Biomimetically Reinforced Polyvinyl Alcohol-Based Hybrid Scaffolds for Cartilage Tissue Engineering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methacrylation of Chondroitin Sulfate and Hyaluronic Acid
2.3. Swelling Ratio Measurement and Mechanical Testing
2.4. Isolation of Rabbit Chondrocytes and Cell Culture
2.5. Photoencapsulation of Chondrocytes in Cartilage-Specific Bioactive Components and Fabrication of Cell-Laden PVA-Based Hybrid Scaffolds
2.6. Cell Viability
2.7. Biochemical Assays
2.8. Quantitative PCR (qPCR)
2.9. Animal Studies
2.10. Histological Analysis
2.11. Statistical Analysis
3. Results and Discussion
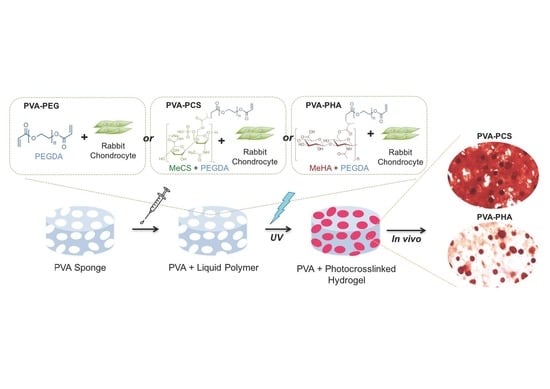
3.1. Development of Novel PVA-Based Hybrid Scaffolds with Photopolymerizable Cell-Laden Cartilage-Specific Bioactive Components
3.2. Effects of the Cartilage-Specific Bioactive Components on Cell Retention and Viability
3.3. Cell-Secreted ECM-Driven Mechanical Reinforcement of PVA-Based Hybrid Scaffolds
3.4. Gene Expression Analysis of Encapsulated Chondrocytes in PVA-Based Hybrid Scaffolds
3.5. In Vivo Cartilage Tissue Formation and Cell-Secreted ECM-Based Mechanical Reinforcement of the PVA-Based Scaffold
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Temenoff, J.S.; Mikos, A.G. Review: Tissue engineering for regeneration of articular cartilage. Biomaterials 2000, 21, 431–440. [Google Scholar] [CrossRef]
- Sophia Fox, A.J.; Bedi, A.; Rodeo, S.A. The basic science of articular cartilage: Structure, composition, and function. Sports Health 2009, 1, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Makris, E.A.; Gomoll, A.H.; Malizos, K.N.; Hu, J.C.; Athanasiou, K.A. Repair and tissue engineering techniques for articular cartilage. Nat. Rev. Rheumatol. 2015, 11, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.A.; Varghese, S.; Sharma, B.; Strehin, I.; Fermanian, S.; Gorham, J.; Fairbrother, D.H.; Cascio, B.; Elisseeff, J.H. Multifunctional chondroitin sulphate for cartilage tissue-biomaterial integration. Nat. Mater. 2007, 6, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Hwang, N.S.; Varghese, S.; Lee, H.J.; Zhang, Z.; Ye, Z.; Bae, J.; Cheng, L.; Elisseeff, J. In vivo commitment and functional tissue regeneration using human embryonic stem cell-derived mesenchymal cells. Proc. Natl. Acad. Sci. USA 2008, 105, 20641–20646. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, K.; Kim, S.; Jung, Y. Enhanced cartilaginous tissue formation with a cell aggregate-fibrin-polymer scaffold complex. Polymers 2017, 9, 348. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Hydrogels for tissue engineering. Chem. Rev. 2001, 101, 1869–1880. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kim, U.J.; Blasioli, D.J.; Kim, H.J.; Kaplan, D.L. In vitro cartilage tissue engineering with 3d porous aqueous-derived silk scaffolds and mesenchymal stem cells. Biomaterials 2005, 26, 7082–7094. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, B.V.; Brock, J.L.; Silver, J.S.; Leight, J.L.; Randolph, M.A.; Anseth, K.S. Development of a cellularly degradable PEG hydrogel to promote articular cartilage extracellular matrix deposition. Adv. Healthc. Mater. 2015, 4, 702–713. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, B.V.; Dailing, E.A.; Brock, J.L.; Stansbury, J.W.; Randolph, M.A.; Anseth, K.S. A biosynthetic scaffold that facilitates chondrocyte-mediated degradation and promotes articular cartilage extracellular matrix deposition. Regen. Eng. Transl. Med. 2015, 1, 11–21. [Google Scholar] [CrossRef] [PubMed]
- O'Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar]
- Drury, J.L.; Mooney, D.J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef]
- Saavedra, Y.G.; Mateescu, M.A.; Averill-Bates, D.A.; Denizeau, F. Polyvinylalcohol three-dimensional matrices for improved long-term dynamic culture of hepatocytes. J. Biomed. Mater. Res. A 2003, 66, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Bichara, D.A.; Bodugoz-Sentruk, H.; Ling, D.; Malchau, E.; Bragdon, C.R.; Muratoglu, O.K. Osteochondral defect repair using a polyvinyl alcohol-polyacrylic acid (pva-paac) hydrogel. Biomed. Mater. 2014, 9, 045012. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-T.; Kung, P.-H.; Lee, Y.-D. Preparation of poly(vinyl alcohol)-chondroitin sulfate hydrogel as matrices in tissue engineering. Carbohydr. Polym. 2005, 61, 348–354. [Google Scholar] [CrossRef]
- Baker, M.I.; Walsh, S.P.; Schwartz, Z.; Boyan, B.D. A review of polyvinyl alcohol and its uses in cartilage and orthopedic applications. J. Biomed. Mater. Res. B 2012, 100, 1451–1457. [Google Scholar] [CrossRef] [PubMed]
- Daley, W.P.; Peters, S.B.; Larsen, M. Extracellular matrix dynamics in development and regenerative medicine. J. Cell Sci. 2008, 121, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Varghese, S.; Hwang, N.S.; Canver, A.C.; Theprungsirikul, P.; Lin, D.W.; Elisseeff, J. Chondroitin sulfate based niches for chondrogenic differentiation of mesenchymal stem cells. Matrix Biol. 2008, 27, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Bian, L.; Guvendiren, M.; Mauck, R.L.; Burdick, J.A. Hydrogels that mimic developmentally relevant matrix and n-cadherin interactions enhance MSC chondrogenesis. Proc. Natl. Acad. Sci. USA 2013, 110, 10117–10122. [Google Scholar] [CrossRef] [PubMed]
- Highley, C.B.; Prestwich, G.D.; Burdick, J.A. Recent advances in hyaluronic acid hydrogels for biomedical applications. Curr. Opin. Biotechnol. 2016, 40, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Han, M.-E.; Kang, B.J.; Kim, S.-H.; Kim, H.D.; Hwang, N.S. Gelatin-based extracellular matrix cryogels for cartilage tissue engineering. J. Ind. Eng. Chem. 2017, 45, 421–429. [Google Scholar] [CrossRef]
- Han, M.E.; Kim, S.H.; Kim, H.D.; Yim, H.G.; Bencherif, S.A.; Kim, T.I.; Hwang, N.S. Extracellular matrix-based cryogels for cartilage tissue engineering. Int. J. Biol. Macromol. 2016, 93, 1410–1419. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-W.; Yoon, J.-R.; Lee, J.-S.; Kim, H.J.; Lim, H.-W.; Lim, H.C.; Park, J.-H.; Kim, B.-S. The use of poly(lactic-co-glycolic acid) microspheres as injectable cell carriers for cartilage regeneration in rabbit knees. J. Biomater. Sci. Polym. Ed. 2006, 17, 925–939. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.; Sangaj, N.; Varghese, S. Interconnected macroporous poly(ethylene glycol) cryogels as a cell scaffold for cartilage tissue engineering. Tissue Eng. A 2010, 16, 3033–3041. [Google Scholar] [CrossRef] [PubMed]
- Farndale, R.; Buttle, D.; Barrett, A. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim. Biophys. Acta 1986, 883, 173–177. [Google Scholar] [CrossRef]
- Eyre, D.R.; Koob, T.J.; van Ness, K.P. Quantitation of hydroxypyridinium crosslinks in collagen by high-performance liquid chromatography. Anal. Biochem. 1984, 137, 380–388. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative pcr and the 2(-delta delta c(t)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Meneghello, G.; Parker, D.J.; Ainsworth, B.J.; Perera, S.P.; Chaudhuri, J.B.; Ellis, M.J.; De Bank, P.A. Fabrication and characterization of poly(lactic-co-glycolic acid)/polyvinyl alcohol blended hollow fibre membranes for tissue engineering applications. J. Membr. Sci. 2009, 344, 55–61. [Google Scholar] [CrossRef]
- Schmedlen, R.H.; Masters, K.S.; West, J.L. Photocrosslinkable polyvinyl alcohol hydrogels that can be modified with cell adhesion peptides for use in tissue engineering. Biomaterials 2002, 23, 4325–4332. [Google Scholar] [CrossRef]
- Hwang, Y.; Zhang, C.; Varghese, S. Poly(ethylene glycol) cryogels as potential cell scaffolds: Effect of polymerization conditions on cryogel microstructure and properties. J. Mater. Chem. 2010, 20, 345–351. [Google Scholar] [CrossRef]
- Annabi, N.; Nichol, J.W.; Zhong, X.; Ji, C.; Koshy, S.; Khademhosseini, A.; Dehghani, F. Controlling the porosity and microarchitecture of hydrogels for tissue engineering. Tissue Eng. Part B 2010, 16, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.L.; Mow, V.C. Biomechanics of articular cartilage and determination of material properties. Med. Sci. Sports Exerc. 2008, 40, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Sobral, J.M.; Caridade, S.G.; Sousa, R.A.; Mano, J.F.; Reis, R.L. Three-dimensional plotted scaffolds with controlled pore size gradients: Effect of scaffold geometry on mechanical performance and cell seeding efficiency. Acta Biomater. 2011, 7, 1009–1018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levett, P.A.; Hutmacher, D.W.; Malda, J.; Klein, T.J. Hyaluronic acid enhances the mechanical properties of tissue-engineered cartilage constructs. PLoS ONE 2014, 9, e113216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kloxin, A.M.; Kloxin, C.J.; Bowman, C.N.; Anseth, K.S. Mechanical properties of cellularly responsive hydrogels and their experimental determination. Adv. Mater. 2010, 22, 3484–3494. [Google Scholar] [CrossRef] [PubMed]
- Ikegawa, S.; Sano, M.; Koshizuka, Y.; Nakamura, Y. Isolation, characterization and mapping of the mouse and human prg4 (proteoglycan 4) genes. Cytogenet. Genome Res. 2000, 90, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Jay, G.D.; Tantravahi, U.; Britt, D.E.; Barrach, H.J.; Cha, C.J. Homology of lubricin and superficial zone protein (szp): Products of megakaryocyte stimulating factor (msf) gene expression by human synovial fibroblasts and articular chondrocytes localized to chromosome 1q25. J. Orthop. Res. 2001, 19, 677–687. [Google Scholar] [CrossRef]
- Flannery, C.R.; Hughes, C.E.; Schumacher, B.L.; Tudor, D.; Aydelotte, M.B.; Kuettner, K.E.; Caterson, B. Articular cartilage superficial zone protein (szp) is homologous to megakaryocyte stimulating factor precursor and is a multifunctional proteoglycan with potential growth-promoting, cytoprotective, and lubricating properties in cartilage metabolism. Biochem. Biophys. Res. Commun. 1999, 254, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Jay, G.D.; Hong, B.-S. Characterization of a bovine synovial fluid lubricating factor. Ii. Comparison with purified ocular and salivary mucin. Connect. Tissue Res. 1992, 28, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Jay, G.D.; Haberstroh, K.; Cha, C.-J. Comparison of the boundary-lubricating ability of bovine synovial fluid, lubricin, and healon. J. Biomed. Mater. Res. 1998, 40, 414–418. [Google Scholar] [CrossRef]
- Grad, S.; Lee, C.R.; Gorna, K.; Gogolewski, S.; Wimmer, M.A.; Alini, M. Surface motion upregulates superficial zone protein and hyaluronan production in chondrocyte-seeded three-dimensional scaffolds. Tissue Eng. 2005, 11, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Swann, D.A.; Hendren, R.B.; Radin, E.L.; Sotman, S.L. The lubricating activity of synovial fluid glycoproteins. Arthritis Rheum. 1981, 24, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Kou, I.; Ikegawa, S. Sox9-dependent and -independent transcriptional regulation of human cartilage link protein. J. Biol. Chem. 2004, 279, 50942–50948. [Google Scholar] [CrossRef] [PubMed]
- Ohno, S.; Tanimoto, K.; Fujimoto, K.; Ijuin, C.; Honda, K.; Tanaka, N.; Doi, T.; Nakahara, M.; Tanne, K. Molecular cloning of rabbit hyaluronic acid synthases and their expression patterns in synovial membrane and articular cartilage. Biochim. Biophys. Acta 2001, 1520, 71–78. [Google Scholar] [CrossRef]
- Tomkoria, S.; Patel, R.V.; Mao, J.J. Heterogeneous nanomechanical properties of superficial and zonal regions of articular cartilage of the rabbit proximal radius condyle by atomic force microscopy. Med. Eng. Phys. 2004, 26, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Burdick, J.A.; Prestwich, G.D. Hyaluronic acid hydrogels for biomedical applications. Adv. Mater. 2011, 23, H41–H56. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.; Chung, C.; Khetan, S.; Burdick, J.A. Hydrolytically degradable hyaluronic acid hydrogels with controlled temporal structures. Biomacromolecules 2008, 9, 1088–1092. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.; Burdick, J.A. Influence of three-dimensional hyaluronic acid microenvironments on mesenchymal stem cell chondrogenesis. Tissue Eng. A 2009, 15, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Knudson, C.B.; Knudson, W. Hyaluronan and CD44: Modulators of chondrocyte metabolism. Clin. Orthop. Relat. Res. 2004, S152–S162. [Google Scholar] [CrossRef]
- Sato, E.; Ando, T.; Ichikawa, J.; Okita, G.; Sato, N.; Wako, M.; Ohba, T.; Ochiai, S.; Hagino, T.; Jacobson, R.; et al. High molecular weight hyaluronic acid increases the differentiation potential of the murine chondrocytic atdc5 cell line. J. Orthop. Res. 2014, 32, 1619–1627. [Google Scholar] [CrossRef] [PubMed]
- Kabra, H.; Hwang, Y.; Lim, H.L.; Kar, M.; Arya, G.; Varghese, S. Biomimetic material-assisted delivery of human embryonic stem cell derivatives for enhanced in vivo survival and engraftment. ACS Biomater. Sci. Eng. 2015, 1, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Jeuken, R.; Roth, A.; Peters, R.; van Donkelaar, C.; Thies, J.; van Rhijn, L.; Emans, P. Polymers in cartilage defect repair of the knee: Current status and future prospects. Polymers 2016, 8, 219. [Google Scholar] [CrossRef]
- Kim, H.D.; Heo, J.; Hwang, Y.; Kwak, S.Y.; Park, O.K.; Kim, H.; Varghese, S.; Hwang, N.S. Extracellular-matrix-based and arg-gly-asp-modified photopolymerizing hydrogels for cartilage tissue engineering. Tissue Eng. A 2015, 21, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Lai, J.H.; Yang, F. Effects of hydrogel stiffness and extracellular compositions on modulating cartilage regeneration by mixed populations of stem cells and chondrocytes in vivo. Tissue Eng. A 2016, 22, 1348–1356. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Lai, J.H.; Han, L.-H.; Tong, X.; Yang, F. Modulating stem cell-chondrocyte interactions for cartilage repair using combinatorial extracellular matrix-containing hydrogels. J. Mater. Chem. B 2016, 4, 7641–7650. [Google Scholar] [CrossRef]






© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.D.; Lee, Y.; Kim, Y.; Hwang, Y.; Hwang, N.S. Biomimetically Reinforced Polyvinyl Alcohol-Based Hybrid Scaffolds for Cartilage Tissue Engineering. Polymers 2017, 9, 655. https://doi.org/10.3390/polym9120655
Kim HD, Lee Y, Kim Y, Hwang Y, Hwang NS. Biomimetically Reinforced Polyvinyl Alcohol-Based Hybrid Scaffolds for Cartilage Tissue Engineering. Polymers. 2017; 9(12):655. https://doi.org/10.3390/polym9120655
Chicago/Turabian StyleKim, Hwan D., Yunsup Lee, Yunhye Kim, Yongsung Hwang, and Nathaniel S. Hwang. 2017. "Biomimetically Reinforced Polyvinyl Alcohol-Based Hybrid Scaffolds for Cartilage Tissue Engineering" Polymers 9, no. 12: 655. https://doi.org/10.3390/polym9120655
APA StyleKim, H. D., Lee, Y., Kim, Y., Hwang, Y., & Hwang, N. S. (2017). Biomimetically Reinforced Polyvinyl Alcohol-Based Hybrid Scaffolds for Cartilage Tissue Engineering. Polymers, 9(12), 655. https://doi.org/10.3390/polym9120655