogen-containing carbon/graphene hybrids and their robust lithium storage performances. RSC Adv. 2015, 5, 62624–62629. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, C.; Kang, F. Nitrogen-enriched porous carbon coating for manganese oxide nanostructures toward high-performance lithium-ion batteries. ACS Appl. Mater. Interfaces 2015, 7, 9185–9194. [Google Scholar] [CrossRef] [PubMed]







© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Han, X.; Du, Y.; Zhang, B.; Xu, P. Heteroatom-Doped Carbon Nanostructures Derived from Conjugated Polymers for Energy Applications. Polymers 2016, 8, 366. https://doi.org/10.3390/polym8100366
He Y, Han X, Du Y, Zhang B, Xu P. Heteroatom-Doped Carbon Nanostructures Derived from Conjugated Polymers for Energy Applications. Polymers. 2016; 8(10):366. https://doi.org/10.3390/polym8100366
Chicago/Turabian StyleHe, Yanzhen, Xijiang Han, Yunchen Du, Bin Zhang, and Ping Xu. 2016. "Heteroatom-Doped Carbon Nanostructures Derived from Conjugated Polymers for Energy Applications" Polymers 8, no. 10: 366. https://doi.org/10.3390/polym8100366
APA StyleHe, Y., Han, X., Du, Y., Zhang, B., & Xu, P. (2016). Heteroatom-Doped Carbon Nanostructures Derived from Conjugated Polymers for Energy Applications. Polymers, 8(10), 366. https://doi.org/10.3390/polym8100366








