Co-Assembly of Graphene Oxide and Albumin/Photosensitizer Nanohybrids towards Enhanced Photodynamic Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
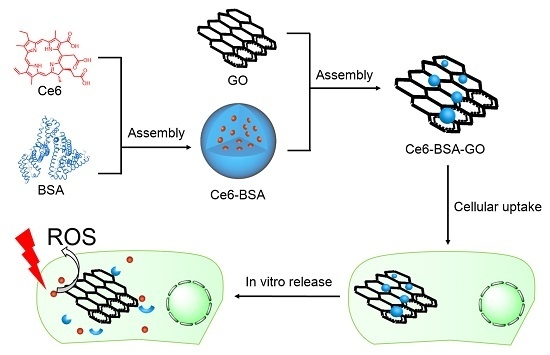
2.2. Preparation of the Nanohybrids
2.3. Characterization of the Nanohybrids
2.4. ROS Generation
2.5. Cell Culture
2.6. In Vitro Imaging
2.7. In Vitro PDT
3. Results and Discussion
3.1. Preparation and Characterization
3.2. Fluorescence and ROS
3.3. Cellular Uptake and In Vitro Release
3.4. In Vitro PDT
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| PDT | Photodynamic therapy |
| GO | Graphene oxide |
| Ce6 | Chlorin e6 |
| BSA | Bovine serum albumin |
| ROS | Reactive oxygen species |
| EPR | Enhanced permeability and retention |
| MTT | Thiazolyl blue tetrazolium bromide |
| FBS | Fetal bovine serum |
| TEM | Transmission electron microscope |
| AFM | Atomic-force microscope |
| DLS | Dynamic laser scattering |
| ADPA | 9,10-anthracene dipropionic acid |
| PBS | Phosphate buffered saline |
| CLSM | Confocal laser scanning microscopy |
References
- Castano, A.P.; Mroz, P.; Hamblin, M.R. Photodynamic therapy and anti-tumour immunity. Nat. Rev. Cancer 2006, 6, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Yano, S.; Hirohara, S.; Obata, M.; Hagiya, Y.; Ogura, S.; Ikeda, A.; Kataoka, H.; Tanaka, M.; Joh, T. Current states and future views in photodynamic therapy. J. Photochem. Photobiol. C 2011, 12, 46–67. [Google Scholar] [CrossRef]
- Kachynski, A.V.; Pliss, A.; Kuzmin, A.N.; Ohulchanskyy, T.Y.; Baev, A.; Qu, J.; Prasad, P.N. Photodynamic therapy by in situ nonlinear photon conversion. Nat. Photonics 2014, 8, 455–461. [Google Scholar] [CrossRef]
- Plaetzer, K.; Krammer, B.; Berlanda, J.; Berr, F.; Kiesslich, T. Photophysics and photochemistry of photodynamic therapy: fundamental aspects. Lasers Med. Sci. 2009, 24, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Ethirajan, M.; Chen, Y.H.; Joshi, P.; Pandey, R.K. The role of porphyrin chemistry in tumor imaging and photodynamic therapy. Chem. Soc. Rev. 2011, 40, 340–362. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.C.; Fei, J.B.; Wang, A.H.; Yang, Y.; Li, J.B. Rational assembly of a biointerfaced core@shell nanocomplex towards selective and highly efficient synergistic photothermal/photodynamic therapy. Nanoscale 2015, 7, 20197–20210. [Google Scholar] [CrossRef] [PubMed]
- Zou, Q.L.; Zhao, H.Y.; Zhao, Y.X.; Fang, Y.Y.; Chen, D.F.; Ren, J.; Wang, X.P.; Wang, Y.; Gu, Y.; Wu, F.P. Effective two-photon excited photodynamic therapy of xenograft tumors sensitized by water-soluble bis(arylidene)cycloalkanone photosensitizers. J. Med. Chem. 2015, 58, 7949–7958. [Google Scholar] [CrossRef] [PubMed]
- Xing, R.R.; Liu, K.; Jiao, T.F.; Zhang, N.; Ma, K.; Zhang, R.Y.; Zou, Q.L.; Ma, G.H.; Yan, X.H. An injectable self-assembling collagen-gold hybrid hydrogel for combinatorial antitumor photothermal/photodynamic therapy. Adv. Mater. 2016. [Google Scholar] [CrossRef] [PubMed]
- Xing, R.R.; Jiao, T.F.; Yan, L.Y.; Ma, G.H.; Liu, L.; Dai, L.R.; Li, J.B.; Mohwald, H.; Yan, X.H. Colloidal gold-collagen protein core-shell nanoconjugate: One-step biomimetic synthesis, layer-by-layer assembled film, and controlled cell growth. ACS Appl. Mater. Interfaces 2015, 7, 24733–24740. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.A.; Kuthati, Y.; Kankala, R.K.; Chang, Y.C.; Liu, C.L.; Weng, C.F.; Mou, C.Y.; Lee, C.H. Encapsulation of palladium porphyrin photosensitizer in layered metal oxide nanoparticles for photodynamic therapy against skin melanoma. Sci. Technol. Adv. Mater. 2015, 16, 054205. [Google Scholar] [CrossRef]
- Kankala, R.K.; Kuthati, Y.; Liu, C.L.; Lee, C.H. Hierarchical coated metal hydroxide nanoconstructs as potential controlled release carriers of photosensitizer for skin melanoma. RSC Adv. 2015, 5, 42666–42680. [Google Scholar] [CrossRef]
- Orenstein, A.; Kostenich, G.; Roitman, L.; Shechtman, Y.; Kopolovic, Y.; Ehrenberg, B.; Malik, Z. A comparative study of tissue distribution and photodynamic therapy selectivity of chlorin e6, Photofrin II and ALA-induced protoporphyrin IX in a colon carcinoma model. Br. J. Cancer 1996, 73, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Lucky, S.S.; Soo, K.C.; Zhang, Y. Nanoparticles in photodynamic therapy. Chem. Rev. 2015, 115, 1990–2042. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Abbas, M.; Zhang, M.; Zou, Q.L.; Shen, G.Z.; Chen, C.J.; Peng, H.S.; Yan, X.H. One-Step nanoengineering of hydrophobic photosensitive drugs for the photodynamic therapy. J. Nanosci. Nanotechnol. 2015, 15, 10141–10148. [Google Scholar] [CrossRef] [PubMed]
- Zou, Q.L.; Zhang, L.; Yan, X.H.; Wang, A.H.; Ma, G.H.; Li, J.B.; Mohwald, H.; Mann, S. Multifunctional porous microspheres based on peptide-porphyrin hierarchical co-assembly. Angew. Chem. Int. Ed. 2014, 53, 2366–2370. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Xing, R.R.; Chen, C.J.; Shen, G.Z.; Yan, L.Y.; Zou, Q.L.; Ma, G.H.; Mohwald, H.; Yan, X.H. Peptide-induced hierarchical long-range order and photocatalytic activity of porphyrin assemblies. Angew. Chem. Int. Ed. 2015, 54, 500–505. [Google Scholar] [CrossRef]
- Yin, P.T.; Shah, S.; Chhowalla, M.; Lee, K.B. Design, Synthesis, and characterization of graphene-nanoparticle hybrid materials for bioapplications. Chem. Rev. 2015, 115, 2483–2531. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.M.; Honma, I.; Paek, S.M.; Akada, M.; Hill, J.P.; Vinu, A.; Ariga, K. Layer-by-Layer films of graphene and ionic liquids for highly selective gas sensing. Angew. Chem. Int. Ed. 2010, 49, 9737–9739. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Vijayaraghavan, A.; Erni, R.; Ariga, K.; Khalakhan, I.; Hill, J.P. High purity graphenes prepared by a chemical intercalation method. Nanoscale 2010, 2, 2139–2143. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.M.; Kang, J.H.; Hong, B.H. Graphene-based nanomaterials for versatile imaging studies. Chem. Soc. Rev. 2015, 44, 4835–4852. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Tan, C.L.; Zhang, H.; Wang, L.Z. Two-dimensional graphene analogues for biomedical applications. Chem. Soc. Rev. 2015, 44, 2681–2701. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, D.R.; Todd, A.D.; Bielawski, C.W. Harnessing the chemistry of graphene oxide. Chem. Soc. Rev. 2014, 43, 5288–5301. [Google Scholar] [CrossRef] [PubMed]
- Jiao, T.F.; Zhao, H.; Zhou, J.X.; Zhang, Q.R.; Luo, X.N.; Hu, J.; Peng, Q.M.; Yan, X.H. Self-assembly reduced graphene oxide nanosheet hydrogel fabrication by anchorage of chitosan/silver and its potential efficient application toward dye degradation for wastewater treatments. ACS Sustain. Chem. Eng. 2015, 3, 3130–3139. [Google Scholar] [CrossRef]
- Cai, P.; Feng, X.Y.; Fei, J.B.; Li, G.L.; Li, J.; Huang, J.G.; Li, J.B. Co-assembly of photosystem II/reduced graphene oxide multilayered biohybrid films for enhanced photocurrent. Nanoscale 2015, 7, 10908–10911. [Google Scholar] [CrossRef] [PubMed]
- Jiao, T.F.; Liu, Y.Z.; Wu, Y.T.; Zhang, Q.R.; Yan, X.H.; Gao, F.M.; Bauer, A.J. P.; Liu, J.Z.; Zeng, T.Y.; Li, B.B. Facile and scalable preparation of graphene oxide-based magnetic hybrids for fast and highly efficient removal of organic dyes. Sci. Rep. 2015, 5, 12451. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, G.; Vila, M.; Portoles, M.T.; Vallet-Regi, M.; Gracio, J.; Marques, P.A.A.P. Nano-graphene oxide: A potential multifunctional platform for cancer therapy. Adv. Healthc. Mater. 2013, 2, 1072–1090. [Google Scholar] [CrossRef] [PubMed]
- Miao, W.; Shim, G.; Lee, S.; Lee, S.; Choe, Y.S.; Oh, Y.K. Safety and tumor tissue accumulation of pegylated graphene oxide nanosheets for co-delivery of anticancer drug and photosensitizer. Biomaterials 2013, 34, 3402–3410. [Google Scholar] [CrossRef] [PubMed]
- Sahu, A.; Choi, W.I.; Lee, J.H.; Tae, G. Graphene oxide mediated delivery of methylene blue for combined photodynamic and photothermal therapy. Biomaterials 2013, 34, 6239–6248. [Google Scholar] [CrossRef] [PubMed]
- Petros, R.A.; DeSimone, J.M. Strategies in the design of nanoparticles for therapeutic applications. Nat. Rev. Drug Discov. 2010, 9, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Park, S.; Ling, D.; Park, W.; Han, J.Y.; Na, K.; Char, K. Hyaluronic acid-conjugated graphene oxide/photosensitizer nanohybrids for cancer targeted photodynamic therapy. J. Mater. Chem. B 2013, 1, 1678–1686. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Yin, J.L.; Peng, C.; Hu, W.Q.; Zhu, Z.Y.; Li, W.X.; Fan, C.H.; Huang, Q. Distribution and biocompatibility studies of graphene oxide in mice after intravenous administration. Carbon 2011, 49, 986–995. [Google Scholar] [CrossRef]
- Kratz, F. A clinical update of using albumin as a drug vehicle—A commentary. J. Control. Release 2014, 190, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.F.; Shen, G.Z.; Chen, C.J.; Xing, R.R.; Zou, Q.L.; Ma, G.H.; Yan, X.H. Nanoengineering of stimuli-responsive protein-based biomimetic protocells as versatile drug delivery tools. Chem. Eur. J. 2014, 20, 6880–6887. [Google Scholar] [CrossRef] [PubMed]
- Schoonen, L.; van Hest, J.C. Functionalization of protein-based nanocages for drug delivery applications. Nanoscale 2014, 6, 7124–7141. [Google Scholar] [CrossRef] [PubMed]
- Kratz, F. Albumin as a drug carrier: Design of prodrugs, drug conjugates and nanoparticles. J. Control. Release 2008, 132, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Tehle, G.; Sinn, H.; Wunder, A.; Schrenk, H.H.; Stewart, J.C.M.; Hartung, G.; MaierBorst, W.; Heene, D.L. Plasma protein (albumin) catabolism by the tumor itself—Implications for tumor metabolism and the genesis of cachexia. Crit. Rev. Oncol. Hemat. 1997, 26, 77–100. [Google Scholar]
- Langer, K.; Anhorn, M.G.; Steinhauser, I.; Dreis, S.; Celebi, D.; Schrickel, I.; Faust, S.; Vogel, V. Human serum albumin (HSA) nanoparticles: Reproducibility of preparation process and kinetics of enzymatic degradation. Int. J. Pharm. 2008, 347, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Ariga, K.; Li, J.B.; Fei, J.B.; Ji, Q.M.; Hill, J.P. Nanoarchitectonics for dynamic functional materials from atomic-/molecular-level manipulation to macroscopic action. Adv. Mater. 2016, 28, 1251–1286. [Google Scholar] [CrossRef] [PubMed]
- Aono, M.; Ariga, K. The way to nanoarchitectonics and the way of nanoarchitectonics. Adv. Mater. 2016, 28, 989–992. [Google Scholar] [CrossRef] [PubMed]
- Ariga, K.; Ji, Q.M.; Nakanishi, W.; Hill, J.P.; Aono, M. Nanoarchitectonics: A new materials horizon for nanotechnology. Mater. Horiz. 2015, 2, 406–413. [Google Scholar] [CrossRef]
- Celli, J.P.; Spring, B.Q.; Rizvi, I.; Evans, C.L.; Samkoe, K.S.; Verma, S.; Pogue, B.W.; Hasan, T. Imaging and photodynamic therapy: Mechanisms, monitoring, and optimization. Chem. Rev. 2010, 110, 2795–2838. [Google Scholar] [CrossRef] [PubMed]
- Lui, K.; Xing, R.R.; Zou, Q.L.; Ma, G.H.; Mohwald, H.; Yan, X.H. Simple peptide-tuned self-assembly of photosensitizers towards anticancer photodynamic therapy. Angew. Chem. Int. Ed. 2016, 55, 3036–3039. [Google Scholar]
- Kuchlyan, J.; Kundu, N.; Banik, D.; Roy, A.; Sarkar, N. Spectroscopic and fluorescence lifetime imaging microscopy to probe the interaction of bovine serum albumin with graphene oxide. Langmuir 2015, 31, 13793–13801. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.; Son, S.; Kim, Y.; Chae, B.J.; Ku, B.C.; Lee, T.S. Preparation of conjugated polymer dots as a fluorescence turn-on assay for bovine serum albumin by interaction with graphene oxide. Mol. Cryst. Liq. Cryst. 2014, 600, 170–178. [Google Scholar] [CrossRef]
- Adhao, M.; Ahirkar, P.; Kumar, H.; Joshi, R.; Meitei, O.R.; Ghosh, S.K. Surfactant induced aggregation-disaggregation of photodynamic active chlorin e6 and its relevant interaction with DNA alkylating quinone in a biomimic micellar microenvironment. RSC Adv. 2015, 5, 81449–81460. [Google Scholar]
- Chin, W.W.L.; Praveen, T.; Heng, P.W.S.; Olivo, M. Effect of polyvinylpyrrolidone on the interaction of chlorin e6 with plasma proteins and its subcellular localization. Eur. J. Pharm. Biopharm. 2010, 76, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Zou, Q.L.; Liu, K.; Abbas, M.; Yan, X.H. Peptide-modulated self-assembly of chromophores toward biomimetic light-harvesting nanoarchitectonics. Adv. Mater. 2016, 28, 1031–1043. [Google Scholar] [CrossRef] [PubMed]
- Lindig, B.A.; Rodgers, M.A.J.; Schaap, A.P. Determination of the lifetime of singlet oxygen in D2O using 9,10-anthracenedipropionic acid, a water-soluble probe. J. Am. Chem. Soc. 1980, 102, 5590–5593. [Google Scholar] [CrossRef]
- Bern, M.; Sand, K.M.K.; Nilsen, J.; Sandlie, I.; Andersen, J.T. The role of albumin receptors in regulation of albumin homeostasis: Implications for drug delivery. J. Control. Release 2015, 211, 144–162. [Google Scholar] [CrossRef] [PubMed]







© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, R.; Jiao, T.; Liu, Y.; Ma, K.; Zou, Q.; Ma, G.; Yan, X. Co-Assembly of Graphene Oxide and Albumin/Photosensitizer Nanohybrids towards Enhanced Photodynamic Therapy. Polymers 2016, 8, 181. https://doi.org/10.3390/polym8050181
Xing R, Jiao T, Liu Y, Ma K, Zou Q, Ma G, Yan X. Co-Assembly of Graphene Oxide and Albumin/Photosensitizer Nanohybrids towards Enhanced Photodynamic Therapy. Polymers. 2016; 8(5):181. https://doi.org/10.3390/polym8050181
Chicago/Turabian StyleXing, Ruirui, Tifeng Jiao, Yamei Liu, Kai Ma, Qianli Zou, Guanghui Ma, and Xuehai Yan. 2016. "Co-Assembly of Graphene Oxide and Albumin/Photosensitizer Nanohybrids towards Enhanced Photodynamic Therapy" Polymers 8, no. 5: 181. https://doi.org/10.3390/polym8050181
APA StyleXing, R., Jiao, T., Liu, Y., Ma, K., Zou, Q., Ma, G., & Yan, X. (2016). Co-Assembly of Graphene Oxide and Albumin/Photosensitizer Nanohybrids towards Enhanced Photodynamic Therapy. Polymers, 8(5), 181. https://doi.org/10.3390/polym8050181