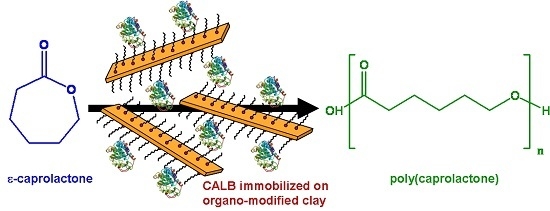
Nanoclays for Lipase Immobilization: Biocatalyst Characterization and Activity in Polyester Synthesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Surface Modification of Clay Minerals
2.2.2. Immobilization of Lipase
2.2.3. Glutaraldehyde Treatment
2.2.4. Determination of Protein Content
2.2.5. Measurement of Lipase Hydrolytic Activity
2.2.6. Applicability of Immobilized Lipase for Esterification Reactions: ε-CL Polymerization Set-Up
2.3. Characterization Techniques
2.3.1. Nuclear Magnetic Resonance (NMR)
2.3.2. Scanning Electron Microscopy (SEM)
2.3.3. X-ray Diffraction Analysis (XRD)
3. Results and Discussion
3.1. CALB Immobilization: Influence of the Type of Clay Support
3.2. Influence of Glutaraldehyde Treatment
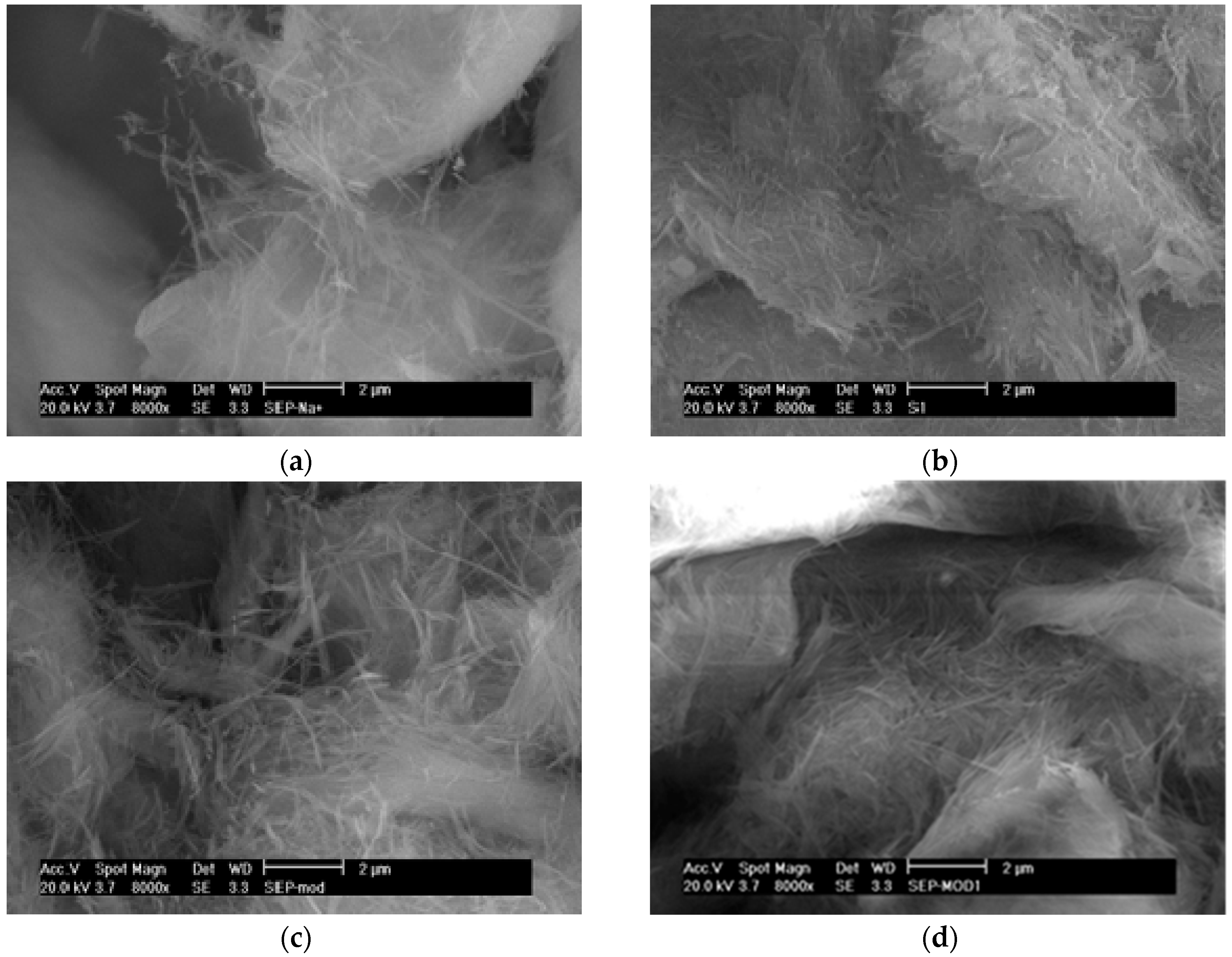
3.3. Surface Characterization of CALB/Clay Catalysts by SEM
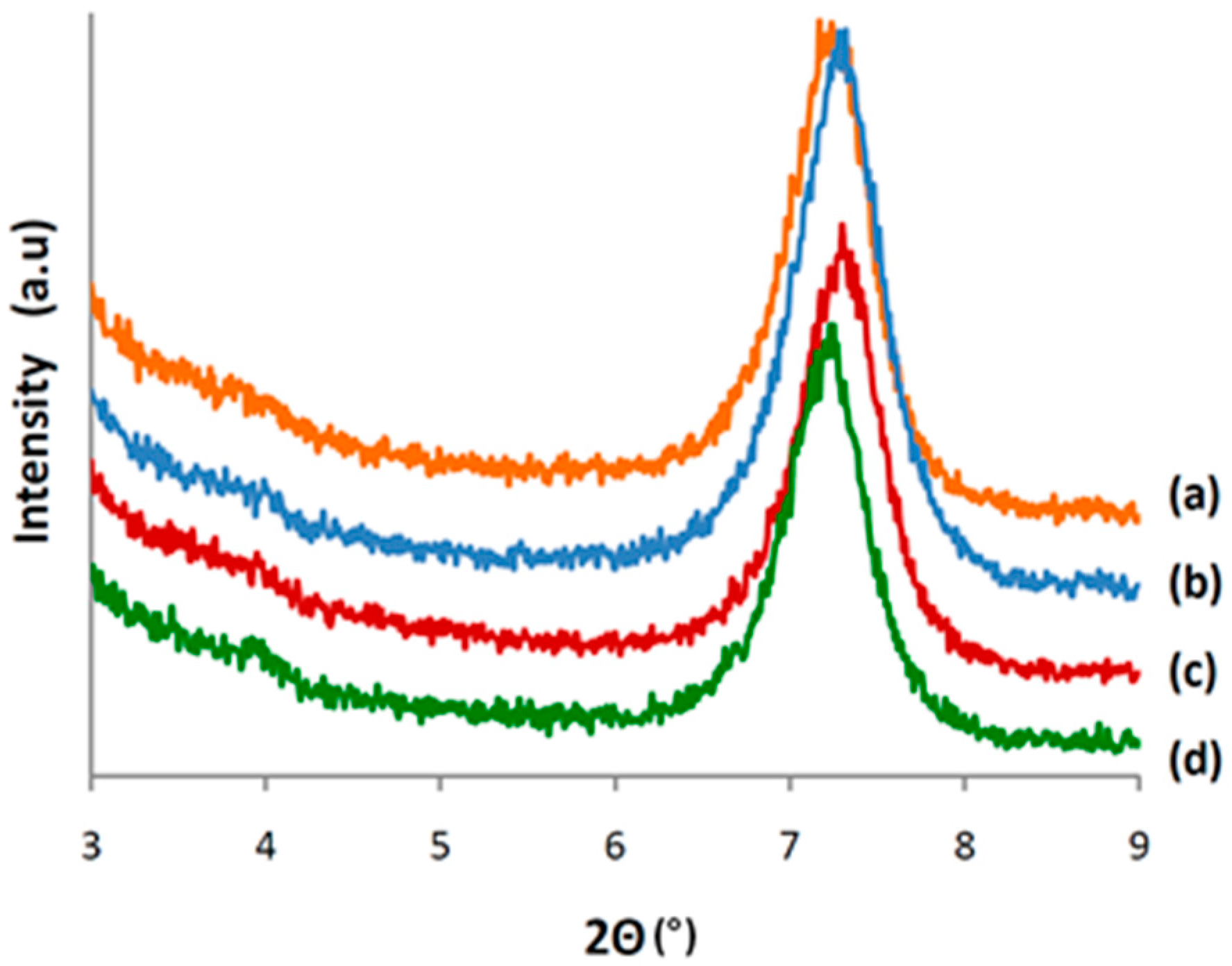
3.4. Characterization of CALB/Clay Catalysts by XRD
3.5. CALB Immobilized on Organo-Modified Clays: Catalytic Performance Study
3.5.1. Influence of Lyophilization on Hydrolytic Activity
3.5.2. Esterification Activity Test: Polymerization of ε-Caprolactone
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| BSA | Bovine serum albumin |
| pNP | p-nitrophenol |
| pNPB | p-nitrophenyl butyrate |
| GA | glutaraldehyde |
| CALB | Candida antarctica lipase B |
| NOV-435 | Novozym®-435, an acrylic resin-immobilized form of Candida antarctica lipase B |
| MMT | neat montmorillonite |
| MMTL | lipase (CALB) immobilized on neat montmorillonite |
| MMTMOD | organo-modified montmorillonite |
| MMTMODL | lipase (CALB) immobilized on organo-modified montmorillonite |
| MMTMODL-Aq | immobilization of CALB on organo-modified montmorillonite performed in aqueous medium |
| MMTMODL-Org | immobilization of CALB on organo-modified montmorillonite performed in organic solvent |
| SEP | neat sepiolite |
| SEPL | lipase (CALB) immobilized on neat sepiolite |
| SEPMOD | organo-modified sepiolite |
| SEPMODL | lipase (CALB) immobilized on organo-modified sepiolite |
| ε-CL | ε-caprolactone |
| PCL | poly(caprolactone) |
| ROP | ring opening polymerization |
| eROP | enzymatic ring opening polymerization |
| NMR | nuclear magnetic resonance |
| SEM | scanning electron microscopy |
| XRD | X-ray diffraction |
References
- Schmid, R.; Verger, R. Lipases: Interfacial enzymes with attractive applications. Angew. Chem. Int. Ed. 1998, 37, 1608–1633. [Google Scholar] [CrossRef]
- Albertsson, A.C.; Srivastava, R.K. Recent developments in enzyme-catalyzed ring-opening polymerization. Adv. Drug. Deliv. Rev. 2008, 60, 1077–1093. [Google Scholar] [CrossRef] [PubMed]
- Varma, I.K.; Albertsson, A.C.; Rajkhowa, R.; Srivastava, R.K. Enzyme catalyzed synthesis of polyesters. Prog. Polym. Sci. 2005, 30, 949–981. [Google Scholar] [CrossRef]
- Loos, K. Biocatalysis in Polymer Chemistry; Wiley-VCH Verlag, GmbH&Co. KGaA: Weinheim, Germany, 2011. [Google Scholar]
- Gitlesen, T.; Bauer, M.; Adlercreutz, P.A. Adsorption of lipase on polypropylene powder. Biochim. Biophys. Acta 1997, 1345, 188–196. [Google Scholar] [CrossRef]
- Cao, L. Carrier-Bound Immobilized Enzymes. Principles, Applications and Design; Wiley-VCH Verlag, GmbH&Co. KGaA: Weinheim, Germany, 2005. [Google Scholar]
- Yu, W.H.; Li, N.; Tong, D.S.; Zhou, C.H.; Lin, C.X.; Xu, C.Y. Adsorption of proteins and nucleic acids on clay minerals and their interactions: A review. Appl. Clay Sci. 2013, 80–81, 443–452. [Google Scholar] [CrossRef]
- Blanco, R.M.; Terreros, P.; Perez, M.F.; Otero, C.; Gonzalez, G. Functionalization of mesoporous silica for lipase immobilization characterization of the support and the catalysts. J. Mol. Catal. B Enzym. 2004, 30, 83–93. [Google Scholar] [CrossRef]
- Trodler, P.; Pleiss, J. Modeling structure and flexibility of Candida antarctica lipase B in organic solvents. BMC Struct. Biol. 2008, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Foresti, M.L.; Ferreira, M.L. Solvent-free ethyl oleate synthesis mediated by lipase from Candida antarctica B adsorbed on polypropylene powder. Catal. Today 2005, 107–108, 23–30. [Google Scholar] [CrossRef]
- Hernandez, K.; Garcia-Galan, C.; Fernandez-Lafuente, R. Simple and efficient immobilization of lipase B from Candida antarctica on porous styrene–divinylbenzene beads. Enzym. Microb. Technol. 2011, 49, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Jiang, Y.; Zhou, L.; Gao, J. Immobilization of Candida antarctica lipase B by adsorption in organic medium. New Biotechnol. 2010, 27, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Forde, J.; Vakurov, A.; Gibson, T.D.; Millner, P.; Whelehan, M.; Marison, I.W.; Ó’Fágáin, C. Chemical modification and immobilisation of lipase B from Candida antarctica onto mesoporous silicates. J. Mol. Catal. B Enzym. 2010, 66, 203–209. [Google Scholar] [CrossRef]
- Arroyo, M.; Sanchez-Montero, J.M.; Sinisterra, J.V. Thermal stabilization of immobilized lipase B from Candida antarctica on different supports: Effect of water activity on enzymatic activity in organic media. Enzym. Microb. Technol. 1999, 24, 3–12. [Google Scholar] [CrossRef]
- Rodrigues, D.S.; Cavalcante, G.P.; Ferreira, A.L.O.; Gonçalves, L.R.B. Immobilization of Candida antarctica lipase type B by adsorption on activated carbon. Chem. Biochem. Eng. Q. 2008, 22, 125–133. [Google Scholar]
- Rodrigues, D.S.; Mendes, A.A.; Adriano, W.S.; Goncalves, L.R.B.; Giordano, R.L.C. Multipoint covalent immobilization of microbial lipase on chitosan and agarose activated by different methods. J. Mol. Catal. B Enzym. 2008, 51, 100–109. [Google Scholar] [CrossRef]
- Fernandez-Lafuente, R.; Armisen, P.; Sabuquillo, P.; Fernandez-Lorente, G.; Guisan, J.M. Immobilization of lipases by selective adsorption on hydrophobic supports. Chem. Phys. Lipids 1998, 93, 185–197. [Google Scholar] [CrossRef]
- Sedaghat, M.E.; Ghiaci, M.; Aghaei, H.; Soleimanian-Zad, S. Enzyme immobilization. Part 4. Immobilization of alkaline phosphatase on Na-sepiolite and modified sepiolite. Appl. Clay Sci. 2009, 46, 131–135. [Google Scholar] [CrossRef]
- Fuentes, I.E.; Viseras, C.A.; Ubiali, D.; Terreni, M.; Alcantara, A.R. Different phyllosilicates as supports for lipase immobilisation. J. Mol. Catal. B Enzym. 2001, 11, 657–663. [Google Scholar] [CrossRef]
- Cengiz, S.; Çavaş, L.; Yurdakoç, K. Bentonite and sepiolite as supporting media: Immobilization of catalase. Appl. Clay Sci. 2012, 65–66, 114–120. [Google Scholar] [CrossRef]
- Gopinath, S.; Sugunan, S. Enzymes immobilized on montmorillonite K 10: Effect of adsorption and grafting on the surface properties and the enzyme activity. Appl. Clay Sci. 2007, 35, 67–75. [Google Scholar] [CrossRef]
- Scherer, R.; Oliveira, J.V.; Pergher, S.; De Oliveira, D. Screening of supports for immobilization of commercial porcine pancreatic lipase. Mater. Res. 2011, 14, 483–492. [Google Scholar] [CrossRef]
- Seleci, M.; Ağ, D.; Yalçinkaya, E.E.; Demirkol, D.O.; Güler, Ç.; Timur, S. Amine-intercalated montmorillonite matrices for enzyme immobilization and biosensing applications. RSC Adv. 2012, 2, 2112–2118. [Google Scholar] [CrossRef]
- Reshmi, R.; Sugunan, S. Superior activities of lipase immobilized on pure and hydrophobic clay supports: Characterization and catalytic activity studies. J. Mol. Catal. B Enzym. 2013, 97, 36–44. [Google Scholar] [CrossRef]
- Tzialla, A.A.; Pavlidis, I.V.; Felicissimo, M.P.; Rudolf, P.; Gournis, D.; Stamatis, H. Lipase immobilization on smectite nanoclays: Characterization and application to the epoxidation of α-pinene. Bioresour. Technol. 2010, 101, 1587–1594. [Google Scholar] [CrossRef] [PubMed]
- Ghiaci, M.; Aghaei, H.; Soleimanian, S.; Sedaghat, M.E. Enzyme immobilization: Part 1. Modified bentonite as a new and efficient support for immobilization of Candida rugosa lipase. Appl. Clay Sci. 2009, 43, 289–295. [Google Scholar] [CrossRef]
- Ghiaci, M.; Aghaei, H.; Soleimanian, S.; Sedaghat, M.E. Enzyme immobilization: Part 2. Immobilization of alkaline phosphatase on Na-bentonite and modified bentonite. Appl. Clay Sci. 2009, 43, 308–316. [Google Scholar] [CrossRef]
- Dong, H.; Li, J.; Li, Y.; Hu, L.; Luo, D. Improvement of catalytic activity and stability of lipase by immobilization on organobentonite. Chem. Eng. J. 2012, 181–182, 590–596. [Google Scholar] [CrossRef]
- Díaz Ramos, M.; Giraldo Gómez, G.I.; Sanabria González, N. Immobilization of Candida rugosa lipase on bentonite modified with benzyltriethylammonium chloride. J. Mol. Catal. B 2014, 99, 79–84. [Google Scholar] [CrossRef]
- Öztürk Düşkünkorur, H.; Pollet, E.; Phalip, V.; Güvenilir, Y.; Avérous, L. Lipase catalyzed synthesis of polycaprolactone and clay-based nanohybrids. Polymer 2014, 55, 1648–1655. [Google Scholar] [CrossRef]
- Öztürk Düşkünkorur, H.; Bégué, A.; Pollet, E.; Phalip, V.; Güvenilir, Y.; Avérous, L. Enzymatic ring-opening (co)polymerization of lactide stereoisomers catalyzed by lipases. Toward the in situ synthesis of organic/inorganic nanohybrids. J. Mol. Catal. B 2015, 115, 20–28. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Mei, Y.; Miller, L.; Gao, W.; Gross, R.A. Imaging the distribution and secondary structure of immobilized enzymes using infrared microspectroscopy. Biomacromolecules 2003, 4, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Lorente, G.; Cabrera, Z.; Godoy, C.; Fernandez-Lafuente, R.; Palomo, J.M.; Guisan, J.M. Interfacially activated lipases against hydrophobic supports: Effect of the support nature on the biocatalytic properties. Process Biochem. 2008, 43, 1061–1067. [Google Scholar] [CrossRef]
- Migneault, I.; Dartiguenave, C.; Bertrand, M.J.; Waldron, K.C. Glutaraldehyde: Behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. Biotechniques 2004, 37, 790–802. [Google Scholar] [PubMed]
- Avrameas, S. Coupling of enzymes to proteins with glutaraldehyde. Use of the conjugates for the detection of antigens and antibodies. Immunochemistry 1969, 6, 43–52. [Google Scholar] [CrossRef]
- Lopez-Gallego, F.; Betancor, L.; Mateo, C.; Hidalgo, A.; Alonso-Morales, N.; Dellamora-Ortiz, G.; Guisan, J.M.; Fernandez-Lafuente, R. Enzyme stabilization by glutaraldehyde crosslinking of adsorbed proteins on aminated supports. J. Biotechnol. 2005, 119, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Chivrac, F.; Pollet, E.; Schmutz, M.; Avérous, L. Starch nano-biocomposites based on needle-like sepiolite clays. Carbohydr. Polym. 2010, 80, 145–153. [Google Scholar] [CrossRef]
- Bedu-Addo, F.K. Understanding lyophilization formulation development. Pharm. Technol. 2004, 2, 10–18. [Google Scholar]
- Öztürk, H.; Pollet, E.; Hébraud, A.; Avérous, L. Lipase catalyzed synthesis of biopolyester and related clay-based nanohybrids. In Biodegradable Polymers and Sustainable Polymers; Jimenez, A., Zaikov, G.E., Eds.; Nova Publishers: New York, NY, USA, 2011. [Google Scholar]
- Xin, J.Y.; Li, S.; Xu, Y.; Wang, L.S.; Yu, C. Study on the immobilization and stability of lipase in organic media. J. Mol. Catal. 1999, 13, 103–108. [Google Scholar]
- Poojari, Y.; Clarson, S.J. Thermal stability of Candida antarctica lipase B immobilized on macroporous acrylic resin particles in organic media. Biocatal. Agric. Biotechnol. 2013, 2, 7–11. [Google Scholar] [CrossRef]


| Catalyst | Loading (mg added protein/g clay) | Immobilization efficiency (%) 1 | Hydrolytic activity (U/mg CALB) 2 | Hydrolytic activity after GA treatment (U/mg CALB) |
|---|---|---|---|---|
| SEPL1a | 7.5 | 88 | 37 | 13 |
| SEPL2a | 13.5 | 74 | 45 | 14 |
| SEPL3a | 27.0 | 56 | 35 | 16 |
| SEPMODL1a | 7.5 | 96 | 210 | 241 |
| SEPMODL2a | 13.5 | 98 | 199 | 222 |
| SEPMODL3a | 27.0 | 96 | 132 | 200 |
| MMTL1a | 7.5 | 99 | 117 | 132 |
| MMTL2a | 13.5 | 90 | 93 | 99 |
| MMTL3a | 27.0 | 73 | 91 | 80 |
| MMTMODL1a | 7.5 | 95 | 165 | 283 |
| MMTMODL2a | 13.5 | 97 | 145 | 246 |
| MMTMODL3a | 27.0 | 67 | 145 | 217 |
| Free CALB | n/a | n/a | 117 | 257 |
| Catalyst | Loading (mg added protein/g clay) | Immobilization efficiency (%) 2 | Hydrolytic activity before lyophilization (U/mg CALB) 3 | Hydrolytic activity after lyophilization (U/mg CALB) 3 |
|---|---|---|---|---|
| SEPMODL1b | 7.5 | 98 | 210 | 60 |
| SEPMODL1b G 1 | 7.5 | 98 | 287 | 117 |
| MMTMODL1b | 7.5 | 98 | 174 | 130 |
| MMTMODL1b G 1 | 7.5 | 98 | 318 | 177 |
| Catalytic system | CALB/ε-CL ratio (wt %) | Monomer conversion (%) 1 | Mn (g/mol) 2 |
|---|---|---|---|
| NOV-435 | 0.17 | 93 | 16,000 |
| NOV-435 in presence of MMTMOD | 0.17 | 92 | 9,000 |
| MMTMODL1b G | 0.08 | 70 | 8,500 |
| SEPMODL1b G | 0.07 | 15 | 1,700 |
| Catalyst | Hydrolytic activity 1 (U/mg CALB) | Reaction time (h) | Monomer conversion 2 (%) | Mn (g/mol) 3 |
|---|---|---|---|---|
| CALB | 170 | n/a | n/a | n/a |
| CALB LYOPH | 127 | n/a | n/a | n/a |
| NOV-435 in presence of MMTMOD | n/d | 24 | 100 | 11,000 |
| MMTMODL-Aq lyoph | 126 | 48 | 95 | 8,000 |
| MMTMODL-Aq dried | 173 | 48 | 90 | 3,500 |
| MMTMODL-Org dried | 179 | 48 | 86 | 3,000 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Öztürk, H.; Pollet, E.; Phalip, V.; Güvenilir, Y.; Avérous, L. Nanoclays for Lipase Immobilization: Biocatalyst Characterization and Activity in Polyester Synthesis. Polymers 2016, 8, 416. https://doi.org/10.3390/polym8120416
Öztürk H, Pollet E, Phalip V, Güvenilir Y, Avérous L. Nanoclays for Lipase Immobilization: Biocatalyst Characterization and Activity in Polyester Synthesis. Polymers. 2016; 8(12):416. https://doi.org/10.3390/polym8120416
Chicago/Turabian StyleÖztürk, Hale, Eric Pollet, Vincent Phalip, Yüksel Güvenilir, and Luc Avérous. 2016. "Nanoclays for Lipase Immobilization: Biocatalyst Characterization and Activity in Polyester Synthesis" Polymers 8, no. 12: 416. https://doi.org/10.3390/polym8120416
APA StyleÖztürk, H., Pollet, E., Phalip, V., Güvenilir, Y., & Avérous, L. (2016). Nanoclays for Lipase Immobilization: Biocatalyst Characterization and Activity in Polyester Synthesis. Polymers, 8(12), 416. https://doi.org/10.3390/polym8120416