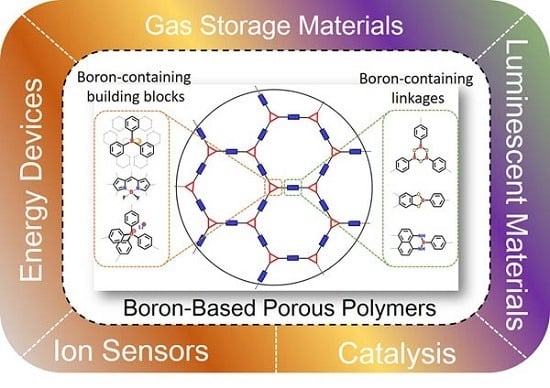
Recent Advances in Boron-Containing Conjugated Porous Polymers
Abstract
Share and Cite
Qiu, F.; Zhao, W.; Han, S.; Zhuang, X.; Lin, H.; Zhang, F. Recent Advances in Boron-Containing Conjugated Porous Polymers. Polymers 2016, 8, 191. https://doi.org/10.3390/polym8050191
Qiu F, Zhao W, Han S, Zhuang X, Lin H, Zhang F. Recent Advances in Boron-Containing Conjugated Porous Polymers. Polymers. 2016; 8(5):191. https://doi.org/10.3390/polym8050191
Chicago/Turabian StyleQiu, Feng, Wuxue Zhao, Sheng Han, Xiaodong Zhuang, Hualin Lin, and Fan Zhang. 2016. "Recent Advances in Boron-Containing Conjugated Porous Polymers" Polymers 8, no. 5: 191. https://doi.org/10.3390/polym8050191
APA StyleQiu, F., Zhao, W., Han, S., Zhuang, X., Lin, H., & Zhang, F. (2016). Recent Advances in Boron-Containing Conjugated Porous Polymers. Polymers, 8(5), 191. https://doi.org/10.3390/polym8050191