Novel Polyamides with 5H-Dibenzo[b,f]azepin-5-yl-Substituted Triphenylamine: Synthesis and Visible-NIR Electrochromic Properties
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Measurements
2.3. Synthesis of Monomers
2.3.1. 5-(4-Nitrophenyl)-5H-dibenzo[b,f]azepine (M1)
2.3.2. 4-(5H-Dibenzo[b,f]azepin-5-yl)aniline (M2)
2.3.3. 4,4′-Dinitro-4″-(5H-dibenzo[b,f]azepin-5-yl)triphenylamine (M3)
2.3.4. 4,4′-Diamino-4″-(5H-dibenzo[b,f]azepin-5-yl)triphenylamine (M4)
2.4. Synthesis of PAs
3. Results and Discussion
3.1. Monomer and Polyamide Synthesis
3.2. Solubility and Thermal Properties
3.3. Optical Properties and Electrochromic Properties
3.4. Quantum Chemistry Calculation
3.5. Spectroelectrochemical and Electrochromic Properties
3.6. Photoelectrical Properties
3.7. Electrofluorochromic Performance
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A







Appendix B


Appendix C

Appendix D
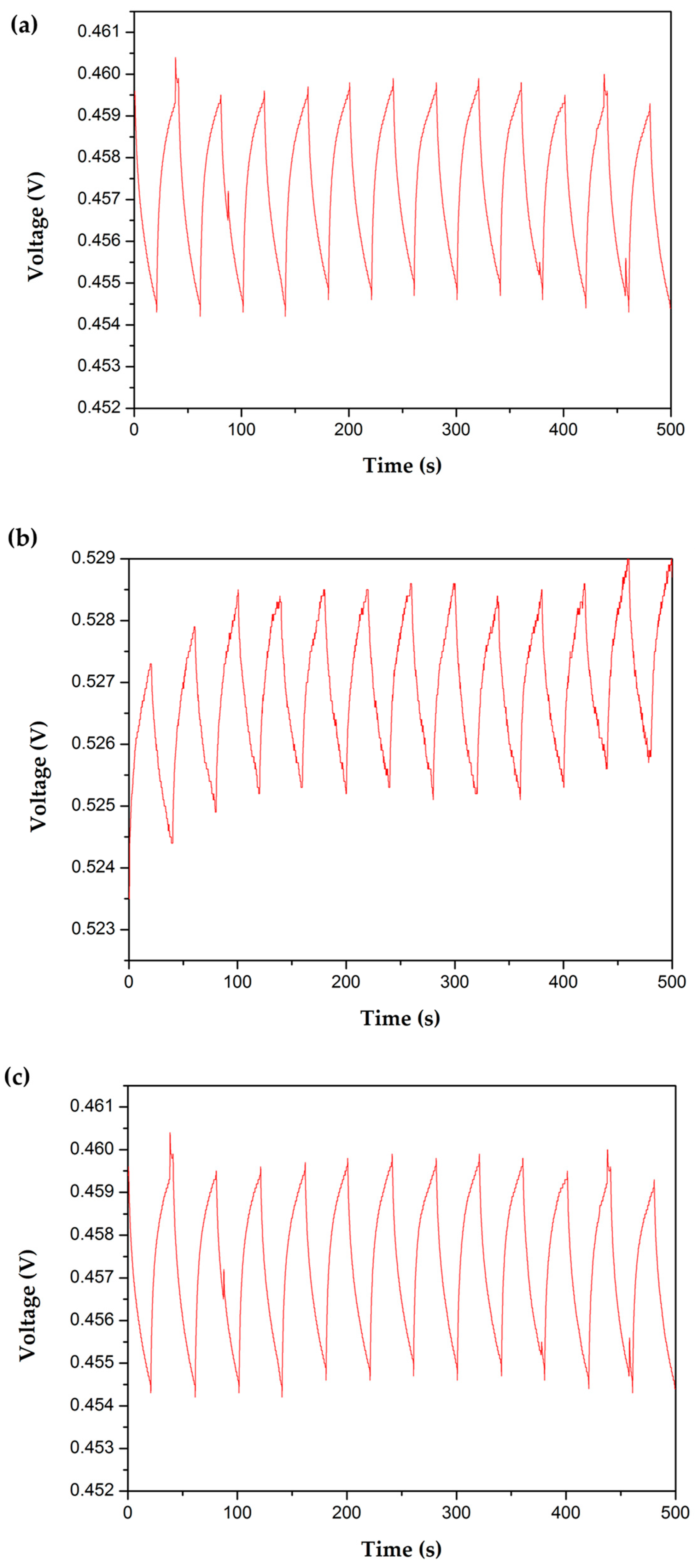
Appendix E

References
- Hsiao, S.H.; Chang, P.C.; Wang, H.M.; Kung, Y.R.; Lee, T.M. Synthesis of a new class of triphenylamine-containing poly(ether-imide)s for electrochromic applications. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 825–838. [Google Scholar] [CrossRef]
- Yen, H.J.; Tsai, C.L.; Chen, S.H.; Liou, G.S. Electrochromism and nonvolatile memory device derived from triphenylamine-based polyimides with pendant viologen units. Macromol. Rapid Commun. 2017, 38, 1600715. [Google Scholar] [CrossRef] [PubMed]
- Beaujuge, P.M.; Reynolds, J.R. Color control in π-conjugated organic polymers for use in electrochromic devices. Chem. Rev. 2010, 110, 268–320. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, S.H.; Chen, Y.Z. Electrochemical synthesis of stable ambipolar electrochromic polyimide film from a bis(triphenylamine) perylene diimide. J. Electroanal. Chem. 2017, 799, 417–423. [Google Scholar] [CrossRef]
- Mortimer, R.J.; Dyer, A.L.; Reynolds, J.R. Electrochromic organic and polymeric materials for display applications. Displays 2006, 27, 2–18. [Google Scholar] [CrossRef]
- Hsiao, S.H.; Han, J.S. Solution-processable transmissive-to-green switching electrochromic polyamides bearing 2,7-bis(diphenylamino)naphthalene units. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 1409–1421. [Google Scholar] [CrossRef]
- Montazami, R.; Jain, V.; Heflin, J.R. High contrast asymmetric solid state electrochromic devices based on layer-by-layer deposition of polyaniline and poly(aniline sulfonic acid). Electrochim. Acta 2010, 56, 990–994. [Google Scholar] [CrossRef]
- Sun, N.; Feng, F.; Wang, D.; Zhou, Z.; Guan, Y.; Dang, G.; Zhou, H.; Chen, C.; Zhao, X. Novel polyamides with fluorene-based triphenylamine: Electrofluorescence and electrochromic properties. RSC Adv. 2015, 5, 88181–88190. [Google Scholar] [CrossRef]
- Sun, J.; Lv, X.; Wang, P.; Zhang, Y.; Dai, Y.; Wu, Q.; Ouyang, M.; Zhang, C. A donor–acceptor cruciform p-system: High contrast mechanochromic properties and multicolour electrochromic behavior. J. Mater. Chem. C 2014, 2, 5365–5371. [Google Scholar] [CrossRef]
- Lv, X.; Li, W.; Ouyang, M.; Zhang, Y.; Wright, D.S.; Zhang, C. Polymeric electrochromic materials with donor–acceptor structures. J. Mater. Chem. C 2017, 5, 12–28. [Google Scholar] [CrossRef]
- Sroog, C.E. Polyimides. Prog. Polym. Sci. 1991, 16, 561–694. [Google Scholar] [CrossRef]
- Garcia, J.M.; Garcia, F.C.; Serna, F.; Pena, J.L. High-performance aromatic polyamides. Prog. Polym. Sci. 2010, 35, 623–686. [Google Scholar] [CrossRef]
- Cai, J.; Niu, H.; Zhao, P.; Ji, Y.; Ma, L.; Wang, C.; Bai, X.; Wang, W. Multicolored near-infrared electrochromic polyimides: Synthesis, electrochemical, and electrochromic properties. Dyes Pigments 2013, 99, 1124–1131. [Google Scholar] [CrossRef]
- Chern, Y.T.; Tsai, J.Y.; Wang, J.J. High Tg and high organosolubility of novel unsymmetric polyimides. J. Polym. Sci. A Polym. Chem. 2009, 47, 2443–2452. [Google Scholar] [CrossRef]
- Hou, Y.J.; Chen, G.F.; Pei, X.L.; Fang, X.Z. Synthesis and properties of transparent polyimides derived from trans- and cis-1,4-bis(3,4-dicarboxyphenoxy)cyclohexane dianhydrides. J. Polym. Res. 2013, 20, 159. [Google Scholar] [CrossRef]
- Hsiao, S.H.; Wang, H.M.; Chen, W.J.; Lee, T.M.; Leu, C.M. Synthesis and properties of novel triptycene-based polyimides. J. Polym. Sci. A Polym. Chem. 2011, 49, 3109–3120. [Google Scholar] [CrossRef]
- Hsiao, S.H.; Hsiao, Y.H.; Kung, Y.R. Synthesis and characterization of new redox-active and electrochromic polyimides with (4-morpholinyl)triphenylamine units. J. Electroanal. Chem. 2016, 764, 31–37. [Google Scholar] [CrossRef]
- Hsiao, S.H.; Cheng, S.L. New electroactive and electrochromic aromatic polyamides with ether-linked bis(triphenylamine) units. J. Polym. Sci. A Polym. Chem. 2015, 53, 496–510. [Google Scholar] [CrossRef]
- Hsiao, S.H.; Han, J.S. Redox-active and electrochromic polymers from triarylamine endcapped, 2,7-bis(diphenylamino)naphthalene-cored dicarboxamides. Eur. Polym. J. 2017, 90, 122–135. [Google Scholar] [CrossRef]
- Liu, H.S.; Pan, B.C.; Huang, D.C.; Kung, Y.R.; Leu, C.M.; Liou, G.S. Highly transparent to truly black electrochromic devices based on an ambipolar system of polyamides and viologen. NPG Asia Mater. 2017, 9, e388. [Google Scholar] [CrossRef]
- Yen, H.J.; Lin, J.H.; Su, Y.O.; Liou, G.S. Novel triarylamine-based aromatic polyamides bearing secondary amines: Synthesis and redox potential inversion characteristics induced by pyridines. J. Mater. Chem. C. 2016, 4, 10381–10385. [Google Scholar] [CrossRef]
- Yen, H.J.; Chen, C.J.; Wu, J.H.; Liou, G.S. High performance polymers and their PCBM hybrids for memory device application. J. Polym. Sci. A Polym. Chem. 2015, 6, 7464–7469. [Google Scholar] [CrossRef]
- Yen, H.J.; Liou, G.S. Solution-processable triarylamine-based electroactive high performance polymers for anodically electrochromic applications. J. Polym. Sci. A Polym. Chem. 2012, 3, 255–264. [Google Scholar] [CrossRef]
- Ji, Y.; Zhang, C.; Niu, H.; Zhao, X.; Wang, C.; Qin, C.; Wang, W.; Bai, X. Preparation and electrochromic properties of two series of polyurethanes containing separated triphenylamine moiety with different blocks. Dyes Pigments 2016, 125, 106–115. [Google Scholar] [CrossRef]
- Sun, N.; Zhou, Z.; Meng, S.; Chao, D.; Chu, X.; Zhao, X.; Wang, D.; Zhou, H.; Chen, C. Aggregation-enhanced emission (AEE)-active polyamides with methylsulfonyltriphenylamine units for electrofluorochromic applications. Dyes Pigments 2017, 141, 356–362. [Google Scholar] [CrossRef]
- Chang, H.W.; Lin, K.H.; Chueh, C.C.; Liou, G.S.; Chen, W.C. New p-type of poly(4-methoxy-triphenylamine)s derived by coupling reactions: Synthesis, electrochromic behaviors, and hole mobility. J. Polym. Sci. A Polym. Chem. 2009, 47, 4037–4050. [Google Scholar] [CrossRef]
- Jin, G.; Xia, L.; Liu, Z.; Lin, H.; Ling, J.; Wu, H.; Hou, L.; Mo, Y. Highly efficient and stable blue polymer light emitting diodes based on polysilafluorenes with pendent hole transporting groups. J. Mater. Chem. C. 2016, 4, 905–913. [Google Scholar] [CrossRef]
- Yen, H.J.; Lin, K.Y.; Liou, G.S. High Tg, ambipolar, andnear-Infrared electrochromic anthraquinone-based aramids with intervalence charge-transfer behavior. J. Polym. Sci. A Polym. Chem. 2012, 50, 61–69. [Google Scholar] [CrossRef]
- Iwanaga, T.; Ogawa, M.; Yamauchi, T.; Toyota, S. Intramolecular charge-transfer interaction of donor–acceptor–donor arrays based on anthracene bisimide. J. Org. Chem. 2016, 81, 4076–4080. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.H.; Liou, G.S. Substituent and charge transfer effects on memory behavior of the ambipolar poly(triphenylamine)s. ACS Appl. Mater. Interfaces 2015, 7, 15988–15994. [Google Scholar] [CrossRef] [PubMed]
- Moss, K.C.; Bourdakos, K.N.; Bhalla, V.; Kamtekar, K.T.; Bryce, M.R.; Fox, M.A.; Vaughan, H.L.; Dias, F.B.; Monkman, A.P. Tuning the intramolecular charge transfer emission from deep blue to green in ambipolar systems based on dibenzothiophene s,s-dioxide by manipulation of conjugation and strength of the electron donor units. J. Org. Chem. 2010, 75, 6771–6781. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, S.H.; Liou, G.S.; Kung, Y.C.; Yen, H.J. High contrast ratio and rapid switching electrochromic polymeric films based on 4-(dimethylamino)triphenylamine-functionalized aromatic Polyamides. Macromolecules 2008, 41, 2800–2808. [Google Scholar] [CrossRef]
- Ko, Y.G.; Kwon, W.; Yen, H.J.; Chang, C.W.; Kim, D.M.; Kim, K.; Hahm, S.G.; Lee, T.J.; Liou, G.S.; Ree, M. Various digital memory behaviors of functional aromatic polyimides based on electron donor and acceptor substituted triphenylamines. Macromolecules 2012, 45, 3749–3758. [Google Scholar] [CrossRef]
- Zhou, Y.; Lee, N.A.; Ngo, K.T.; Peng, X.; Feng, Y.; Rochford, J. Rigid triarylamine donor–π–acceptor porphyrin dyes and their application in dye-sensitized solar cells. RSC Adv. 2015, 41193–41202. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, C.; Chen, J.; Qi, H.; Hua, J.; Liu, Y.; Baranoff, E.; Tan, H.; Fan, J.; Zhu, W. Influence of the donor size in panchromatic D-π-A-π-A dyes bearing 5-phenyl-5H-dibenzo-[b,f]azepine units for dye-sensitized solar cells. Dyes Pigments 2016, 127, 204–212. [Google Scholar] [CrossRef]
- Niu, H.; Cai, J.; Zhao, P.; Wang, C.; Bai, X.; Wang, W. Simple approach to regulate the spectra of novel kinds of polyazomethines containing bulky triphenylamine: Electrochemistry, electrochromism and photophysical responsive to environment. Dyes Pigments. 2013, 96, 158–169. [Google Scholar] [CrossRef]
- Niu, H.; Kang, H.; Cai, J.; Wang, C.; Bai, X.; Wang, W. Novel soluble polyazomethines with pendant carbazole and triphenylamine derivatives: Preparation, characterization, and optical, electrochemical and electrochromic properties. J. Polym. Sci. A Polym. Chem. 2011, 2, 2804–2817. [Google Scholar] [CrossRef]
- Wang, H.M.; Hsiao, S.H. Ambipolar, multi-electrochromic polypyromellitimides and polynaphthalimides containing di(tert-butyl)-substituted bis(triarylamine) units. J. Mater. Chem. C 2014, 2, 1553–1564. [Google Scholar] [CrossRef]
- Ma, X.; Wu, Y.; Wen, H.; Niu, H.; Wang, C.; Qin, C.; Bai, X.; Lei, L.; Wang, W. Immobilized polyazomethines containing triphenylamine groups on ITO: Synthesis and acidochromic, electrochemical, electrochromic and photoelectronic propertie. RSC Adv. 2016, 6, 4564–4575. [Google Scholar] [CrossRef]











| Polymer Code | Td (°C) 10% | Char Yield (%) | Mw (Da) | Mn (Da) | PDI |
|---|---|---|---|---|---|
| PA–a | 326 | 64 | 1.40 × 104 | 1.11 × 104 | 1.26 |
| PA–b | 326 | 58 | 1.33 × 104 | 1.05 × 104 | 1.27 |
| PA–c | 294 | 55 | 1.58 × 104 | 1.34 × 104 | 1.18 |
| PA–d | 326 | 59 | 1.34 × 104 | 1.12 × 104 | 1.20 |
| Polymer Code | In Solution λ (nm) | As Film λ (nm) | |||
|---|---|---|---|---|---|
| Abs Max | PL Max | ФPL (%) | Abs Max | Abs Onset | |
| PA–a | 299 | 415 | 14.94 | 332 | 394 |
| PA–b | 293 | 354 | 0.93 | 304 | 468 |
| PA–c | 291 | 352 | 1.12 | 352 | 436 |
| PA–d | 293 | 352 | 0.96 | 333 | 430 |
| Polymer Code | Oxidation Potential (V) | Egopt (eV) c | Energy Levels (eV) | |||
|---|---|---|---|---|---|---|
| Eonset | E1/2ox1 | E1/2ox2 | HOMO a | LUMO b | ||
| PA–a | 0.37 | 0.52 | 0.98 | 3.15 | −4.87 | −1.72 |
| PA–b | 0.39 | 0.54 | 1.02 | 2.65 | −4.89 | −2.24 |
| PA–c | 0.42 | 0.56 | 1.02 | 2.84 | −4.91 | −2.07 |
| PA–d | 0.42 | 0.56 | 1.02 | 2.88 | −4.91 | −2.03 |
| Polymer Code | λmax (nm) | ΔT (%) | Response Time | ΔOD | Qd (mC cm−2) | CE (cm2 C−1) | |
|---|---|---|---|---|---|---|---|
| tc (s) | tb (s) | ||||||
| PA–a | 918 | 61 | 5.4 | 4.2 | 0.580 | 3.059 | 190 |
| PA–b | 914 | 74 | 5.7 | 4.7 | 0.820 | 3.987 | 206 |
| PA–c | 910 | 58 | 4.9 | 5.8 | 0.599 | 2.636 | 227 |
| PA–d | 912 | 70 | 6.1 | 5.5 | 0.822 | 3.172 | 259 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Q.; Cai, W.; Niu, H.; Wang, W.; Bai, X.; Hou, Y. Novel Polyamides with 5H-Dibenzo[b,f]azepin-5-yl-Substituted Triphenylamine: Synthesis and Visible-NIR Electrochromic Properties. Polymers 2017, 9, 542. https://doi.org/10.3390/polym9100542
Lu Q, Cai W, Niu H, Wang W, Bai X, Hou Y. Novel Polyamides with 5H-Dibenzo[b,f]azepin-5-yl-Substituted Triphenylamine: Synthesis and Visible-NIR Electrochromic Properties. Polymers. 2017; 9(10):542. https://doi.org/10.3390/polym9100542
Chicago/Turabian StyleLu, Qingyi, Wanan Cai, Haijun Niu, Wen Wang, Xuduo Bai, and Yanjun Hou. 2017. "Novel Polyamides with 5H-Dibenzo[b,f]azepin-5-yl-Substituted Triphenylamine: Synthesis and Visible-NIR Electrochromic Properties" Polymers 9, no. 10: 542. https://doi.org/10.3390/polym9100542
APA StyleLu, Q., Cai, W., Niu, H., Wang, W., Bai, X., & Hou, Y. (2017). Novel Polyamides with 5H-Dibenzo[b,f]azepin-5-yl-Substituted Triphenylamine: Synthesis and Visible-NIR Electrochromic Properties. Polymers, 9(10), 542. https://doi.org/10.3390/polym9100542





