Structural Features and the Anti-Inflammatory Effect of Green Tea Extract-Loaded Liquid Crystalline Systems Intended for Skin Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Methods
2.2.1. Extract
2.2.2. Construction of Ternary Phase Diagram: Development of Liquid-Crystalline Systems
2.2.3. Characterization of Systems
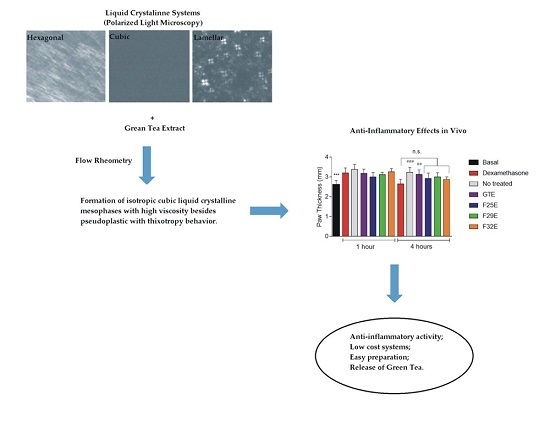
Polarized Light Microscopy (PLM)
Flow Rheometry
Oscillatory Rheometry
Texture Profile Analysis (TPA)
In Vitro Evaluation of the Bioadhesive Force
2.2.4. Physico-Chemical Stability Studies
2.2.5. Evaluation of Anti-Inflammatory Effects In Vivo
3. Results and Discussion
3.1. Construction of Ternary Phase Diagram: Development of Liquid-Crystalline Systems
3.2. Characterization of Systems
3.2.1. Polarized Light Microscopy (PLM)
3.2.2. Rheological Studies
Flow Rheometry
Oscillatory Rheology
3.2.3. Texture Profile Analyses (TPA)
3.2.4. In Vitro Evaluation of the Bioadhesive Force
3.3. Physico-Chemical Stability Studies
3.4. Evaluation of Anti-Inflammatory Effects In Vivo
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Crespy, V.; Williamson, G.A. A review of the health effects of green tea catechins in vivo animal models. J. Nutr. 2004, 134, S3431–S3440. [Google Scholar]
- Lee, L.-S.; Kim, S.-H.; Kim, Y.-B.; Kim, Y.-C. Quantitative analysis of major constituents in green tea with different plucking periods and their antioxidant activity. Molecules 2014, 19, 9173–9186. [Google Scholar] [CrossRef] [PubMed]
- Külkamp, I.C.; Rabelo, B.D.; Berlitz, S.J.; Isoppo, M.; Bianchin, M.D.; Schaffazick, S.R.; Pohlmann, A.R.; Guterres, S.S. Nanoencapsulation improves the in vitro antioxidant activity of lipoic acid. J. Biomed. Nanotechnol. 2011, 7, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, A.T.; Martinez, R.M.; Pinho-Ribeiro, F.A.; da Silva, A.A.L.D.; Bracat, M.M.; Georgetti, S.R.; Verri, W.A., Jr.; Chorilli, M. Resveratrol-loaded liquid-crystalline system inhibits UVB-induced skin inflammation and oxidative stress in mice. J. Nat. Prod. 2016, 79, 1329–1338. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Jahn, A.; Cho, S.-J.; Kim, J.S.; Ki, M.-H.; Kim, D.-D. Lyotropic liquid crystal systems in drug delivery: A review. J. Pharm. Investig. 2015, 45, 1–11. [Google Scholar] [CrossRef]
- Rigon, R.B.; Oyafuso, M.H.; Fujimura, A.T.; Gonçalez, M.L.; Prado, A.H.; Gremião, M.P.D.; Chorilli, M. Nanotechnology-based drug delivery systems for melanoma antitumoral therapy: A review. Biomed Res. Int. 2015, 2015, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Siddig, M.A.; Radiman, S.; Jan, L.S.; Muniandy, S.V. Rheological behaviours of the hexagonal and lamellar phases of glucopone (APG) surfactant. Colloids Surf. A Physicochem. Eng. Asp. 2006, 276, 15–21. [Google Scholar] [CrossRef]
- Makai, M.; Csànyi, E.; Németh, Z.S.; Pàlinkàs, J.; Eros, I. Structure and drug release of lamellar liquid crystals containing glycerol. Int. J. Pharm. 2003, 256, 95–107. [Google Scholar] [CrossRef]
- Shaw, D.J. Reologia. In Introdução à Química dos Colóides e de Superficies; Edgard Blücher, Ed.; da Universidade de São Paulo: São Paulo, Brazil, 1975; pp. 143–152. [Google Scholar]
- Fonseca-Santos, B.; Santos, A.M.; Rodero, C.F.; Gremião, M.P.D.; Chorilli, M. Design, characterization, and biological evaluation of curcumin-loaded surfactant-based systems for topical drug delivery. Int. J. Nanomed. 2016, 11, 4553–4562. [Google Scholar] [CrossRef] [PubMed]
- Chorilli, M.; Rigon, R.B.; Calixto, G.; Cartezani, P.M.F.; Ribeiro, M.C.A.P.; Polacow, M.L.O.; Cerri, P.S.; Sarmento, V.H.V.; Scarpa, M.V. Rheological characterization and safety evaluation of non-ionic lamellar liquid crystalline systems containing retinyl palmitate. J. Biomed. Nanotechnol. 2016, 12, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.B.; Souza, P.C.; Calixto, G.M.F.; Lopes, E.O.; Frem, R.C.G.; Godoy Netto, A.V.; Mauro, A.E.; Pavan, F.R.; Chorilli, M. In vitro activity of copper(II) complexes, loaded or unloaded into a nanostructured lipid system against Mycobacterium tuberculosis. Int. J. Mol. Sci. 2016, 17, 745. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.A.; Santana, D.P.; Bedor, D.G.C.; Borba, V.F.C.; Lira, A.A.M.; Egito, E.S.T. Estudo de liberação e permeação in vitro do diclofenaco de dietilamônio em microemulsão gel-like. Quim. Nova 2009, 32, 1389–1393. [Google Scholar] [CrossRef]
- Salmazi, R.; Calixto, G.; Bernegossi, J.; Ramos, M.A.; Bauab, T.M.; Chorilli, M. A curcumin-loaded liquid crystal precursor mucoadhesive system for the treatment of vaginal candidiasis. Int. J. Nanomed. 2015, 10, 4815–4824. [Google Scholar]
- Jones, D.S.; Woolfson, A.D.; Brown, A.F. Textural, viscoelastic and mucoadhesive properties of pharmaceutical gels composed of cellulose polymers. Int. J. Pharm. 1997, 151, 223–233. [Google Scholar] [CrossRef]
- Carvalho, F.C.; Calixto, G.; Hatakeyama, I.N.; Luz, G.M.; Daflon, M.P.G.; Chorilli, M. Rheological, mechanical, and bioadhesive behavior of hydrogels to optimize skin delivery systems. Drug Dev. Ind. Pharm. 2013, 39, 1750–1757. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, M.J.; Rees, G.D. Microemulsion based media as novel drug delivery systems. Adv. Drug Deliv. Rev. 2000, 45, 89–121. [Google Scholar] [CrossRef]
- Formariz, T.P.; Cocenza Urban, M.C.; Da Silva-Júnior, A.A.; Daflon Gremião, M.P.; De Oliveira, A.G. Microemulsões e fases líquidas cristalinas como sistema de liberação de fármacos. Rev. Bras. Cienc. Farm. 2005, 41, 301–313. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, W. Lamellar liquid crystals of Brij97 aqueous solutions containing different additives. J. Solut. Chem. 2009, 38, 659–668. [Google Scholar] [CrossRef]
- Tadesse, A.; Hymete, A.; Bekhit, A.A.; Mohammed, S.F. Quantification of total polyphenols, catechin, caffeine, l-theanine, determination of antioxidant activity and effect on antileishmanial drugs of Ethiopian tea leaves extracts. Pharmacogn. Res. 2015, 7, S7–S14. [Google Scholar]
- Mezzenga, R. Physics of self-assembly of lyotropic liquid crystals. In Self-Assembled Supramolecular Architectures: Lyotropic Liquid Crystals; Garti, N., Somasundaran, P., Mezzenga, R., Eds.; Wiley: Hoboken, NJ, USA, 2012; pp. 1–20. [Google Scholar]
- Savic, M.; Lukic, M.; Jaksic, I.; Reichl, S.; Tamburic, S.; Müller-Goymann, C. An alkyl polyglucoside-mixed emulsifier as stabilizer of emulsion systems: The influence of colloidal structure on emulsions skin hydration potential. J. Colloid Interface Sci. 2011, 358, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Pestana, K.C.; Formariz, T.P.; Franzini, C.M.; Sarmento, V.H.V.; Chiavacci, L.A.; Scarpar, M.V.; Egito, E.S.T.; Oliveira, A.G. Oil-in-water lecithin-based microemulsions as a potential delivery system for amphotericin B. Colloids Surf. B Biointerfaces 2008, 66, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Cintra, G.A.S.; Pinto, L.A.; Calixto, G.M.F.; Soares, C.P.; von Zuben, E.S.; Scarpa, M.V.; Gremião, M.P.D.; Chorilli, M. Bioadhesive surfactant systems for methotrexate skin delivery. Molecules 2016, 21, 231. [Google Scholar] [CrossRef] [PubMed]
- Calixto, G.; Yoshii, A.C.; Rocha e Silva, H.; Stringhetti Ferreira, B.C.; Chorilli, M. Polyacrylic acid polymers hydrogels intended to topical drug delivery: Preparation and characterization. Pharm. Dev. Technol. 2015, 20, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Pénzes, T.; Csóka, I.; Erõs, I. Rheological analysis of the structural properties effecting the percutaneous absorption and stability in pharmaceutical organogels. Rheol. Acta 2004, 43, 457–463. [Google Scholar] [CrossRef]
- Bernegossi, J.; Calixto, G.; Sanches, P.; Fontana, C.; Cilli, E.; Garrido, S.; Chorilli, M. Peptide KSL-W loaded mucoadhesive liquid crystalline vehicle as an alternative treatment for multispecies oral biofilm. Molecules 2016, 21, 37. [Google Scholar] [CrossRef] [PubMed]
- Souza Ferreira, S.B.; Moço, T.D.; Borghi-Pangoni, F.B.; Junqueira, M.V.; Bruschi, M.L. Rheological, mucoadhesive and textural properties of thermoresponsive polymer blends for biomedical applications. J. Mech. Behav. Biomed. Mater. 2015, 55, 164–178. [Google Scholar] [CrossRef] [PubMed]
- Lucero, M.J.; Ferris, C.; Sánchez-Gutiérrez, C.A.; Jiménez-Castellanos, M.R.; De-Paz, M.V. Novel aqueous chitosan-based dispersions as efficient drug delivery systems for topical use. Rheological, textural and release studies. Carbohydr. Polym. 2016, 151, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Loudet, J.-C.; Philippe, B.; Philippe, P. Colloidal ordering from phase separation in a liquid-crystalline continuous phase. Nature 2000, 407, 611–613. [Google Scholar] [PubMed]
- Kapnistos, M.; Hinrichs, A.; Vlassopoulos, D.; Anastasiadis, S.H.; Stammer, A.; Wolf, B.A. Rheology of a lower critical solution temperature binary polymer blend in the homogeneous, phase-separated, and transitional regimes. Macromolecules 1996, 29, 7155–7163. [Google Scholar] [CrossRef]
- Nórlen, L. Skin barrier formation: The membrane folding model. J. Investig. Dermatol. 2001, 117, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Namdeo, A.; Jain, N. Liquid crystalline pharmacogel based enhanced transdermal delivery of propranolol hydrochloride. J. Control. Release 2002, 82, 223–236. [Google Scholar] [CrossRef]
- Lopes, L.B.; Ferreira, D.A.; de Paul, D.; Garcia, M.T.; Thomazini, J.A.; Fantini, M.C.; Betley, M.V. Reverse hexagonal phase nanodispersion of monoolein and oleic acid for topical delivery of peptides: In vitro and in vivo skin penetration of cyclosporin A. Pharm. Res. 2006, 23, 1332–1342. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Yamashita, J.; Todo, H.; Miyamoto, K.; Hashimoto, S.; Tokudome, Y.; Hashimoto, F.; Sugibayashi, K. Preparation and evaluation of liquid-crystal formulations with skin-permeation-enhancing abilities for entrapped drugs. J. Oleo Sci. 2011, 60, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Lopes, L.B.; Speretta, F.F.F.; Bentley, M.V.L.B. Enhancement of skin penetration of vitamin K using monoolein-based liquid crystalline systems. Eur. J. Pharm. Sci. 2007, 32, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Bruschi, M.L.; Jones, D.S.; Panzeri, H.; Gremião, M.P.D.; Freitas, O.; Lara, E.H.G. Semisolid systems containing propolis for the treatment of periodontal disease: In vitro release kinetics, syringeability, rheological, textural, and mucoadhesive properties. J. Pharm. Sci. 2007, 96, 2074–2089. [Google Scholar] [CrossRef] [PubMed]
- Bruschi, M.L.; De Freitas, O.; Lara, E.H.; Panzei, H.; Gremião, M.P.D.; Jones, D.S. Precursor system of liquid crystalline phase with propolis-containing microparticles for use in the treatment of periodontal disease. Drug Dev. Ind. Pharm. 2008, 34, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.C.; Campos, M.L.; Peccinini, R.G.; Gremião, M.P. Nasal administration of liquid crystal precursor mucoadhesive vehicle as an alternative antiretroviral therapy. Eur. J. Pharm. Biopharm. 2013, 84, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.C.; Bruschi, M.L.; Evangelista, R.C.; Gremião, M.P.D. Mucoadhesive drug delivery systems. Braz. J. Pharm. Sci. 2010, 46, 1–17. [Google Scholar] [CrossRef]
- Cho, C.-W.; Choi, J.-S.; Shin, S.-C. Enhanced local anesthetic action of mepivacaine from the bioadhesive gels. Pak. J. Pharm. Sci. 2011, 24, 87–93. [Google Scholar] [PubMed]
- Jain, S.K.; Puri, R. Development, characterization and in vivo localization study of topical 5-fluorouracil gels: A comparative study with conventional formulation. Curr. Drug Deliv. 2014, 11, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Sing, S.; Gaira, B.; Rawat, M.; Muthu, M.S. Enhanced transdermal delivery of ketoprofen from bioadhesive gels. Pak. J. Pharm. Sci. 2009, 22, 193–198. [Google Scholar]







| Formulation | Oleic acid (%) | Avocado oil (%) | Water (%) | O:S ratio |
|---|---|---|---|---|
| F25 | 40.0 | 40.0 | 20.0 | 1.00 |
| F29 | 50.0 | 30.0 | 20.0 | 0.67 |
| F32 | 60.0 | 20.0 | 20.0 | 0.33 |
| Formulations | K | n |
|---|---|---|
| F25 | 0.52 | 1.63 |
| F29 | 1168.21 | 0.49 |
| F32 | 0.98 | 1.00 |
| F25E | 3455.21 | 0.10 |
| F29E | 5504.35 | 0.06 |
| F32E | 3213.89 | 0.007 |
| Formulations | G’ | S | n |
|---|---|---|---|
| F25 | 1567.08 | 466.32 | 0.39 |
| F29 | 69,432.90 | 62,048.60 | 0.04 |
| F32 | 2.77 | 0.00 | 0.00 |
| F25E | 63,430.82 | 46,021.71 | 0.11 |
| F29E | 101,038.9 | 86,767.83 | 0.05 |
| F32E | 86,293.23 | 86,651.92 | 0.001 |
| Formulations | pH |
|---|---|
| F25 | 6.67 |
| F25E | 3.94 |
| F29 | 6.56 |
| F29E | 4.01 |
| F32 | 6.15 |
| F32E | 4.20 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bento da Silva, P.; Fioramonti Calixto, G.M.; Oshiro Júnior, J.A.; Bombardelli, R.L.Á.; Fonseca-Santos, B.; Rodero, C.F.; Chorilli, M. Structural Features and the Anti-Inflammatory Effect of Green Tea Extract-Loaded Liquid Crystalline Systems Intended for Skin Delivery. Polymers 2017, 9, 30. https://doi.org/10.3390/polym9010030
Bento da Silva P, Fioramonti Calixto GM, Oshiro Júnior JA, Bombardelli RLÁ, Fonseca-Santos B, Rodero CF, Chorilli M. Structural Features and the Anti-Inflammatory Effect of Green Tea Extract-Loaded Liquid Crystalline Systems Intended for Skin Delivery. Polymers. 2017; 9(1):30. https://doi.org/10.3390/polym9010030
Chicago/Turabian StyleBento da Silva, Patricia, Giovana Maria Fioramonti Calixto, João Augusto Oshiro Júnior, Raisa Lana Ávila Bombardelli, Bruno Fonseca-Santos, Camila Fernanda Rodero, and Marlus Chorilli. 2017. "Structural Features and the Anti-Inflammatory Effect of Green Tea Extract-Loaded Liquid Crystalline Systems Intended for Skin Delivery" Polymers 9, no. 1: 30. https://doi.org/10.3390/polym9010030
APA StyleBento da Silva, P., Fioramonti Calixto, G. M., Oshiro Júnior, J. A., Bombardelli, R. L. Á., Fonseca-Santos, B., Rodero, C. F., & Chorilli, M. (2017). Structural Features and the Anti-Inflammatory Effect of Green Tea Extract-Loaded Liquid Crystalline Systems Intended for Skin Delivery. Polymers, 9(1), 30. https://doi.org/10.3390/polym9010030