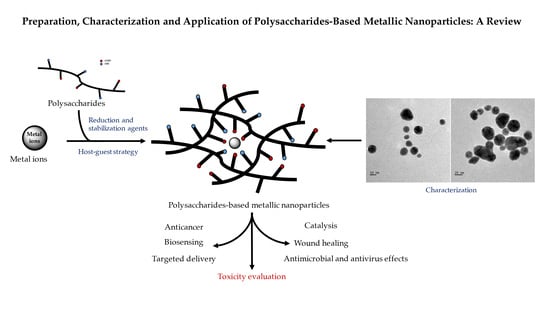
Preparation, Characterization and Application of Polysaccharide-Based Metallic Nanoparticles: A Review
Abstract
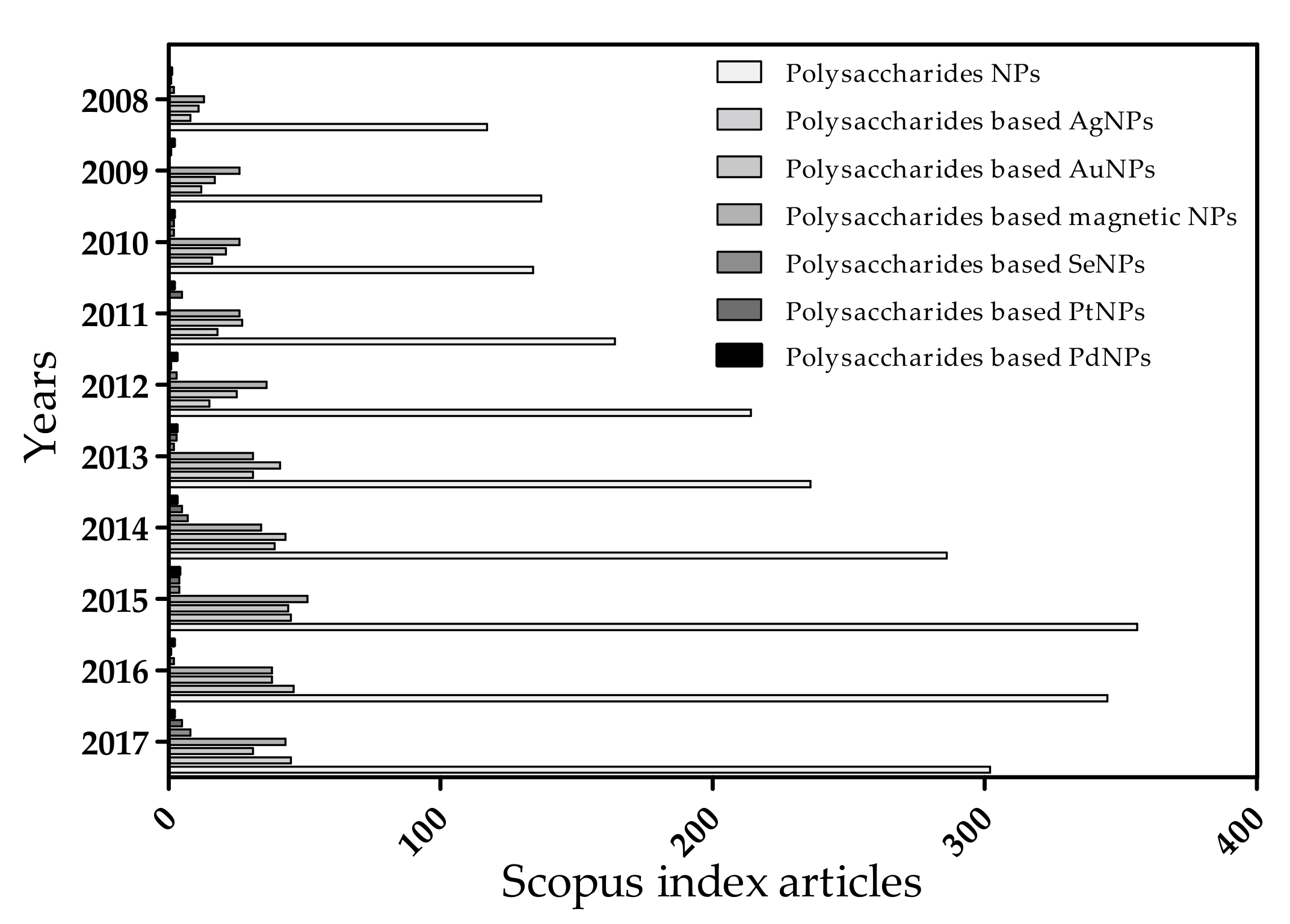
:1. Introduction
2. Preparation of PMNPs
3. Characterization of PMNPs
4. Application of PMNPs
4.1. Antimicrobial and Antiviral Property of PMNPs
4.2. Anticancer Property of PMNPs
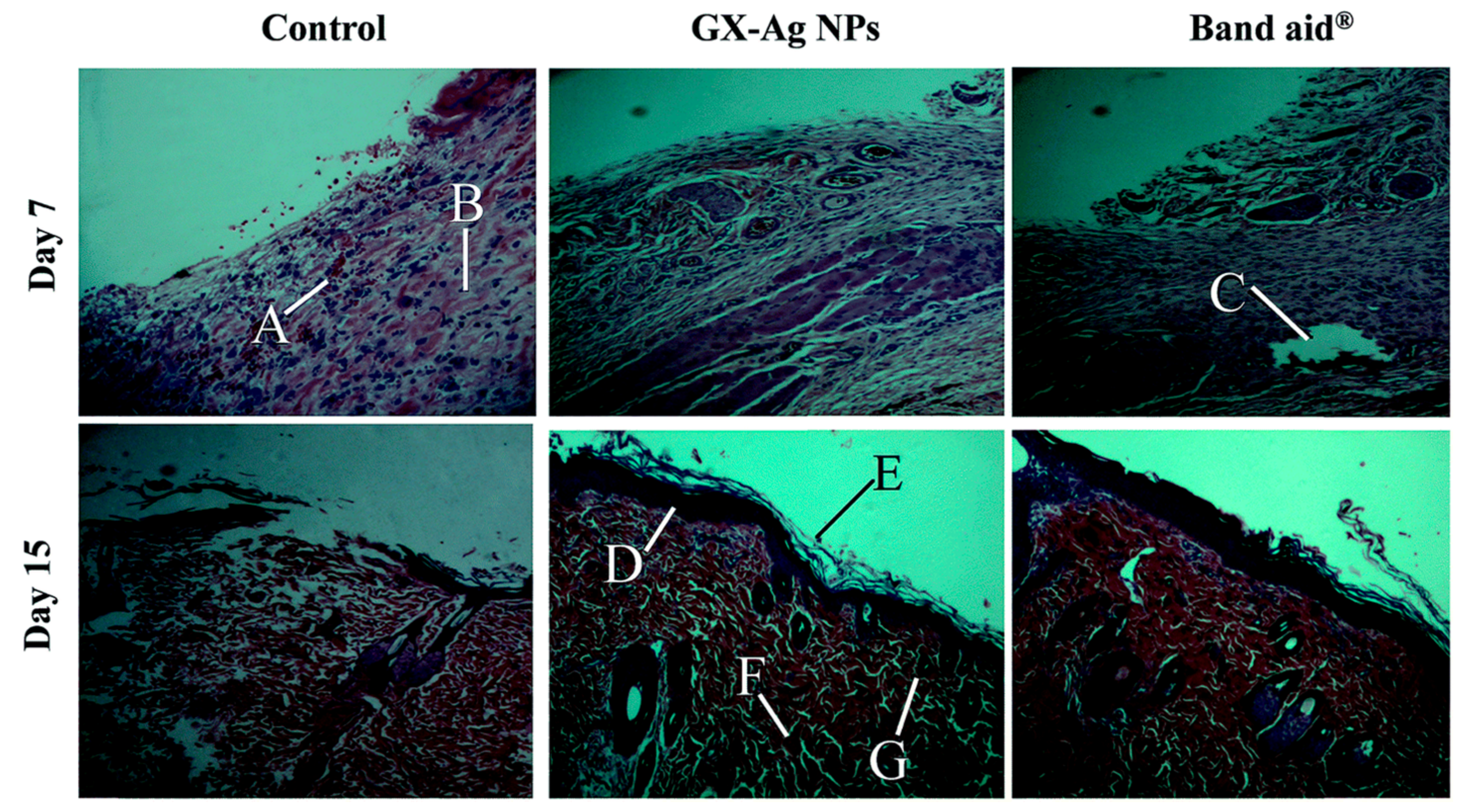
4.3. Wound Healing Property of PMNPs
4.4. PMNPs in Targeted Delivery
4.5. PMNPs for Biosensing
4.6. PMNPs in Catalytic Application
5. Toxicity of PMNPs
6. Conclusions and Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
References
- Datta, K.K.R.; Reddy, B.V.S.; Zboril, R. Polysaccharides as functional scaffolds for noble metal nanoparticles and their catalytic applications. In Encyclopedia of Nanoscience and Nanotechnology; American Scientific Publishers: Valencia, CA, USA, 2016; pp. 1–20. ISBN 1588831590. [Google Scholar]
- Huang, H.; Yuan, Q.; Yang, X. Preparation and characterization of metal-chitosan nanocomposites. Colloids Surf. B Biointerfaces 2004, 39, 31–37. [Google Scholar]
- Huang, X.; Jain, P.K.; El-Sayed, I.H.; El-Sayed, M.A. Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Lasers Med. Sci. 2008, 23, 217–228. [Google Scholar]
- Sengupta, S.; Eavarone, D.; Capila, I.; Zhao, G.; Watson, N.; Kiziltepe, T.; Sasisekharan, R. Temporal targeting of tumour cells and neovasculature with a nanoscale delivery system. Nature 2005, 436, 568–572. [Google Scholar]
- Neville, F.; Pchelintsev, N.A.; Broderick, M.J.F.; Gibson, T.; Millner, P.A. Novel one-pot synthesis and characterization of bioactive thiol-silicate nanoparticles for biocatalytic and biosensor applications. Nanotechnology 2009, 20, 55612. [Google Scholar]
- Ahmed, S.; Ahmad, M.; Swami, B.L.; Ikram, S. Green synthesis of silver nanoparticles using Azadirachta indica aqueous leaf extract. J. Radiat. Res. Appl. Sci. 2016, 9, 1–7. [Google Scholar]
- Medina-Ramirez, I.; Bashir, S.; Luo, Z.; Liu, J.L. Green synthesis and characterization of polymer-stabilized silver nanoparticles. Colloids Surf. B Biointerfaces 2009, 73, 185–191. [Google Scholar]
- Zhao, C.; Li, J.; He, B.; Zhao, L. Fabrication of hydrophobic biocomposite by combining cellulosic fibers with polyhydroxyalkanoate. Cellulose 2017, 24, 2265–2274. [Google Scholar]
- Siqueira, G.; Bras, J.; Dufresne, A. Cellulosic bionanocomposites: A review of preparation, properties and applications. Polymers 2010, 2, 728–765. [Google Scholar]
- Pauly, M.; Keegstra, K. Cell-wall carbohydrates and their modification as a resource for biofuels. Plant J. 2008, 54, 559–568. [Google Scholar]
- Emna, C.; Fatma, G.; Satinder, K.B. Biopolymers Synthesis and Application. In Biotransformation of Waste Biomass into High Value Biochemicals; Springer: New York, NY, USA, 2014; Chapter 17; pp. 415–443. ISBN 9781461480051. [Google Scholar]
- Pasqui, D.; De Cagna, M.; Barbucci, R. Polysaccharide-based hydrogels: The key role of water in affecting mechanical properties. Polymers 2012, 4, 1517–1534. [Google Scholar]
- Liu, Z.; Jiao, Y.; Wang, Y.; Zhou, C.; Zhang, Z. Polysaccharides-based nanoparticles as drug delivery systems. Adv. Drug Deliv. Rev. 2008, 60, 1650–1662. [Google Scholar]
- Yang, J.; Han, S.; Zheng, H.; Dong, H.; Liu, J. Preparation and application of micro/nanoparticles based on natural polysaccharides. Carbohydr. Polym. 2015, 123, 53–66. [Google Scholar]
- Lee, J.W.; Park, J.H.; Robinson, J.R. Bioadhesive-based dosage forms: The next generation. J. Pharm. Sci. 2000, 89, 850–866. [Google Scholar]
- Debele, T.A.; Mekuria, S.L.; Tsai, H.C. Polysaccharide based nanogels in the drug delivery system: Application as the carrier of pharmaceutical agents. Mater. Sci. Eng. C 2016, 68, 964–981. [Google Scholar]
- Wang, J.; Chen, H.; Wang, Y.; Xing, L. Synthesis and characterization of a new Inonotus obliquus polysaccharide-iron(III) complex. Int. J. Biol. Macromol. 2015, 75, 210–217. [Google Scholar]
- Li, W.; Yuan, G.; Pan, Y.; Wang, C.; Chen, H. Network Pharmacology Studies on the Bioactive Compounds and Action Mechanisms of Natural Products for the Treatment of Diabetes Mellitus: A Review. Front. Pharmacol. 2017, 8, 1–10. [Google Scholar]
- Wang, C.; Chen, Z.; Pan, Y.; Gao, X.; Chen, H. Anti-diabetic effects of Inonotus obliquus polysaccharides-chromium(III) complex in type 2 diabetic mice and its sub-acute toxicity evaluation in normal mice. Food Chem. Toxicol. 2017, 108, 498–509. [Google Scholar]
- Rao, K.M.; Kumar, A.; Haider, A.; Han, S.S. Polysaccharides based antibacterial polyelectrolyte hydrogels with silver nanoparticles. Mater. Lett. 2016, 184, 189–192. [Google Scholar]
- Kim, C.; Tonga, G.Y.; Yan, B.; Kim, C.S.; Kim, S.T.; Park, M.-H.; Zhu, Z.; Duncan, B.; Creran, B.; Rotello, V.M. Regulating exocytosis of nanoparticles via host–guest chemistry. Org. Biomol. Chem. 2015, 13, 2474–2479. [Google Scholar]
- Dhar, S.; Maheswara Reddy, E.; Shiras, A.; Pokharkar, V.; Prasad, B.L. Natural gum reduced/stabilized gold nanoparticles for drug delivery formulations. Chemistry 2008, 14, 10244–10250. [Google Scholar]
- Tran, H.V.; Tran, L.D.; Ba, C.T.; Vu, H.D.; Nguyen, T.N.; Pham, D.G.; Nguyen, P.X. Synthesis, characterization, antibacterial and antiproliferative activities of monodisperse chitosan- based silver nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2010, 360, 32–40. [Google Scholar]
- Narayanan, G.; Aguda, R.; Hartman, M.; Chung, C.C.; Boy, R.; Gupta, B.S.; Tonelli, A.E. Fabrication and Characterization of Poly(ε-caprolactone)/α-Cyclodextrin Pseudorotaxane Nanofibers. Biomacromolecules 2016, 17, 271–279. [Google Scholar]
- Moreno-Trejo, M.B.; Sánchez-Domínguez, M. Mesquite gum as a novel reducing and stabilizing agent for modified tollens synthesis of highly concentrated Ag nanoparticles. Materials 2016, 9, 817. [Google Scholar]
- Chen, W.H.; Lei, Q.; Luo, G.F.; Jia, H.Z.; Hong, S.; Liu, Y.X.; Cheng, Y.J.; Zhang, X.Z. Rational Design of Multifunctional Gold Nanoparticles via Host-Guest Interaction for Cancer-Targeted Therapy. ACS Appl. Mater. Interfaces 2015, 7, 17171–17180. [Google Scholar]
- Noël, S.; Léger, B.; Ponchel, A.; Philippot, K.; Denicourt-Nowicki, A.; Roucoux, A.; Monflier, E. Cyclodextrin-based systems for the stabilization of metallic(0) nanoparticles and their versatile applications in catalysis. Catal. Today 2014, 235, 20–32. [Google Scholar] [Green Version]
- Yao, X.; Zhu, Q.; Li, C.; Yuan, K.; Che, R.; Zhang, P.; Yang, C.; Lu, W.; Wu, W.; Jiang, X. Carbamoylmannose enhances the tumor targeting ability of supramolecular nanoparticles formed through host–guest complexation of a pair of homopolymers. J. Mater. Chem. B 2017, 5, 834–848. [Google Scholar]
- Nair, L.S.; Laurencin, C.T. Silver nanoparticles: Synthesis and therapeutic applications. J. Biomed. Nanotechnol. 2007, 3, 301–316. [Google Scholar]
- Sharma, D.; Kanchi, S.; Bisetty, K. Biogenic synthesis of nanoparticles: A review. Arab. J. Chem. 2015. [Google Scholar] [CrossRef]
- Ahmed, S.; Ahmad, M.; Swami, B.L.; Ikram, S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: A green expertise. J. Adv. Res. 2016, 7, 17–28. [Google Scholar]
- Kaliaraj, G.S.; Subramaniyan, B.; Manivasagan, P. Green Synthesis of Metal Nanoparticles Using Seaweed Polysaccharides. In Seaweed Polysaccharides; Elsevier: Amsterdam, The Netherlands, 2017; Chapter 7; pp. 101–109. ISBN 9780128098165. [Google Scholar]
- Shukla, A.K.; Iravani, S. Metallic nanoparticles: Green synthesis and spectroscopic characterization. Environ. Chem. Lett. 2017, 15, 223–231. [Google Scholar]
- Singh, P.; Kim, Y.J.; Zhang, D.; Yang, D.C. Biological Synthesis of Nanoparticles from Plants and Microorganisms. Trends Biotechnol. 2016, 34, 588–599. [Google Scholar]
- Thakkar, K.N.; Mhatre, S.S.; Parikh, R.Y. Biological synthesis of metallic nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 257–262. [Google Scholar]
- Mittal, A.K.; Chisti, Y.; Banerjee, U.C. Synthesis of metallic nanoparticles using plant extracts. Biotechnol. Adv. 2013, 31, 346–356. [Google Scholar]
- Yip, J.; Liu, L.; Wong, K.H.; Leung, P.H.M.; Yuen, C.W.M.; Cheung, M.C. Investigation of antifungal and antibacterial effects of fabric padded with highly stable selenium nanoparticles. J. Appl. Polym. Sci. 2014, 131, 8886–8893. [Google Scholar]
- Li, H.; Yang, Y.W. Gold nanoparticles functionalized with supramolecular macrocycles. Chin. Chem. Lett. 2013, 24, 545–552. [Google Scholar]
- Cram, D.J.; Cram, J.M. Host-Guest Chemistry. Science 1974, 183, 803–809. [Google Scholar]
- Niu, Z.; Li, Y. Removal and utilization of capping agents in nanocatalysis. Chem. Mater. 2014, 26, 72–83. [Google Scholar]
- Chan, H.K.; Kwok, P.C.L. Production methods for nanodrug particles using the bottom-up approach. Adv. Drug Deliv. Rev. 2011, 63, 406–416. [Google Scholar]
- Raghunandan, D.; Basavaraja, S.; Mahesh, B.; Balaji, S.; Manjunath, S.Y.; Venkataraman, A. Biosynthesis of stable polyshaped gold nanoparticles from microwave-exposed aqueous extracellular anti-malignant guava (Psidium guajava) leaf extract. Nanobiotechnology 2009, 5, 34–41. [Google Scholar]
- Hussain, M.A.; Shah, A.; Jantan, I.; Shah, M.R.; Tahir, M.N.; Ahmad, R.; Bukhari, S.N.A. Hydroxypropylcellulose as a novel green reservoir for the synthesis, stabilization, and storage of silver nanoparticles. Int. J. Nanomed. 2015, 10, 2079–2088. [Google Scholar]
- Ehmann, H.M.A.; Breitwieser, D.; Winter, S.; Gspan, C.; Koraimann, G.; Maver, U.; Sega, M.; Köstler, S.; Stana-Kleinschek, K.; Spirk, S.; et al. Gold nanoparticles in the engineering of antibacterial and anticoagulant surfaces. Carbohydr. Polym. 2015, 117, 34–42. [Google Scholar]
- Abedini, A.; Daud, A.; Abdul Hamid, M.; Kamil Othman, N.; Saion, E. A review on radiation-induced nucleation and growth of colloidal metallic nanoparticles. Nanoscale Res. Lett. 2013, 8, 474. [Google Scholar]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.; Zolfaghari, B. Synthesis of silver nanoparticles: Chemical, physical and biological methods. Res. Pharm. Sci. 2014, 9, 385–406. [Google Scholar]
- Sokolov, S.V.; Batchelor-Mcauley, C.; Tschulik, K.; Fletcher, S.; Compton, R.G. Are Nanoparticles Spherical or Quasi-Spherical? Chemistry 2015, 21, 10741–10746. [Google Scholar] [Green Version]
- Lechner, M.; Mächtle, W. Characterization of nanoparticles. Macromol. Symp. 1999, 7, 1–7. [Google Scholar]
- Hall, J.B.; Dobrovolskaia, M.A.; Patri, A.K.; McNeil, S.E. Characterization of nanoparticles for therapeutics. Nanomedicine 2007, 2, 789–803. [Google Scholar]
- Bootz, A.; Vogel, V.; Schubert, D.; Kreuter, J. Comparison of scanning electron microscopy, dynamic light scattering and analytical ultracentrifugation for the sizing of poly(butyl cyanoacrylate) nanoparticles. Eur. J. Pharm. Biopharm. 2004, 57, 369–375. [Google Scholar]
- Lin, P.C.; Lin, S.; Wang, P.C.; Sridhar, R. Techniques for physicochemical characterization of nanomaterials. Biotechnol. Adv. 2014, 32, 711–726. [Google Scholar]
- Dobrovolskaia, M.A.; Patri, A.K.; Zheng, J.; Clogston, J.D.; Ayub, N.; Aggarwal, P.; Neun, B.W.; Hall, J.B.; McNeil, S.E. Interaction of colloidal gold nanoparticles with human blood: Effects on particle size and analysis of plasma protein binding profiles. Nanomed. Nanotechnol. Biol. Med. 2009, 5, 106–117. [Google Scholar]
- Powers, K.W.; Palazuelos, M.; Moudgil, B.M.; Roberts, S.M. Characterization of the size, shape, and state of dispersion of nanoparticles for toxicological studies. Nanotoxicology 2007, 1, 42–51. [Google Scholar]
- Sanyasi, S.; Majhi, R.K.; Kumar, S.; Mishra, M.; Ghosh, A.; Suar, M.; Satyam, P.V.; Mohapatra, H.; Goswami, C.; Goswami, L. Polysaccharide-capped silver Nanoparticles inhibit biofilm formation and eliminate multi-drug-resistant bacteria by disrupting bacterial cytoskeleton with reduced cytotoxicity towards mammalian cells. Sci. Rep. 2016, 6, 24929. [Google Scholar]
- Berne, B.J.; Pecora, R. Dynamic Light Scattering: With Applications to Chemistry, Biology, and Physics, 1st ed.; Dover Publications: New York, NY, USA, 2000; pp. 3–24. ISBN 0486411559. [Google Scholar]
- Ito, T.; Sun, L.; Bevan, M.A.; Crooks, R.M. Comparison of nanoparticle size and electrophoretic mobility measurements using a carbon-nanotube-based coulter counter, dynamic light scattering, transmission electron microscopy, and phase analysis light scattering. Langmuir 2004, 20, 6940–6945. [Google Scholar]
- Upstone, S. Ultraviolet/visible light absorption spectrophotometry in clinical chemistry. Encycl. Anal. Chem. 2000, 1699–1714. [Google Scholar] [CrossRef]
- Liu, X.M.; Sheng, G.P.; Luo, H.W.; Zhang, F.; Yuan, S.J.; Xu, J.; Zeng, R.J.; Wu, J.G.; Yu, H.Q. Contribution of extracellular polymeric substances (EPS) to the sludge aggregation. Environ. Sci. Technol. 2010, 44, 4355–4360. [Google Scholar]
- Khorsand Zak, A.; Abd. Majid, W.H.; Abrishami, M.E.; Yousefi, R. X-ray analysis of ZnO nanoparticles by Williamson-Hall and size-strain plot methods. Solid State Sci. 2011, 13, 251–256. [Google Scholar]
- Sapsford, K.E.; Tyner, K.M.; Dair, B.J.; Deschamps, J.R.; Medintz, I.L. Analyzing nanomaterial bioconjugates: A review of current and emerging purification and characterization techniques. Anal. Chem. 2011, 83, 4453–4488. [Google Scholar]
- Caminade, A.M.; Laurent, R.; Majoral, J.P. Characterization of dendrimers. Adv. Drug Deliv. Rev. 2005, 57, 2130–2146. [Google Scholar]
- Stanjek, H.; Häusler, W. Basics of X-ray diffraction. Hyperfine Interact. 2004, 154, 107–119. [Google Scholar]
- Bindu, P.; Thomas, S. Estimation of lattice strain in ZnO nanoparticles: X-ray peak profile analysis. J. Theor. Appl. Phys. 2014, 8, 123–134. [Google Scholar]
- Tadic, M.; Panjan, M.; Damnjanovic, V.; Milosevic, I. Magnetic properties of hematite (α-Fe2O3) nanoparticles prepared by hydrothermal synthesis method. Appl. Surf. Sci. 2014, 320, 183–187. [Google Scholar]
- Das, T.; Yeasmin, S.; Khatua, S.; Acharya, K.; Bandyopadhyay, A. Influence of a blend of guar gum and poly(vinyl alcohol) on long term stability, and antibacterial and antioxidant efficacies of silver nanoparticles. RSC Adv. 2015, 5, 54059–54069. [Google Scholar]
- Gupta, D.; Singh, D.; Kothiyal, N.C.; Saini, A.K.; Singh, V.P.; Pathania, D. Synthesis of chitosan-g-poly(acrylamide)/ZnS nanocomposite for controlled drug delivery and antimicrobial activity. Int. J. Biol. Macromol. 2015, 74, 547–557. [Google Scholar]
- Vidya, S.M.; Mutalik, S.; Bhat, K.U.; Huilgol, P.; Avadhani, K. Preparation of gold nanoparticles by novel bacterial exopolysaccharide for antibiotic delivery. Life Sci. 2016, 153, 171–179. [Google Scholar]
- El-Rafie, M.H.; Mohamed, A.A.; Shaheen, T.I.; Hebeish, A. Antimicrobial effect of silver nanoparticles produced by fungal process on cotton fabrics. Carbohydr. Polym. 2010, 80, 779–782. [Google Scholar]
- Ma, Y.; Liu, C.; Qu, D.; Chen, Y.; Huang, M.; Liu, Y. Antibacterial evaluation of sliver nanoparticles synthesized by polysaccharides from Astragalus membranaceus roots. Biomed. Pharmacother. 2017, 89, 351–357. [Google Scholar]
- Kanmani, P.; Lim, S.T. Synthesis and characterization of pullulan-mediated silver nanoparticles and its antimicrobial activities. Carbohydr. Polym. 2013, 97, 421–428. [Google Scholar]
- Chopra, I. The increasing use of silver-based products as antimicrobial agents: A useful development or a cause for concern? J. Antimicrob. Chemother. 2007, 59, 587–590. [Google Scholar]
- Gwinn, E.G.; O’Neill, P.; Guerrero, A.J.; Bouwmeester, D.; Fygenson, D.K. Sequence-dependent fluorescence of DNA-hosted silver nanoclusters. Adv. Mater. 2008, 20, 279–283. [Google Scholar]
- Pallavicini, P.; Arciola, C.R.; Bertoglio, F.; Curtosi, S.; Dacarro, G.; D’Agostino, A.; Ferrari, F.; Merli, D.; Milanese, C.; Rossi, S.; et al. Silver nanoparticles synthesized and coated with pectin: An ideal compromise for anti-bacterial and anti-biofilm action combined with wound-healing properties. J. Colloid Interface Sci. 2017, 498, 271–281. [Google Scholar]
- Thaya, R.; Malaikozhundan, B.; Vijayakumar, S.; Sivakamavalli, J.; Jeyasekar, R.; Shanthi, S.; Vaseeharan, B.; Ramasamy, P.; Sonawane, A. Chitosan coated Ag/ZnO nanocomposite and their antibiofilm, antifungal and cytotoxic effects on murine macrophages. Microb. Pathog. 2016, 100, 124–132. [Google Scholar]
- Sathiyanarayanan, G.; Vignesh, V.; Saibaba, G.; Vinothkanna, A.; Dineshkumar, K.; Viswanathan, M.B.; Selvin, J. Synthesis of carbohydrate polymer encrusted gold nanoparticles using bacterial exopolysaccharide: A novel and greener approach. RSC Adv. 2014, 4, 22817–22827. [Google Scholar]
- Geraldo, D.A.; Needhan, P.; Chandia, N.; Arratia-Pérez, R.; Mora, G.C.; Villagra, N. Green synthesis of polysaccharides-based gold and silver nanoparticles and their promissory biological activity. Biointerface Res. Appl. Chem. 2016, 6, 1263–1271. [Google Scholar]
- Valodkar, M.; Rathore, P.S.; Jadeja, R.N.; Thounaojam, M.; Devkar, R.V.; Thakore, S. Cytotoxicity evaluation and antimicrobial studies of starch capped water soluble copper nanoparticles. J. Hazard. Mater. 2012, 201–202, 244–249. [Google Scholar]
- Rajeshkumar, S. Phytochemical constituents of fucoidan (Padina tetrastromatica) and its assisted AgNPs for enhanced antibacterial activity. IET Nanobiotechnol. 2016, 11, 292–299. [Google Scholar]
- Goyal, G.; Hwang, J.; Aviral, J.; Seo, Y.; Jo, Y.; Son, J.; Choi, J. Green synthesis of silver nanoparticles using β-glucan, and their incorporation into doxorubicin-loaded water-in-oil nanoemulsions for antitumor and antibacterial applications. J. Ind. Eng. Chem. 2017, 47, 179–186. [Google Scholar]
- Yumei, L.; Yamei, L.; Qiang, L.; Jie, B. Rapid Biosynthesis of Silver Nanoparticles Based on Flocculation and Reduction of an Exopolysaccharide from Arthrobacter sp. B4: Its Antimicrobial Activity and Phytotoxicity. J. Nanomater. 2017, 2017, 9703614. [Google Scholar]
- Chen, X.; Yan, J.-K.; Wu, J.-Y. Characterization and antibacterial activity of silver nanoparticles prepared with a fungal exopolysaccharide in water. Food Hydrocoll. 2015, 53, 69–74. [Google Scholar]
- Emam, H.E.; Zahran, M.K. Ag0 nanoparticles containing cotton fabric: Synthesis, characterization, color data and antibacterial action. Int. J. Biol. Macromol. 2015, 75, 106–114. [Google Scholar]
- Xu, W.; Jin, W.; Lin, L.; Zhang, C.; Li, Z.; Li, Y.; Song, R.; Li, B. Green synthesis of xanthan conformation-based silver nanoparticles: Antibacterial and catalytic application. Carbohydr. Polym. 2014, 101, 961–967. [Google Scholar]
- Ghasemzadeh, H.; Mahboubi, A.; Karimi, K.; Hassani, S. Full polysaccharide chitosan-CMC membrane and silver nanocomposite: Synthesis, characterization, and antibacterial behaviors. Polym. Adv. Technol. 2016, 27, 1204–1210. [Google Scholar]
- Rasulov, B.; Rustamova, N.; Yili, A.; Zhao, H.Q.; Aisa, H.A. Synthesis of silver nanoparticles on the basis of low and high molar mass exopolysaccharides of Bradyrhizobium japonicum 36 and its antimicrobial activity against some pathogens. Folia Microbiol. 2016, 61, 283–293. [Google Scholar]
- Baldi, F.; Daniele, S.; Gallo, M.; Paganelli, S.; Battistel, D.; Piccolo, O.; Faleri, C.; Puglia, A.M.; Gallo, G. Polysaccharide-based silver nanoparticles synthesized by Klebsiella oxytoca DSM 29614 cause DNA fragmentation in E. coli cells. BioMetals 2016, 29, 321–331. [Google Scholar]
- Manna, D.K.; Mandal, A.K.; Sen, I.K.; Maji, P.K.; Chakraborti, S.; Chakraborty, R.; Islam, S.S. Antibacterial and DNA degradation potential of silver nanoparticles synthesized via green route. Int. J. Biol. Macromol. 2015, 80, 455–459. [Google Scholar]
- Sen, I.K.; Mandal, A.K.; Chakraborti, S.; Dey, B.; Chakraborty, R.; Islam, S.S. Green synthesis of silver nanoparticles using glucan from mushroom and study of antibacterial activity. Int. J. Biol. Macromol. 2013, 62, 439–449. [Google Scholar]
- Kanmani, P.; Lim, S.T. Synthesis and structural characterization of silver nanoparticles using bacterial exopolysaccharide and its antimicrobial activity against food and multidrug resistant pathogens. Process Biochem. 2013, 48, 1099–1106. [Google Scholar]
- Iconaru, S.L.; Prodan, A.M.; Motelica-Heino, M.; Sizaret, S.; Predoi, D. Synthesis and characterization of polysaccharide-maghemite composite nanoparticles and their antibacterial properties. Nanoscale Res. Lett. 2012, 7, 576. [Google Scholar] [Green Version]
- White, R.J.; Budarin, V.L.; Moir, J.W.B.; Clark, J.H. A sweet killer: Mesoporous polysaccharide confined silver nanoparticles for antibacterial applications. Int. J. Mol. Sci. 2011, 12, 5782–5796. [Google Scholar]
- Kora, A.; Beedu, S.; Jayaraman, A. Size-controlled green synthesis of silver nanoparticles mediated by gum ghatti (Anogeissus latifolia) and its biological activity. Org. Med. Chem. Lett. 2012, 2, 17. [Google Scholar]
- El-Rafie, H.M.; El-Rafie, M.H.; Zahran, M.K. Green synthesis of silver nanoparticles using polysaccharides extracted from marine macro algae. Carbohydr. Polym. 2013, 96, 403–410. [Google Scholar]
- Selvakumar, R.; Aravindh, S.; Ashok, A.M.; Balachandran, Y.L. A facile synthesis of silver nanoparticle with SERS and antimicrobial activity using Bacillus subtilis exopolysaccharides. J. Exp. Nanosci. 2013, 8080, 1–13. [Google Scholar]
- Kora, A.J.; Sashidhar, R.B.; Arunachalam, J. Gum kondagogu (Cochlospermum gossypium): A template for the green synthesis and stabilization of silver nanoparticles with antibacterial application. Carbohydr. Polym. 2010, 82, 670–679. [Google Scholar]
- Venkatpurwar, V.; Pokharkar, V. Green synthesis of silver nanoparticles using marine polysaccharide: Study of in-vitro antibacterial activity. Mater. Lett. 2011, 65, 999–1002. [Google Scholar]
- Anuradha, K.; Bangal, P.; Madhavendra, S.S. Macromolecular arabinogalactan polysaccharide mediated synthesis of silver nanoparticles, characterization and evaluation. Macromol. Res. 2016, 24, 152–162. [Google Scholar]
- Gostin, L.O.; Hodge, J.G. Zika virus and global health security. Lancet Infect. Dis. 2016, 16, 1099–1100. [Google Scholar]
- Koonin, E.V.; Senkevich, T.G.; Dolja, V.V. The ancient Virus World and evolution of cells. Biol. Direct 2006, 1, 29. [Google Scholar]
- Zheng, L.; Wei, J.; Lv, X.; Bi, Y.; Wu, P.; Zhang, Z.; Wang, P.; Liu, R.; Jiang, J.; Cong, H.; et al. Detection and differentiation of influenza viruses with glycan-functionalized gold nanoparticles. Biosens. Bioelectron. 2017, 91, 46–52. [Google Scholar]
- Wei, J.; Zheng, L.; Lv, X.; Bi, Y.; Chen, W.; Zhang, W.; Shi, Y.; Zhao, L.; Sun, X.; Wang, F.; et al. Analysis of Influenza Virus Receptor Specificity Using Glycan-Functionalized Gold Nanoparticles. ACS Nano 2014, 8, 4600–4607. [Google Scholar]
- Yan, J.-K.; Ma, H.-L.; Cai, P.-F.; Wu, J.-Y. Highly selective and sensitive nucleic acid detection based on polysaccharide-functionalized silver nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 134, 17–21. [Google Scholar]
- Speshock, J.L.; Murdock, R.C.; Braydich-Stolle, L.K.; Schrand, A.M.; Hussain, S.M. Interaction of silver nanoparticles with Tacaribe virus. J. Nanobiotechnol. 2010, 8, 19. [Google Scholar]
- Rogers, J.V.; Parkinson, C.V.; Choi, Y.W.; Speshock, J.L.; Hussain, S.M. A preliminary assessment of silver nanoparticle inhibition of monkeypox virus plaque formation. Nanoscale Res. Lett. 2008, 3, 129–133. [Google Scholar]
- Chen, Y.-S.; Hung, Y.-C.; Lin, W.-H.; Huang, G.S. Assessment of gold nanoparticles as a size-dependent vaccine carrier for enhancing the antibody response against synthetic foot-and-mouth disease virus peptide. Nanotechnology 2010, 21, 195101. [Google Scholar]
- Zheng, Y.; Wang, W.; Li, Y. Antitumor and immunomodulatory activity of polysaccharide isolated from Trametes orientalis. Carbohydr. Polym. 2015, 131, 248–254. [Google Scholar]
- Tietze, R.; Zaloga, J.; Unterweger, H.; Lyer, S.; Friedrich, R.P.; Janko, C.; Pöttler, M.; Dürr, S.; Alexiou, C. Magnetic nanoparticle-based drug delivery for cancer therapy. Biochem. Biophys. Res. Commun. 2015, 468, 463–470. [Google Scholar]
- Wicki, A.; Witzigmann, D.; Balasubramanian, V.; Huwyler, J. Nanomedicine in Cancer Therapy: Challenges, Opportunities, and Clinical Applications. J. Control. Release 2014, 200, 138–157. [Google Scholar]
- Joseph, M.M.; Aravind, S.R.; George, S.K.; Pillai, K.R.; Mini, S.; Sreelekha, T.T. Antitumor activity of galactoxyloglucan-gold nanoparticles against murine ascites and solid carcinoma. Colloids Surf. B Biointerfaces 2014, 116, 219–227. [Google Scholar]
- Suganya, K.S.U.; Govindaraju, K.; Kumar, V.G.; Karthick, V.; Parthasarathy, K. Pectin mediated gold nanoparticles induces apoptosis in mammary adenocarcinoma cell lines. Int. J. Biol. Macromol. 2016, 93, 1030–1040. [Google Scholar]
- Joseph, M.M.; Aravind, S.R.; Varghese, S.; Mini, S.; Sreelekha, T.T. PST-Gold nanoparticle as an effective anticancer agent with immunomodulatory properties. Colloids Surf. B Biointerfaces 2013, 104, 32–39. [Google Scholar]
- Tengdelius, M.; Gurav, D.; Konradsson, P.; Påhlsson, P.; Griffith, M.; Oommen, O.P. Synthesis and anticancer properties of fucoidan-mimetic glycopolymer coated gold nanoparticles. Chem. Commun. 2015, 2, 8532–8535. [Google Scholar]
- Chen, T.; Wong, Y.S. Selenocystine induces apoptosis of A375 human melanoma cells by activating ROS-mediated mitochondrial pathway and p53 phosphorylation. Cell. Mol. Life Sci. 2008, 65, 2763–2775. [Google Scholar]
- Nie, T.; Wu, H.; Wong, K.-H.; Chen, T. Facile synthesis of highly uniform selenium nanoparticles using glucose as the reductant and surface decorator to induce cancer cell apoptosis. J. Mater. Chem. B 2016, 4, 2351–2358. [Google Scholar]
- Budihardjo, I.; Oliver, H.; Lutter, M.; Luo, X.; Wang, X. Biochemical pathways of caspase activation during apoptosis. Annu. Rev. Cell Dev. Biol. 1999, 15, 269–290. [Google Scholar]
- Namvar, F.; Rahman, H.S.; Mohamad, R.; Baharara, J.; Mahdavi, M.; Amini, E.; Chartrand, M.S.; Yeap, S.K. Cytotoxic effect of magnetic iron oxide nanoparticles synthesized via seaweed aqueous extract. Int. J. Nanomed. 2014, 9, 2479–2488. [Google Scholar]
- Wu, H.; Zhu, H.; Li, X.; Liu, Z.; Zheng, W.; Chen, T.; Yu, B.; Wong, K.H. Induction of apoptosis and cell cycle arrest in A549 human lung adenocarcinoma cells by surface-capping selenium nanoparticles: An effect enhanced by polysaccharide-protein complexes from Polyporus rhinocerus. J. Agric. Food Chem. 2013, 61, 9859–9866. [Google Scholar]
- Raveendran, S.; Chauhan, N.; Palaninathan, V.; Nagaoka, Y.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Extremophilic polysaccharide for biosynthesis and passivation of gold nanoparticles and photothermal ablation of cancer cells. Part. Part. Syst. Charact. 2015, 32, 54–64. [Google Scholar]
- Liu, C.P.; Lin, F.S.; Chien, C.T.; Tseng, S.Y.; Luo, C.W.; Chen, C.H.; Chen, J.K.; Tseng, F.G.; Hwu, Y.; Lo, L.W.; et al. In-situ formation and assembly of gold nanoparticles by gum Arabic as efficient photothermal agent for killing cancer cells. Macromol. Biosci. 2013, 13, 1314–1320. [Google Scholar]
- Medhat, D.; Hussein, J.; El-Naggar, M.E.; Attia, M.F.; Anwar, M.; Latif, Y.A.; Booles, H.F.; Morsy, S.; Farrag, A.R.; Khalil, W.K.B.; et al. Effect of Au-dextran NPs as anti-tumor agent against EAC and solid tumor in mice by biochemical evaluations and histopathological investigations. Biomed. Pharmacother. 2017, 91, 1006–1016. [Google Scholar]
- Arjunan, N.; Kumari, H.L.J.; Singaravelu, C.M.; Kandasamy, R.; Kandasamy, J. Physicochemical investigations of biogenic chitosan-silver nanocomposite as antimicrobial and anticancer agent. Int. J. Biol. Macromol. 2016, 92, 77–87. [Google Scholar]
- Estrela-Llopis, V.R.; Chevichalova, A.V.; Trigubova, N.A.; Ryzhuk, E.V. Heterocoagulation of polysaccharide-coated platinum nanoparticles with ovarian-cancer cells. Colloid J. 2014, 76, 609–621. [Google Scholar]
- Jia, X.; Liu, Q.; Zou, S.; Xu, X.; Zhang, L. Construction of selenium nanoparticles/β-glucan composites for enhancement of the antitumor activity. Carbohydr. Polym. 2015, 117, 434–442. [Google Scholar]
- Ren, Y.; Zhao, T.; Mao, G.; Zhang, M.; Li, F.; Zou, Y.; Yang, L.; Wu, X. Antitumor activity of hyaluronic acid-selenium nanoparticles in Heps tumor mice models. Int. J. Biol. Macromol. 2013, 57, 57–62. [Google Scholar]
- Chen, T.; Wong, Y.S.; Zheng, W.; Bai, Y.; Huang, L. Selenium nanoparticles fabricated in Undaria pinnatifida polysaccharide solutions induce mitochondria-mediated apoptosis in A375 human melanoma cells. Colloids Surf. B Biointerfaces 2008, 67, 26–31. [Google Scholar]
- Yang, F.; Tang, Q.; Zhong, X.; Bai, Y.; Chen, T.; Zhang, Y.; Li, Y.; Zheng, W. Surface decoration by Spirulina polysaccharide enhances the cellular uptake and anticancer efficacy of selenium nanoparticles. Int. J. Nanomed. 2012, 7, 835–844. [Google Scholar]
- Wu, H.; Li, X.; Liu, W.; Chen, T.; Li, Y.; Zheng, W.; Man, C.W.-Y.; Wong, M.-K.; Wong, K.-H. Surface decoration of selenium nanoparticles by mushroom polysaccharides–protein complexes to achieve enhanced cellular uptake and antiproliferative activity. J. Mater. Chem. 2012, 22, 9602–9610. [Google Scholar]
- Martin, C.; Low, W.L.; Amin, M.C.I.M.; Radecka, I.; Raj, P.; Kenward, K. Current trends in the development of wound dressings, biomaterials and devices. Pharm. Pat. Anal. 2013, 2, 341–359. [Google Scholar]
- El-Feky, G.S.; Sharaf, S.S.; El Shafei, A.; Hegazy, A.A. Using chitosan nanoparticles as drug carriers for the development of a silver sulfadiazine wound dressing. Carbohydr. Polym. 2017, 158, 11–19. [Google Scholar]
- Kamoun, E.A.; Chen, X.; Mohy Eldin, M.S.; Kenawy, E.R.S. Crosslinked poly(vinyl alcohol) hydrogels for wound dressing applications: A review of remarkably blended polymers. Arab. J. Chem. 2015, 8, 1–14. [Google Scholar]
- Keleştemur, S.; Kilic, E.; Uslu, Ü.; Cumbul, A.; Ugur, M.; Akman, S.; Culha, M. Wound healing properties of modified silver nanoparticles and their distribution in mouse organs after topical application. Nano Biomed. Eng. 2012, 4, 170–176. [Google Scholar]
- Mugade, M.; Patole, M.; Pokharkar, V. Bioengineered mannan sulphate capped silver nanoparticles for accelerated and targeted wound healing: Physicochemical and biological investigations. Biomed. Pharmacother. 2017, 91, 95–110. [Google Scholar]
- Huang, J.; Ren, J.; Chen, G.; Deng, Y.; Wang, G.; Wu, X. Evaluation of the Xanthan-Based Film Incorporated with Silver Nanoparticles for Potential Application in the Nonhealing Infectious Wound. J. Nanomater. 2017, 2017, 6802397. [Google Scholar]
- Singla, R.; Soni, S.; Patial, V.; Kulurkar, P.M.; Kumari, A.; Mahesh, S.; Padwad, Y.S.; Yadav, S.K. In vivo diabetic wound healing potential of nanobiocomposites containing bamboo cellulose nanocrystals impregnated with silver nanoparticles. Int. J. Biol. Macromol. 2017, 105, 45–55. [Google Scholar] [CrossRef]
- Haseeb, M.T.; Hussain, M.A.; Abbas, K.; Youssif, B.G.M.; Bashir, S.; Yuk, S.H.; Bukhari, S.N.A. Linseed hydrogel-mediated green synthesis of silver nanoparticles for antimicrobial and wound-dressing applications. Int. J. Nanomed. 2017, 12, 2845–2855. [Google Scholar]
- Gupta, A.; Low, W.L.; Radecka, I.; Britland, S.T.; Mohd Amin, M.C.I.; Martin, C. Characterisation and in vitro antimicrobial activity of biosynthetic silver-loaded bacterial cellulose hydrogels. J. Microencapsul. 2016, 33, 725–734. [Google Scholar]
- Muhammad, G.; Hussain, M.A.; Amin, M.; Hussain, S.Z.; Hussain, I.; Abbas Bukhari, S.N.; Naeem-ul-Hassan, M. Glucuronoxylan-mediated silver nanoparticles: Green synthesis, antimicrobial and wound healing applications. RSC Adv. 2017, 7, 42900–42908. [Google Scholar]
- Ding, L.; Shan, X.; Zhao, X.; Zha, H.; Chen, X.; Wang, J.; Cai, C.; Wang, X.; Li, G.; Hao, J.; et al. Spongy bilayer dressing composed of chitosan–Ag nanoparticles and chitosan–Bletilla striata polysaccharide for wound healing applications. Carbohydr. Polym. 2017, 157, 1538–1547. [Google Scholar]
- Mulens, V.; Morales, M.D.P.; Barber, D.F.; Barber, D.F. Development of Magnetic Nanoparticles for Cancer Gene Therapy: A Comprehensive Review. ISRN Nanomater. 2013, 2013, 1–14. [Google Scholar]
- Venkatesan, J.; Anil, S.; Kim, S.-K.; Shim, M. Seaweed Polysaccharide-Based Nanoparticles: Preparation and Applications for Drug Delivery. Polymers 2016, 8, 30. [Google Scholar]
- Maiyo, F.; Singh, M. Selenium nanoparticles: Potential in cancer gene and drug delivery. Nanomedicine 2017, 12, 1075–1089. [Google Scholar] [CrossRef]
- Manivasagan, P.; Bharathiraja, S.; Bui, N.Q.; Jang, B.; Oh, Y.O.; Lim, I.G.; Oh, J. Doxorubicin-loaded fucoidan capped gold nanoparticles for drug delivery and photoacoustic imaging. Int. J. Biol. Macromol. 2016, 91, 578–588. [Google Scholar]
- Manivasagan, P.; Bharathiraja, S.; Bui, N.Q.; Lim, I.G.; Oh, J. Paclitaxel-loaded chitosan oligosaccharide-stabilized gold nanoparticles as novel agents for drug delivery and photoacoustic imaging of cancer cells. Int. J. Pharm. 2016, 511, 367–379. [Google Scholar]
- Li, N.; Chen, Y.; Zhang, Y.-M.; Yang, Y.; Su, Y.; Chen, J.-T.; Liu, Y. Polysaccharide-Gold Nanocluster Supramolecular Conjugates as a Versatile Platform for the Targeted Delivery of Anticancer Drugs. Sci. Rep. 2015, 4, 4164. [Google Scholar]
- Aryal, S.; Grailer, J.J.; Pilla, S.; Steeber, D.A.; Gong, S. Doxorubicin conjugated gold nanoparticles as water-soluble and pH-responsive anticancer drug nanocarriers. J. Mater. Chem. 2009, 19, 7879–7884. [Google Scholar]
- Reddy, P.R.S.; Eswaramma, S.; Rao, K.S.V.K.; Lee, Y.I. Dual responsive pectin hydrogels and their silver nanocomposites: Swelling studies, controlled drug delivery and antimicrobial applications. Bull. Korean Chem. Soc. 2014, 35, 2391–2399. [Google Scholar]
- Rau, L.R.; Tsao, S.W.; Liaw, J.W.; Tsai, S.W. Selective Targeting and Restrictive Damage for Nonspecific Cells by Pulsed Laser-Activated Hyaluronan-Gold Nanoparticles. Biomacromolecules 2016, 17, 2514–2521. [Google Scholar]
- Jiang, W.; Fu, Y.; Yang, F.; Yang, Y.; Liu, T.; Zheng, W.; Zeng, L.; Chen, T. Gracilaria lemaneiformis polysaccharide as integrin-targeting surface decorator of selenium nanoparticles to achieve enhanced anticancer efficacy. ACS Appl. Mater. Interfaces 2014, 6, 13738–13748. [Google Scholar]
- Silva-Cunha, A.; Chéron, M.; Grossiord, J.L.; Puisieux, F.; Seiller, M. W/O/W multiple emulsions of insulin containing a protease inhibitor and an absorption enhancer: Biological activity after oral administration to normal and diabetic rats. Int. J. Pharm. 1998, 169, 33–44. [Google Scholar]
- Bhumkar, D.R.; Joshi, H.M.; Sastry, M.; Pokharkar, V.B. Chitosan reduced gold nanoparticles as novel carriers for transmucosal delivery of insulin. Pharm. Res. 2007, 24, 1415–1426. [Google Scholar]
- Kievit, F.M.; Veiseh, O.; Bhattarai, N.; Fang, C.; Gunn, J.W.; Lee, D.; Ellenbogen, R.G.; Olson, J.M.; Zhang, M. PEI-PEG-chitosan-copolymer-coated iron oxide nanoparticles for safe gene delivery: Synthesis, complexation, and transfection. Adv. Funct. Mater. 2009, 19, 2244–2251. [Google Scholar]
- Chen, T.; Xu, S.; Zhao, T.; Zhu, L.; Wei, D.; Li, Y.; Zhang, H.; Zhao, C. Gold nanocluster-conjugated amphiphilic block copolymer for tumor-targeted drug delivery. ACS Appl. Mater. Interfaces 2012, 4, 5766–5774. [Google Scholar]
- Safari, D.; Marradi, M.; Chiodo, F.; Th Dekker, H.A.; Shan, Y.; Adamo, R.; Oscarson, S.; Rijkers, G.T.; Lahmann, M.; Kamerling, J.P.; et al. Gold nanoparticles as carriers for a synthetic Streptococcus pneumoniae type 14 conjugate vaccine. Nanomedicine 2012, 7, 651–662. [Google Scholar]
- Rastegari, B.; Karbalaei-Heidari, H.R.; Zeinali, S.; Sheardown, H. The enzyme-sensitive release of prodigiosin grafted β-cyclodextrin and chitosan magnetic nanoparticles as an anticancer drug delivery system: Synthesis, characterization and cytotoxicity studies. Colloids Surf. B Biointerfaces 2017, 158, 589–601. [Google Scholar]
- Pooja, D.; Panyaram, S.; Kulhari, H.; Reddy, B.; Rachamalla, S.S.; Sistla, R. Natural polysaccharide functionalized gold nanoparticles as biocompatible drug delivery carrier. Int. J. Biol. Macromol. 2015, 80, 48–56. [Google Scholar]
- Cai, H.; Yao, P. In situ preparation of gold nanoparticle-loaded lysozyme–dextran nanogels and applications for cell imaging and drug delivery. Nanoscale 2013, 5, 2892–2900. [Google Scholar]
- Vu-Quang, H.; Yoo, M.K.; Jeong, H.J.; Lee, H.J.; Muthiah, M.; Rhee, J.H.; Lee, J.H.; Cho, C.S.; Jeong, Y.Y.; Park, I.K. Targeted delivery of mannan-coated superparamagnetic iron oxide nanoparticles to antigen-presenting cells for magnetic resonance-based diagnosis of metastatic lymph nodes in vivo. Acta Biomater. 2011, 7, 3935–3945. [Google Scholar]
- Yoo, M.K.; Park, I.Y.K.; Kim, I.Y.; Kwon, J.S.; Jeong, H.J.; Jeong, Y.Y.; Cho, C.S. Superparamagnetic iron oxide nanoparticles coated with mannan for macrophage targeting. J. Nanosci. Nanotechnol. 2008, 8, 5196–5202. [Google Scholar]
- Chichova, M.; Shkodrova, M.; Vasileva, P.; Kirilova, K.; Doncheva-Stoimenova, D. Influence of silver nanoparticles on the activity of rat liver mitochondrial ATPase. J. Nanopart. Res. 2014, 16, 2243. [Google Scholar]
- Mahdavinia, G.R.; Mosallanezhad, A.; Soleymani, M.; Sabzi, M. Magnetic- and pH-responsive κ-carrageenan/chitosan complexes for controlled release of methotrexate anticancer drug. Int. J. Biol. Macromol. 2017, 97, 209–217. [Google Scholar]
- Assa, F.; Jafarizadeh-Malmiri, H.; Ajamein, H.; Anarjan, N.; Vaghari, H.; Sayyar, Z.; Berenjian, A. A biotechnological perspective on the application of iron oxide nanoparticles. Nano Res. 2016, 9, 2203–2225. [Google Scholar]
- Taton, T.A. Scanometric DNA Array Detection with Nanoparticle Probes. Science 2000, 289, 1757–1760. [Google Scholar]
- Tagad, C.K.; Kim, H.U.; Aiyer, R.C.; More, P.; Kim, T.; Moh, S.H.; Kulkarni, A.; Sabharwal, S.G. A sensitive hydrogen peroxide optical sensor based on polysaccharide stabilized silver nanoparticles. RSC Adv. 2013, 3, 22940–22943. [Google Scholar]
- Tagad, C.K.; Dugasani, S.R.; Aiyer, R.; Park, S.; Kulkarni, A.; Sabharwal, S. Green synthesis of silver nanoparticles and their application for the development of optical fiber based hydrogen peroxide sensor. Sens. Actuators B Chem. 2013, 183, 144–149. [Google Scholar]
- Narayanan, K.B.; Han, S.S. Colorimetric detection of manganese (II) ions using alginate-stabilized silver nanoparticles. Res. Chem. Intermed. 2017, 43, 5665–5674. [Google Scholar]
- Bankura, K.; Rana, D.; Mollick, M.M.R.; Pattanayak, S.; Bhowmick, B.; Saha, N.R.; Roy, I.; Midya, T.; Barman, G.; Chattopadhyay, D. Dextrin-mediated synthesis of Ag NPs for colorimetric assays of Cu2+ ion and Au NPs for catalytic activity. Int. J. Biol. Macromol. 2015, 80, 309–316. [Google Scholar]
- Guan, H.; Yu, J.; Chi, D. Label-free colorimetric sensing of melamine based on chitosan-stabilized gold nanoparticles probes. Food Control 2013, 32, 35–41. [Google Scholar]
- Narasimhan, L.R.; Goodman, W.; Patel, C.K.N. Correlation of breath ammonia with blood urea nitrogen and creatinine during hemodialysis. Proc. Natl. Acad. Sci. USA 2001, 98, 4617–4621. [Google Scholar]
- Pandey, S.; Goswami, G.K.; Nanda, K.K. Green synthesis of biopolymer-silver nanoparticle nanocomposite: An optical sensor for ammonia detection. Int. J. Biol. Macromol. 2012, 51, 583–589. [Google Scholar]
- Pandey, S.; Goswami, G.K.; Nanda, K.K. Green synthesis of polysaccharide/gold nanoparticle nanocomposite: An efficient ammonia sensor. Carbohydr. Polym. 2013, 94, 229–234. [Google Scholar]
- Pandey, S.; Nanda, K.K. Au Nanocomposite Based Chemiresistive Ammonia Sensor for Health Monitoring. ACS Sens. 2016, 1, 55–62. [Google Scholar]
- Dai, Z.; Xu, L.; Duan, G.; Li, T.; Zhang, H.; Li, Y.; Wang, Y.; Wang, Y.; Cai, W. Fast-Response, Sensitivitive and Low-Powered Chemosensors by Fusing Nanostructured Porous Thin Film and IDEs-Microheater Chip. Sci. Rep. 2013, 3, 1669. [Google Scholar]
- Gattu, K.P.; Kashale, A.A.; Ghule, K.; Ingole, V.H.; Sharma, R.; Deshpande, N.G.; Ghule, A.V. NO2 sensing studies of bio-green synthesized Au-doped SnO2. J. Mater. Sci. Mater. Electron. 2017, 28, 13209–13216. [Google Scholar]
- Tagad, C.K.; Rajdeo, K.S.; Kulkarni, A.; More, P.; Aiyer, R.C.; Sabharwal, S.; Hu, J.; Cai, W.; Cai, W.; Calderer, J. Green synthesis of polysaccharide stabilized gold nanoparticles: Chemo catalytic and room temperature operable vapor sensing application. RSC Adv. 2014, 4, 24014–24019. [Google Scholar]
- Davidović, S.; Lazić, V.; Vukoje, I.; Papan, J.; Anhrenkiel, S.P.; Dimitrijević, S.; Nedeljković, J.M. Dextran coated silver nanoparticles—Chemical sensor for selective cysteine detection. Colloids Surf. B Biointerfaces 2017, 160. [Google Scholar] [CrossRef]
- Lee, K.C.; Chiang, H.L.; Chiu, W.R.; Chen, Y.C. Molecular recognition between insulin and dextran encapsulated gold nanoparticles. J. Mol. Recognit. 2016, 29, 528–535. [Google Scholar]
- Lai, C.; Zeng, G.M.; Huang, D.L.; Zhao, M.H.; Wei, Z.; Huang, C.; Xu, P.; Li, N.J.; Zhang, C.; Chen, M.; et al. Synthesis of gold-cellobiose nanocomposites for colorimetric measurement of cellobiase activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 132, 369–374. [Google Scholar]
- Shen, M.Y.; Chao, C.F.; Wu, Y.J.; Wu, Y.H.; Huang, C.P.; Li, Y.K. A design for fast and effective screening of hyaluronidase inhibitor using gold nanoparticles. Sens. Actuators B Chem. 2013, 181, 605–610. [Google Scholar]
- Li, Q.; Sun, A.; Si, Y.; Chen, M.; Wu, L. One-Pot Synthesis of Polysaccharide-Diphenylalanine Ensemble with Gold Nanoparticles and Dye for Highly Efficient Detection of Glutathione. Chem. Mater. 2017, 29, 6758–6765. [Google Scholar]
- Chen, Z.; Zhang, X.; Cao, H.; Huang, Y. Chitosan-capped silver nanoparticles as a highly selective colorimetric probe for visual detection of aromatic ortho-trihydroxy phenols. Analyst 2013, 138, 2343–2349. [Google Scholar]
- Sergeev, A.A.; Mironenko, A.Y.; Nazirov, A.E.; Leonov, A.A.; Voznesenskii, S.S. Nanocomposite Polymer Structures for Optical Sensors of Hydrogen Sulfide. Tech. Phys. 2017, 62, 1277–1280. [Google Scholar]
- Rastogi, P.K.; Ganesan, V.; Krishnamoorthi, S. Palladium nanoparticles decorated gaur gum based hybrid material for electrocatalytic hydrazine determination. Electrochim. Acta 2014, 125, 593–600. [Google Scholar]
- Luo, Y.; Shen, S.; Luo, J.; Wang, X.; Sun, R. Green synthesis of silver nanoparticles in xylan solution via Tollens reaction and their detection for Hg2+. Nanoscale 2015, 7, 690–700. [Google Scholar]
- Su, H.; Liu, Y.; Wang, D.; Wu, C.; Xia, C.; Gong, Q.; Song, B.; Ai, H. Amphiphilic starlike dextran wrapped superparamagnetic iron oxide nanoparticle clsuters as effective magnetic resonance imaging probes. Biomaterials 2013, 34, 1193–1203. [Google Scholar]
- Hemmati, B.; Javanshir, S.; Dolatkhah, Z. Hybrid magnetic Irish moss/Fe3O4 as a nano-biocatalyst for synthesis of imidazopyrimidine derivatives. RSC Adv. 2016, 6, 50431–50436. [Google Scholar]
- Lee, J.S.; Saka, S. Biodiesel production by heterogeneous catalysts and supercritical technologies. Bioresour. Technol. 2010, 101, 7191–7200. [Google Scholar]
- Grunes, J.; Zhu, J.; Somorjai, G.A. Catalysis and nanoscience. Chem. Commun. 2003, 18, 2257–2260. [Google Scholar]
- Králik, M.; Biffis, A. Catalysis by metal nanoparticles supported on functional organic polymers. J. Mol. Catal. A Chem. 2001, 177, 113–138. [Google Scholar]
- Chang, Y.C.; Chen, D.H. Catalytic reduction of 4-nitrophenol by magnetically recoverable Au nanocatalyst. J. Hazard. Mater. 2009, 165, 664–669. [Google Scholar]
- Aditya, T.; Pal, A.; Pal, T. Nitroarene reduction: A trusted model reaction to test nanoparticle catalysts. Chem. Commun. 2015, 51, 9410–9431. [Google Scholar]
- Rode, C.V.; Vaidya, M.J.; Chaudhari, R.V. Synthesis of p-Aminophenol by Catalytic Hydrogenation of Nitrobenzene. Org. Process Res. Dev. 1999, 3, 465–470. [Google Scholar]
- Zhou, J.; Gao, J.; Xu, X.; Hong, W.; Song, Y.; Xue, R.; Zhao, H.; Liu, Y.; Qiu, H. Synthesis of porous Bi@Cs networks by a one-step hydrothermal method and their superior catalytic activity for the reduction of 4-nitrophenol. J. Alloys Compd. 2017, 709, 206–212. [Google Scholar]
- Zheng, Z.; Huang, Q.; Guan, H.; Liu, S. In situ synthesis of silver nanoparticles dispersed or wrapped by a Cordyceps sinensis exopolysaccharide in water and their catalytic activity. RSC Adv. 2015, 5, 69790–69799. [Google Scholar]
- Gao, Z.; Su, R.; Huang, R.; Qi, W.; He, Z. Glucomannan-mediated facile synthesis of gold nanoparticles for catalytic reduction of 4-nitrophenol. Nanoscale Res. Lett. 2014, 9, 404. [Google Scholar]
- Maity, S.; Sen, I.K.; Islam, S.S. Green synthesis of gold nanoparticles using gum polysaccharide of Cochlospermum religiosum (katira gum) and study of catalytic activity. Phys. E Low-Dimens. Syst. Nanostruct. 2012, 45, 130–134. [Google Scholar]
- Tripathy, T.; Kolya, H.; Jana, S.; Senapati, M. Green synthesis of Ag-Au bimetallic nanocomposites using a biodegradable synthetic graft copolymer; hydroxyethyl starch-g-poly(acrylamide-co-acrylic acid) and evaluation of their catalytic activities. Eur. Polym. J. 2017, 87, 113–123. [Google Scholar]
- Yang, Y.; Buchwald, S.L. Ligand-controlled palladium-catalyzed regiodivergent suzuki-miyaura cross-coupling of allylboronates and aryl halides. J. Am. Chem. Soc. 2013, 135, 10642–10645. [Google Scholar]
- Elazab, H.A.; Siamaki, A.R.; Moussa, S.; Gupton, B.F.; El-Shall, M.S. Highly efficient and magnetically recyclable graphene-supported Pd/Fe3O4 nanoparticle catalysts for Suzuki and Heck cross-coupling reactions. Appl. Catal. A Gen. 2015, 491, 58–69. [Google Scholar]
- Chen, W.; Zhong, L.; Peng, X.; Lin, J.; Sun, R. Xylan-type hemicelluloses supported terpyridine-palladium(II) complex as an efficient and recyclable catalyst for Suzuki-Miyaura reaction. Cellulose 2014, 21, 125–137. [Google Scholar]
- Chtchigrovsky, M.; Lin, Y.; Ouchaou, K.; Chaumontet, M.; Robitzer, M.; Quignard, F.; Taran, F. Dramatic effect of the gelling cation on the catalytic performances of alginate-supported palladium nanoparticles for the Suzuki-Miyaura reaction. Chem. Mater. 2012, 24, 1505–1510. [Google Scholar]
- Arcon, I.; Paganelli, S.; Piccolo, O.; Gallo, M.; Vogel-Mikus, K.; Baldi, F. XAS analysis of iron and palladium bonded to a polysaccharide produced anaerobically by a strain of Klebsiella oxytoca. J. Synchrotron Radiat. 2015, 22, 1215–1226. [Google Scholar]
- Paganelli, S.; Piccolo, O.; Baldi, F.; Tassini, R.; Gallo, M.; La Sorella, G. Aqueous biphasic hydrogenations catalyzed by new biogenerated Pd-polysaccharide species. Appl. Catal. A Gen. 2013, 451, 144–152. [Google Scholar]
- Chook, S.W.; Chia, C.H.; Chan, C.H.; Chin, S.X.; Zakaria, S.; Sajab, M.S.; Huang, N.M. A porous aerogel nanocomposite of silver nanoparticles-functionalized cellulose nanofibrils for SERS detection and catalytic degradation of rhodamine B. RSC Adv. 2015, 5, 88915–88920. [Google Scholar]
- Lin, S.T.; Thirumavalavan, M.; Jiang, T.Y.; Lee, J.F. Synthesis of ZnO/Zn nano photocatalyst using modified polysaccharides for photodegradation of dyes. Carbohydr. Polym. 2014, 105, 1–9. [Google Scholar]
- Cheryl-Low, Y.L.; Theam, K.L.; Lee, H.V. Alginate-derived solid acid catalyst for esterification of low-cost palm fatty acid distillate. Energy Convers. Manag. 2015, 106, 932–940. [Google Scholar]
- Sherly, K.B.; Rakesh, K. Synthesis and catalytic activity of polysaccharide templated nanocrystalline sulfated zirconia. AIP Conf. Proc. 2014, 128, 128–131. [Google Scholar]
- Behar, S.; Gonzalez, P.; Agulhon, P.; Quignard, F.; Świerczyński, D. New synthesis of nanosized Cu-Mn spinels as efficient oxidation catalysts. Catal. Today 2012, 189, 35–41. [Google Scholar]
- Porta, F.; Rossi, M. Gold nanostructured materials for the selective liquid phase catalytic oxidation. J. Mol. Catal. A Chem. 2003, 204–205, 553–559. [Google Scholar]
- Wang, Y.; Kong, Q.; Ding, B.; Chen, Y.; Yan, X.; Wang, S.; Chen, F.; You, J.; Li, C. Bioinspired catecholic activation of marine chitin for immobilization of Ag nanoparticles as recyclable pollutant nanocatalysts. J. Colloid Interface Sci. 2017, 505, 220–229. [Google Scholar]
- Pourjavadi, A.; Motamedi, A.; Marvdashti, Z.; Hosseini, S.H. Magnetic nanocomposite based on functionalized salep as a green support for immobilization of palladium nanoparticles: Reusable heterogeneous catalyst for Suzuki coupling reactions. Catal. Commun. 2017, 97, 27–31. [Google Scholar]
- Li, Y.; Li, G.; Li, W.; Yang, F.; Liu, H. Greenly Synthesized Gold–Alginate Nanocomposites Catalyst for Reducing Decoloration of Azo-Dyes. Nano 2015, 10, 1550108. [Google Scholar]
- Lee, Y.J.; Cha, S.-H.; Lee, K.J.; Kim, Y.S.; Cho, S.; Park, Y. Plant Extract (Bupleurum falcatum) as a Green Factory for Biofabrication of Gold Nanoparticles. Nat. Prod. Commun. 2015, 10, 1593–1596. [Google Scholar]
- Ahmed, K.B.A.; Kalla, D.; Uppuluri, K.B.; Anbazhagan, V. Green synthesis of silver and gold nanoparticles employing levan, a biopolymer from Acetobacter xylinum NCIM 2526, as a reducing agent and capping agent. Carbohydr. Polym. 2014, 112, 539–545. [Google Scholar]
- Thirumavalavan, M.; Yang, F.M.; Lee, J.F. Investigation of preparation conditions and photocatalytic efficiency of nano ZnO using different polysaccharides. Environ. Sci. Pollut. Res. 2013, 20, 5654–5664. [Google Scholar]
- Sen, I.K.; Maity, K.; Islam, S.S. Green synthesis of gold nanoparticles using a glucan of an edible mushroom and study of catalytic activity. Carbohydr. Polym. 2013, 91, 518–528. [Google Scholar]
- Budarin, V.L.; Clark, J.H.; Luque, R.; Macquarrie, D.J.; White, R.J. Palladium nanoparticles on polysaccharide-derived mesoporous materials and their catalytic performance in C–C coupling reactions. Green Chem. 2008, 10, 382–387. [Google Scholar]
- Yah, C.S.; Iyuke, S.E.; Simate, G.S. A review of nanoparticles toxicity and their routes of exposures. Iran. J. Pharm. Sci. 2012, 8, 299–314. [Google Scholar]
- Medina, C.; Santos-Martinez, M.J.; Radomski, A.; Corrigan, O.I.; Radomski, M.W. Nanoparticles: Pharmacological and toxicological significance. Br. J. Pharmacol. 2009, 150, 552–558. [Google Scholar]
- Lam, C.-W.; James, J.T.; McCluskey, R.; Hunter, R.L. Pulmonary toxicity of single-wall carbon nanotubes in mice 7 and 90 days after intratracheal instillation. Toxicol. Sci. 2004, 77, 126–134. [Google Scholar]
- Radomski, A.; Jurasz, P.; Alonso-Escolano, D.; Drews, M.; Morandi, M.; Malinski, T.; Radomski, M.W. Nanoparticle-induced platelet aggregation and vascular thrombosis. Br. J. Pharmacol. 2005, 146, 882–893. [Google Scholar]
- Hussain, S.M.; Javorina, A.K.; Schrand, A.M.; Duhart, H.M.H.M.; Ali, S.F.; Schlager, J.J. The interaction of manganese nanoparticles with PC-12 cells induces dopamine depletion. Toxicol. Sci. 2006, 92, 456–463. [Google Scholar]
- Xie, J.-H.; Jin, M.-L.; Morris, G.A.; Zha, X.-Q.; Chen, H.-Q.; Yi, Y.; Li, J.-E.; Wang, Z.-J.; Gao, J.; Nie, S.-P.; et al. Advances on Bioactive Polysaccharides from Medicinal Plants. Crit. Rev. Food Sci. Nutr. 2016, 56, S60–S84. [Google Scholar]
- Berry, J.; Arnoux, B.; Stanislas, G.; Galle, P.; Chretien, J. A microanalytic study of particles transport across the alveoli: Role of blood platelets. Biomedicine 1977, 27, 354–357. [Google Scholar]
- Nemmar, A.; Hoet, P.H.M.; Vanquickenborne, B.; Dinsdale, D.; Thomeer, M.; Hoylaerts, M.F.; Vanbilloen, H.; Mortelmans, L.; Nemery, B. Passage of inhaled particles into the blood circulation in humans. Circulation 2002, 105, 411–414. [Google Scholar]
- Pooja, D.; Panyaram, S.; Kulhari, H.; Rachamalla, S.S.; Sistla, R. Xanthan gum stabilized gold nanoparticles: Characterization, biocompatibility, stability and cytotoxicity. Carbohydr. Polym. 2014, 110, 1–9. [Google Scholar]
- Asharani, P.V.; Sethu, S.; Vadukumpully, S.; Zhong, S.; Lim, C.T.; Hande, M.P.; Valiyaveettil, S. Investigations on the structural damage in human erythrocytes exposed to silver, gold, and platinum nanoparticles. Adv. Funct. Mater. 2010, 20, 1233–1242. [Google Scholar]
- Dhar, S.; Murawala, P.; Shiras, A.; Pokharkar, V.; Prasad, B.L.V. Gellan gum capped silver nanoparticle dispersions and hydrogels: Cytotoxicity and in vitro diffusion studies. Nanoscale 2012, 4, 563–567. [Google Scholar]
- Amorim, M.O.R.; Gomes, D.L.; Dantas, L.A.; Viana, R.L.S.; Chiquetti, S.C.; Almeida-Lima, J.; Silva Costa, L.; Rocha, H.A.O. Fucan-coated silver nanoparticles synthesized by a green method induce human renal adenocarcinoma cell death. Int. J. Biol. Macromol. 2016, 93, 57–65. [Google Scholar]
- Venkatpurwar, V.; Mali, V.; Bodhankar, S.; Pokharkar, V. In vitro cytotoxicity and in vivo sub-acute oral toxicity assessment of porphyran reduced gold nanoparticles. Toxicol. Environ. Chem. 2012, 94, 1357–1367. [Google Scholar]
- Reena, K.; Balashanmugam, P.; Gajendiran, M.; Antony, S.A. Synthesis of Leucas Aspera Extract Loaded Gold-PLA-PEG-PLA Amphiphilic Copolymer Nanoconjugates: In Vitro Cytotoxicity and Anti-Inflammatory Activity Studies. J. Nanosci. Nanotechnol. 2016, 16, 4762–4770. [Google Scholar]
- Worthington, K.L.S.; Adamcakova-Dodd, A.; Wongrakpanich, A.; Mudunkotuwa, I.A.; Mapuskar, K.A.; Joshi, V.B.; Allan Guymon, C.; Spitz, D.R.; Grassian, V.H.; Thorne, P.S.; et al. Chitosan coating of copper nanoparticles reduces in vitro toxicity and increases inflammation in the lung. Nanotechnology 2013, 24, 395101. [Google Scholar]
- Li, J.J.; Muralikrishnan, S.; Ng, C.T.; Yung, L.Y.; Bay, B.H. Nanoparticle-induced pulmonary toxicity. Exp. Biol. Med. 2010, 235, 1025–1033. [Google Scholar]
- Hwang, P.A.; Lin, X.Z.; Kuo, K.L.; Hsu, F.Y. Fabrication and cytotoxicity of fucoidan-cisplatin nanoparticles for macrophage and tumor cells. Materials 2017, 10, 291. [Google Scholar]
- Braydich-Stolle, L.K.; Breitner, E.K.; Comfort, K.K.; Schlager, J.J.; Hussain, S.M. Dynamic characteristics of silver nanoparticles in physiological fluids: Toxicological implications. Langmuir 2014, 30, 15309–15316. [Google Scholar]
- Borysov, A.; Krisanova, N.; Chunihin, O.; Ostapchenko, L.; Pozdnyakova, N.; Borisova, T. A comparative study of neurotoxic potential of synthesized polysaccharide-coated and native ferritin-based magnetic nanoparticles. Croat. Med. J. 2014, 55, 195–205. [Google Scholar]
- Iram, F.; Iqbal, M.S.; Athar, M.M.; Saeed, M.Z.; Yasmeen, A.; Ahmad, R. Glucoxylan-mediated green synthesis of gold and silver nanoparticles and their phyto-toxicity study. Carbohydr. Polym. 2014, 104, 29–33. [Google Scholar]
- Dhar, S.; Mali, V.; Bodhankar, S.; Shiras, A.; Prasad, B.L.V.; Pokharkar, V. Biocompatible gellan gum-reduced gold nanoparticles: Cellular uptake and subacute oral toxicity studies. J. Appl. Toxicol. 2011, 31, 411–420. [Google Scholar]
- Devendiran, R.M.; Chinnaiyan, S. kumar; Yadav, N.K.; Moorthy, G.K.; Ramanathan, G.; Singaravelu, S.; Sivagnanam, U.T.; Perumal, P.T. Green synthesis of folic acid-conjugated gold nanoparticles with pectin as reducing/stabilizing agent for cancer theranostics. RSC Adv. 2016, 6, 29757–29768. [Google Scholar]
- Baroli, B.; Ennas, M.G.; Loffredo, F.; Isola, M.; Pinna, R.; López-Quintela, M.A. Penetration of metallic nanoparticles in human full-thickness skin. J. Investig. Dermatol. 2007, 127, 1701–1712. [Google Scholar]





| Resource | Polysaccharides | Metals | Diameter (nm) | Shape | Antimicrobial Strains | References |
|---|---|---|---|---|---|---|
| Lactobacillus plantarum | Exopolysaccharides | Au | 10.0–20.0 | Spherical/ellipsoidal | E. coli, S. aureus, K. pneumoniae | [25] |
| Pleurotus tuber-regium | Polysaccharides-protein complexes | Se | 122.0 | - | Staphylococcus, T. rubrum | [39] |
| - | Hydroxypropylcellulose | Ag | 25.0–55.0 | Spherical | E. coli, B. subtilis, S. aureus, P. aeruginosa, S. epidermidis, A. niger, Actinomycetes | [43] |
| - | 6-O-chitosan sulfate | Au | 15.0 | Spherical | E. coli | [44] |
| Tamarind | Carboxymethyl polysaccharides | Ag | 20.0–40.0 | Spherical/polygonal | E. coli, B. subtilis, S. typhimurium | [54] |
| - | Agarose/dextran/gelatin | Fe2O3 | 10.0 | Dumbbell shape | S. aureus, A. hydrophila, S. pyogenes, P. aeruginosa | [64] |
| - | Guar gum | Ag | 16.0 | Spherical | B. subtilis | [65] |
| - | Chitosan-g-poly(acrylamide) | ZnS | 19.0–26.0 | Triangular | E. coli | [66] |
| Astragalus membranaceus root | Crude polysaccharides | Ag | 65.1 | Spherical | S. aureus, E. coli, S. epidermidis, P. aeruginosa | [69] |
| - | Pullulan | Ag | 2.0–30.0 | Spherical/ oval-shaped | E. coli, K. pneumoniae, L. monocytogenes, P. aeruginosa, Aspergillus spp., Penicillum spp. | [70] |
| - | Pectin | Ag | 5.4–10.6 | Spherical | E. coli, S. epidermidis | [73] |
| - | Chitosan | Ag/ZnO | 10.0–65.0 | Spherical/Uneven distribution | E. coil, P. aeruginosa, L. fermentum, E. faecium, S. aureus, B. licheniformis, B. subtilis, B. cereus, V. parahaemolyticus, P. vulgaris | [23,74] |
| Bacillus megaterium | Exopolysaccharides | Au | 5.0–20.0 | Spherical | E. coli, B. cereus, S. aureus, S. epidermidis, K. pneumoniae, S. typhi, P. aeruginosa, V. cholerae, S. pneumoniae | [75] |
| Seaweed Chondracanthuschamissoi, LessoniaSpicata, Ulvasp | Polysaccharides | Ag/Au | 10.0/25.0 | Spherical | P. aeruginosa, S. typhimurium | [76] |
| - | Starch | Cu(NO3)2 | 5.0–12.0 | Spherical | E. coli, S. aureus, Salmonella typhi | [77] |
| Padina tetrastromatica | Fucoidan | Ag | 17.0 | Spherical | B. subtilis, Bacillus sp. K. planticola, K. pneumoniae, S. nematodiphila, Streptococcus sp. | [78] |
| - | β-glucan | Ag | 15.0 | - | E. coli, Methylobacterium spp., Sphingomonas spp. | [79] |
| Arthrobacter sp. B4 | Exopolysaccharides | Ag | 9.0–72.0 | Face-centred-cubic | P. aeruginosa, S. aureus, C. albicans, F. oxysporum | [80] |
| Cordyceps sinensis (Berk.) | Exopolysaccharides | Ag | 50.0 | Spherical | E. coil, S. aureus | [81] |
| - | Xanthan gum/chitosan | Ag | 5.0–20.0 | Spherical | E. coil, S. aureus | [22,82,83] |
| - | Chitosan-carboxymethyl cellulose | Ag | 5.0–20.0 | Irregular shape | E. coli, S. aureus, P. aeruginosa | [84] |
| Bradyrhizobium japonicum 36 | Exopolysaccharides | Ag | 5.0–50.0 | Rod/oval-shaped structures | E. coli, S. aureus | [85] |
| Klebsiella oxytoca | Exopolysaccharides | Ag | 6.0–16.0 | Spherical | E. coli, K. rhizophila | [86] |
| Lentinus squarrosulus (Mont.) | Hetero polysaccharides | Ag | 1.3–4.5 | Spherical | E. coli | [87] |
| Pleurotus florida | Glucan | Ag | 1.3–2.5 | Spherical | K. pneumoniae | [88] |
| Lactic acid bacterium | Exopolysaccharides | Ag | 2.0–15.0 | Spherical/triangular | E. coli, K. pneumoniae, L. monocytogenes, P. aeruginosa | [89] |
| - | Dextran/sucrose | Fe | 5.8/7.3 | Spherical | E. coli, P. aeruginosa, E. faecalis, C. krusei | [90] |
| - | Mesoporous starch | Ag | 5.0–25.0 | Spherical | E. coli, S. aureus | [91] |
| Anogeissus latifolia | Gum ghatti | Ag | 5.5–5.9 | Uneven shape | E. coli, S. aureus, P. aeruginosa | [92] |
| Marine macro algae (U. faciata, P. capillacae, J. rubins, C. sinusa) | Polysaccharides | Ag | 7.0–20.0 | Spherical | E. coli, S. aureus | [93] |
| Bacillus subtilis | Exopolysaccharides | Ag | 1.1–6.7 | Spherical | S. aureus, P. aeruginosa | [94] |
| Cochlospermum gossypium | Gum kondagogu | Ag | 18.9–55.0 | Spherical | E. coli, S. aureus, P. aeruginosa | [95] |
| Porphyra vietnamensis | Sulfated polysaccharides | Ag | 10.0–16.0 | Spherical | E. coli, S. aureus | [96] |
| Portulaca | Arabinogalactan | Ag | 20.0–35.0 | Spherical | C. albicans, S. cerevisiae, A. niger, A. flavus | [97] |
| Resource | Polysaccharides | Metals | Diameter (nm) | Shape | Cancer types | References |
|---|---|---|---|---|---|---|
| Tamarindus indica | Galactoxyloglucan polysaccharides | Au | 20.0 | Spherical | Murine cancer cells (DLA, EAC) | [109] |
| Musa paradisiaca/ Ganoderma lucidum | Pectin | Au | 8.0 | Spherical | Human breast adenocarcinoma cells (MCF-7/MDA-MB-231) | [110] |
| Tamarindus indica | Polysaccharides PST001 | Au | 15.0–20.0 | Circular | Breast cancer cells (MCF7), Leukemia cells (K562) | [111] |
| - | Fucoidan-mimetic glycopolymer | Au | 20.0–55.0 | Spherical | Human colon cancer cells (HCT116) | [112] |
| Sargassum muticum | Aqueous extract | Fe3O4 | - | - | HepG2, MCF-7, HeLa, Jurkat | [116] |
| Polyporus rhinocerus | Polysaccharide–protein complexes | Se | 50.0 | Spherical | Human lung adenocarcinoma cells (A549) | [117] |
| Halomonas maura | Sulfated exopolysaccharides | Au | 70.0–107.0 | Quasi-spherical | Breast cancer cells (MCF7) Glioma cells (GI-1) | [118] |
| - | Gum arabic | Au | 0.9–2.3 | Spherical | Human breast adenocarcinoma cells (MDA-MB-231) | [119] |
| Leuconostoc spp. | Dextran | Au | 49.0 | Spherical | Ehrlich ascites carcinoma (in vivo) | [120] |
| - | Chitosan | Ag | 5.0–15.0 | Spherical | A549, HepG2, Lu, KB, MCF-7 | [23,121] |
| Chlorella vulgaris LARG-3 | Polysaccharides | Pt | 18.0–38.0 | Quasi-spherical | Ovarian cancer A2780 | [122] |
| Lentinus edodes | Lentinan | Se | 28.0 | Spherical | Human cervix carcinoma cells (HeLa) | [123] |
| - | Hyaluronic acid | Se | 66.8 | Spherical | Heps solid tumor (in vivo) | [124] |
| Undaria pinnatifida | Polysaccharides | Se | 59.0 | Spherical | Human melanoma cells (A375) | [125] |
| Spirulina | Polysaccharides | Se | 20.0–50.0 | Spherical | Human melanoma cells (A375) | [126] |
| Pleurotus tuber-regium | Polysaccharide–protein complexes | Se | 44.0–220.0 | Spherical | Human breast carcinoma (MCF-7) | [127] |
| Resource | Polysaccharides | Metals | Diameter (nm) | Shape | Targeted delivery | References |
|---|---|---|---|---|---|---|
| Sphingomonas elodea | Gellan gum | Au | 12.0–14.0 | Spherical | Doxorubicin hydrochloride delivery | [24] |
| Lactobacillus plantarum | Exopolysaccharides | Au | 20.0–30.0 | Spherical/ellipsoidal | Levofloxacin, cefotaxime, ceftriaxone, ciprofloxacin delivery | [25] |
| - | Mannan sulfate | Ag | 17.0–23.0 | Spherical | Targeting in cellular uptake (J774A.1, TE 353.Sk and HaCaT cells) | [132] |
| Fucus vesiculosus | Fucoidan | Au | 73.0–96.0 | Spherical | Doxorubicin delivery | [142] |
| - | Chitosan-oligosaccharide | Au | 58.8–64.8 | Spherical | Paclitaxel delivery | [143] |
| - | β-cyclodextrin-hyaluronic acid | Au | 2.2 | Spherical | Doxorubicin hydrochloride, paclitaxel, topotecan hydrochloride, camptothecin, irinotecan hydrochloride delivery | [144] |
| - | Poly(acrylamidoglycolic acid-co-vinylcaprolactam)-pectin | Ag | 50.0–100.0 | Spherical | 5-fluorouracil delivery | [146] |
| - | Hyaluronic acid | Au | 50.8–56.0 | - | Binding with receptor CD44 | [147] |
| Gracilaria lemaneiformis | Polysaccharides | Se | 50.0 | Near-spherical | αvβ3 integrin receptor mediated endocytosis | [148] |
| - | Chitosan | Au | 10.0–50.0 | - | Insulin delivery, bioadhesive and intestinal barrier bypass characters | [150] |
| Gynostemma pentaphyllum Makino | Folate-conjugated sulfated polysaccharides | Au | 4.0–6.0 | Spherical | Camptothecin delivery | [152] |
| Musa paradisiaca | Gal-Glc-[Gal-]GlcNAc | Au | 1.7–1.9 | Spherical | Polysaccharides of Targeting in Streptococcus pneumoniae type 14 | [153] |
| - | β-cyclodextrin/ chitosan | Fe | 8.4–16.3 | Spherical | Prodigiosin delivery | [154] |
| - | Gum karaya | Au | 20.0–25.0 | Spherical | Gemcitabine hydrochloride delivery | [155] |
| - | Dextran-lysozyme | Au | 2.5–15.8 | Spherical | Doxorubicin delivery | [156] |
| Saccharomyces cerevisiae | Mannan | Fe3O4 | 21.2–48.1 | Ellipsoidal | Targeting in antigen-presenting cells/macrophage | [157,158] |
| - | Starch | Ag | 11.5–19.3 | Spherical | Targeting in mitochondrial membrane | [159] |
| k-carrageenan | Fe3O4 | 4.0 | Spherical | Methotrexate | [160] |
| Resource | Polysaccharides | Metals | Diameter (nm) | Shape | Biosensing applications | References |
|---|---|---|---|---|---|---|
| Ceratonia siliqua | Locust bean gum | Au-SnO2/Ag | 16.0–28.0 | Spherical | Ethanol vapor sensing/hydrogen peroxide sensing | [163,164,174] |
| - | Alginate | Ag | 10.0–20.0 | Spherical | Detection of manganese (II) ions | [165] |
| - | Dextrin | Ag | 15.0–28.0 | Spherical | Detection of copper (II) ions | [166] |
| - | Chitosan | Ag/Au | 7.3–8.8 | Spherical | Detection of aromatic ortho-trihydroxy phenols/hydrogen sulfide/melamine | [167,180,181] |
| - | Guar gum | Au/Pd/Ag | 6.0–10.0 | Spherical | Sensor for the detection of ammonia level/electrocatalytic hydrazine | [169,171,182] |
| Cyamopsis tetragonaloba | Polysaccharides | Au | 6.5 | Spherical | Sensor for the detection of ammonia | [170] |
| C. arietinum L. | Water extracts | Au-SnO2 | 25.0 | Spherical | Sensor for the detection of NO2 | [173] |
| Leuconostoc mesenteroides T3 | Dextran | Ag/Au | 9.9–13.9 | Spherical | Sensor for the detection of cysteine/insulin | [175,176] |
| - | Cellobiose | Au | 10.7–33.5 | - | Measurement of cellobiase activity | [177] |
| - | Hyaluronic acid | Au | 14.0–19.0 | Spherical | Hyaluronidase inhibitor screening | [178] |
| Bagasse | Xylan | Ag | 20.0–35.0 | Spherical | Detection of Hg2+ | [183] |
| - | β-cyclodextrin-dextran-g-stearic acid | Fe3O4 | 59.0–149.0 | Micelles | Magnetic resonance imaging for monitoring cancer cells | [184] |
| Resource | Polysaccharides | Metals | Diameter (nm) | Shape | Reaction types | Reference |
|---|---|---|---|---|---|---|
| - | Xanthan | Ag | 5.0–40.0 | Spherical | 4-NP reduction | [83] |
| Portulaca | Arabinogalactan | Ag | 20.0–30.0 | Spherical | 4-NP reduction | [97] |
| - | Dextrin | Ag/Au | 8.0–28.0 | Spherical | 4-NP reduction | [166] |
| Ceratonia siliqua | Locust bean gum | Au | - | Spherical | 4-NP reduction | [174] |
| Chondrus crispus | Irish moss | Fe3O4 | - | Homogeneous | Imidazopyrimidine derivatives synthesis | [185] |
| - | Alginate | Bi | 5.0–8.0 | Porous | 4-NP reduction | [192] |
| Cordyceps sinensis | Exopolysaccharides | Ag | 5.0 | Spherical | 4-NP reduction | [193] |
| - | Glucomannan | Au | 12.0–31.0 | Spherical | 4-NP reduction | [194] |
| Cochlospermum religiosum | Katira gum | Au | 6.9 | Spherical | 4-NP reduction | [195] |
| - | Starch-g-poly | Ag-Au | 11.1 | - | 4-NP reduction | [196] |
| - | Xylan-type hemicellulose | Terpyridine-Pd | 10.0–20.0 | Particle | Suzuki–Miyaura reaction | [199] |
| - | Alginate | Pd-Cu | >10 | Fibrils network | Suzuki–Miyaura reaction | [200] |
| Klebsiella oxytoca BAS-10 | Exopolysaccharides | Fe/Fe-Pd | 1.0–1.5 | Cluster | Hydrodechlorination reaction | [201] |
| Klebsiella oxytoca BAS-10 | Exopolysaccharides | Pd | 30.0–550.0 | Jagged undefined structures | Aqueous biphasic hydrogenation | [202] |
| - | Cellulose nanofibrils | Ag | 25.2–18.0 | Porous | Rhodamine B degradation | [203] |
| Corn | Crosslinked carboxymethyl starch/cellulose | ZnO/Zn | 20.0–100.0 | Spherical | Photodegradation of dyes | [204] |
| Algae | Algin | Al | 4.0–5.0 | Rough with wrinkled surface | Esterification reaction | [205] |
| - | Chitosan | ZrO | 9.0 | - | Benzylation of o-xylene | [206] |
| - | Sodium alginate | Cu-Mn | 10.0–20.0 | Spherical | Toluene oxidation | [207] |
| - | Dextrin | Au | 8.4–12.0 | - | Liquid phase oxidation of ethylene glycol | [208] |
| - | Chitin | Ag | 5.5–15.2 | Mesoporous, fibrous | p-NP reduction | [209] |
| - | Salep | Pd (II) | - | Rough | Suzuki coupling reaction | [210] |
| - | Alginate | Au | 20.0–40.0 | Centered cubic crystal lattice | Decoloration of Azo-Dyes | [211] |
| Bupleurum falcatum | Water extract | Au | 8.2–12.8 | Spherical | 4-NP reduction | [212] |
| Acetobacter xylinum NCIM2526 | Levan | Ag/Au | 5.0–12.0 | Spherical | 4-NP reduction | [213] |
| - | Chitosan/ corn starch/ sodium alginate | ZnO | 8.3–11.3 | Hexagonal phase with Wurtzite structure | Photocatalytic reaction | [214] |
| Pleurotus florida | Glucan | Au | 19.0–27.2 | Spherical | 4-NP reduction | [215] |
| - | Starch | Pd | 1.5–4.5 | Spherical | Heck reaction, Suzuki reaction, Sonogashira reaction | [216] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Gao, X.; Chen, Z.; Chen, Y.; Chen, H. Preparation, Characterization and Application of Polysaccharide-Based Metallic Nanoparticles: A Review. Polymers 2017, 9, 689. https://doi.org/10.3390/polym9120689
Wang C, Gao X, Chen Z, Chen Y, Chen H. Preparation, Characterization and Application of Polysaccharide-Based Metallic Nanoparticles: A Review. Polymers. 2017; 9(12):689. https://doi.org/10.3390/polym9120689
Chicago/Turabian StyleWang, Cong, Xudong Gao, Zhongqin Chen, Yue Chen, and Haixia Chen. 2017. "Preparation, Characterization and Application of Polysaccharide-Based Metallic Nanoparticles: A Review" Polymers 9, no. 12: 689. https://doi.org/10.3390/polym9120689
APA StyleWang, C., Gao, X., Chen, Z., Chen, Y., & Chen, H. (2017). Preparation, Characterization and Application of Polysaccharide-Based Metallic Nanoparticles: A Review. Polymers, 9(12), 689. https://doi.org/10.3390/polym9120689