Opening New Gates for the Modification of PVC or Other PVC Derivatives: Synthetic Strategies for the Covalent Binding of Molecules to PVC
Abstract
:1. Introduction
2. Materials and Methods
3. Results
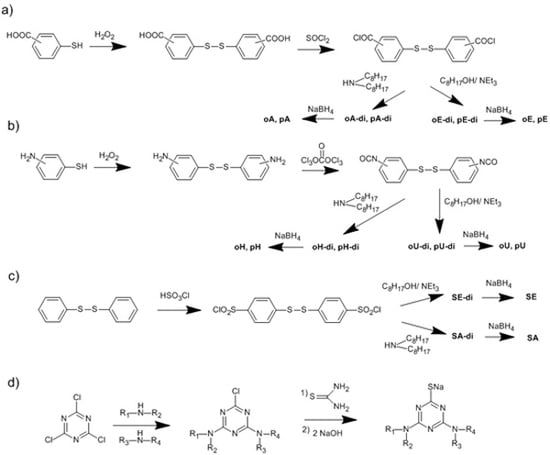
3.1. Use of Substituted Thiols
3.2. Trichlorotriazine-Based Thiols
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jakoby, R. Marketing and sales in the chemical industry. In Plastics and Rubbers, 2nd ed.; Wiley: New York, NY, USA, 2002. [Google Scholar]
- Braun, D. Special Issue: Macromolecules in the 21st Century—Otto Vogl. J. Polym. Sci. A Polym. Chem. 2004, 42, 578–586. [Google Scholar] [CrossRef]
- Fasano, E.; Cirillo, T.; Esposito, F.; Lacorte, S. Growth and acidification performance of probiotics in pure culture and co-culture with Streptococcus thermophilus: The effect of inulin. LWT Food Sci. Technol. 2015, 64, 1015–1021. [Google Scholar] [CrossRef]
- Sampson, J.; de Korte, D. DEHP-plasticised PVC: Relevance to blood services. Transfus. Med. 2011, 21, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, D.K.; Lawrence, W.H.; Turner, J.E.; Autian, J. Effects of parenteral di-(2-ethylhexyl)phthalate (DEHP) on gonadal biochemistry, pathology, and reproductive performance of mice. J. Toxicol. Environ. Health 1989, 26, 39–59. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.T.; Giovanoulis, G.; Palm Cousins, A.; Magnér, J.; Cousins, I.T.; de Wit, C.A. Human exposure, hazard and risk of alternative plasticizers to phthalate esters. Sci. Total Environ. 2016, 541, 451–467. [Google Scholar] [CrossRef] [PubMed]
- Bourdeauxa, D.; Yessaada, M.; Chennell, P.; Larbrea, V.; Eljezi, T.; Bernarda, L.; Sautou, V. Analysis of PVC plasticizers in medical devices and infused solutions by GC-MS. J. Pharm. Biomed. Anal. 2016, 118, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, S.; Jayakrishnan, A. Photocross-linking of dithiocarbamate-substituted PVC reduces plasticizer migration. Polymer 1998, 39, 151–157. [Google Scholar] [CrossRef]
- Ito, R.; Seshimo, F.; Haishima, Y.; Hasegawa, C.; Isama, K.; Yagami, T.; Nakahashi, K.; Yamazaki, H.; Inoue, K.; Yoshimura, Y.; et al. Reducing the migration of di-2-ethylhexyl phthalate from polyvinyl chloride medical devices. Int. J. Pharm. 2005, 303, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, S.; Jayakrishnan, A. Synthesis, surface properties and performance of thiosulphate-substituted plasticized poly(vinyl chloride). Biomaterials 2002, 23, 4855–4862. [Google Scholar] [CrossRef]
- Jayakrishnan, A.; Sunny, M.C. Phase transfer catalysed surface modification of plasticized poly(vinyl chloride) in aqueous media to retard plasticizer migration. Polymer 1996, 37, 5213–5218. [Google Scholar] [CrossRef]
- Kalliyana Krishnan, V.; Jayakrishnan, A.; Francis, J.D. Radiation grafting of hydrophilic monomers on to plasticized poly(vinyl chloride) sheets: II. Migration behaviour of the plasticizer from N-vinyl pyrrolidone grafted sheets. Biomaterials 1991, 12, 489–492. [Google Scholar] [CrossRef]
- Itoa, R.; Miuraa, N.; Ushiroa, M.; Kawaguchi, M.; Nakamuraa, H.; Iguchi, H.; Ogino, J.; Oishi, M.; Wakui, N.; Iwasaki, Y.; et al. Effect of γ-ray irradiation on degradation of di(2-ethylhexyl)phthalate in polyvinyl chloride sheet. Int. J. Pharm. 2009, 376, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Jayakrishnan, A.; Lakshmi, S. Immobile plasticizer in flexible PVC. Nature 1998, 396, 638. [Google Scholar] [CrossRef] [PubMed]
- Min Lim, K.; Yern Chee Ching, S. Effect of palm oil bio-based plasticizer on the morphological, thermal and mechanical properties of poly(vinyl chloride). Polymers 2015, 7, 2031–2043. [Google Scholar] [CrossRef]
- Earla, A.; Braslau, R. Covalently linked plasticizers: Triazole analogues of phthalate plasticizers prepared by mild copper-free “click” reactions with azide-functionalized PVC. Macromol. Rapid Commun. 2014, 35, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Navarro, R.; Perez Perrino, M.; Gomez Tardajos, M.; Reinecke, H. Phthalate plasticizers covalently bound to pvc: plasticization with suppressed migration. Macromolecules 2010, 43, 2377–2381. [Google Scholar] [CrossRef]
- Navarro, R.; Gallardo, A.; Pérez, M.; Reinecke, H. Thiolate als Nicht-Migrierende PVC-Weichmacher. European Patent EP2492259-A1, 25 February 2011. [Google Scholar]
- Reinecke, H.; Navarro, R.; Pérez, M. Nuevos aditivos aditivos basados en ftalatos para la plastificación interna de polímeros clorados. Spanish Patent ES2341524 A1, 18 December 2008. [Google Scholar]
- Navarro, R.; Pérez Perrino, M.; García, C.; Elvira, C.; Gallardo, A.; Reinecke, H. Highly flexible PVC materials without plasticizer migration as obtained by efficient one-pot procedure using trichlorotriazine chemistry. Macromolecules 2016, 49, 2224–2227. [Google Scholar] [CrossRef]
- Moulay, A. Chemical modification of poly(vinyl chloride)—Still on the run. Prog. Polym. Sci. 2010, 35, 303–331. [Google Scholar] [CrossRef]
- Domagala, J.M.; Bader, J.P.; Gogliotti, R.D.; Sanchez, J.P.; Stier, M.A.; Song, Y.; Vara Prasad, J.V.N.; Tummino, P.J.; Scholten, J.; Harvey, P.; et al. A new class of anti-HIV-1 agents targeted toward the nucleocapsid protein NCp7: The 2,2′-dithiobisbenzamides. Bioorg. Med. Chem. 1997, 5, 569–579. [Google Scholar] [CrossRef]
- Ossowska-Chruściel, M.D.; Chruście, J. Mesomorphic properties of (S)-MHPSBOn series. Thermochim. Acta 2010, 502, 51–59. [Google Scholar] [CrossRef]
- Bubert, C.; Blacker, J.; Brown, S.M.; Crosby, J.; Fitzjohn, S.; Muxworthy, J.P.; Thorpe, T.; Williams, J.M.J. Synthesis of water-soluble aminosulfonamide ligands and their application in enantioselective transfer hydrogenation. Tetrahedron Lett. 2001, 42, 4037–4039. [Google Scholar] [CrossRef]
- Blotny, G. Recent applications of 2,4,6-trichloro-1,3,5-triazine and its derivatives in organic synthesis. Tetrahedron 2006, 62, 9507–9522. [Google Scholar] [CrossRef]
- Stoyanov, S.; Petkov, I.; Antonov, L.; Stoyanova, T.; Karagiannidis, P.; Aslanidis, P. Thione–thiol tautomerism and stability of 2- and 4-mercaptopyridines and 2-mercaptopyrimidines. Can. J. Chem. 1990, 68, 1482–1489. [Google Scholar] [CrossRef]







| Compound | Amount of modifier in the mixture with PVC (wt %) | Covalently bound modifier (wt %) | Degree of anchorage A (%) | Tg (°C) |
|---|---|---|---|---|
| oE | 20 | 14.4 | 72.0 | 55 |
| 40 | 34.1 | 85.2 | 35 | |
| oA | 20 | 18.5 | 92.5 | 56 |
| 40 | 37.8 | 94.5 | 37 | |
| pE | 20 | 18.7 | 93.5 | 51 |
| 40 | 38.8 | 97.0 | 29 | |
| pA | 20 | 18.6 | 93.0 | 53 |
| 40 | 38.8 | 97.0 | 32 | |
| oU | 20 | 13.9 | 69.5 | 54 |
| 40 | 32.2 | 77.0 | 38 | |
| oH | 20 | 14.8 | 74.0 | 62 |
| 40 | 30.8 | 77.0 | 41 | |
| pU | 20 | 13.9 | 69.5 | 53 |
| 40 | 31.2 | 78.0 | 39 | |
| pH | 20 | 17.2 | 86.1 | 60 |
| 40 | 35.1 | 87.8 | 33 | |
| SE | 20 | - | - | - |
| 40 | - | - | - | |
| SA | 20 | 15.1 | 75.5 | 49 |
| 40 | 36.3 | 90.8 | 28 |
| Y1 | R1 | Y2 | R2 | Modifier in the mixture (wt %) | Covalently bound modifier (wt %) | Degree of anchorage (%) | Tg (°C) | |
|---|---|---|---|---|---|---|---|---|
| 6 | N(C8H17) | C8H17 | N(C8H17) | C8H17 | 20 | 19.5 | 97 | 42 |
| 40 | 39.2 | 98 | 35 | |||||
| 7 | N(C4H9) | C4H9 | N(C4H9) | C4H9 | 20 | 19.6 | 98 | 72 |
| 40 | 39.6 | 98 | 55 | |||||
| 8 | O | C2H5 | N(C8H17) | C8H17 | 20 | 19.9 | 98 | 43 |
| 40 | 39.8 | 98 | 37 | |||||
| 9 | O | C8H17 | O | C8H17 | 20 | <8 | - | - |
| 40 | <10 | - | - | |||||
| 10 | S | C6H13 | S | C6H13 | 20 | <15 | - | - |
| 40 | <31 | - | - |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarro, R.; Perrino, M.P.; García, C.; Elvira, C.; Gallardo, A.; Reinecke, H. Opening New Gates for the Modification of PVC or Other PVC Derivatives: Synthetic Strategies for the Covalent Binding of Molecules to PVC. Polymers 2016, 8, 152. https://doi.org/10.3390/polym8040152
Navarro R, Perrino MP, García C, Elvira C, Gallardo A, Reinecke H. Opening New Gates for the Modification of PVC or Other PVC Derivatives: Synthetic Strategies for the Covalent Binding of Molecules to PVC. Polymers. 2016; 8(4):152. https://doi.org/10.3390/polym8040152
Chicago/Turabian StyleNavarro, Rodrigo, Mónica Pérez Perrino, Carolina García, Carlos Elvira, Alberto Gallardo, and Helmut Reinecke. 2016. "Opening New Gates for the Modification of PVC or Other PVC Derivatives: Synthetic Strategies for the Covalent Binding of Molecules to PVC" Polymers 8, no. 4: 152. https://doi.org/10.3390/polym8040152
APA StyleNavarro, R., Perrino, M. P., García, C., Elvira, C., Gallardo, A., & Reinecke, H. (2016). Opening New Gates for the Modification of PVC or Other PVC Derivatives: Synthetic Strategies for the Covalent Binding of Molecules to PVC. Polymers, 8(4), 152. https://doi.org/10.3390/polym8040152